Exploring the Complex Relationship between Diabetes and Cardiovascular Complications: Understanding Diabetic Cardiomyopathy and Promising Therapies
Abstract
1. Introduction
2. Epidemiology
3. Progression of DCM
4. Cardiac Structural and Functional Anomalies in DCM
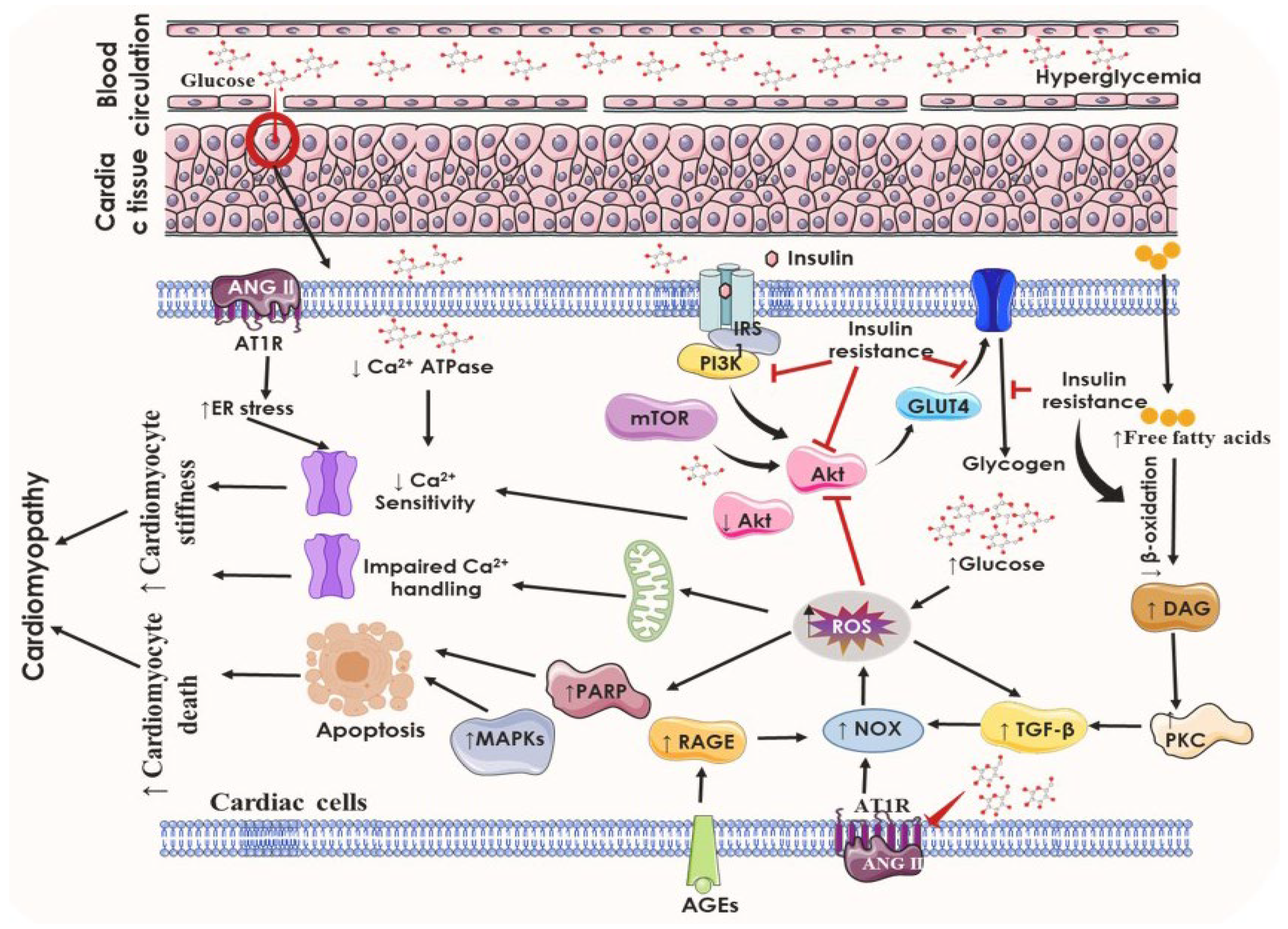
5. Pathophysiological Anomalies Underlying DCM
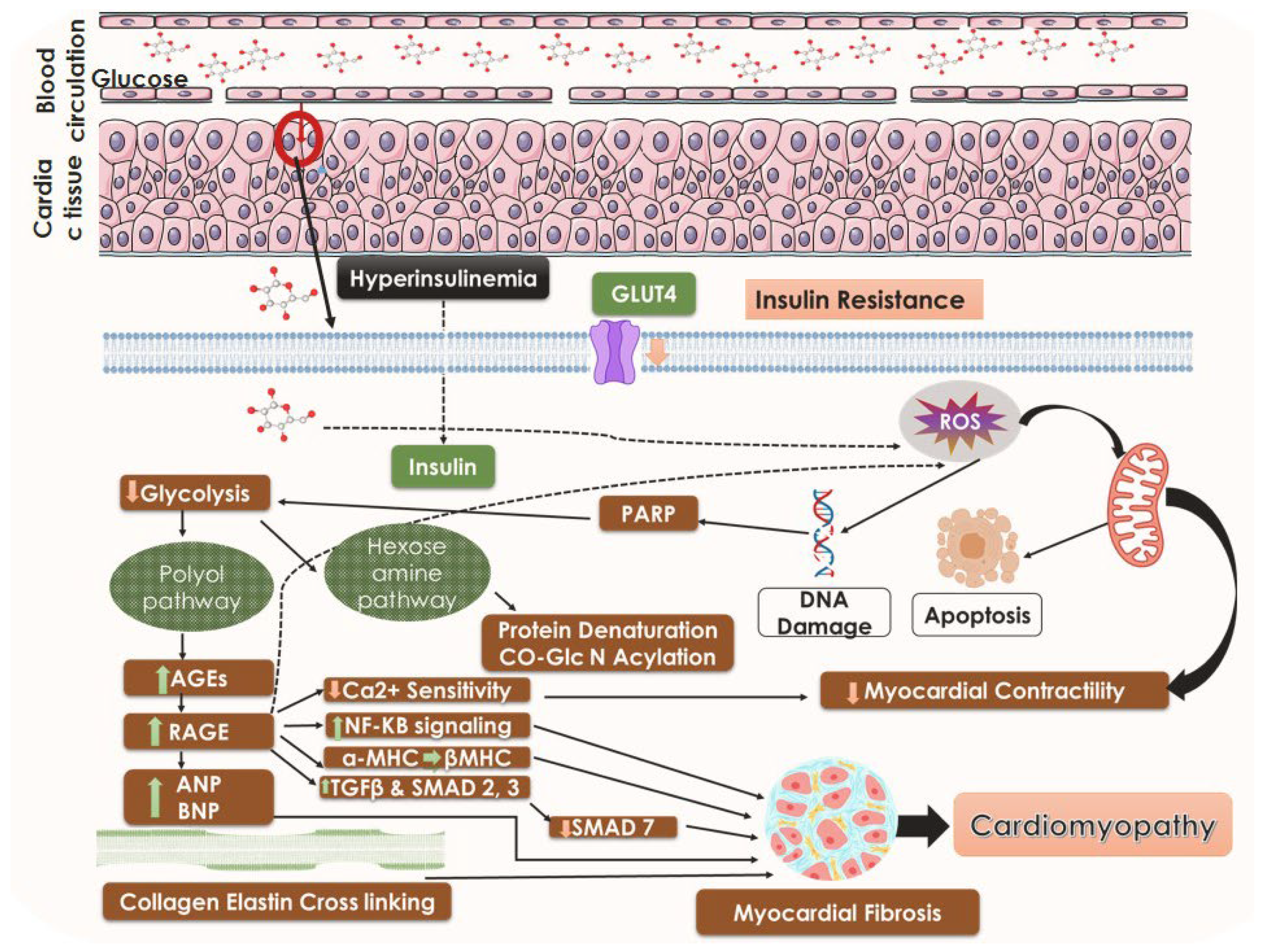
5.1. Insulin Resistance and the State of Hyperglycemia
5.2. Altered Insulin Signaling Cascades
5.3. Hyperinsulinemia
5.4. Altered Metabolic Cascades: Lipotoxicity and Glucotoxicity
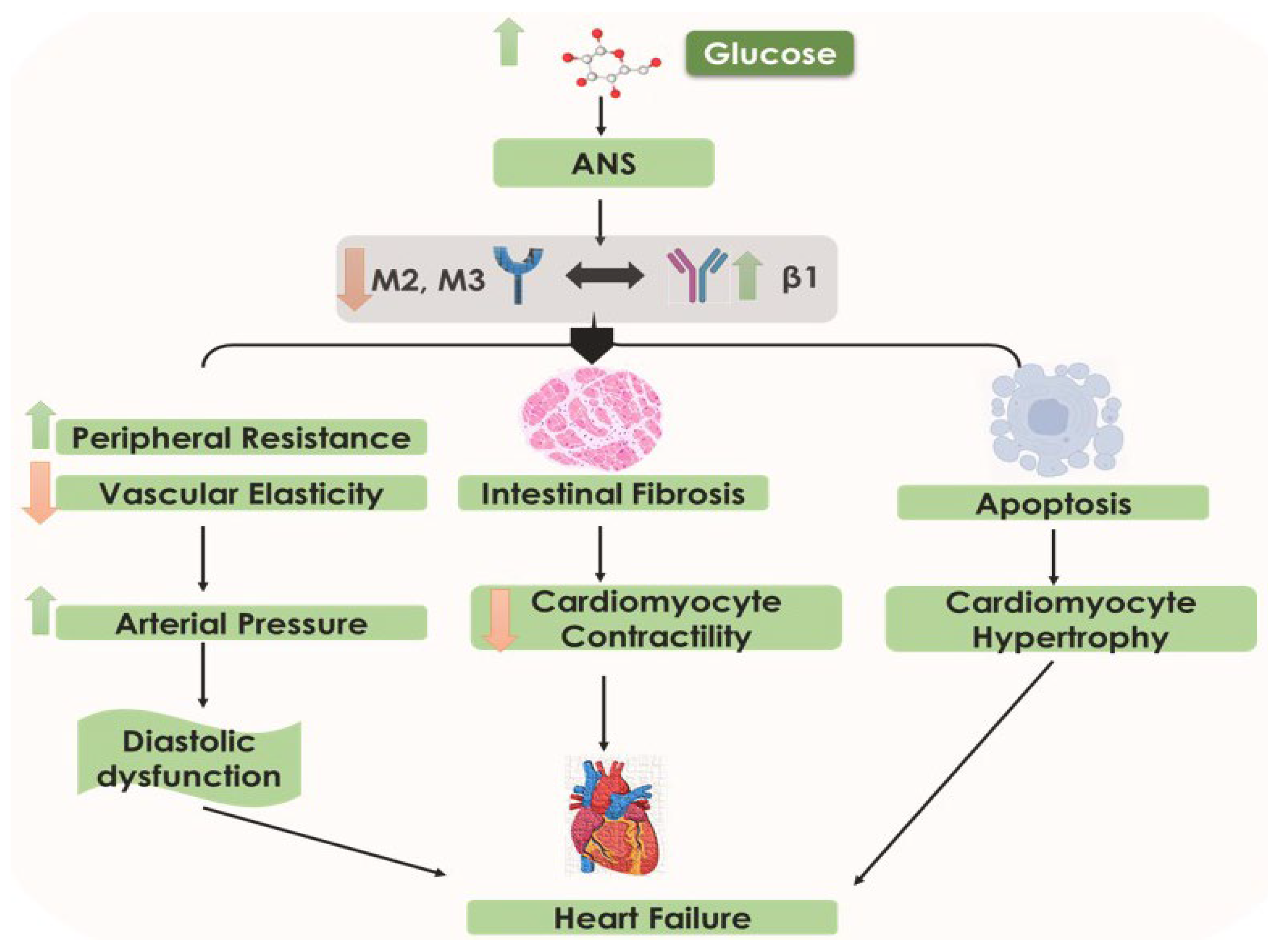
5.5. Altered Neurohumoral Activation: Cardiac Autonomic Neuropathy in DCM
5.6. Altered RAAS: Neurohormonal Abnormalities
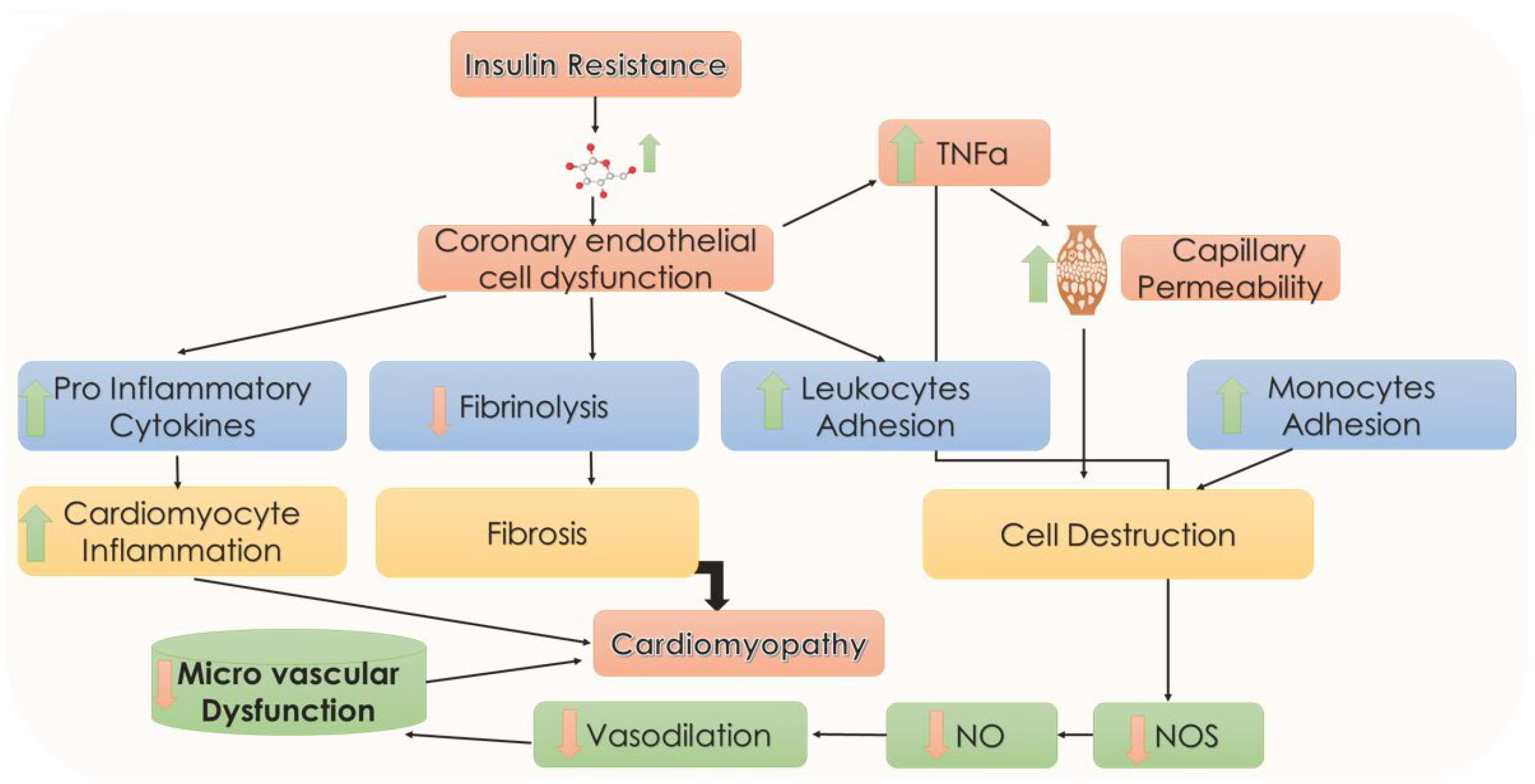
5.7. Endothelial Abnormalities
5.8. Mitochondrial Maladaptive Role in DCM
5.9. Dysfunctional Immune Responses
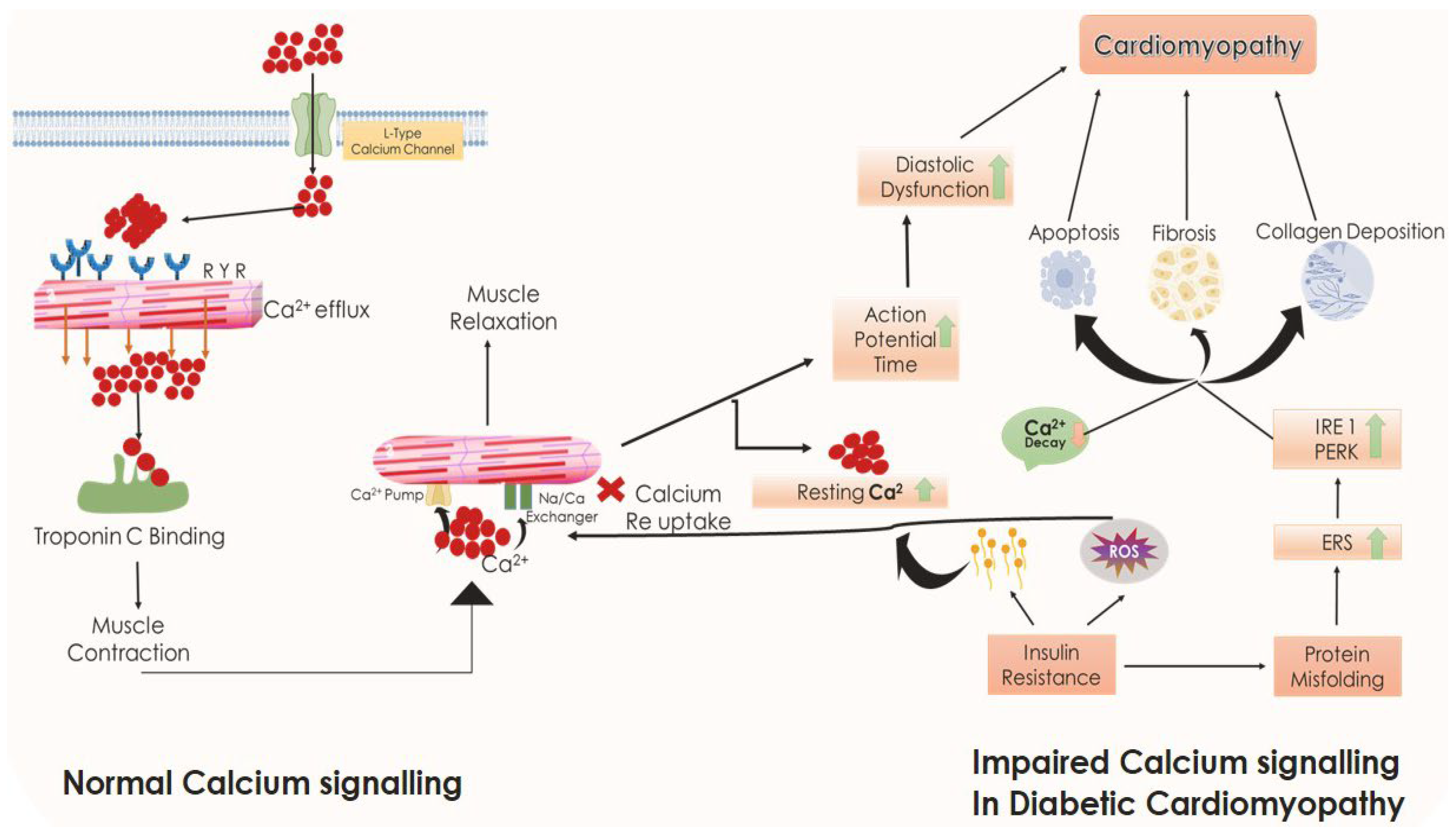
5.10. Role of ERS, Impaired Calcium Handling, and Cardiomyocyte Mass Depletion in DCM
6. Existing Therapeutic Approaches and Their Limitations in the Management of DCM
7. Emerging Pharmacotherapies
7.1. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors
7.2. Glucagon-Like Peptide-1 (GLP-1) Mimetics
7.3. DPP-4 Inhibitors
7.4. Neprilysin Pathway Inhibitors
7.5. SERCA as a Promising Target Therapy
7.6. Application of Adrenomedullin (ADM) as a Potent Endogenous Vasodilator
7.7. Antioxidant Strategies in the Management of DCM
7.8. Glucose-Dependent Insulinotropic Polypeptide (GIP) Agonists
7.9. Imeglimin
7.10. Miscellaneous Targets
8. MicroRNAs (miRs) as Potential Biomarkers in DCM
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end products |
| AGT II | angiotensin II |
| ATP | adenosine triphosphate |
| BNP | brain-derived natriuretic peptide |
| cAMP | cyclic adenosine monophosphate |
| CAN | cardiac autonomic neuropathy |
| CD163 | cluster of differentiation 163 |
| cGMP | cyclic guanosine monophosphate |
| CVD | cardiovascular disease |
| DCM | diabetic cardiomyopathy |
| DPP-4 | dipeptidyl peptidase-4 |
| EDRF | endothelium-derived relaxation factor |
| ER | endoplasmic reticulum |
| FFA | free fatty acid |
| FPG | fasting plasma glucose |
| GLP-1 | glucagon-like peptide-1 |
| GLUT | glucose transporters |
| HbA1c | glycated hemoglobin |
| HDL | high-density lipoprotein |
| IL | interleukin |
| IRS-1 | insulin receptor substrate 1 |
| JAK | Janus kinase |
| LV | left ventricle |
| LVH | left ventricular hypertrophy |
| MAPK | mitogen-activated protein kinases |
| MHC | myosin heavy chain |
| MMP | matrix metalloproteinase |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NHE3 | sodium-hydrogen exchanger 3 |
| NOS | nitric oxide synthases |
| NPY | neuropeptide Y |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NRG | neuregulins |
| PARP | poly (ADP-ribose) polymerase enzymes |
| PI3K | phosphoinositide 3-kinases |
| PKB | protein kinase B |
| PKC | protein kinase C |
| PKG | protein Kinase G |
| PPAR | peroxisome proliferator-activated receptors |
| RAAS | renin–angiotensin–aldosterone system |
| RAGE | receptor for advanced glycation end products |
| ROS | reactive oxygen species |
| SERCA | sarcoplasmic/endoplasmic reticulum Ca2+ ATPase |
| SGLT2 | sodium glucose cotransporter 2 |
| SS31 | Szeto–Schiller peptide 31 |
| T2DM | type 2 diabetes mellitus |
| TGF | transforming growth factor |
| TGs | triglycerides |
| TIMP | tissue inhibitor of metalloproteinase |
| TZD | thiazolidinediones |
| TNF | tumor necrosis factor |
| TXNIP | thioredoxin-interacting protein. |
References
- Rosano, G.M.C.; Vitale, C.; Seferovic, P. Heart Failure in Patients with Diabetes Mellitus. Card. Fail. Rev. 2017, 3, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between Insulin Resistance and the Development of Cardiovascular Disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Ren, J.; Wu, N.N.; Wang, S.; Sowers, J.R.; Zhang, Y. Obesity Cardiomyopathy: Evidence, Mechanisms, and Therapeutic Implications. Physiol. Rev. 2021, 101, 1745–1807. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, M.; Zhang, Y.; Guo, H.; Ding, W.; Sun, T. Nlrp3 Deficiency Alleviates Angiotensin II-Induced Cardiomyopathy by Inhibiting Mitochondrial Dysfunction. Oxid. Med. Cell. Longev. 2021, 2021, 6679100. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Dua, T.K.; Khanra, R.; Joardar, S.; Nandy, A.; Saha, A.; De Feo, V.; Dewanjee, S. Protocatechuic Acid, a Phenolic from Sansevieria roxburghiana Leaves, Suppresses Diabetic Cardiomyopathy via Stimulating Glucose Metabolism, Ameliorating Oxidative Stress, and Inhibiting Inflammation. Front. Pharmacol. 2017, 8, 251. [Google Scholar] [CrossRef]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic Cardiomyopathy: A Hyperglycaemia-and Insulin-Resistance-Induced Heart Disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Cañizo-Gómez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef]
- Johnson, J.D. On the Causal Relationships between Hyperinsulinaemia, Insulin Resistance, Obesity and Dysglycaemia in Type 2 Diabetes. Diabetologia 2021, 64, 2138–2146. [Google Scholar] [CrossRef]
- International Diabetes Federation. Diabetes Facts & Figures. Available online: https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 12 December 2022).
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global Estimates for the Prevalence of Diabetes for 2015 and 2040. Diabetes Res. Clin. Pr. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pr. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of Cardiovascular Disease in Type 2 Diabetes: A Systematic Literature Review of Scientific Evidence from across the World in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Givertz, M.M.; Aguilar, D.; Allen, L.A.; Chan, M.; Desai, A.S.; Deswal, A.; Dickson, V.V.; Kosiborod, M.N.; Lekavich, C.L. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement from the American Heart Association and the Heart Failure Society of America: This Statement Does Not Represent an Update of the 2017 ACC/AHA/HFSA Heart Failure Guideline Update. Circulation 2019, 140, e294–e324. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef]
- Kaviarasan, V.; Mohammed, V.; Veerabathiran, R. Genetic Predisposition Study of Heart Failure and Its Association with Cardiomyopathy. Egypt. Heart J. 2022, 74, 5. [Google Scholar] [CrossRef]
- Tran, D.H.; Wang, Z. Glucose Metabolism in Cardiac Hypertrophy and Heart Failure. J. Am. Heart Assoc. 2019, 8, e012673. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Khanra, R.; Dua, T.K.; Das, S.; De, B.; Zia-Ul-Haq, M.; De Feo, V.; Dewanjee, S. Sansevieria roxburghiana Schult. & Schult. F. (Family: Asparagaceae) Attenuates Type 2 Diabetes and Its Associated Cardiomyopathy. PLoS ONE 2016, 11, e0167131. [Google Scholar]
- Makrecka-Kuka, M.; Liepinsh, E.; Murray, A.J.; Lemieux, H.; Dambrova, M.; Tepp, K.; Puurand, M.; Käämbre, T.; Han, W.H.; de Goede, P. Altered Mitochondrial Metabolism in the Insulin-resistant Heart. Acta Physiol. 2020, 228, e13430. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of Diabetic Cardiomyopathy and Potential Therapeutic Strategies: Preclinical and Clinical Evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Kusirisin, P.; Chattipakorn, S.C.; Chattipakorn, N. Contrast-Induced Nephropathy and Oxidative Stress: Mechanistic Insights for Better Interventional Approaches. J. Transl. Med. 2020, 18, 400. [Google Scholar] [CrossRef] [PubMed]
- Kaludercic, N.; di Lisa, F. Mitochondrial ROS Formation in the Pathogenesis of Diabetic Cardiomyopathy. Front. Cardiovasc. Med. 2020, 7, 12. [Google Scholar] [CrossRef]
- Seferovic, J.P.; Tesic, M.; Seferovic, P.M.; Lalic, K.; Jotic, A.; Biering-Sørensen, T.; Giga, V.; Stankovic, S.; Milic, N.; Lukic, L. Increased Left Ventricular Mass Index Is Present in Patients with Type 2 Diabetes without Ischemic Heart Disease. Sci. Rep. 2018, 8, 926. [Google Scholar] [CrossRef]
- Aroor, A.R.; Mummidi, S.; Lopez-Alvarenga, J.C.; Das, N.; Habibi, J.; Jia, G.; Lastra, G.; Chandrasekar, B.; DeMarco, V.G. Sacubitril/Valsartan Inhibits Obesity-Associated Diastolic Dysfunction through Suppression of Ventricular-Vascular Stiffness. Cardiovasc. Diabetol. 2021, 20, 80. [Google Scholar] [CrossRef]
- Arrigo, M.; Jessup, M.; Mullens, W.; Reza, N.; Shah, A.M.; Sliwa, K.; Mebazaa, A. Acute Heart Failure. Nat. Rev. Dis. Prim. 2020, 6, 16. [Google Scholar] [CrossRef]
- Schmitt, V.H.; Billaudelle, A.-M.; Schulz, A.; Keller, K.; Hahad, O.; Tröbs, S.-O.; Koeck, T.; Michal, M.; Schuster, A.K.; Toenges, G. Disturbed Glucose Metabolism and Left Ventricular Geometry in the General Population. J. Clin. Med. 2021, 10, 3851. [Google Scholar] [CrossRef]
- Mohan, M.; Dihoum, A.; Mordi, I.R.; Choy, A.-M.; Rena, G.; Lang, C.C. Left Ventricular Hypertrophy in Diabetic Cardiomyopathy: A Target for Intervention. Front. Cardiovasc. Med. 2021, 8, 746382. [Google Scholar] [CrossRef]
- Poudel, A.; Zhou, J.Y.; Story, D.; Li, L. Diabetes and Associated Cardiovascular Complications in American Indians/Alaskan Natives: A Review of Risks and Prevention Strategies. J. Diabetes Res. 2018, 2018, 2742565. [Google Scholar] [CrossRef]
- Yeo, J.L.; Brady, E.M.; McCann, G.P.; Gulsin, G.S. Sex and Ethnic Differences in the Cardiovascular Complications of Type 2 Diabetes. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211034296. [Google Scholar] [CrossRef]
- Ponte, C.M.M.; Fernandes, V.O.; Liberato, C.B.R.; Montenegro, A.P.D.R.; Batista, L.A.; Gurgel, M.H.C.; de Azevedo Karbage, L.B.; Vasconcelos, I.T.G.F.; d’Alva, C.B.; Montenegro Júnior, R.M. Association between Cardiovascular Autonomic Neuropathy and Left Ventricular Hypertrophy in Young Patients with Congenital Generalized Lipodystrophy. Diabetol. Metab. Syndr. 2019, 11, 53. [Google Scholar] [CrossRef]
- Kruszewska, J.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Remodeling and Fibrosis of the Cardiac Muscle in the Course of Obesity—Pathogenesis and Involvement of the Extracellular Matrix. Int. J. Mol. Sci. 2022, 23, 4195. [Google Scholar] [CrossRef]
- Nair, N. Epidemiology and Pathogenesis of Heart Failure with Preserved Ejection Fraction. Rev. Cardiovasc. Med. 2020, 21, 531–540. [Google Scholar]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Dong, S.; Qian, L.; Cheng, Z.; Chen, C.; Wang, K.; Hu, S.; Zhang, X.; Wu, T. Lactate and Myocadiac Energy Metabolism. Front. Physiol. 2021, 12, 715081. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular Death, Reactive Oxygen Species (ROS) and Diabetic Complications. Cell Death. Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Prandi, F.R.; Evangelista, I.; Sergi, D.; Palazzuoli, A.; Romeo, F. Mechanisms of Cardiac Dysfunction in Diabetic Cardiomyopathy: Molecular Abnormalities and Phenotypical Variants. Heart Fail Rev. 2022. [CrossRef]
- Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G.; Menini, S. Diabetic Complications and Oxidative Stress: A 20-Year Voyage Back in Time and Back to the Future. Antioxidants 2021, 10, 727. [Google Scholar] [CrossRef]
- Takawale, A.; Sakamuri, S.S.; Kassiri, Z. Extracellular matrix communication and turnover in cardiac physiology and pathology. Compr. Physiol. 2015, 5, 687–719. [Google Scholar]
- Stöhr, R.; Kappel, B.A.; Carnevale, D.; Cavalera, M.; Mavilio, M.; Arisi, I.; Fardella, V.; Cifelli, G.; Casagrande, V.; Rizza, S.; et al. TIMP3 interplays with apelin to regulate cardiovascular metabolism in hypercholesterolemic mice. Mol. Metab. 2015, 4, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, G.; von Lewinski, D.; Eaton, D.M.; Sourij, H.; Houser, S.R.; Wallner, M. Diabetic Cardiomyopathy: Current and Future Therapies. Beyond Glycemic Control. Front. Physiol. 2018, 9, 1514. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; TsouhFokou, P.V.; Azzini, E.; Peluso, I. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057. [Google Scholar] [CrossRef]
- Fruchart, J.-C.; Hermans, M.P.; Fruchart-Najib, J. Selective Peroxisome Proliferator–Activated Receptor Alpha Modulators (SPPARMα): New Opportunities to Reduce Residual Cardiovascular Risk in Chronic Kidney Disease? Curr. Atheroscler. Rep. 2020, 22, 43. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Y.; Jian, L.; Cheung, C.W.; Zhang, L.; Xia, Z. Impact of Peroxisome Proliferator-Activated Receptor-α on Diabetic Cardiomyopathy. Cardiovasc. Diabetol. 2021, 20, 2. [Google Scholar] [CrossRef]
- Ye, X.; Kong, W.; Zafar, M.I.; Chen, L.-L. Serum Triglycerides as a Risk Factor for Cardiovascular Diseases in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Prospective Studies. Cardiovasc. Diabetol. 2019, 18, 48. [Google Scholar] [CrossRef]
- Alexopoulos, A.-S.; Qamar, A.; Hutchins, K.; Crowley, M.J.; Batch, B.C.; Guyton, J.R. Triglycerides: Emerging Targets in Diabetes Care? Review of Moderate Hypertriglyceridemia in Diabetes. Curr. Diab. Rep. 2019, 19, 13. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Zhang, Y.; Wei, J.; Wang, Y. Plin5, a New Target in Diabetic Cardiomyopathy. Oxid. Med. Cell Longev. 2022, 2022, 2122856. [Google Scholar] [CrossRef]
- Villasante, F.A.; Iacobellis, G. Epicardial adipose tissue: Clinical biomarker of cardio-metabolic risk. Int. J. Mol. Sci. 2019, 20, 5989. [Google Scholar] [CrossRef]
- SalgadoSomoza, A.; Teijeira Fernández, E.; Rubio, J.; Couso, E.; González-Juanatey, J.R.; Eiras, S. Coronary artery disease is associated with higher epicardial retinol-binding protein 4 (RBP4) and lower glucose transporter (GLUT) 4 levels in epicardial and subcutaneous adipose tissue. Clin. Endocrinol. Oxf. 2012, 76, 51–58. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Arora, R.; Buckley, U.; Shivkumar, K. Autonomic Nervous System Dysfunction: JACC Focus Seminar. J. Am. Coll. Cardiol. 2019, 73, 1189–1206. [Google Scholar] [CrossRef]
- Agashe, S.; Petak, S. Cardiac Autonomic Neuropathy in Diabetes Mellitus. Methodist Debakey Cardiovasc. J. 2018, 14, 251. [Google Scholar] [CrossRef]
- Long, W.; Zhou, T.; Xuan, X.; Cao, Q.; Luo, Z.; Qin, Y.; Ning, Q.; Luo, X.; Xie, X. IUGR with Catch-up Growth Programs Impaired Insulin Sensitivity through LRP6/IRS-1 in Male Rats. Endocr. Connect. 2022, 11, e210203. [Google Scholar] [CrossRef]
- Sultan, F.; Kaur, R.; Tarfain, N.U.; Mir, A.H.; Dumka, V.K.; Sharma, S.K.; Singh Saini, S.P. Protective Effect of Rosuvastatin Pretreatment against Acute Myocardial Injury by Regulating Nrf2, Bcl-2/Bax, INOS, and TNF-α Expressions Affecting Oxidative/Nitrosative Stress and Inflammation. Hum. Exp. Toxicol. 2022, 41, 09603271211066065. [Google Scholar] [CrossRef]
- Angolano, C.; Kaczmarek, E.; Essayagh, S.; Daniel, S.; Choi, L.Y.; Tung, B.; Sauvage, G.; Lee, A.; Kipper, F.C.; Arvelo, M.B. A20/TNFAIP3 Increases ENOS Expression in an ERK5/KLF2-Dependent Manner to Support Endothelial Cell Health in the Face of Inflammation. Front. Cardiovasc. Med. 2021, 8, 651230. [Google Scholar] [CrossRef]
- D’Amario, D.; Migliaro, S.; Borovac, J.A.; Restivo, A.; Vergallo, R.; Galli, M.; Leone, A.M.; Montone, R.A.; Niccoli, G.; Aspromonte, N. Microvascular Dysfunction in Heart Failure with Preserved Ejection Fraction. Front. Physiol. 2019, 10, 1347. [Google Scholar] [CrossRef]
- Premont, R.T.; Reynolds, J.D.; Zhang, R.; Stamler, J.S. Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle. Circ. Res. 2020, 126, 129–158. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Kansakar, U.; Varzideh, F.; Wilson, S.; Mone, P.; Lombardi, A.; Gambardella, J.; Santulli, G. Heart Failure in Diabetes. Metabolism 2021, 125, 154910. [Google Scholar] [CrossRef]
- Ritchie, R.H.; Abel, E.D. Basic Mechanisms of Diabetic Heart Disease. Circ. Res. 2020, 126, 1501–1525. [Google Scholar] [CrossRef]
- Wenzl, F.A.; Ambrosini, S.; Mohammed, S.A.; Kraler, S.; Lüscher, T.F.; Costantino, S.; Paneni, F. Inflammation in Metabolic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 742178. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, S. Mitochondrial Uncoupling, ROS Generation and Cardioprotection. Biochim. Biophys. Acta. Bioenerg. 2018, 1859, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial Uncoupling: A Key Controller of Biological Processes in Physiology and Diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Aroor, A.R.; Hill, M.A.; Sowers, J.R. Role of Renin-Angiotensin-Aldosterone System Activation in Promoting Cardiovascular Fibrosis and Stiffness. Hypertension 2018, 72, 537–548. [Google Scholar] [CrossRef]
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V.S. Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair. Cells 2020, 10, 51. [Google Scholar] [CrossRef]
- Wahid, A.; Chen, W.; Wang, X.; Tang, X. High-Mobility Group Box 1 Serves as an Inflammation Driver of Cardiovascular Disease. Biomed. Pharmacother 2021, 139, 111555. [Google Scholar] [CrossRef]
- Navya, P.D.; Kaarthikeyan, G.; Raj, J.S.; Alamoudi, A.; Bahammam, M.A.; Zidane, B.; Bahammam, H.A.; Bahammam, S.A.; Hassan, A.A.A.; Kamil, M.A.; et al. Suppression of Tumorigenicity 2 Pro-Inflammatory Biomarker Linking Diabetes Mellitus and Periodontitis: A Pilot Study. Med. Sci. Monit. 2022, 28, e938218. [Google Scholar] [CrossRef]
- Homsak, E.; Gruson, D. Soluble ST2: A complex and diverse role in several diseases. Clin. Chim. Acta 2020, 507, 75–87. [Google Scholar] [CrossRef]
- Cardellini, M.; Rizza, S.; Casagrande, V.; Cardolini, I.; Ballanti, M.; Davato, F.; Porzio, O.; Canale, M.P.; Legramante, J.M.; Mavilio, M.; et al. Soluble ST2 is a biomarker for cardiovascular mortality related to abnormal glucose metabolism in high-risk subjects. Acta Diabetol. 2019, 56, 273–280. [Google Scholar] [CrossRef]
- Amen, O.M.; Sarker, S.D.; Ghildyal, R.; Arya, A. Endoplasmic Reticulum Stress Activates Unfolded Protein Response Signaling and Mediates Inflammation, Obesity, and Cardiac Dysfunction: Therapeutic and Molecular Approach. Front. Pharmacol. 2019, 10, 977. [Google Scholar] [CrossRef]
- Adams, C.J.; Kopp, M.C.; Larburu, N.; Nowak, P.R.; Ali, M.M.U. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front. Mol. Biosci. 2019, 6, 11. [Google Scholar] [CrossRef]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell. 2018, 69, 169–181. [Google Scholar] [CrossRef]
- Eisner, D.A.; Caldwell, J.L.; Trafford, A.W.; Hutchings, D.C. The Control of Diastolic Calcium in the Heart: Basic Mechanisms and Functional Implications. Circ. Res. 2020, 126, 395–412. [Google Scholar] [CrossRef]
- Federico, M.; Valverde, C.A.; Mattiazzi, A.; Palomeque, J. Unbalance between Sarcoplasmic Reticulum Ca2+ Uptake and Release: A First Step toward Ca2+ Triggered Arrhythmias and Cardiac Damage. Front. Physiol. 2020, 1630. [Google Scholar] [CrossRef]
- Banerjee, D.; Winocour, P.; Chowdhury, T.A.; De, P.; Wahba, M.; Montero, R.; Fogarty, D.; Frankel, A.H.; Karalliedde, J.; Mark, P.B. Management of Hypertension and Renin-Angiotensin-Aldosterone System Blockade in Adults with Diabetic Kidney Disease: Association of British Clinical Diabetologists and the Renal Association UK Guideline Update 2021. BMC Nephrol. 2022, 23, 9. [Google Scholar] [CrossRef]
- Parsamanesh, N.; Karami-Zarandi, M.; Banach, M.; Penson, P.E.; Sahebkar, A. Effects of Statins on Myocarditis: A Review of Underlying Molecular Mechanisms. Prog. Cardiovasc. Dis. 2021, 67, 53–64. [Google Scholar] [CrossRef]
- Lee, W.-S.; Kim, J. Diabetic Cardiomyopathy: Where We Are and Where We Are Going. Korean. J. Intern. Med. 2017, 32, 404–421. [Google Scholar] [CrossRef]
- Gui, F.; You, Z.; Fu, S.; Wu, H.; Zhang, Y. Endothelial Dysfunction in Diabetic Retinopathy. Front. Endocrinol. Lausanne 2020, 11, 591. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Longo, M.; Scappaticcio, L.; Cirillo, P.; Maio, A.; Carotenuto, R.; Maiorino, M.I.; Bellastella, G.; Esposito, K. Glycemic Control and the Heart: The Tale of Diabetic Cardiomyopathy Continues. Biomolecules 2022, 12, 272. [Google Scholar] [CrossRef]
- Gutiérrez-Cuevas, J.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Monroy-Ramírez, H.C.; Galicia-Moreno, M.; García-Bañuelos, J.; Santos, A.; Armendariz-Borunda, J. Molecular Mechanisms of Obesity-Linked Cardiac Dysfunction: An up-Date on Current Knowledge. Cells 2021, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity: Mechanistic Insights and Clinical Implications. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-ProBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef] [PubMed]
- Lamichane, S.; DahalLamichane, B.; Kwon, S.-M. Pivotal Roles of Peroxisome Proliferator-Activated Receptors (PPARs) and Their Signal Cascade for Cellular and Whole-Body Energy Homeostasis. Int. J. Mol. Sci. 2018, 19, 949. [Google Scholar] [CrossRef] [PubMed]
- Filardi, T.; Ghinassi, B.; di Baldassarre, A.; Tanzilli, G.; Morano, S.; Lenzi, A.; Basili, S.; Crescioli, C. Cardiomyopathy Associated with Diabetes: The Central Role of the Cardiomyocyte. Int. J. Mol. Sci. 2019, 20, 3299. [Google Scholar] [CrossRef]
- Chu, C.; Lu, Y.-P.; Yin, L.; Hocher, B. The SGLT2 Inhibitor Empagliflozin Might Be a New Approach for the Prevention of Acute Kidney Injury. Kidney Blood Press Res. 2019, 44, 149–157. [Google Scholar] [CrossRef]
- Kosiborod, M.; Cavender, M.A.; Fu, A.Z.; Wilding, J.P.; Khunti, K.; Holl, R.W.; Norhammar, A.; Birkeland, K.I.; Jørgensen, M.E.; Thuresson, M. Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation 2017, 136, 249–259. [Google Scholar]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An Update on Sodium-Glucose Co-Transporter-2 Inhibitors for the Treatment of Diabetes Mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Bjornstad, P.; Udell, J.A.; Lovshin, J.A.; Cherney, D.Z.I. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation 2017, 136, 1643–1658. [Google Scholar] [CrossRef]
- Garcia-Ropero, A.; Santos-Gallego, C.G.; Zafar, M.U.; Badimon, J.J. Metabolism of the Failing Heart and the Impact of SGLT2 Inhibitors. Expert. Opin. Drug. Metab. Toxicol. 2019, 15, 275–285. [Google Scholar] [CrossRef]
- Dyck, J.R.B.; Sossalla, S.; Hamdani, N.; Coronel, R.; Weber, N.C.; Light, P.E.; Zuurbier, C.J. Cardiac Mechanisms of the Beneficial Effects of SGLT2 Inhibitors in Heart Failure: Evidence for Potential off-Target Effects. J. Mol. Cell Cardiol. 2022, 167, 17–31. [Google Scholar] [CrossRef]
- Staels, B. Cardiovascular Protection by Sodium Glucose Cotransporter 2 Inhibitors: Potential Mechanisms. Am. J. Cardiol. 2017, 120, S28–S36. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J. SGLT2 Inhibitors and Mechanisms of Cardiovascular Benefit: A State-of-the-Art Review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Liontos, A.; Papakitsou, I.; Elisaf, M.S. SGLT2 Inhibitors and Cardioprotection: A Matter of Debate and Multiple Hypotheses. Postgrad Med. 2019, 131, 82–88. [Google Scholar] [CrossRef]
- Kim, A.H.; Jang, J.E.; Han, J. Current Status on the Therapeutic Strategies for Heart Failure and Diabetic Cardiomyopathy. Biomed. Pharmacother 2022, 145, 112463. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- McMurray, J.J.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W. SGLT2 Inhibitors in Patients with Heart Failure with Reduced Ejection Fraction: A Meta-Analysis of the EMPEROR-Reduced and DAPA-HF Trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Khan, M.S.; Marx, N.; Lam, C.S.P.; Schnaidt, S.; Ofstad, A.P.; Brueckmann, M.; Jamal, W. Effect of Empagliflozin on Cardiovascular and Renal Outcomes in Patients with Heart Failure by Baseline Diabetes Status: Results from the EMPEROR-Reduced Trial. Circulation 2021, 143, 337–349. [Google Scholar] [CrossRef]
- Zilliox, L.A.; Chadrasekaran, K.; Kwan, J.Y.; Russell, J.W. Diabetes and Cognitive Impairment. Curr. Diab. Rep. 2016, 16, 87. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Sathyapalan, T.; Rizzo, M.; Sahebkar, A. Cognitive benefits of Sodium-Glucose Co-Transporters-2 Inhibitors in the Diabetic Milieu. Curr. Med. Chem. 2023. [CrossRef] [PubMed]
- Zarkasi, K.A.; Abdul Murad, N.A.; Ahmad, N.; Jamal, R.; Abdullah, N. Coronary Heart Disease in Type 2 Diabetes Mellitus: Genetic Factors and Their Mechanisms, Gene-Gene, and Gene-Environment Interactions in the Asian Populations. Int. J. Environ. Res. Public Health 2022, 19, 647. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, Z.; Ilyas, I.; Little, P.J.; Kamato, D.; Sahebka, A.; Chen, Z.; Luo, S.; Zheng, X.; Weng, J. GLP-1 Receptor Agonists (GLP-1RAs): Cardiovascular Actions and Therapeutic Potential. Int. J. Biol. Sci. 2021, 17, 2050. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A.F.H. The Evolving Story of Incretins (GIP and GLP-1) in Metabolic and Cardiovascular Disease: A Pathophysiological Update. Diabetes Obes. Metab. 2021, 23, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Ussher, J.R.; McLean, B.A.; Cao, X.; Kabir, M.G.; Mulvihill, E.E.; Mighiu, A.S.; Zhang, H.; Ludwig, A.; Seeley, R.J. The Autonomic Nervous System and Cardiac GLP-1 Receptors Control Heart Rate in Mice. Mol. Metab. 2017, 6, 1339–1349. [Google Scholar] [CrossRef]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. Lausanne 2018, 9, 672. [Google Scholar] [CrossRef]
- Sposito, A.C.; Berwanger, O.; de Carvalho, L.S.F.; Saraiva, J.F.K. GLP-1RAs in Type 2 Diabetes: Mechanisms That Underlie Cardiovascular Effects and Overview of Cardiovascular Outcome Data. Cardiovasc. Diabetol. 2018, 17, 157. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F. Glucagon-like Peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Li, J.; Zheng, J.; Wang, S.; Lau, H.K.; Fathi, A.; Wang, Q. Cardiovascular Benefits of Native GLP-1 and Its Metabolites: An Indicator for GLP-1-Therapy Strategies. Front. Physiol. 2017, 8, 15. [Google Scholar] [CrossRef]
- Natali, A.; Nesti, L.; Tricò, D.; Ferrannini, E. Effects of GLP-1 Receptor Agonists and SGLT-2 Inhibitors on Cardiac Structure and Function: A Narrative Review of Clinical Evidence. Cardiovasc. Diabetol. 2021, 20, 196. [Google Scholar] [CrossRef]
- Husain, M.; Bain, S.C.; Jeppesen, O.K.; Lingvay, I.; Sørrig, R.; Treppendahl, M.B.; Vilsbøll, T. Semaglutide (SUSTAIN and PIONEER) Reduces Cardiovascular Events in Type 2 Diabetes across Varying Cardiovascular Risk. Diabetes. Obes. Metab. 2020, 22, 442–451. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef]
- Giglio, R.V.; Patti, A.M.; Rizvi, A.A.; Stoian, A.P.; Ciaccio, M.; Papanas, N.; Janez, A.; Sonmez, A.; Banach, M.; Sahebkar, A.; et al. Advances in the Pharmacological Management of Diabetic Nephropathy: A 2022 International Update. Biomedicines 2023, 11, 291. [Google Scholar] [CrossRef]
- Bechlioulis, A.; Markozannes, G.; Chionidi, I.; Liberopoulos, E.; Naka, K.K.; Ntzani, E.E.; Liatis, S.; Rizzo, M.; Rizos, E.C. The effect of SGLT2 inhibitors, GLP1 agonists, and their sequential combination on cardiometabolic parameters: A randomized, prospective, intervention study. J. Diabetes Complicat. 2023, 37, 108436. [Google Scholar] [CrossRef]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front. Endocrinol. Lausanne 2020, 11, 178. [Google Scholar] [CrossRef]
- Takahashi, A.; Asakura, M.; Ito, S.; Min, K.-D.; Shindo, K.; Yan, Y.; Liao, Y.; Yamazaki, S.; Sanada, S.; Asano, Y. Dipeptidyl-Peptidase IV Inhibition Improves Pathophysiology of Heart Failure and Increases Survival Rate in Pressure-Overloaded Mice. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1361–H1369. [Google Scholar] [CrossRef]
- Deedwania, P.; Acharya, T. Cardiovascular Protection with Anti-Hyperglycemic Agents. Am. J. Cardiovasc. Drugs 2019, 19, 249–257. [Google Scholar] [CrossRef]
- Furukawa, N.; Koitabashi, N.; Matsui, H.; Sunaga, H.; Umbarawan, Y.; Syamsunarno, M.R.A.A.; Yamaguchi, A.; Obokata, M.; Hanaoka, H.; Yokoyama, T. DPP-4 Inhibitor Induces FGF21 Expression via Sirtuin 1 Signaling and Improves Myocardial Energy Metabolism. Heart Vessel. 2021, 36, 136–146. [Google Scholar] [CrossRef]
- Salazar, J.; Rojas-Quintero, J.; Cano, C.; Pérez, J.L.; Ramírez, P.; Carrasquero, R.; Torres, W.; Espinoza, C.; Chacín-González, M.; Bermúdez, V. Neprilysin: A Potential Therapeutic Target of Arterial Hypertension? Curr. Cardiol. Rev. 2020, 16, 25–35. [Google Scholar]
- Arendse, L.B.; Danser, A.H.J.; Poglitsch, M.; Touyz, R.M.; Burnett, J.C.; Llorens-Cortes, C.; Ehlers, M.R.; Sturrock, E.D. Novel Therapeutic Approaches Targeting the Renin-Angiotensin System and Associated Peptides in Hypertension and Heart Failure. Pharmacol. Rev. 2019, 71, 539–570. [Google Scholar] [CrossRef]
- da Silva, G.J.J.; Altara, R.; Booz, G.W.; Cataliotti, A. Atrial Natriuretic Peptide31–67: A Novel Therapeutic Factor for Cardiovascular Diseases. Front. Physiol. 2021, 12, 691407. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Zraika, S. Neprilysin Inhibition: A New Therapeutic Option for Type 2 Diabetes? Diabetologia 2019, 62, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE−/− and LDLr−/− Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Rubattu, S.; Battistoni, A. ARNi: A Novel Approach to Counteract Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 2092. [Google Scholar] [CrossRef]
- Campbell, D.J. Neprilysin Inhibitors and Bradykinin. Front. Med. Lausanne 2018, 5, 257. [Google Scholar] [CrossRef]
- Seferovic, J.P.; Claggett, B.; Seidelmann, S.B.; Seely, E.W.; Packer, M.; Zile, M.R.; Rouleau, J.L.; Swedberg, K.; Lefkowitz, M.; Shi, V.C. Effect of Sacubitril/Valsartan versus Enalapril on Glycaemic Control in Patients with Heart Failure and Diabetes: A Post-Hoc Analysis from the PARADIGM-HF Trial. Lancet. Diabetes Endocrinol. 2017, 5, 333–340. [Google Scholar] [CrossRef]
- Dargad, R.R.; Prajapati, M.R.; Dargad, R.R.; Parekh, J.D. Sacubitril/Valsartan: A Novel Angiotensin Receptor-Neprilysin Inhibitor. Indian Heart J. 2018, 70, S102–S110. [Google Scholar] [CrossRef]
- Jordan, J.; Stinkens, R.; Jax, T.; Engeli, S.; Blaak, E.E.; May, M.; Havekes, B.; Schindler, C.; Albrecht, D.; Pal, P. Improved Insulin Sensitivity with Angiotensin Receptor Neprilysin Inhibition in Individuals with Obesity and Hypertension. Clin. Pharmacol. Ther. 2017, 101, 254–263. [Google Scholar] [CrossRef]
- Primeau, J.O.; Armanious, G.P.; Fisher, M.E.; Young, H.S. The Sarcoendoplasmic Reticulum Calcium ATPase. Subcell. Biochem. 2018, 87, 229–258. [Google Scholar]
- Demkes, E.J.; Wenker, S.; Silvis, M.J.M.; van Nieuwburg, M.M.J.; Visser, M.J.; Jansen, M.S.; Brans, M.A.D.; Velema, E.; Sluijter, J.P.G.; Hoefer, I.E. Neutral Effects of Combined Treatment with GLP-1R Agonist Exenatide and MR Antagonist Potassium Canrenoate on Cardiac Function in Porcine and Murine Chronic Heart Failure Models. Front. Pharmacol. 2021, 12, 702326. [Google Scholar] [CrossRef]
- Chen, Y.; Xin, Y.; Cheng, Y.; Liu, X. Mitochondria-Endoplasmic Reticulum Contacts: The Promising Regulators in Diabetic Cardiomyopathy. Oxid. Med. Cell Longev. 2022, 2022, 2531458. [Google Scholar] [CrossRef]
- Sivasankar, D.; George, M.; Sriram, D.K. Novel Approaches in the Treatment of Diabetic Cardiomyopathy. Biomed. Pharm. 2018, 106, 1039–1045. [Google Scholar] [CrossRef]
- Lu, J.; Dai, Q.M.; Ma, G.S.; Zhu, Y.H.; Chen, B.; Li, B.; Yao, Y.Y. Erythropoietin Attenuates Cardiac Dysfunction in Rats by Inhibiting Endoplasmic Reticulum Stress-Induced Diabetic Cardiomyopathy. Cardiovasc. Drugs Ther. 2017, 31, 367–379. [Google Scholar] [CrossRef]
- Voors, A.A.; Kremer, D.; Geven, C.; terMaaten, J.M.; Struck, J.; Bergmann, A.; Pickkers, P.; Metra, M.; Mebazaa, A.; Düngen, H. Adrenomedullin in Heart Failure: Pathophysiology and Therapeutic Application. Eur. J. Heart Fail. 2019, 21, 163–171. [Google Scholar] [CrossRef]
- Kita, T.; Kitamura, K. Translational Studies of Adrenomedullin and Related Peptides Regarding Cardiovascular Diseases. Hypertens Res. 2022, 45, 389–400. [Google Scholar] [CrossRef]
- Khanra, R.; Dewanjee, S.; Dua, T.K.; Sahu, R.; Gangopadhyay, M.; De Feo, V.; Zia-Ul-Haq, M. Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J. Transl. Med. 2015, 13, 6. [Google Scholar] [CrossRef]
- Byrne, N.J.; Rajasekaran, N.S.; Abel, E.D.; Bugger, H. Therapeutic Potential of Targeting Oxidative Stress in Diabetic Cardiomyopathy. Free Radic. Biol. Med. 2021, 169, 317–342. [Google Scholar] [CrossRef]
- de Geest, B.; Mishra, M. Role of Oxidative Stress in Diabetic Cardiomyopathy. Antioxidants 2022, 11, 784. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Koh, H.M.; Lim, S.Y.; Choudhury, H.; Pandey, M. Molecular and Biochemical Pathways of Catalpol in Alleviating Diabetes Mellitus and Its Complications. Biomolecules 2021, 11, 323. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Li, Q.; Gao, X.; Sugano, E.; Tomita, H.; Yang, L.; Shi, S. Thioredoxin 2 Offers Protection against Mitochondrial Oxidative Stress in H9c2 Cells and against Myocardial Hypertrophy Induced by Hyperglycemia. Int. J. Mol. Sci. 2017, 18, 1958. [Google Scholar] [CrossRef]
- Stancill, J.S.; Corbett, J.A. The Role of Thioredoxin/Peroxiredoxin in the β-Cell Defense Against Oxidative Damage. Front. Endocrinol. Lausanne 2021, 12, 718235. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.M.; Abd El-Ghafar, O.A.M.; Ahmed, M.A.; Sayed, A.M.; Gad-Elrab, W.M.; Ajarem, J.S.; Allam, A.A.; Mahmoud, A.M. Edaravone and Acetovanillone Upregulate Nrf2 and PI3K/Akt/MTOR Signaling and Prevent Cyclophosphamide Cardiotoxicity in Rats. Drug. Des. Devel. Ther. 2020, 14, 5275. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zeng, Y.-Q.; Gu, J.-H.; Song, R.; Cang, P.-H.; Xu, Y.-X.; Shao, X.; Pu, L.-J.; Luo, H.-Y.; Zhou, X.-F. Novel Oral Edaravone Attenuates Diastolic Dysfunction of Diabetic Cardiomyopathy by Activating the Nrf2 Signaling Pathway. Eur. J. Pharmacol. 2022, 920, 174846. [Google Scholar] [CrossRef] [PubMed]
- Albadrani, G.M.; BinMowyna, M.N.; Bin-Jumah, M.N.; El–Akabawy, G.; Aldera, H.; Al-Farga, A.M. Quercetin Prevents Myocardial Infarction Adverse Remodeling in Rats by Attenuating TGF-Β1/Smad3 Signaling: Different Mechanisms of Action. Saudi. J. Biol. Sci. 2021, 28, 2772–2782. [Google Scholar] [CrossRef]
- Chen, W.-J.; Cheng, Y.; Li, W.; Dong, X.-K.; Wei, J.; Yang, C.-H.; Jiang, Y.-H. Quercetin Attenuates Cardiac Hypertrophy by Inhibiting Mitochondrial Dysfunction through SIRT3/PARP-1 Pathway. Front. Pharmacol. 2021, 12, 739615. [Google Scholar] [CrossRef]
- Mattera, R.; Benvenuto, M.; Giganti, M.G.; Tresoldi, I.; Pluchinotta, F.R.; Bergante, S.; Tettamanti, G.; Masuelli, L.; Manzari, V.; Modesti, A. Effects of Polyphenols on Oxidative Stress-Mediated Injury in Cardiomyocytes. Nutrients 2017, 9, 523. [Google Scholar] [CrossRef]
- Komici, K.; Conti, V.; Davinelli, S.; Bencivenga, L.; Rengo, G.; Filippelli, A.; Ferrara, N.; Corbi, G. Cardioprotective Effects of Dietary Phytochemicals on Oxidative Stress in Heart Failure by a Sex-Gender-Oriented Point of View. Oxid. Med. Cell Longev. 2020, 2020, 2176728. [Google Scholar] [CrossRef]
- Barteková, M.; Adameová, A.; Görbe, A.; Ferenczyová, K.; Pecháňová, O.; Lazou, A.; Dhalla, N.S.; Ferdinandy, P.; Giricz, Z. Natural and Synthetic Antioxidants Targeting Cardiac Oxidative Stress and Redox Signaling in Cardiometabolic Diseases. Free Radic. Biol. Med. 2021, 169, 446–477. [Google Scholar] [CrossRef]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin Protects against Diabetic Cardiomyopathy by Inhibiting NF-ΚB-Mediated Inflammation and Activating the Nrf2-Mediated Antioxidant Responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef]
- Chen, X.; Qian, J.; Wang, L.; Li, J.; Zhao, Y.; Han, J.; Khan, Z.; Chen, X.; Wang, J.; Liang, G. Kaempferol Attenuates Hyperglycemia-Induced Cardiac Injuries by Inhibiting Inflammatory Responses and Oxidative Stress. Endocrine 2018, 60, 83–94. [Google Scholar] [CrossRef]
- Du, Y.; Han, J.; Zhang, H.; Xu, J.; Jiang, L.; Ge, W. Kaempferol Prevents against Ang II-Induced Cardiac Remodeling through Attenuating Ang II-Induced Inflammation and Oxidative Stress. J. Cardiovasc. Pharmacol. 2019, 74, 326. [Google Scholar] [CrossRef]
- Escribano-Lopez, I.; Diaz-Morales, N.; Iannantuoni, F.; Lopez-Domenech, S.; de Marañon, A.M.; Abad-Jimenez, Z.; Bañuls, C.; Rovira-Llopis, S.; Herance, J.R.; Rocha, M. The Mitochondrial Antioxidant SS-31 Increases SIRT1 Levels and Ameliorates Inflammation, Oxidative Stress and Leukocyte-Endothelium Interactions in Type 2 Diabetes. Sci. Rep. 2018, 8, 15862. [Google Scholar] [CrossRef]
- Escribano-López, I.; de Marañon, A.M.; Iannantuoni, F.; López-Domènech, S.; Abad-Jiménez, Z.; Díaz, P.; Solá, E.; Apostolova, N.; Rocha, M.; Víctor, V.M. The Mitochondrial Antioxidant SS-31 Modulates Oxidative Stress, Endoplasmic Reticulum Stress, and Autophagy in Type 2 Diabetes. J. Clin. Med. 2019, 8, 1322. [Google Scholar] [CrossRef]
- Gao, L.; Yao, R.; Liu, Y.; Wang, Z.; Huang, Z.; Du, B.; Zhang, D.; Wu, L.; Xiao, L.; Zhang, Y. Isorhamnetin Protects against Cardiac Hypertrophy through Blocking PI3K–AKT Pathway. Mol. Cell Biochem. 2017, 429, 167–177. [Google Scholar] [CrossRef]
- Aonuma, K.; Ferdousi, F.; Xu, D.; Tominaga, K.; Isoda, H. Effects of Isorhamnetin in Human Amniotic Epithelial Stem Cells in vitro and Its Cardioprotective Effects in vivo. Front. Cell Dev. Biol. 2020, 8, 578197. [Google Scholar] [CrossRef]
- Duan, J.-Y.; Lin, X.; Xu, F.; Shan, S.-K.; Guo, B.; Li, F.-X.-Z.; Wang, Y.; Zheng, M.-H.; Xu, Q.-S.; Lei, L.-M. Ferroptosis and Its Potential Role in Metabolic Diseases: A Curse or Revitalization? Front. Cell Dev. Biol. 2021, 9, 701788. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Zhou, W.; Men, H.; Bao, T.; Sun, Y.; Wang, Q.; Tan, Y.; Keller, B.B.; Tong, Q. Ferroptosis Is Essential for Diabetic Cardiomyopathy and Is Prevented by Sulforaphane via AMPK/NRF2 Pathways. Acta Pharm. Sin. B 2022, 12, 708–722. [Google Scholar] [CrossRef]
- Nauck, M.A.; D‘Alessio, D.A. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc. Diabetol. 2022, 21, 169. [Google Scholar] [CrossRef]
- Hallakou-Bozec, S.; Kergoat, M.; Fouqueray, P.; Bolze, S.; Moller, D.E. Imeglimin amplifies glucose-stimulated insulin release from diabetic islets via a distinct mechanism of action. PLoS ONE 2021, 16, e0241651. [Google Scholar] [CrossRef]
- Hallakou-Bozec, S.; Vial, G.; Kergoat, M.; Fouqueray, P.; Bolze, S.; Borel, A.L.; Fontaine, E.; Moller, D.E. Mechanism of action of Imeglimin: A novel therapeutic agent for type 2 diabetes. Diabetes Obes. Metab. 2021, 23, 664–673. [Google Scholar] [CrossRef]
- Prakoso, D.; de Blasio, M.J.; Tate, M.; Kiriazis, H.; Donner, D.G.; Qian, H.; Nash, D.; Deo, M.; Weeks, K.L.; Parry, L.J. Gene Therapy Targeting Cardiac Phosphoinositide 3-Kinase (P110α) Attenuates Cardiac Remodeling in Type 2 Diabetes. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H840–H852. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Katare, R. Molecular Mechanism of Diabetic Cardiomyopathy and Modulation of MicroRNA Function by Synthetic Oligonucleotides. Cardiovasc. Diabetol. 2018, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Guarini, G.; Huqi, A.; Morrone, D.; Capozza, P.F.G.; Marzilli, M. Trimetazidine and Other Metabolic Modifiers. Eur. Cardiol. 2018, 13, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Schwemmlein, J.; Maack, C.; Bertero, E. Mitochondria as Therapeutic Targets in Heart Failure. Curr. Heart Fail Rep. 2022, 19, 27–37. [Google Scholar] [CrossRef]
- Tang, S.-G.; Liu, X.-Y.; Wang, S.-P.; Wang, H.-H.; Jovanović, A.; Tan, W. Trimetazidine Prevents Diabetic Cardiomyopathy by Inhibiting Nox2/TRPC3-Induced Oxidative Stress. J. Pharmacol. Sci. 2019, 139, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fu, J.; Liu, B.; Wang, R.; You, T. A Synthetic Curcuminoid Analog,(2 E, 6 E)-2, 6-Bis (2-(Trifluoromethyl) Benzylidene) Cyclohexanone, Ameliorates Impaired Wound Healing in Streptozotocin-Induced Diabetic Mice by Increasing MiR-146a. Molecules 2020, 25, 920. [Google Scholar] [CrossRef]
- Liu, P.; Liu, J.; Wu, Y.; Xi, W.; Wei, Y.; Yuan, Z.; Zhuo, X. Zinc Supplementation Protects against Diabetic Endothelial Dysfunction via GTP Cyclohydrolase 1 Restoration. Biochem. Biophys. Res. Commun. 2020, 521, 1049–1054. [Google Scholar] [CrossRef]
- Gu, J.; Yan, X.; Dai, X.; Wang, Y.; Lin, Q.; Xiao, J.; Zhou, S.; Zhang, J.; Wang, K.; Zeng, J. Metallothionein Preserves Akt2 Activity and Cardiac Function via Inhibiting TRB3 in Diabetic Hearts. Diabetes 2018, 67, 507–517. [Google Scholar] [CrossRef]
- Shukla, S.K.; Rafiq, K. Proteasome Biology and Therapeutics in Cardiac Diseases. Transl. Res. 2019, 205, 64–76. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Wiersma, M.; Brundel, B.J.J.M. Role of Autophagy in Proteostasis: Friend and Foe in Cardiac Diseases. Cells 2018, 7, 279. [Google Scholar] [CrossRef]
- Li, C.; Miao, X.; Lou, Y.; Lu, Z.; Adhikari, B.K.; Wang, Y.; Liu, Q.; Sun, J.; Wang, Y. Cardioprotective Effects of the Novel Curcumin Analogue C66 in Diabetic Mice Is Dependent on JNK 2 Inactivation. J. Cell Mol. Med. 2018, 22, 6314–6326. [Google Scholar] [CrossRef]
- Huang, S.; Wang, J.; Men, H.; Tan, Y.; Lin, Q.; Gozal, E.; Zheng, Y.; Cai, L. Cardiac Metallothionein Overexpression Rescues Diabetic Cardiomyopathy in Akt2-knockout Mice. J. Cell Mol. Med. 2021, 25, 6828–6840. [Google Scholar] [CrossRef]
- Gumà, A.; Díaz-Sáez, F.; Camps, M.; Zorzano, A. Neuregulin, an Effector on Mitochondria Metabolism That Preserves Insulin Sensitivity. Front. Physiol. 2020, 11, 696. [Google Scholar] [CrossRef]
- de Keulenaer, G.W.; Feyen, E.; Dugaucquier, L.; Shakeri, H.; Shchendrygina, A.; Belenkov, Y.N.; Brink, M.; Vermeulen, Z.; Segers, V.F.M. Mechanisms of the Multitasking Endothelial Protein NRG-1 as a Compensatory Factor during Chronic Heart Failure. Circ. Heart Fail 2019, 12, e006288. [Google Scholar] [CrossRef]
- Ahmed, U.; Ashfaq, U.A.; Qasim, M.; Ahmad, I.; Ahmad, H.U.; Tariq, M.; Masoud, M.S.; Khaliq, S. Dysregulation of Circulating MiRNAs Promotes the Pathogenesis of Diabetes-Induced Cardiomyopathy. PLoS ONE 2021, 16, e0250773. [Google Scholar] [CrossRef]
- Guo, R.; Nair, S. Role of microRNA in diabetic cardiomyopathy: From mechanism to intervention. Biochim. Biophys. Acta Mol. Basis. Dis. 2017, 1863, 2070–2077. [Google Scholar] [CrossRef]
- Rawal, S.; Manning, P.; Katare, R. Cardiovascular microRNAs: As modulators and diagnostic biomarkers of diabetic heart disease. Cardiovasc. Diabetol. 2014, 13, 44. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, X.; Chen, X.; Yang, S.; Chen, H. Inhibition of MiR-223 Attenuates the NLRP3 Inflammasome Activation, Fibrosis, and Apoptosis in Diabetic Cardiomyopathy. Life Sci. 2020, 256, 117980. [Google Scholar] [CrossRef]

| Sl. No. | Agents | Mechanisms | References |
|---|---|---|---|
| 1 | Coenzyme Q10 | It elicits protection for cardiomyocyte function by augmenting antioxidant properties. It reduces cardiac inflammation, fibrosis, and hypertrophy induced by T1DM and T2DM. | [138,139] |
| 2 | Catalase | Upregulation of catalase causes improvement in the cardiac morphology, mitochondrial, and myofibrillar characters and cardiomyocyte contractility with a significant reduction in the levels of ROS. It has been found to ameliorate diabetes-induced autophagy by increasing NF-κB activity. | [61,140] |
| 3 | Thioredoxin | Thioredoxin 2 acts like a mitochondrial antioxidant that offers protection against oxidative stress. Overexpression of thioredoxin 2 has been reported to reduce high glucose-induced mitochondrial oxidative damage along with decreasing expression of ANP and BNP. Loss of thioredoxin 2 has been found to induce cardiomyocyte hypertrophy. | [141,142] |
| 4 | Edaravone | Edaravone inhibits fibrosis and cardiac apoptosis by activating Nrf2, NADP quinone oxidoreductase and heme oxygenase. Increased activity of sirtuin 1 and PGC-1α by edaravone has been reported. Additionally, it reduces apoptosis by increasing Bcl-2 expression and reducing Bax and caspase-3 expressions in cardiomyocytes. | [143,144] |
| 5 | Quercetin | Quercetin prevents cardiac remodeling by promoting Nrf-2 and inhibiting NF-κB signaling. It inhibits the RAAS pathway, decreases expression of TGF-β1, and subsequently reduces deposition of the ECM. Additionally, it modulates the sirtuin 3/PARP-1 pathway and inhibits cardiac hypertrophy. | [145,146] |
| 6 | Taxifolin | Taxifolin exerts an antifibrotic effect by inhibiting TGF-β/SMAD signaling. It has been found to improve diastolic dysfunction, ameliorate myocardium structure abnormality, and enhance endogenous antioxidant enzyme activities. It also reduces angiotensin II levels in the myocardium and inhibits NADPH oxidase activity. It has been found to prevent myocyte apoptosis by inhibiting caspase-3 and caspase-9 activation and restoring mitochondrial membrane potential. | [147,148] |
| 7 | Luteolin | Luteolin protects against DCM by inhibiting NF-κB and activating the Nrf2-mediated antioxidant responses. It inhibits TGF-β1, NOX4, and NOX2. | [149,150] |
| 8 | Kaempferol | Kaempferol inhibits NF-κB and endorses Nrf-2 activation. It prevents diabetes-induced cardiac fibrosis and apoptosis. | [151,152] |
| 9 | Szeto–Schiller peptide (SS31) | SS31 is a positively charged free radical scavenger that accumulates in the mitochondria and prevents diastolic dysfunction, myocardial fibrosis, and subsequent cardiac hypertrophy. SS31 has been reported to mitigate oxidative stress, autophagy, and ER stress. | [153,154] |
| 10 | Isorhamnetin | Isorhamnetin reduces cardiac fibrosis and hypertrophy. It improves insulin signaling and restores the arrangement of myofibrils. It upregulated Akt-2, microRNA (miR)-1, and miR-3163 expression in skeletal muscle and adipose tissue. | [155,156] |
| 11 | Sulphoraphane | Sulforaphane prevents DCM via the upregulation of Nrf2 and metallothionein. It also prevents ferroptosis and associated pathogenesis via AMPK-mediated NRF2 activation. | [157,158] |
| Sl no. | Targets/Agents | Mechanisms | References |
|---|---|---|---|
| 1 | Cardiac PI3K (p110α) signaling pathway | Increased activation of the p110α pathway leads to improved diastolic dysfunction, cardiomyocyte hypertrophy, myocardial fibrosis, and programmed cell death in diabetic subjects, thus preserving ventricular function as well as augmenting cardiac structural remodeling. The beneficial effect of recombinant adeno-associated viral vectors carrying a constitutively active PI3K construct (rAAV6-caPI3K) in T2DM animals was studied. rAAV6-caPI3K gene-bearing animals showed a reduction in diabetes-induced cardiac remodeling by preventing cardiac fibrosis and cardiomyocyte hypertrophy. Additionally, LV reactive oxygen species and ER stress were reduced. | [22,162,163] |
| 2 | Long-chain 3-ketoacyl-CoA thiolase inhibitors | Trimetazidine (TMZ) is a competitive inhibitor of the long-chain 3-ketoacyl-CoA thiolase involved in the β-oxidation of fatty acids, which potentially improves myocardial metabolic or substrate utilization and reduces calcium overload, and ROS-induced cell injury. It reduces FFA utilization and enhances glucose oxidation along with decreasing insulin resistance. TMZ has been shown to be cardioprotective in DCM. TMZ treatment reciprocated LV dysfunction, cardiac hypertrophy, fibrosis, inflammation, and oxidative stress in the myocardium. Additionally, TMZ treatment inhibited diabetes-associated structural and functional alterations by inhibiting NADPH oxidase 2 and transient receptor potential channel 3. Furthermore, the administration of TMZ in an early stage of diabetes may inhibit the progression of DCM by inhibiting myocardial fibrosis and cardiomyocyte apoptosis and enhancing autophagy. TMZ has been found to reverse myocardial remodeling and reduce the deposition of collagen I and III content. | [164,165,166] |
| 3 | Metallothioneins (MTs) | MTs involved in the regulation of the intracellular zinc concentration have received significant attention due to the fact that supplementation with zinc has been found to be beneficial in the management of T2DM. MTs are considered a key regulator of zinc metabolism, and the redox process controlled by them causes the simultaneous release and regeneration of zinc-binding capacity. MTs have been found to be involved in the attenuation of oxidative stress by the scavenging of superoxide and hydroxyl radicals. MT prevents DCM and increases the expression of proteins associated with glucose metabolism. MT has been found to preserve Akt2 activity and cardiac function by inhibiting tribbles pseudokinase 3 (TRB3). Cardiac MT overexpression in Akt2 knockout mice was found to prevent pathological changes associated with DCM. | [167,168,169] |
| 4 | E3 ubiquitin ligase | The E3 ubiquitin ligases (E3s), the components of the ubiquitin-proteasome system, are believed to play a key role in the progression of DCM due to their involvement in cardiac hypertrophy, increased apoptosis, fibrosis, and altered insulin metabolism. In animal models of T2DM, it has been seen that there is an increase in the expression of E3s in the cardiac tissue, leading to proteasomal degradation of the insulin receptor and insulin receptor substrates, converging to a state of insulin resistance. | [170,171] |
| 5 | A novel curcumin analog, C66, in the management of DCM | C66[(2E,6E)-2,6-bis(2-(trifluoromethyl)benzylidene)cyclohexanone], a curcumin analogue, could be beneficial in the management of DCM. It has been reported to reduce hypertriglyceridemia in diabetic animals, along with reducing plasma and cardiac triglyceride levels. Additionally, it also inhibits Jun NH2-terminal kinase (JNK) and NF-κB activation in the heart. | [172,173] |
| 6 | Neuregulins (NRG) | NRG-1 is involved in cardiac damage adaptability, as well as maintenance of the shape of cardiomyocytes to limit apoptosis and increase cardiomyocyte proliferation. NRG-1 promotes mitochondrial homeostasis and stability and is considered to be an agent with the potential to ameliorate heart failure and other metabolic dysregulation and inflammation-related diseases such as obesity and T2DM. | [96,174,175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, N.; Chacko, L.; Bhattacharya, H.; Vallamkondu, J.; Nag, S.; Dey, A.; Karmakar, T.; Reddy, P.H.; Kandimalla, R.; Dewanjee, S. Exploring the Complex Relationship between Diabetes and Cardiovascular Complications: Understanding Diabetic Cardiomyopathy and Promising Therapies. Biomedicines 2023, 11, 1126. https://doi.org/10.3390/biomedicines11041126
Ghosh N, Chacko L, Bhattacharya H, Vallamkondu J, Nag S, Dey A, Karmakar T, Reddy PH, Kandimalla R, Dewanjee S. Exploring the Complex Relationship between Diabetes and Cardiovascular Complications: Understanding Diabetic Cardiomyopathy and Promising Therapies. Biomedicines. 2023; 11(4):1126. https://doi.org/10.3390/biomedicines11041126
Chicago/Turabian StyleGhosh, Nilanjan, Leena Chacko, Hiranmoy Bhattacharya, Jayalakshmi Vallamkondu, Sagnik Nag, Abhijit Dey, Tanushree Karmakar, P. Hemachandra Reddy, Ramesh Kandimalla, and Saikat Dewanjee. 2023. "Exploring the Complex Relationship between Diabetes and Cardiovascular Complications: Understanding Diabetic Cardiomyopathy and Promising Therapies" Biomedicines 11, no. 4: 1126. https://doi.org/10.3390/biomedicines11041126
APA StyleGhosh, N., Chacko, L., Bhattacharya, H., Vallamkondu, J., Nag, S., Dey, A., Karmakar, T., Reddy, P. H., Kandimalla, R., & Dewanjee, S. (2023). Exploring the Complex Relationship between Diabetes and Cardiovascular Complications: Understanding Diabetic Cardiomyopathy and Promising Therapies. Biomedicines, 11(4), 1126. https://doi.org/10.3390/biomedicines11041126










