Abscopal Effect on Bone Metastases from Solid Tumors: A Systematic Review and Retrospective Analysis of Challenge within a Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Studies Selection
2.2. Data Extraction and Methodological Quality Assessment of the Included Studies
2.3. Retrospective Analysis of Our Patient Population
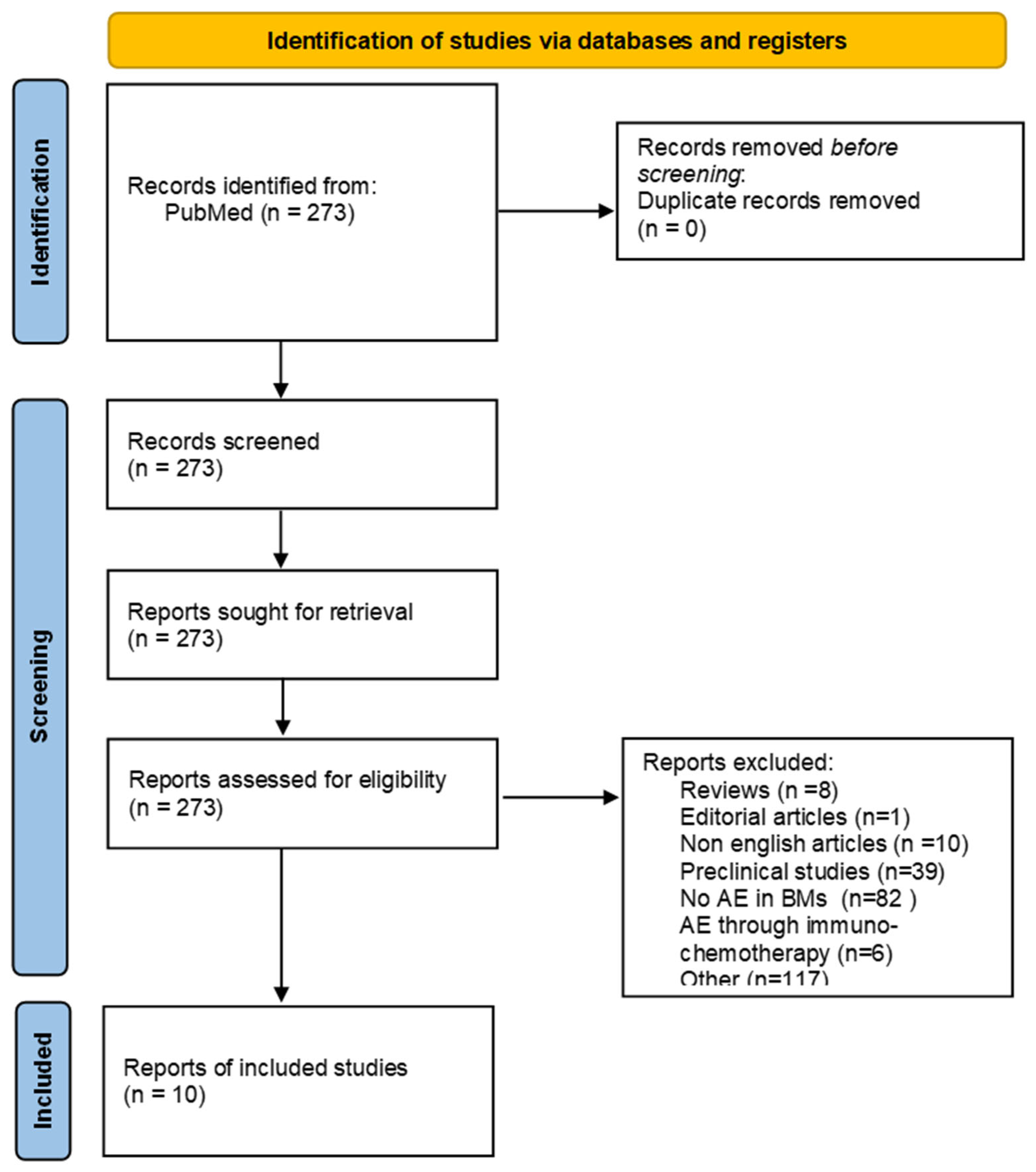
3. Results
3.1. Systematic Review Analysis
3.2. Methodological Quality of the Retrieved Studies
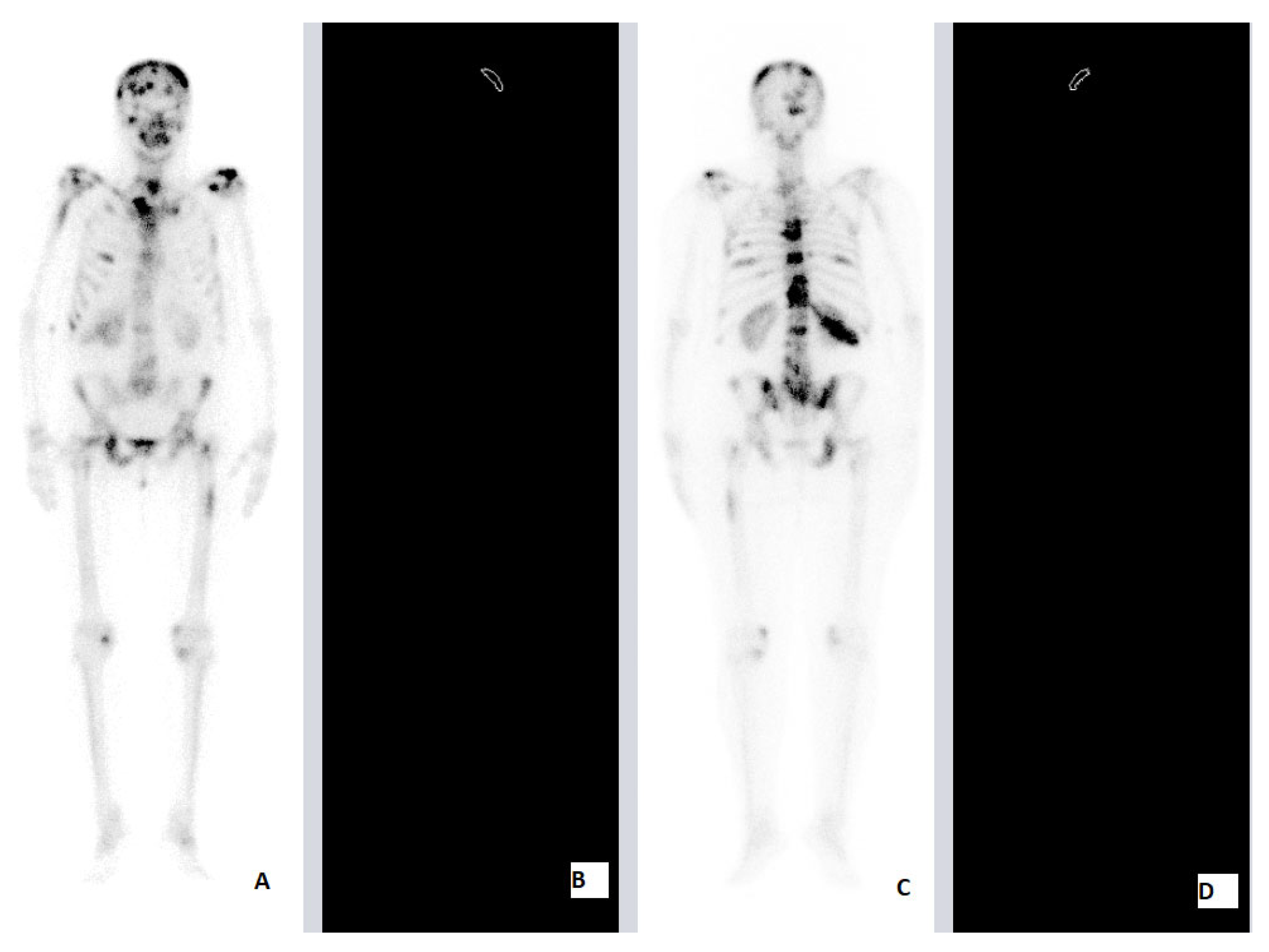
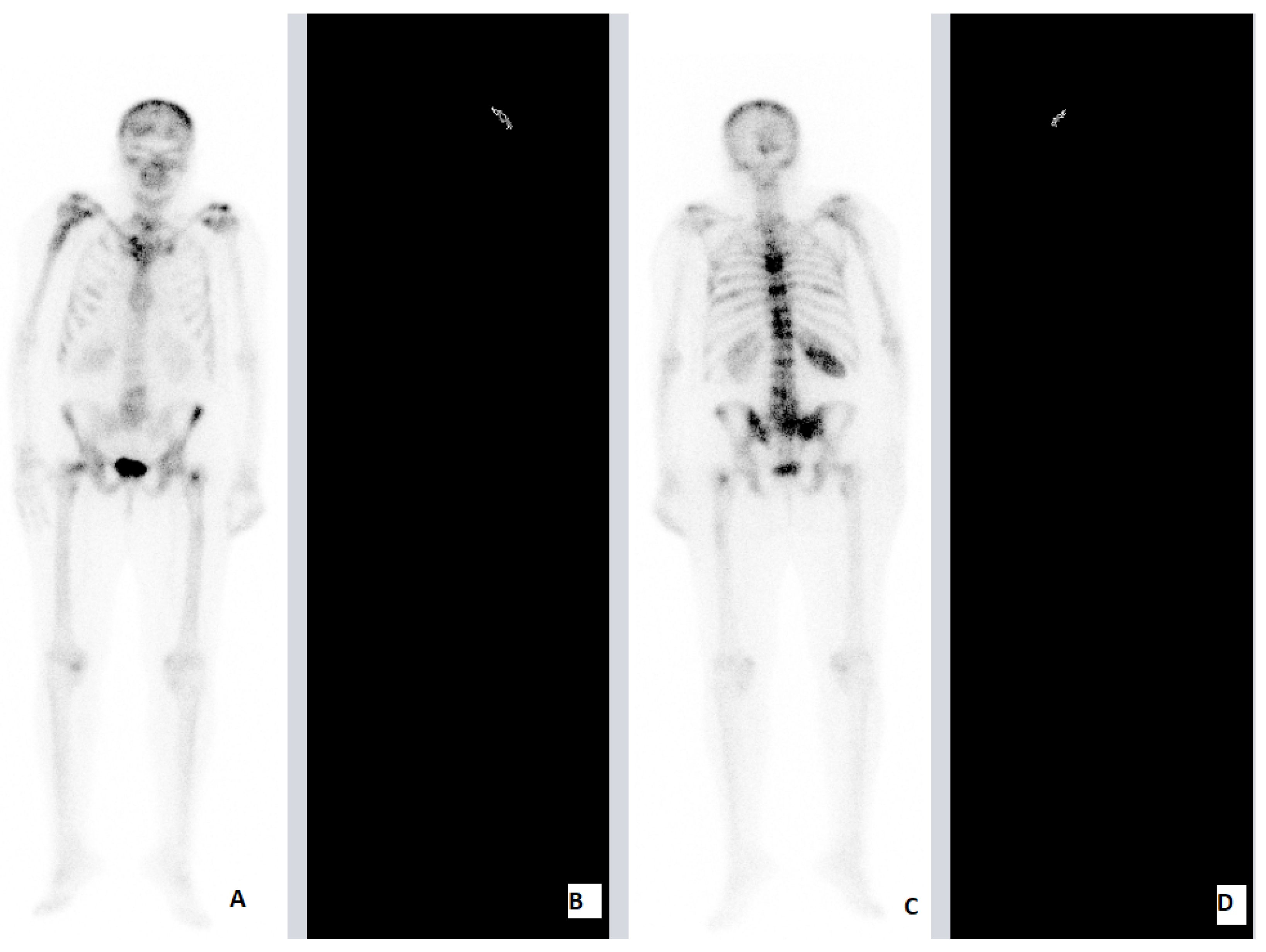
Our Retrospective Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.-W. Biological Response of Cancer Cells to Radiation Treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef]
- Demaria, S.; Formenti, S.C. The Abscopal Effect 67 Years Later: From a Side Story to Center Stage. Br. J. Radiol. 2020, 93, 20200042. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing Radiation Inhibition of Distant Untreated Tumors (Abscopal Effect) Is Immune Mediated. Int. J. Radiat. Oncol. *Biol. *Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef]
- Demaria, S.; Guha, C.; Schoenfeld, J.; Morris, Z.; Monjazeb, A.; Sikora, A.; Crittenden, M.; Shiao, S.; Khleif, S.; Gupta, S.; et al. Radiation Dose and Fraction in Immunotherapy: One-Size Regimen Does Not Fit All Settings, so How Does One Choose? J. Immunother. Cancer 2021, 9, e002038. [Google Scholar] [CrossRef] [PubMed]
- Abuodeh, Y.; Venkat, P.; Kim, S. Systematic Review of Case Reports on the Abscopal Effect. Curr. Probl. Cancer 2016, 40, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Dagoglu, N.; Karaman, S.; Caglar, H.B.; Oral, E.N. Abscopal Effect of Radiotherapy in the Immunotherapy Era: Systematic Review of Reported Cases. Cureus 2019, 11, e4103. [Google Scholar] [CrossRef] [PubMed]
- Bang, A.; Schoenfeld, J.D. Immunotherapy and Radiotherapy for Metastatic Cancers. Ann. Palliat. Med. 2019, 8, 312–325. [Google Scholar] [CrossRef]
- Xing, D.; Siva, S.; Hanna, G.G. The Abscopal Effect of Stereotactic Radiotherapy and Immunotherapy: Fool’s Gold or El Dorado? Clin. Oncol. 2019, 31, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Azami, A.; Suzuki, N.; Azami, Y.; Seto, I.; Sato, A.; Takano, Y.; Abe, T.; Teranishi, Y.; Tachibana, K.; Ohtake, T. Abscopal Effect following Radiation Monotherapy in Breast Cancer: A Case Report. Mol. Clin. Oncol. 2018, 9, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, C.; Wu, S.; Liu, Z.; Liu, L.; Li, L.; Li, S. Abscopal Effect Induced by Modulated Radiation Therapy and Pembrolizumab in a Patient with Pancreatic Metastatic Lung Squamous Cell Carcinoma. Thorac. Cancer 2020, 11, 2014–2017. [Google Scholar] [CrossRef]
- Yano, M.; Aso, S.; Sato, M.; Aoyagi, Y.; Matsumoto, h.; Nasu, K. Pembrolizumab and Radiotherapy for Platinum-Refractory Recurrent Uterine Carcinosarcoma with an Abscopal Effect: A Case Report. Anticancer Res. 2020, 40, 4131–4135. [Google Scholar] [CrossRef]
- Watanabe, T.; Firat, E.; Scholber, J.; Gaedicke, S.; Heinrich, C.; Luo, R.; Ehrat, N.; Multhoff, G.; Schmitt-Graeff, A.; Grosu, A.-L.; et al. Deep Abscopal Response to Radiotherapy and Anti-PD-1 in an Oligometastatic Melanoma Patient with Unfavorable Pretreatment Immune Signature. Cancer Immunol. Immunother. 2020, 69, 1823–1832. [Google Scholar] [CrossRef]
- Igarashi, H.; Fukuda, M.; Konno, Y.; Takano, H. Abscopal Effect of Radiation Therapy after Nivolumab Monotherapy in a Patient with Oral Mucosal Melanoma: A Case Report. Oral Oncol. 2020, 108, 104919. [Google Scholar] [CrossRef]
- Kuhara, Y.; Ninomiya, M.; Hirahara, S.; Doi, H.; Kenji, S.; Toyota, K.; Yano, R.; Kobayashi, H.; Hashimoto, Y.; Yokoyama, Y.; et al. A Long-Term Survival Case of Unresectable Gastric Cancer with Multidisciplinary Therapy Including Immunotherapy and Abscopal Effect. Int. Cancer Conf. J. 2020, 9, 193–198. [Google Scholar] [CrossRef]
- Kareff, S.A.; Lischalk, J.W.; Krochmal, R.; Kim, C. Abscopal Effect in Pulmonary Carcinoid Tumor following Ablative Stereotactic Body Radiation Therapy: A Case Report. J. Med. Case Rep. 2020, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Ogawa, S.; Aikawa, G. Abscopal Effect of Pelvic Intensity Modulated Radiation Therapy on Lung Metastases in a Patient with Recurrent Endometrial Cancer. Adv. Radiat. Oncol. 2021, 6, 100563. [Google Scholar] [CrossRef]
- Mampuya, W.A.; Bouchaab, H.; Schaefer, N.; Kinj, R.; La Rosa, S.; Letovanec, I.; Ozsahin, M.; Bourhis, J.; Coukos, G.; Peters, S.; et al. Abscopal Effect in a Patient with Malignant Pleural Mesothelioma Treated with Palliative Radiotherapy and Pembrolizumab. Clin. Transl. Radiat. Oncol. 2021, 27, 85–88. [Google Scholar] [CrossRef]
- Hotta, T.; Okuno, T.; Nakao, M.; Amano, Y.; Isobe, T.; Tsubata, Y. Reproducible Abscopal Effect in a Patient with Lung Cancer Who Underwent Whole-brain Irradiation and Atezolizumab Administration. Thorac. Cancer 2021, 12, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Mazzaschi, G.; Tommasi, C.; Pietri, E.; Corcione, L.; De Giorgi, A.; Bini, P.; Bui, S. Abscopal Effect as Part of Treatment of Oligometastatic Head and Neck Cancer: A Case Report. Clin. Case Rep. 2021, 9, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Chang, J.S. Abscopal Effect after Palliative Five-Fraction Radiation Therapy on Bone and Lymph Node Metastases from Luminal B Breast Cancer: A Case Report and Clinical Implications for Palliative Radiation Therapy. Radiat. Oncol. J. 2021, 39, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, F.; Belluomini, L.; Giuliani, J.; Urbini, B.; Milella, M.; Frassoldati, A.; Pilotto, S.; Giorgi, C. Abscopal Effect and Resistance Reversion in Nivolumab-Treated Non-Small-Cell Lung Cancer Undergoing Palliative Radiotherapy: A Case Report. Immunotherapy 2021, 13, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Nakata, H.; Ishigaki, K.; Tachibana, S.; Yoshida, M.; Muto, M.; Yanagawa, N.; Okumura, T. Successful Treatment of Advanced Gastric Cancer with Brain Metastases through an Abscopal Effect by Radiation and Immune Checkpoint Inhibitor Therapy. J. Gastric Cancer 2021, 21, 319. [Google Scholar] [CrossRef] [PubMed]
- Vilinovszki, O.; Andratschke, N.; Huellner, M.; Curioni-Fontecedro, A.; Kroeze, S.G.C. True Abscopal Effect in a Patient with Metastatic Non-Small Cell Lung Cancer. Radiat. Oncol. 2021, 16, 194. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liu, Y.; Yang, Y.; Dong, P.; He, L.; Zhou, F. Stereotactic Body Radiotherapy-Induced Abscopal Effect Twice after Pembrolizumab Failure in Hereditary Leiomyomatosis and Renal Cell Carcinoma: A Case Report with Genetic and Immunologic Analysis. Transl. Urol. 2021, 10, 4304–4312. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Fushida, S.; Kinoshita, J.; Saito, H.; Shimada, M.; Terai, S.; Moriyama, H.; Okamoto, K.; Nakamura, K.; Ninomiya, I.; et al. A Case of Primary Malignant Melanoma of the Esophagogastric Junction with Abscopal Effect after Nivolumab Administration. Surg. Case Rep. 2021, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Rittberg, R.; Chan, E.; Yip, S.; Alex, D.; Ho, C. Radiation Induced Abscopal Effect in a Patient with Malignant Pleural Mesothelioma on Pembrolizumab. Cureus 2022, 14, e22159. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Umezawa, R.; Yamamoto, T.; Takahashi, N.; Takeda, K.; Suzuki, Y.; Jingu, K. Differential Abscopal Effect in Extracranial and Intracranial Lesions after Radiotherapy Alone for Vertebral Bone Metastasis of Unknown Primary: A Case Report. J. Med. Case Rep. 2022, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Saijo, A.; Nokihara, H.; Mitsuhashi, A.; Yoneda, H.; Otsuka, K.; Ogino, H.; Bando, Y.; Nishioka, Y. Radiation Therapy Induces an Abscopal Effect and Upregulates Programmed Death-ligand 1 Expression in a Patient with Non-small Cell Lung Cancer. Thorac. Cancer 2022, 13, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Sato, H.; Gao, X.; Ohno, T. Pembrolizumab After Carbon Ion Radiation Therapy for Alveolar Soft Part Sarcoma Shows a Remarkable Abscopal Effect: A Case Report. Adv. Radiat. Oncol. 2022, 7, 100893. [Google Scholar] [CrossRef]
- Aldakhil, S.; Mathieu, D. Abscopal Effect Leading to Complete Disappearance of Extensive Meningiomatosis after Gamma Knife Radiosurgery: Case Report. Front. Surg. 2022, 9, 1004. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Ito, K.; Fujiwara, K.; Nishii, Y.; Ochiai, S.; Nomoto, Y.; Hataji, O. An Oldest-old Non-small Cell Lung Cancer Patient with Abscopal Effect in a Single Lesion. Thorac. Cancer 2022, 13, 2267–2270. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, L.; Wu, S.; Shen, J.; Huang, C.; Chen, Y.; Li, S. Abscopal Effect of Radiation Therapy and Nivolumab in a Patient with Combined Small-Cell Lung Cancer: A Case Report. Immunotherapy 2022, 14, 909–914. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Li, Z.; Xiao, Z.-F.; Li, D.; Liu, W.-Y. Case Report: Radiotherapy plus Pneumococcal Conjugate Vaccine Stimulates Abscopal Immune Response in a Patient with ALK+ NSCLC. Front. Immunol. 2022, 13, 950252. [Google Scholar] [CrossRef] [PubMed]
- Marco, D.F.; Gianluca, A.; Mariagrazia, T.; Luca, E.P. Overcoming Immune-Resistance in Laryngeal Cancer: A Case Report of the Abscopal Effect and Nivolumab beyond Progression. Immunotherapy 2022, 14, 1089–1095. [Google Scholar] [CrossRef]
- Lin, X.; Lu, T.; Xie, Z.; Qin, Y.; Liu, M.; Xie, X.; Li, S.; Zhou, C. Extracranial Abscopal Effect Induced by Combining Immunotherapy with Brain Radiotherapy in a Patient with Lung Adenocarcinoma: A Case Report and Literature Review. Thorac. Cancer 2019, 10, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Garelli, E.; Rittmeyer, A.; Putora, P.M.; Glatzer, M.; Dressel, R.; Andreas, S. Abscopal Effect in Lung Cancer: Three Case Reports and a Concise Review. Immunotherapy 2019, 11, 1445–1461. [Google Scholar] [CrossRef]
- Choi, J.S.; Sansoni, E.R.; Lovin, B.D.; Lindquist, N.R.; Phan, J.; Mayo, L.L.; Ferrarotto, R.; Su, S.Y. Abscopal Effect following Immunotherapy and Combined Stereotactic Body Radiation Therapy in Recurrent Metastatic Head and Neck Squamous Cell Carcinoma: A Report of Two Cases and Literature Review. Ann. Otol. Rhinol. Laryngol. 2020, 129, 517–522. [Google Scholar] [CrossRef]
- Ellerin, B.E.; Demandante, C.G.N.; Martins, J.T. Pure Abscopal Effect of Radiotherapy in a Salivary Gland Carcinoma: Case Report, Literature Review, and a Search for New Approaches. Cancer/Radiothérapie 2020, 24, 226–246. [Google Scholar] [CrossRef]
- Forner, D.; Horwich, P.; Trites, J.R.; Hollenhorst, H.; Bullock, M.; Lamond, N.W.D. The Abscopal Effect in Head-and-Neck Squamous Cell Carcinoma Treated with Radiotherapy and Nivolumab: A Case Report and Literature Review. Curr. Oncol. 2020, 27, 330–335. [Google Scholar] [CrossRef]
- Wu, M.; Liu, J.; Seery, S.; Meng, X.; Yue, J. Cytoreductive Nephrectomy Promoted Abscopal Effect of Camrelizumab Combined with Radiotherapy for Metastatic Renal Cell Carcinoma: A Case Report and Review of the Literature. Front. Immunol. 2021, 12, 646085. [Google Scholar] [CrossRef]
- Zhao, X.; Kang, J.; Zhao, R. Abscopal Effect of Radiation on Lymph Node Metastasis in Esophageal Carcinoma: A Case Report and Literature Review. Oncol. Lett. 2018, 16, 3555–3560. [Google Scholar] [CrossRef]
- Van de Walle, M.; Demol, J.; Staelens, L.; Rottey, S. Abscopal Effect in Metastatic Renal Cell Carcinoma. Acta Clin. Belg. 2017, 72, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Suzuki, Y.; Yoshimoto, Y.; Noda, S.; Murata, K.; Takakusagi, Y.; Okazaki, A.; Sekihara, T.; Nakano, T. An Abscopal Effect in a Case of Concomitant Treatment of Locally and Peritoneally Recurrent Gastric Cancer Using Adoptive T-Cell Immunotherapy and Radiotherapy. Clin. Case Rep. 2017, 5, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.J.; Seid, J.; Verdecchia, K.; Chuba, P. Abscopal Effect after Radiosurgery for Solitary Brain Metastasis from Non-Small Cell Lung Cancer. Cureus 2018, 10, e3777. [Google Scholar] [CrossRef] [PubMed]
- Agyeman, M.B.; Vanderpuye, V.D.; Yarney, J. Abscopal Effect of Radiotherapy in Imatinib-Resistant Dermatofibrosarcoma Protuberans. Cureus 2019, 11, e3857. [Google Scholar] [CrossRef]
- Ebner, D.K.; Kamada, T.; Yamada, S. Abscopal Effect in Recurrent Colorectal Cancer Treated with Carbon-Ion Radiation Therapy: 2 Case Reports. Adv. Radiat. Oncol. 2017, 2, 333–338. [Google Scholar] [CrossRef]
- Cong, Y.; Shen, G.; Wu, S.; Hao, R. Abscopal Regression following SABR for Non-Small-Cell-Lung Cancer: A Case Report. Cancer Biol. 2017, 18, 1–3. [Google Scholar] [CrossRef]
- Desar, I.M.E.; Braam, P.M.; Kaal, S.E.J.; Gerritsen, W.R.; Oyen, W.J.G.; van der Graaf, W.T.A. Abscopal Effect of Radiotherapy in a Patient with Metastatic Diffuse-Type Giant Cell Tumor. Acta Oncol. 2016, 55, 1510–1512. [Google Scholar] [CrossRef]
- Bruton Joe, M.; Truong, P.T. Abscopal Effect after Palliative Radiation Therapy for Metastatic Adenocarcinoma of the Esophagus. Cureus 2018, 10, e3089. [Google Scholar] [CrossRef]
- Joe, M.B.; Lum, J.J.; Watson, P.H.; Tonseth, R.P.; McGhie, J.P.; Truong, P.T. Radiation Generates an Abscopal Response and Complete Resolution of Metastatic Squamous Cell Carcinoma of the Anal Canal: A Case Report. J. Gastrointest. Oncol. 2017, 8, E84–E89. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Higashiyama, M.; Okami, J.; Tokunaga, T.; Inoue, N.; Akazawa, T.; Seya, T. A Possible Abscopal Effect of Post-Irradiation Immunotherapy in Two Patients with Metastatic Lung Tumors. Int. Cancer Conf. J. 2014, 3, 122–127. [Google Scholar] [CrossRef]
- Siva, S.; Callahan, J.; MacManus, M.P.; Martin, O.; Hicks, R.J.; Ball, D.L. Abscopal Effects after Conventional and Stereotactic Lung Irradiation of Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2013, 8, e71–e72. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Lawrence, D.P.; Wargo, J.A.; Oh, K.S.; Gonzalez, R.G.; Piris, A. Case 21-2013. N. Engl. J. Med. 2013, 369, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Takaya, M.; Niibe, Y.; Tsunoda, S.; Jobo, T.; Imai, M.; Kotani, S.; Unno, N.; Hayakawa, K. Abscopal Effect of Radiation on Toruliform Para-Aortic Lymph Node Metastases of Advanced Uterine Cervical Carcinoma—A Case Report. Anticancer Res. 2007, 27, 499–503. [Google Scholar] [PubMed]
- Bitran, J. The Abscopal Effect Exists in Non-Small Cell Lung Cancer: A Case Report and Review of the Literature. Cureus 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Britschgi, C.; Riesterer, O.; Burger, I.A.; Guckenberger, M.; Curioni-Fontecedro, A. Report of an Abscopal Effect Induced by Stereotactic Body Radiotherapy and Nivolumab in a Patient with Metastatic Non-Small Cell Lung Cancer. Radiat. Oncol. 2018, 13, 102. [Google Scholar] [CrossRef]
- Chino, F.; Pollis, K.E.; Choi, S.; Salama, J.K.; Palta, M. Stereotactic Body Radiation Therapy–Induced Abscopal Effect on Hepatocellular Carcinoma After Treatment for Lung Cancer: A Case Report. Hepatology 2018, 68, 1653–1655. [Google Scholar] [CrossRef]
- Barsky, A.R.; Cengel, K.A.; Katz, S.I.; Sterman, D.H.; Simone, C.B. First-Ever Abscopal Effect after Palliative Radiotherapy and Immuno-Gene Therapy for Malignant Pleural Mesothelioma. Cureus 2019, 11, e4102. [Google Scholar] [CrossRef]
- Hidaka, Y.; Takeichi, T.; Ishikawa, Y.; Kawamura, M.; Akiyama, M. Abscopal Effect of Local Irradiation Treatment for Diffuse Large B-Cell Lymphoma. Acta Derm. Venereol. 2017, 97, 1140–1141. [Google Scholar] [CrossRef] [PubMed]
- Brenneman, R.J.; Sharifai, N.; Fischer-Valuck, B.; Hassanzadeh, C.; Guzelian, J.; Chrisinger, J.S.A.; Michalski, J.M.; Oppelt, P.; Baumann, B.C. Abscopal Effect following Proton Beam Radiotherapy in a Patient with Inoperable Metastatic Retroperitoneal Sarcoma. Front. Oncol. 2019, 9, 922. [Google Scholar] [CrossRef]
- Kim, J.O.; Kim, C.A. Abscopal Resolution of a Hepatic Metastasis in a Patient with Metastatic Cholangiocarcinoma following Radical Stereotactic Body Radiotherapy to a Synchronous Early Stage Non-Small Cell Lung Cancer. Cureus 2019, 11, e4082. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.W.; Wang, S.-Y.; Jin-Jhih, H.; Chan, A.L. Abscopal Effect of Radiation on Bone Metastases of Breast Cancer: A Case Report. Cancer Biol. 2018, 19, 20–24. [Google Scholar] [CrossRef]
- Katayama, K.; Tamiya, A.; Koba, T.; Fukuda, S.; Atagi, S. An Abscopal Response to Radiation Therapy in a Patient with Metastatic Non-Small Cell Lung Cancer: A Case Report. J. Cancer Sci. 2017, 9, 365–367. [Google Scholar] [CrossRef]
- Shinde, A.; Novak, J.; Freeman, M.L.; Glaser, S.; Amini, A. Induction of the Abscopal Effect with Immunotherapy and Palliative Radiation in Metastatic Head and Neck Squamous Cell Carcinoma: A Case Report and Review of the Literature. Cureus 2019, 11, e4201. [Google Scholar] [CrossRef]
- Shi, F.; Wang, X.; Teng, F.; Kong, L.; Yu, J. Abscopal Effect of Metastatic Pancreatic Cancer after Local Radiotherapy and Granulocyte-Macrophage Colony-Stimulating Factor Therapy. Cancer Biol. 2017, 18, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Cerbone, L.; Rebuzzi, S.E.; Lattanzi, E.; Gnetti, L.; Iaia, M.L.; D’Abbiero, N.; Buti, S. Abscopal Effect after Hypofractionated Radiotherapy in Metastatic Renal Cell Carcinoma Pretreated with Pazopanib. Immunotherapy 2020, 12, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.T.; Elmali, A.; Yazici, G. Abscopal Effect, From Myth to Reality: From Radiation Oncologists’ Perspective. Cureus 2019, 11, e3860. [Google Scholar] [CrossRef]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Makita, K.; Hamamoto, Y.; Kanzaki, H.; Kataoka, M.; Yamamoto, S.; Nagasaki, K.; Ishikawa, H.; Takata, N.; Tsuruoka, S.; Uwatsu, K.; et al. Local Control of Bone Metastases Treated with External Beam Radiotherapy in Recent Years: A Multicenter Retrospective Study. Radiat. Oncol. 2021, 16, 225. [Google Scholar] [CrossRef]
- Zhu, Y.; Chang, X.; Zhou, R.; Chen, Y.; Ma, H.; Xiao, Z.; Qu, X.; Liu, Y.; Liu, L.; Li, Y.; et al. Bone Metastasis Attenuates Efficacy of Immune Checkpoint Inhibitors and Displays “Cold” Immune Characteristics in Non-Small Cell Lung Cancer. Lung Cancer 2022, 166, 189–196. [Google Scholar] [CrossRef]
- Qin, A.; Zhao, S.; Miah, A.; Wei, L.; Patel, S.; Johns, A.; Grogan, M.; Bertino, E.M.; He, K.; Shields, P.G.; et al. Bone Metastases, Skeletal-Related Events, and Survival in Patients with Metastatic Non–Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. J. Natl. Compr. Cancer Netw. 2021, 19, 915–921. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy Combined with Immunotherapy: The Dawn of Cancer Treatment. Signal Transduct. Target. Ther. 2022, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, M.; Xu, C.; Li, B.; Chen, J.; Chen, J.; Wang, Z. Immune Checkpoint Inhibitor Therapy for Bone Metastases: Specific Microenvironment and Current Situation. J. Immunol. Res. 2021, 2021, 8970173. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, L.; Orione, C.; Bore, P.; Misery, L.; Legoupil, D.; Leclere, J.-C.; Coste, A.; Girault, G.; Sicard-Cras, I.; Kacperek, C.; et al. Abscopal Response in Metastatic Melanoma: Real-World Data of a Retrospective, Multicenter Study. Cancers 2022, 14, 4213. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; HAN, J.Y.; Kim, J.I.; Park, S.H.; Cho, S.H.; Han, N.I.; Yang, J.M.; Kim, J.K.; Choi, S.W.; Lee, Y.S.; et al. Spontaneous Regression of a Large Hepatocellular Carcinoma with Skull Metastasis. J. Gastroenterol. Hepatol. 2005, 20, 488–492. [Google Scholar] [CrossRef]
- Golden, E.B.; Demaria, S.; Schiff, P.B.; Chachoua, A.; Formenti, S.C. An Abscopal Response to Radiation and Ipilimumab in a Patient with Metastatic Non–Small Cell Lung Cancer. Cancer Immunol. Res. 2013, 1, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Masui, K.; Yamazaki, H.; Takenaka, T.; Asai, S.; Taniguchi, H.; Nakamura, T.; Ukimura, O.; Yamada, K. Abscopal Effect of High-Dose-Rate Brachytherapy on Pelvic Bone Metastases from Renal Cell Carcinoma: A Case Report. J. Contemp. Brachytherapy 2019, 11, 458–461. [Google Scholar] [CrossRef]
- Mundy, G.R. Metastasis to Bone: Causes, Consequences and Therapeutic Opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef]
- Morse, M.D.; McNeel, D.G. Prostate Cancer Patients on Androgen Deprivation Therapy Develop Persistent Changes in Adaptive Immune Responses. Hum. Immunol. 2010, 71, 496–504. [Google Scholar] [CrossRef]
- Kingsley, L.A.; Fournier, P.G.J.; Chirgwin, J.M.; Guise, T.A. Molecular Biology of Bone Metastasis. Mol. Cancer 2007, 6, 2609–2617. [Google Scholar] [CrossRef]
- Xiang, L.; Gilkes, D. The Contribution of the Immune System in Bone Metastasis Pathogenesis. Int. J. Mol. Sci. 2019, 20, 999. [Google Scholar] [CrossRef]
- Baschuk, N.; Rautela, J.; Parker, B.S. Bone Specific Immunity and Its Impact on Metastasis. Bonekey Rep. 2015, 4, 665. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.K.; Perlow, H.K.; Raj, R.K.; Nalin, A.P.; Lehrer, E.J.; Kotecha, R.; Trifiletti, D.M.; McClelland, S.; Kendra, K.; Williams, N.; et al. Treatment of Brain Metastases: The Synergy of Radiotherapy and Immune Checkpoint Inhibitors. Biomedicines 2022, 10, 2211. [Google Scholar] [CrossRef]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using Immunotherapy to Boost the Abscopal Effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef]
- Vogler, J.B.; Murphy, W.A. Bone Marrow Imaging. Radiology 1988, 168, 679–693. [Google Scholar] [CrossRef]
- O’Sullivan, G.J. Imaging of Bone Metastasis: An Update. World J. Radiol. 2015, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Frantellizzi, V.; Pani, A.; Ippoliti, M.D.; Farcomeni, A.; Aloise, I.; Colosi, M.; Polito, C.; Pani, R.; Vincentis, G. De Scintigraphic Load of Bone Disease Evaluated by DASciS Software as a Survival Predictor in Metastatic Castration-Resistant Prostate Cancer Patients Candidates to 223RaCl Treatment. Radiol. Oncol. 2019, 54, 40–47. [Google Scholar] [CrossRef] [PubMed]
| Study | Primary Tumor | Age | Gender | RT Type | Total Dose (Dose/Fraction) | Site of Metastasis Different from Bone | Site of RT | Systemic Therapy (Close to RT) | BMs Site of AE | Time for AE (Months) | Follow-Up Duration (Months) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Timing | |||||||||||
| Yano M. et al. (2020) [11] | ECs | 65 | F | HFRT | 30 Gy (10 Gy) | LG, M-LNMs | Pelvis | ICI | Before Concomitant | Scr | 7 | 14 |
| Mazzaschi G. et al. (2021) [19] | H&N | 66 | M | SIB | 70 Gy (2 Gy) | N/A | Oph Hph Ln | CHT | Before | Lhs Hmr | 2 | 72 |
| 40 Gy (2 Gy) | N/A | LNMs | ||||||||||
| Vilinovszki O. et al. (2021) [23] | NSCLC | 81 | F | HFRT | 36 Gy (12 Gy) | Cervical LNMs | LG | -- | -- | 12th TV, 4th LV, LG, LNMs | 0.25 | 25 |
| Ishikawa Y. et al. (2022) [27] | Unknown | 57 | M | HFRT | 39 Gy (13 Gy) | Cervical LNMs | 9th TV | -- | -- | 8th Lhs Rib, Rhs Ilm, LGs, LNMs | 1 | 30 |
| Sakaguchi T. et al. (2022) [31] | SCC | 94 | M | HFRT | 30 Gy (10 Gy) | M-LNMs | IC | -- | -- | Axial vertebrae | 0.25 | NA |
| Siva S. et al. (2013) [52] | NSCLC | 78 | F | SBRT | 26 Gy (26 Gy) | AG | LG | -- | -- | Rhs Hmr, AG | 12 | 15 |
| Leung H. W. et al. (2018) [62] | BCa | 65 | F | SBRT | 225 Gy (15 Gy) | A-LNMs | Breast | -- | -- | 8th TV | 3 | 48 |
| Nam S. W. et al. (2005) [75] | HCC | 64 | M | HFRT | 30 Gy (6 Gy) | N/A | Skull | Skull, Stn, Ribs, Lvr | 3 | 24 | ||
| Golden E. B. et al. (2013) [76] | ADK | 64 | M | Lvr | 30 Gy (NA) | Cervical and M-LNMs | lvr | ICI | Concomitant | Lhs scr, LG, Lvr, Hilar LNMs | 2.5 | 12 |
| Suzuki G. et al. (2019) [77] | Renal cell carcinoma | 30 | F | BRT | 7 Gy + 9.2 Gy + 8.5 Gy + 7.9 Gy | LG, Rhs ovary, Hilar and M-LNMs, lvr | IC | ICI | Before After | TV | 3 | NA |
| Primary Tumor | Age | Gender | RT Type | Total Dose (Dose/Fraction) | Site of Metastasis | Site of RT | Systemic Therapy (Close to RT) | BMs Site of AE | Time for AE (Months) | Follow-Up Duration (Months) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Timing | ||||||||||
| SNCE | 69 | F | HFRT | 25 Gy (2.5 Gy) | Brain 12th TV 1st, 3rd, 5th LV 3rd, 7th Rhs Rib Rhs Tb Lhs Hmr LNMs | Brain | Lhs Hmr 7th Rhs Rib | 4 | 6 | ||
| PCa | 84 | M | HFRT | 20 Gy (4 Gy) | 7th, 8th, 9th, 10th TV Stn Lhs Tb Rhs Tb | 7th, 8th, 9th, 10th TV | ADT | Concomitant | Stn Lhs Tb Rhs Tb | 2 | 5 |
| PCa | 72 | M | HFRT | 20 Gy (4 Gy) | 11th TV Lhs Fmr Rhs Fmr Lhs Hmr Rhs Hmr | 11th TV Lhs Fmr Rhs Fmr | ADT | Concomitant | Lhs Hmr Rhs Hmr | 2 | 6 |
| BCa | 60 | F | HFRT | 20 Gy (4 Gy) | Brain LG 5th, 10th, 11th, 12th TV 1st, 2nd LV Rhs Ischium Pelvis Lhs Fmr Rhs Fmr 12th Rhs Rib Rhs Clc LNMs | 10th, 11th, 12th TV 1st, 2nd LV | SkC 12th Rhs Rib Rhs Clc Lhs Fmr | 2 | 8 | ||
| PCa | 83 | M | HFRT | 20 Gy (4 Gy) | Lhs SJ Stn Lhs Fmr | Lhs SJ | ADT | Concomitant | Stn Lhs Fmr | 1.5 | 4 |
| PCa | 75 | M | HFRT | 20 Gy (4 Gy) | 5th, 6th, 7th, 8th TV 2nd Rhs Rib Rhs SJ | 5th, 6th, 7th, 8th TV | ADT | Concomitant | 2nd Rhs Rib Rhs Fmr | 1.5 | 4 |
| BCa | 58 | F | HFRT | 20 Gy (4 Gy) | Kidney Brain AG 10th, 11th TV Pelvis Lhs Fmr Rhs Fmr SKC | Rhs Fms | SKC | 3 | 5 | ||
| BCa | 37 | F | HFRT | 20 Gy (4 Gy) | 3rd, 4th, 5th CV 4th, 5th LV Stn 9th, 12th Rhs Rib Scr Pelvis LNMs | Sacrum | 4th LV | 3.5 | 6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaciello, M.; Conte, M.; Montinaro, F.R.; Sabatini, A.; Cunicella, G.; Di Giammarco, F.; Tini, P.; Gravina, G.L.; Cortesi, E.; Minniti, G.; et al. Abscopal Effect on Bone Metastases from Solid Tumors: A Systematic Review and Retrospective Analysis of Challenge within a Challenge. Biomedicines 2023, 11, 1157. https://doi.org/10.3390/biomedicines11041157
Tomaciello M, Conte M, Montinaro FR, Sabatini A, Cunicella G, Di Giammarco F, Tini P, Gravina GL, Cortesi E, Minniti G, et al. Abscopal Effect on Bone Metastases from Solid Tumors: A Systematic Review and Retrospective Analysis of Challenge within a Challenge. Biomedicines. 2023; 11(4):1157. https://doi.org/10.3390/biomedicines11041157
Chicago/Turabian StyleTomaciello, Miriam, Miriam Conte, Francesca Romana Montinaro, Arianna Sabatini, Giorgia Cunicella, Federico Di Giammarco, Paolo Tini, Giovanni Luca Gravina, Enrico Cortesi, Giuseppe Minniti, and et al. 2023. "Abscopal Effect on Bone Metastases from Solid Tumors: A Systematic Review and Retrospective Analysis of Challenge within a Challenge" Biomedicines 11, no. 4: 1157. https://doi.org/10.3390/biomedicines11041157
APA StyleTomaciello, M., Conte, M., Montinaro, F. R., Sabatini, A., Cunicella, G., Di Giammarco, F., Tini, P., Gravina, G. L., Cortesi, E., Minniti, G., De Vincentis, G., Frantellizzi, V., & Marampon, F. (2023). Abscopal Effect on Bone Metastases from Solid Tumors: A Systematic Review and Retrospective Analysis of Challenge within a Challenge. Biomedicines, 11(4), 1157. https://doi.org/10.3390/biomedicines11041157










