Cognitive disorders have become a common disease in the elderly and are a common cause of disability and death in this age group. The understanding of neurocognitive disorders is still limited, and there are some problems, such as unclear specific mechanisms and the lack of early diagnosis and interventions. Therefore, developing methods for the early identification of and intervention in patients during the MCI period to avoid the development of dementia is crucial. Previous studies have suggested that T2DM is not only a risk factor for MCI, but also promotes the transformation of MCI into dementia [
31]. It is widely believed that T2DM and MCI share several common abnormal molecular and cellular characteristics, including impaired glucose metabolism, insulin resistance, and increased oxidative stress, which are manifested by persistent hyperinsulinemia and hyperglycemia [
32,
33]. Cerebral insulin resistance seems to be an early and common feature in patients with dementia. Abnormal glucose metabolism may play a leading role in the progression of dementia, but its specific mechanism remains unclear. Exploring the common pathogenesis of MCI and T2DM is conducive to the early identification of cognitive impairment to achieve the purpose of diagnosing and treating the disease.
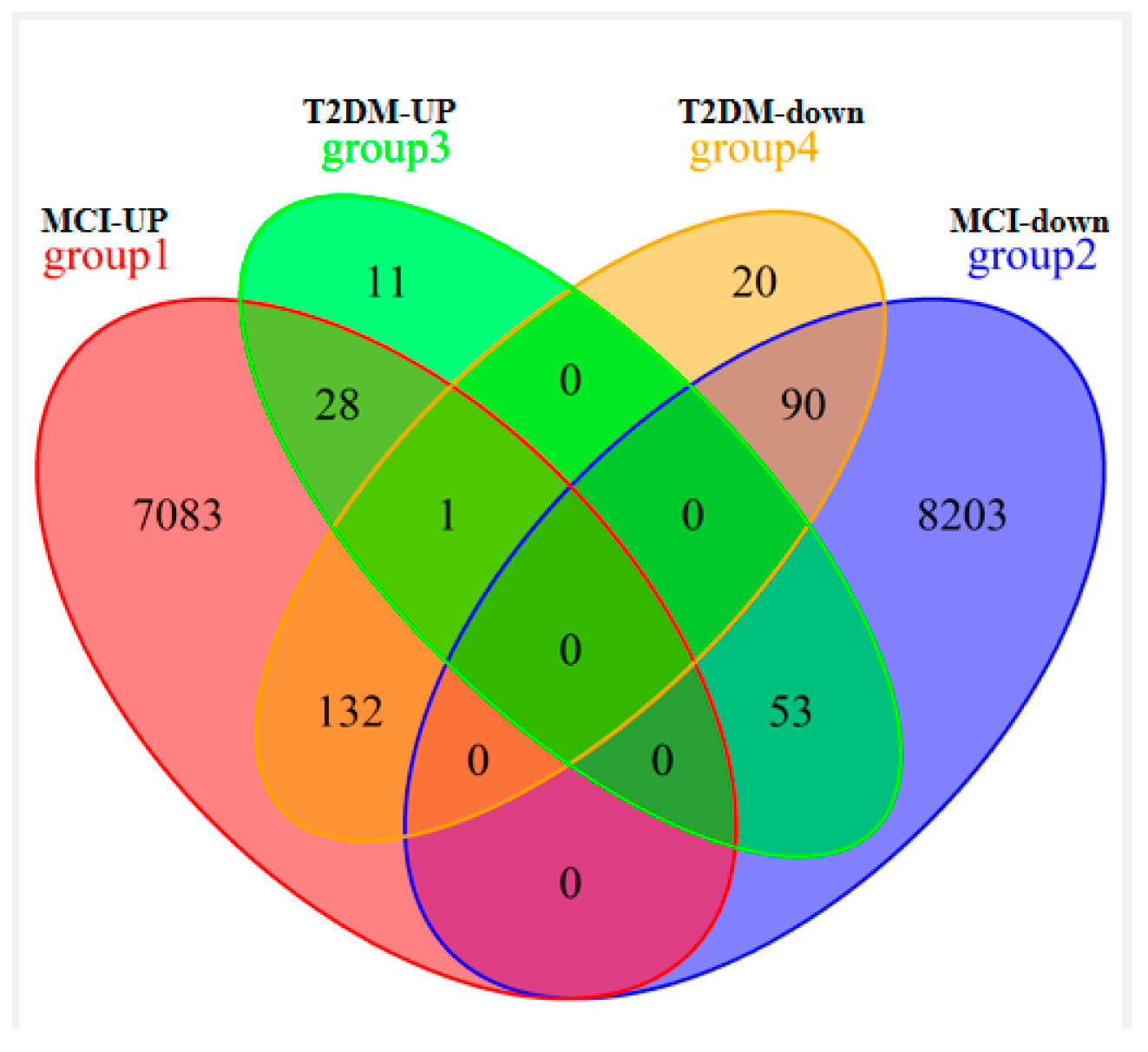
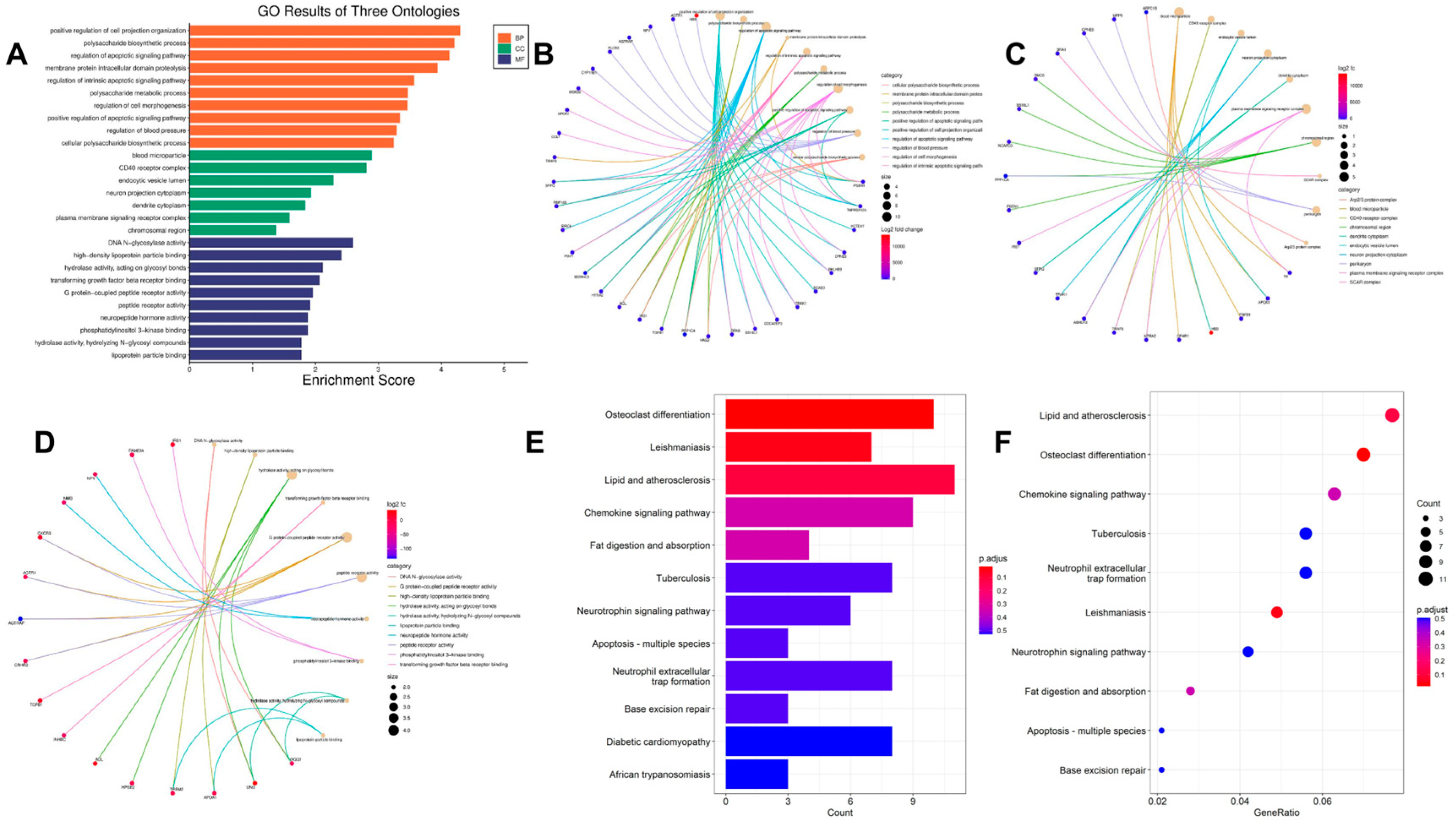
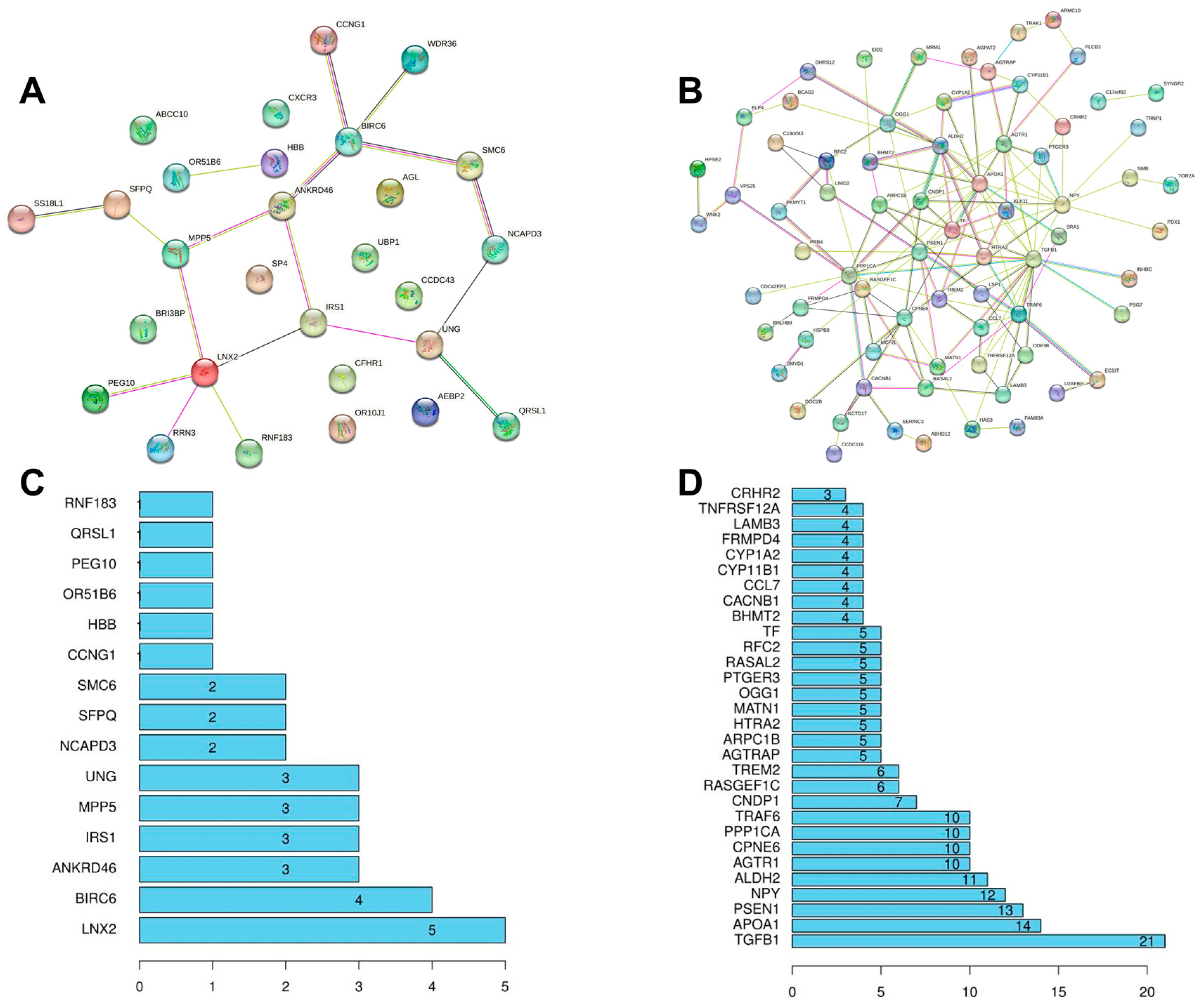
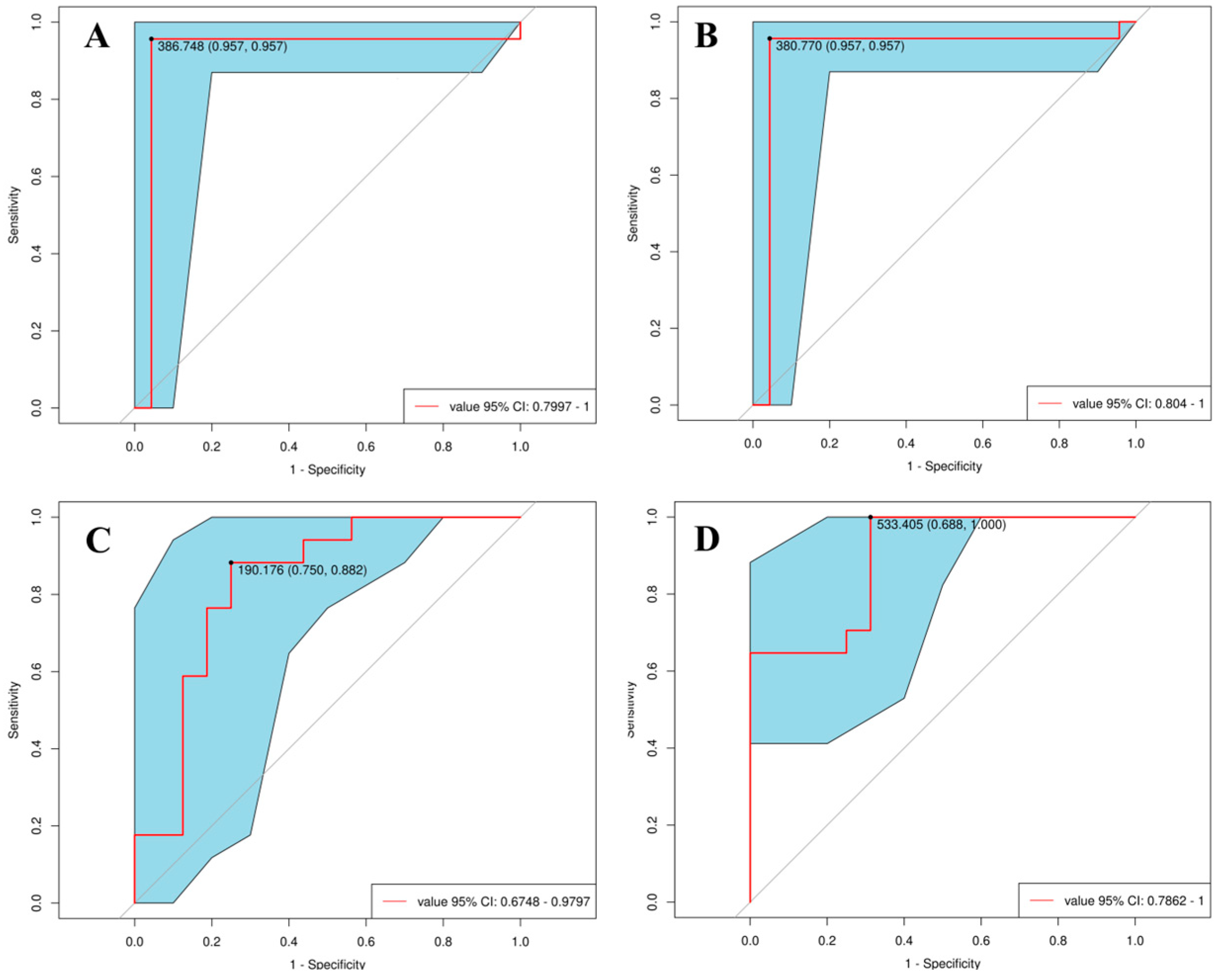
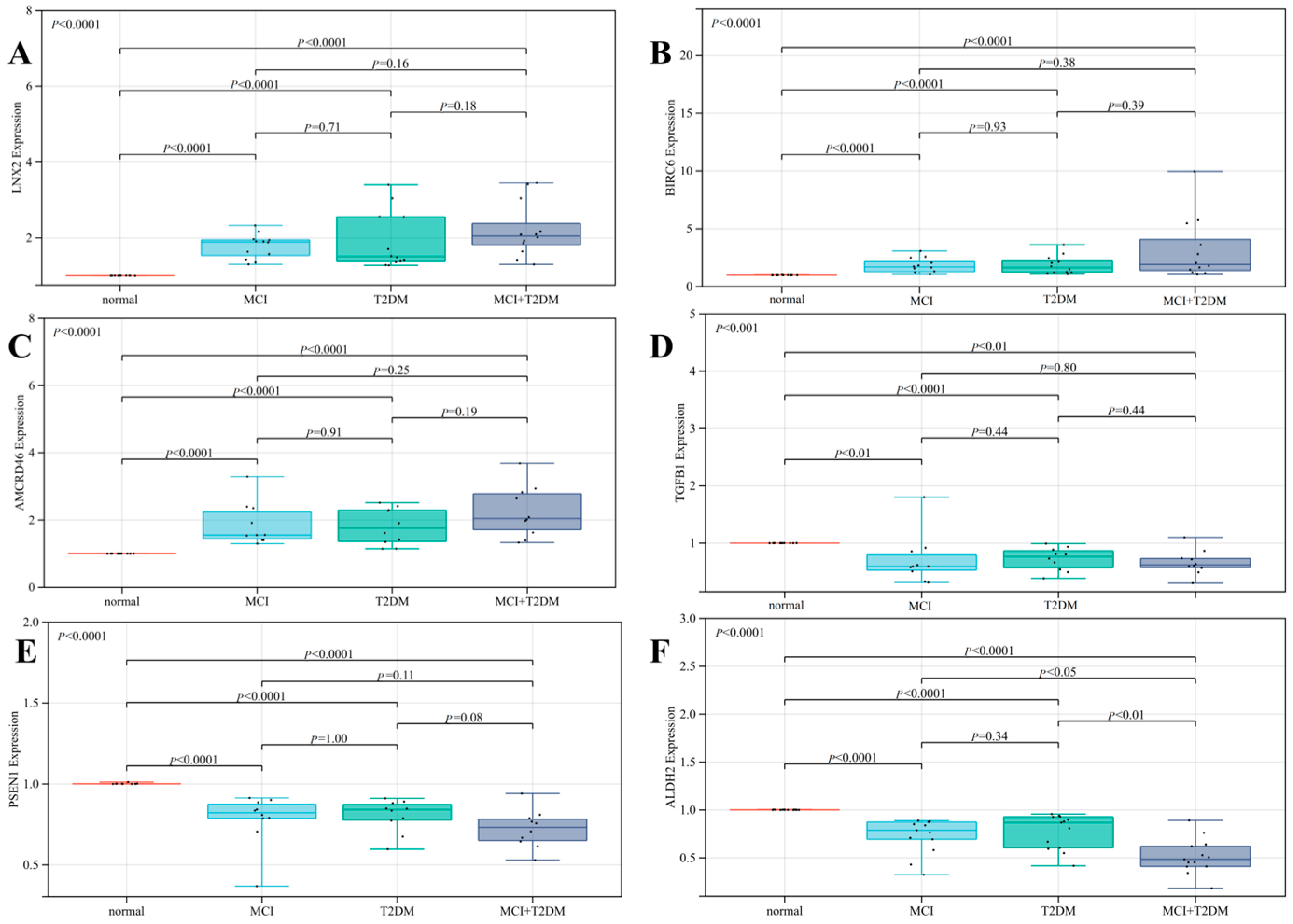
For the first time, we used the blood co-expressed genes of T2DM and MCI as the entry point, performed bioinformatics analysis based on GEO public clinical database, and identified 303 co-expressed genes, 28 up-regulated co-expressed genes, and 90 down-regulated co-expressed genes. We then constructed a KEGG pathway enrichment analysis and PPI network to identify the hub genes in co-DEGs. KEGG pathway analysis of co-DEGs is mainly concentrated in several metabolic diseases and signaling pathways. However, surprisingly, the pathway enrichment analysis, which was mainly concentrated on neurodegenerative diseases, was not statistically significant (p-value > 0.05). Our analysis is mainly due to the low number of co-DEGs, but this does not mean that these co-expressed genes are not associated with neurodegenerative diseases. From the above analysis, we identified the hub genes of the nine co-DEGs, namely LNX 2, BIRC 6, ANKRD46, IRS 1, TGFB1, APOA 1, PSEN 1, NPY, and ALDH 2. The ROC curve analysis found that these nine hub genes had excellent diagnostic values for both MCI and T2DM (0.9 > AUC > 0.7). Based on the results of bioinformatics analysis, on the one hand, we designed a clinical verification to explore the risk factors and correlation analysis of cognitive impairment. The current investigation results showed that MCI was correlated with T2DM, a risk factor for cognitive impairment. The risk of MCI in patients with a history of T2DM was 1.839 times higher than that in patients without diabetes. On the other hand, we designed a qRT-PCR experiment to detect the relative expression of nine hub genes in the blood tissues of the normal group, the MCI group, the T2DM group, and the MCI + T2DM group, respectively. The experimental results showed that the expression trends of LNX2, BIRC6, ANKRD46, PSEN1, and ALDH2 were consistent with our bioinformatics results, while there was no significant difference in the expression of NPY, APOA1, and IRS1, which was considered as being due to its low expression in the blood. Combined with the above findings, we found that these five hub genes play an important role in the progression and diagnosis of MCI and T2DM, but their specific mechanisms need to be further studied. In addition, we found that LNX2 and ALDH2 had better results. We guessed that they may act on the same pathway and may have antagonistic effects, so the expression is highly correlated.
4.1. Potential Diagnostic Markers
Currently, some dementia-related genes (such as APOE, MPKA, and APP/PS1) have been found to have good clinical diagnostic value. However, most patients undergo genetic testing at an advanced stage of the disease, and it does not prevent the disease from developing. We obtained co-expressed genes of MCI and T2DM through bioinformatics analysis, and we can detect co-expressed genes for early diagnosis of the disease. Combined with bioinformatics analysis and our qRT-PCR validation results, we considered that LNX2, BIRC6, ANKRD46, TGFB1, PSEN1, and ALDH2 could be good disease markers.
LNX2 is mainly expressed in the hepatocytes, as well as erythroid cells, B cells, and T cells in the blood. The LNX2 gene is a member of the LNX (numb protein-X ligand) family and mainly encodes LNX2 proteins. LNX proteins usually contain an amino-terminal RING domain adjacent to two or four PDZ domains, which is unique to the LNX family [
34]. The LNX2 gene is expressed in all tissues throughout the body and is strongly expressed in the forebrain and many other regions of the developing embryo. Contact protein-associated protein-4 (CASPR4) is a transmembrane protein that is a member of the neuropathic superfamily. It is expressed in the developing cortex and neural progenitor cells in the SVZ. Some studies have shown that LNX2 binds to the cytoplasmic domain of CASPR4, and both proteins have been found to inhibit proliferation and promote differentiation in cultured neural progenitor cells [
35]. Currently, LNX2 has not been used in studies related to cognitive impairment and T2DM.
The BIRC6 gene is widely expressed in oligodendrocytes, inhibitory neurons, and excitatory neurons, and also in erythroid cells (dendritic cells, B cells, T cells, and erythroid cells). BIRC6 encodes proteins with the BIR (baculovirus inhibiting apoptosis protein repeat) 999 domain and the UBCc (ubiquitin coupling enzyme E2) domain. This protein inhibits apoptosis by promoting ubiquitination to degrade apoptotic proteins. At present, most people are familiar with the BIRC6 gene in tumors, such as non-small cell lung cancer, bladder cancer, colorectal cancer, and others [
36,
37,
38]. Ubiquitin coupling enzyme (BRUCE) containing BIR repeats of the apoptotic protein family (IAP) has been reported to inhibit the regulation of autophagosome and lysosome fusion, and also interacts with syntaxin 17 (STX17), an important mediator of autophagosome–lysosome fusion. BRUCE may affect the development of dystrophic axons in Alzheimer’s disease by regulating the fusion of autophagosomes and lysosomes, thus affecting cognition [
39]. In addition, it has been reported that BIRC6 may be a potential biomarker of T2DM, but its mechanism of action still needs further study [
40].
ANKRD46 was mainly enriched in hepatocytes and thyroid gland cells and encodes proteins containing multiple ankyrin repeats. The ankyrin domain plays a role in protein–protein interactions in various cellular processes. Alternative splicing results in multiple transcript variants. Through the literature search, we found that there were few studies on the ANKRD46 gene, which mainly plays a pathogenic role in tumors, such as nasopharyngeal carcinoma, breast cancer, and gastric cancer [
41].
TGFB1 (TGF-β1) is widely expressed in extravillous trophoblasts, NK cells, dendritic cells, and T cells in humans. The TGFB1 gene encodes a secreted ligand of the transforming growth factor-β (TGF-β) protein superfamily. Ligands of this family bind to various TGF-β receptors, leading to the recruitment and activation of SMAD family transcription factors that regulate gene expression. The TGFB1 gene encodes proteins that regulate cell proliferation, differentiation, and growth, as well as the expression and activation of other growth factors (such as interferon-gamma and tumor necrosis factor α) [
42]. T2DM leads to increased TGFβ signaling, which interferes with VEGFA-induced monocyte migration and leads to monocyte dysfunction. TGFβ signaling can affect monocyte function, causing vascular complications [
43] in T2DM patients. Studies have found that serum TGF-β1 levels in patients with Alzheimer’s disease (AD), vascular dementia (VaD), and Parkinson’s disease dementia (PDD) are significantly increased, but serum TGF-β1 levels in AD and VaD are significantly higher than those in PDD [
44]. TGF-β1 may be a biomarker for assessing the degree of cognitive impairment [
44].
The PSEN 1 gene (PS1) is mainly expressed in oligodendrocytes and encodes for presenilin 1. Most patients with familial hereditary AD carry mutations in the PSEN gene. These disease-associated mutations can lead to increased production of Aβ. Presenolin can regulate APP processing through γ-secretase. Current studies have shown that PSEN1 is mainly associated with Alzheimer’s disease, amyloidosis, cancer-related genes, cardiomyopathy, disease variation, and cognitive disorders [
45,
46]. PSEN1 is a well-known gene associated with MCI and AD [
47]. In addition, studies have found that PSEN1 is also associated with T2DM, and this gene promotes Aβ deposition, which is a factor in the development of diabetes to AD [
48].
The ALDH2 gene is mainly expressed in hepatocytes, and the proximal renal tubular cells, and it is also expressed in blood cells. The ALDH2 gene encodes for the aldehyde dehydrogenase 2 family member, a protein that belongs to the proteins of the aldehyde dehydrogenase family [
49]. Aldehyde dehydrogenase is the second enzyme in the main oxidative pathway of alcohol metabolism. Increased exposure to acetaldehyde in individuals with catalytically active forms may also lead to greater susceptibility to many types of cancer [
50]. Some research teams have reported that patients with T2DM and mutations in the formaldehyde (FA)-degrading enzyme aldehyde dehydrogenase 2 (ALDH2) gene have higher levels of FA and more severe dementia [
51]. Overexpression of ALDH2 reduces FA and reduces hyperglycemia and cognitive deficits in a diabetic mouse model [
52].
In conclusion, combined with the results of the literature search and bioinformatic analysis, we believe that the three genes, BIRC6, TGFB1, and PSEN1, have a clear correlation with T2DM and cognitive impairment, which can be used as target genes for disease prediction in the future. At the same time, more experiments can be designed around these genes to verify the pathogenic mechanism, in order to achieve the purpose of early disease prediction.
4.2. Potential Treatment Options
Although the treatment of dementia has been studied for many years, only two classes of drugs are currently approved for the treatment of AD, namely cholinesterase inhibitors and N-methyl-D-aspartic acid antagonists (NMDA). Although both drugs are clinically effective, they can only improve symptoms, not cure or prevent the disease [
53,
54]. At present, the treatment of MCI mainly focuses on non-pharmaceutical therapy, such as cognitive function training and physical therapy, and there is a lack of specific drug therapies [
55]. Therefore, exploring drugs that enable the early treatment of MCI is particularly important for human social health.
As
Table 3 shows, the lower the CMap connectivity score, the more likely it is to be a potential therapeutic agent. Mycophenol ester is an antimetabolite and potent immunosuppressant used as an adjunct therapy for the prevention of allograft rejection and the treatment of severe autoimmune diseases. It was found that mycophenolate mofetil could be used to treat T2DM and diabetic ketoacidosis and improve insulin resistance [
56]. In addition, some researchers have designed animal experiments to show that mantemycophenol ester has therapeutic effects in diabetic rat models, and has a certain protective effect on experimental diabetic nephropathy. At present, few researchers use mycophenolate mofetil to treat MCI, but it can be used to treat neuropsychiatric symptoms caused by systemic erythema [
57]. Nilotinib, a serine/threonine kinase mammalian sterile 20-like kinase 1 (MST1) inhibitor, is widely used for adjunctive treatment of early breast cancer. MST1 can directly induce β-cell death and impair insulin secretion. The importance of MST1 as a therapeutic target for diabetes has been demonstrated at the β-cell level and in diabetic complications [
58]. Neratinib is a potential β cytoprotective drug, and both in vivo and in vitro experiments have demonstrated that neratinib can treat T1DM and T2DM [
59]. In addition, neratinib can also activate the ataxic telangiectasia mutation (ATM) through reactive oxygen species, which can phosphorylate and activate the AMP-dependent protein kinase (AMPK). By reducing mTOR activity and directly activating ULK1, AMPK also leads to the formation of autophagosomes and the degradation of Tau and APP, thus achieving the purpose of treating dementia. However, no studies have observed the therapeutic effects of Merck60 and benzanthrone in diseases, and more studies may be needed in the future.
At the same time, we have obtained co-expressed important pathogenic genes and identified targeted Chinese medicines according to the target genes. In this paper, hub genes were used to screen traditional Chinese medicines with potential therapeutic effects, such as Grapsidae, Nymphaea tetragona Georgi, Asini Corii Colla, Angelicae Sinensis Radix, Rosa roxburghii, Arnebia euchr0ma (Royle) Johnst, Flos leonuri, Periostracum Serpentis, Semen Euryales, Coptis chinensis Franch, and Folium Mori. Compared with the different concepts of Western medicine, traditional Chinese medicine believes that the etiology and pathology of dementia are complex and diverse, which can be divided into a deficiency of qi and blood, a functional decline of the five viscera, six organs, blood stasis, phlegm turbidity, and blockage [
60,
61]. Some studies have found that bupleurum can significantly inhibit Aβ-induced cell death, increase cell membrane potential and reduce mitochondria-dependent apoptosis induced by Aβ by up-regulating the ratio of Bcl-2/Bax, thus achieving a therapeutic effect on AD [
62]. In addition, some research teams have found that Bupleurum Shugan pills can have a therapeutic effect on diabetic patients with depression by effectively reducing the HbA1c level and depressive symptom score, improving clinical efficacy, and regulating serum 5-HT and NE levels, and are safe and reliable. Bupleurum Shugan pills are a traditional Chinese medicine prescription, and their main components are bupleurum, green peel, tangerine peel, parsnip, Fructus aurantii, Fructus aurantii, Radix xylosa, and Radix Aconitum. Based on UHPLC-Q-Exactive-ObitriapMS, researchers quickly analyzed the chemical components of Bupleurum Shugan pills [
63]. A total of 121 compounds were detected, including flavonoids, terpenoids, phenylpropanes, anthraquinones, and alkaloids. Modern pharmacological studies have shown that flavonoids have the effects of lowering blood sugar, regulating mood, and anti-depression. In addition, it has been proved that puerarin can inhibit tau phosphorylation in the olfactory bulb of AD rats, and its mechanism may be due to its decreased activity level of GSK-3β and its effect on glucose metabolism in diabetic model rats [
63]. Other traditional Chinese medicines, such as Asini Corii Colla, angelica, coptis, and so on, have a good effect on the treatment of cognition-related diseases and diabetes [
64,
65,
66]. These results all indicate that the prediction results of traditional Chinese medicine in this study are consistent with clinical practice. However, the specific mechanisms are still unclear and need to be further explored. However, this is a meaningful research direction.
We expect that exploring the co-expressed genes of MCI and T2DM can clearly determine the co-pathogenesis and further serve as new therapeutic targets for the diagnosis and treatment of diseases. However, some limitations still exist in this study. First, when we selected the target dataset in the GEO database, we could only select from a limited number of datasets, as some dataset sample sizes were too small, while some datasets had no obvious differentially expressed genes (adj p-value < 0.05, and |logFC| ≥ 1.0), such as GSE48350, GSE131617, GSE15993, and GSE161335. We performed a multi-chip co-analysis of the same disease and standardized the dataset to eliminate differences across platforms and individuals. Through the verification of the dataset, we found that our conclusion is still reliable. In addition, the GEO database is still being updated, as are disease-causing genes, and there are still more genes to be discovered. During clinical verification, due to the impact of the COVID-19 pandemic, our team only analyzed the current situation regarding cognitive impairment in Zhuhai City, lacking multicenter verification. Meanwhile, since January 2022, Zhuhai has been affected by the COVID-19 pandemic, hindering the progress of the project, and the sample size is limited. In addition, one of the randomly selected communities was a nursing home, where most of the elderly people have a cognitive impairment, which makes the data biased to a certain extent. In the future, large-sample, multicenter experiments are needed to study the correlation between MCI and T2DM. In the experimental part of qPCR, we expected to include more clinical samples to verify our results, but due to the strict inclusion and exclusion criteria we set, the number of cases meeting the criteria was limited, but we tried to include as many clinical samples as possible to verify our conclusions. In addition, we obtained only a preliminary conclusion, and further verification of cell and animal models needs to be performed. In the future, we will conduct further research around our conclusions.