Pathologic Characteristics of Somatotroph Pituitary Tumors—An Observational Single-Center Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Pathological Evaluation
2.3. Statistical Analysis
- *
- Mann–Whitney’s U test to compare numerical clinical, imaging, or pathological variables between patients with DG and SG tumors, as well as between α-SU-positive and α-SU-negative tumors;
- *
- Kruskal–Wallis H test to compare numerical variables between pure GH(+), mixed GH(+)/PRL(+), and plurihormonal tumors, as well as between 3 Ki-67 index intervals;
- *
- Pearson’s chi-square test to compare categorical variables if all the expected counts were at least equal to 5;
- *
- Fisher’s exact test to compare categorical variables if any expected counts were smaller than 5.
3. Results
3.1. Characteristic of the Study Group
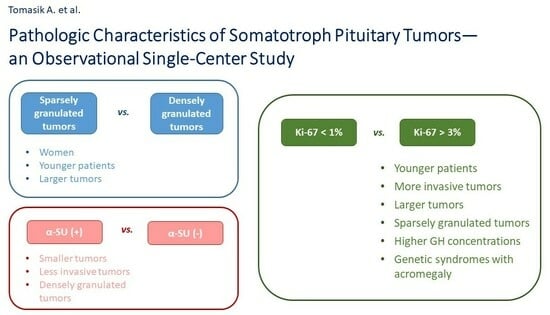
3.2. Comparison of Sparsely and Densely Granulated Tumors
3.3. Comparison of Pure GH(+), GH(+)/PRL(+), and Plurihormonal Tumors
3.4. Comparison of Tumors with Positive and Negative α-SU Staining
3.5. Comparison of Tumors with Various Values of Ki-67 Index
4. Discussion
5. Limitations and Strengths of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GH | growth hormone |
| IGF-1 | insulin-like growth factor 1 |
| WHO | World Health Organization |
| LMWCs | low-molecular-weight cytokeratins |
| SSTR | somatostatin receptor |
| EM | electron microscopy |
| SG | sparsely granulated |
| DG | densely granulated |
| α-SU | α-subunit |
| TSH | thyrotropin-secreting hormone |
| LH | luteinizing hormone |
| FSH | follicle-stimulating hormone |
| IHC | immunohistochemistry |
| PRL | prolactin |
| Mm | millimeters |
| ACTH | adrenocorticotropic hormone |
| Me | median |
| IQR | interquartile range |
| ULN | upper limits of normal |
| MEN 1 | Multiple Endocrine Neoplasia Type 1 |
| FIPA | Familial Isolated Pituitary Adenomas |
References
- Colao, A.; Amato, G.; Pedroncelli, A.M.; Baldelli, R.; Grottoli, S.; Gasco, V.; Petretta, M.; Carella, C.; Pagani, G.; Tamburano, G.; et al. Gender- and age-related differences in the endocrine parameters of acromegaly. J. Endocrinol. Investig. 2002, 25, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wu, Z.B. New pathological classification and clinical implications of pituitary neuroendocrine tumors of the 2022 WHO version. Zhonghua Yi Xue Za Zhi 2022, 102, 3723–3726. [Google Scholar] [PubMed]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Akirov, A.; Asa, S.L.; Amer, L.; Shimon, I.; Ezzat, S. The Clinicopathological Spectrum of Acromegaly. J. Clin. Med. 2019, 8, 1962. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.; Davoodi, Z.; Bidari, F.; Moghaddam, A.M.; Khalili, D.; Bahrami-Motlagh, H.; Jamali, E.; Alamdari, S.; Hosseinpanah, F.; Hedayati, M.; et al. Association of different pathologic subtypes of growth hormone producing pituitary adenoma and remission in acromegaly patients: A retrospective cohort study. BMC Endocr. Disord. 2021, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Kiseljak-Vassiliades, K.; Carlson, N.E.; Borges, M.T.; Kleinschmidt-DeMasters, B.K.; Lillehei, K.O.; Kerr, J.M.; Wierman, M.E. Growth hormone tumor histological subtypes predict response to surgical and medical therapy. Endocrine 2015, 49, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Vantyghem, M.C.; Cortet, C.; Bauters, C.; Gevaert, M.H.; Dewailly, D.; Lefebvre, J.; Mazzucca, M. Immunohistochemical detection of glycoprotein hormone alpha subunit in somatoprolactinic and pure somatotroph adenomas. J. Endocrinol. Investig. 1998, 21, 434–440. [Google Scholar] [CrossRef]
- Trouillas, J.; Jaffrain-Rea, M.L.; Vasiljevic, A.; Raverot, G.; Roncaroli, F.; Villa, C. How to Classify the Pituitary Neuroendocrine Tumors (PitNET)s in 2020. Cancers 2020, 12, 514. [Google Scholar] [CrossRef]
- Landolt, A.M.; Shibata, T.; Kleihues, P. Growth rate of human pituitary adenomas. J. Neurosurg. 1987, 67, 803–806. [Google Scholar] [CrossRef]
- Gerdes, J. Ki-67 and other proliferation markers useful for immunohistological diagnostic and prognostic evaluations in human malignancies. Semin. Cancer Biol. 1990, 1, 199–206. [Google Scholar]
- Cattoretti, G.; Becker, M.H.; Key, G.; Duchrow, M.; Schlüter, C.; Galle, J.; Gerdes, J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J. Pathol. 1992, 168, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Biller, B.M.K.; Freda, P.U.; Gadelha, M.R.; Giustina, A.; Katznelson, L.; Molitch, M.E.; Samson, S.L.; Strasburger, C.J.; van der Lely, A.J.; et al. A Pituitary Society update to acromegaly management guidelines. Pituitary 2021, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jane, J.A., Jr.; Starke, R.M.; Elzoghby, M.A.; Reames, D.L.; Payne, S.C.; Thorner, M.O.; Marshall, J.C.; Laws, E.R., Jr.; Vance, M.L. Endoscopic transsphenoidal surgery for acromegaly: Remission using modern criteria, complications, and predictors of outcome. J. Clin. Endocrinol. Metab. 2011, 96, 2732–2740. [Google Scholar] [CrossRef]
- Coopmans, E.C.; Postma, M.R.; Wolters, T.L.C.; van Meyel, S.W.F.; Netea-Maier, R.; van Beek, A.P.; Neggers, S.J. Predictors for Remission after Transsphenoidal Surgery in Acromegaly: A Dutch Multicenter Study. J. Clin. Endocrinol. Metab. 2021, 106, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Bolanowski, M.; Ruchała, M.; Zgliczyński, W.; Kos-Kudła, B.; Hubalewska-Dydejczyk, A.; Lewiński, A. Diagnostics and treatment of acromegaly—Updated recommendations of the Polish Society of Endocrinology. Endokrynol. Pol. 2019, 70, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Van der Lely, A.J.; Kuhn, E.; Muhammad, A.; Coopmans, E.C.; Neggers, S.J.; Chanson, P. Pegvisomant and not somatostatin receptor ligands (SRLs) is first-line medical therapy for acromegaly. Eur. J. Endocrinol. 2020, 182, D17–D29. [Google Scholar] [CrossRef] [PubMed]
- Mete, O.; Asa, S.L. Clinicopathological correlations in pituitary adenomas. Brain Pathol. 2012, 22, 443–453. [Google Scholar] [CrossRef]
- Sarkar, S.; Chacko, A.G.; Chacko, G. An analysis of granulation patterns, MIB-1 proliferation indices and p53 expression in 101 patients with acromegaly. Acta Neurochir. 2014, 156, 2221–2230; discussion 2230. [Google Scholar] [CrossRef]
- Kontogeorgos, G.; Markussis, V.; Thodou, E.; Kyrodimou, E.; Choreftaki, T.; Nomikos, P.; Lampropoulos, K.I.; Tsagarakis, S. Association of Pathology Markers with Somatostatin Analogue Responsiveness in Acromegaly. Int. J. Endocrinol. 2022, 2022, 8660470. [Google Scholar] [CrossRef]
- Swanson, A.A.; Erickson, D.; Donegan, D.M.; Jenkins, S.M.; Van Gompel, J.J.; Atkinson, J.L.D.; Erickson, B.J.; Giannini, C. Clinical, biological, radiological, and pathological comparison of sparsely and densely granulated somatotroph adenomas: A single center experience from a cohort of 131 patients with acromegaly. Pituitary 2021, 24, 192–206. [Google Scholar] [CrossRef]
- Larkin, S.; Reddy, R.; Karavitaki, N.; Cudlip, S.; Wass, J.; Ansorge, O. Granulation pattern, but not GSP or GHR mutation, is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur. J. Endocrinol. 2013, 168, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Rajaratnam, S.; Chacko, G.; Chacko, A.G. Endocrinological outcomes following endoscopic and microscopic transsphenoidal surgery in 113 patients with acromegaly. Clin. Neurol. Neurosurg. 2014, 126, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Obari, A.; Sano, T.; Ohyama, K.; Kudo, E.; Qian, Z.R.; Yoneda, A.; Rayhan, N.; Mustafizur Rahman, M.; Yamada, S. Clinicopathological features of growth hormone-producing pituitary adenomas: Difference among various types defined by cytokeratin distribution pattern including a transitional form. Endocr. Pathol. 2008, 19, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Heng, L.; Liu, X.; Jia, D.; Guo, W.; Zhang, S.; Gao, G.; Gong, L.; Qu, Y. Preoperative prediction of granulation pattern subtypes in GH-secreting pituitary adenomas. Clin. Endocrinol. 2021, 95, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Akkus, G.; Odabaş, F.; Sözütok, S.; Sert, M.; Ak, N.E.; Evran, M.; Tetiker, T. Novel Classification of Acromegaly in Accordance with Immunohistochemical Subtypes: Is There Really a Clinical Relevance? Horm. Metab. Res. 2022, 54, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgos, G.; Asa, S.L.; Kovacs, K.; Smyth, H.S.; Singer, W. Production of alpha-subunit of glycoprotein hormones by pituitary somatotroph adenomas in vitro. Acta Endocrinol. 1993, 129, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Hasanov, R.; Aydoğan, B.; Kiremitçi, S.; Erden, E.; Güllü, S. The Prognostic Roles of the Ki-67 Proliferation Index, P53 Expression, Mitotic Index, and Radiological Tumor Invasion in Pituitary Adenomas. Endocr. Pathol. 2019, 30, 49–55. [Google Scholar] [CrossRef]
- Stefanidis, P.; Kyriakopoulos, G.; Seretis, A.M.; Korfias, S.; Theocharis, S.; Angelousi, A. Prognostic Factors for Invasiveness and Recurrence of Pituitary Adenomas: A Series of 94 Patients. Diagnostics 2022, 12, 2413. [Google Scholar] [CrossRef]
- Sadeghipour, A.; Mahouzi, L.; Salem, M.M.; Ebrahim-Nejad, S.; Asadi-Lari, M.; Radfar, A.; Filip, I.; Babaheidarian, P. Ki67 Labeling Correlated with Invasion but Not with Recurrence. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 341–345. [Google Scholar] [CrossRef]
- Brito, J.; Sáez, L.; Lemp, M.; Liberman, C.; Michelsen, H.; Araya, A.V. Immunohistochemistry for pituitary hormones and Ki-67 in growth hormone producing pituitary adenomas. Rev. Med. Chil. 2008, 136, 831–836. [Google Scholar]
- Alimohamadi, M.; Ownagh, V.; Mahouzi, L.; Ostovar, A.; Abbassioun, K.; Amirjmshidi, A. The impact of immunohistochemical markers of Ki-67 and p53 on the long-term outcome of growth hormone-secreting pituitary adenomas: A cohort study. Asian J. Neurosurg. 2014, 9, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Kasuki, L.; Wildemberg, L.E.; Neto, L.V.; Marcondes, J.; Takiya, C.M.; Gadelha, M.R. Ki-67 is a predictor of acromegaly control with octreotide LAR independent of SSTR2 status and relates to cytokeratin pattern. Eur. J. Endocrinol. 2013, 169, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Fusco, A.; Zatelli, M.C.; Bianchi, A.; Cimino, V.; Tilaro, L.; Veltri, F.; Angelini, F.; Lauriola, L.; Vellone, V.; Doglietto, F.; et al. Prognostic significance of the Ki-67 labeling index in growth hormone-secreting pituitary adenomas. J. Clin. Endocrinol. Metab. 2008, 93, 2746–2750. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Cui, G.; Lu, C.; Qu, X.; Han, T. Role of Ki-67 in acromegalic patients with hyperprolactinemia: Retrospective analysis in 61 Chinese Patients. Pak. J. Pharm. Sci. 2015, 28 (Suppl. S2), 719–723. [Google Scholar] [PubMed]
- Mohseni, S.; Aboeerad, M.; Sharifi, F.; Tavangar, S.M.; Mohajeri-Tehrani, M. Associations of Ki-67 Labeling Index with Clinical and Paraclinical Features of Growth Hormone-Secreting Pituitary Adenomas: A Single Center Report from Iran. Int. J. Endocrinol. Metab. 2019, 17, e81983. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Mete, O.; Cusimano, M.D.; McCutcheon, I.E.; Perry, A.; Yamada, S.; Nishioka, H.; Casar-Borota, O.; Uccella, S.; La Rosa, S.; et al. Pituitary neuroendocrine tumors: A model for neuroendocrine tumor classification. Mod. Pathol. 2021, 34, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Antunes, X.; Ventura, N.; Camilo, G.B.; Wildemberg, L.E.; Guasti, A.; Pereira, P.J.M.; Camacho, A.H.; Chimelli, L.; Niemeyer, P.; Gadelha, M.R.; et al. Predictors of surgical outcome and early criteria of remission in acromegaly. Endocrine 2018, 60, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Iacovazzo, D.; Carlsen, E.; Lugli, F.; Chiloiro, S.; Piacentini, S.; Bianchi, A.; Giampietro, A.; Mormando, M.; Clear, A.J.; Doglietto, F.; et al. Factors predicting pasireotide responsiveness in somatotroph pituitary adenomas resistant to first-generation somatostatin analogues: An immunohistochemical study. Eur. J. Endocrinol. 2016, 174, 241–250. [Google Scholar] [CrossRef]
- Tomasik, A.; Stelmachowska-Banaś, M.; Maksymowicz, M.; Czajka-Oraniec, I.; Raczkiewicz, D.; Zieliński, G.; Kunicki, J.; Zgliczyński, W. Clinical, hormonal and pathomorphological markers of somatotroph pituitary neuroendocrine tumors predicting the treatment outcome in acromegaly. Front. Endocrinol. 2022, 13, 957301. [Google Scholar] [CrossRef]
| Variable | Unit or Category | N | Statistics | Results |
|---|---|---|---|---|
| Age | years | 120 | Min–Max, Me (IQR) | 23–74, 51 (44–62) |
| Age at diagnosis | years | 120 | Min–Max, Me (IQR) | 15–69, 44 (35–55) |
| Sex | male | 120 | n (%) | 53 (44.17) |
| female | 67 (55.83) | |||
| Diagnostic delay | years | 113 | Min–Max, Me (IQR) | 1–29, 5 (3–10) |
| Fasting GH at diagnosis | µg/L | 106 | Min–Max, Me (IQR) | 0.64–98.30, 11.70 (4.08–26.00) |
| IGF-1 at diagnosis | ng/mL | 103 | Min–Max, Me (IQR) | 294–1600, 811 (618–1054) |
| x ULN | 103 | Min–Max, Me (IQR) | 1.18–6.35, 3.31 (2.55–4.34) | |
| PRL at diagnosis | ng/mL | 89 | Min–Max, Me (IQR) | 2–407, 14.5 (9.0–27.9) |
| Hyperprolactinemia at diagnosis | yes | 89 | n (%) | 29 (32.58) |
| Pituitary axis dysfunction at diagnosis | at least one | 120 | n (%) | 38 (31.67) |
| FSH/LH | 28 (23.33) | |||
| ACTH | 9 (7.50) | |||
| TSH | 9 (7.50) | |||
| Clinical diagnosis of genetic syndrome associated with acromegaly | at least one | 120 | n (%) | 12 (10.00) |
| MEN1 | 6 (5.00) | |||
| FIPA | 4 (3.33) | |||
| McCune–Albright syndrome | 2 (1.67) | |||
| Visual field defects | no | 120 | n (%) | 102 (85.00) |
| small | 10 (8.33) | |||
| quadrantanopia | 8 (6.67) |
| Variable | Unit or Category | N | Statistics | Results |
|---|---|---|---|---|
| Tumor imaging in MRI | microadenoma | 116 | n (%) | 26 (22.41) |
| macroadenoma | 90 (77.59) | |||
| Maximal tumor diameter | mm | 102 | Min–Max, Me (IQR) | 5–51, 19 (11–25) |
| Extrasellar expansion | yes | 116 | n (%) | 60 (51.72) |
| Compression of the optic chiasm | yes | 116 | n (%) | 25 (21.55) |
| Degree of cavernous sinus invasion | no | 100 | n (%) | 49 (49.00) |
| unilateral | 39 (39.00) | |||
| bilateral | 12 (12.00) | |||
| Granulation subtype of tumor | densely | 108 | n (%) | 59 (54.63) |
| sparsely | 49 (45.37) | |||
| Immunohistochemical evaluation of tumor | pure GH(+) | 118 | n (%) | 63 (53.39) |
| GH(+)/PRL(+) | 46 (38.98) | |||
| plurihormonal | 9 (7.63) | |||
| alpha-SU | (−) | 117 | n (%) | 62 (52.99) |
| (+) | 55 (47.01) | |||
| Ki-67 Index | Ki67 < 1% | 116 | n (%) | 89 (76.72) |
| 1% ≤ Ki67 < 3% | 16 (13.79) | |||
| Ki67 ≥ 3% | 11 (9.48) |
| Variable | Unit or Category | Statistics | Sparsely Granulated | Densely Granulated | p | ||
|---|---|---|---|---|---|---|---|
| N | Results | N | Results | ||||
| Age | years | Me (IQR) | 49 | 47 (38–59) | 59 | 58 (45–64) | 0.051 |
| Age at diagnosis | years | Me (IQR) | 49 | 39 (32–49) | 59 | 46 (38–58) | 0.011 * |
| Sex | male | n (%) | 49 | 13 (26.53) | 59 | 35 (59.32) | 0.001 * |
| female | 36 (73.47) | 24 (40.68) | |||||
| Diagnostic delay | years | Me (IQR) | 46 | 5 (3–10) | 55 | 6 (4–10) | 0.201 |
| Fasting GH at diagnosis | µg/L | Me (IQR) | 43 | 12 (5.30–33.60) | 56 | 10.45 (4.00–23.55) | 0.274 |
| IGF-1 at diagnosis | ng/mL | Me (IQR) | 42 | 862 (632–1134) | 52 | 806 (641–1020) | 0.386 |
| x ULN | Me (IQR) | 42 | 3.07 (2.53–4.30) | 52 | 3.46 (2.80–4.36) | 0.491 | |
| PRL at diagnosis | ng/mL | Me (IQR) | 34 | 19.1 (9–34) | 48 | 11.65 (9–24) | 0.281 |
| Hyperprolactinemia at diagnosis | yes | n (%) | 34 | 14 (41.18) | 48 | 13 (27.08) | 0.181 |
| Pituitary axis dysfunction at diagnosis | at least one | n (%) | 49 | 17 (34.69) | 59 | 19 (32.20) | 0.785 |
| FSH/LH | 12 (24.49) | 15 (25.42) | 0.911 | ||||
| ACTH | 3 (6.12) | 5 (8.47) | 0.642 | ||||
| TSH | 9 (18.37) | 0 (0.00) | 0.001 * | ||||
| Clinical diagnosis of genetic syndrome associated with acromegaly | at least one | n (%) | 49 | 6 (12.24) | 59 | 5 (8.47) | 0.519 |
| Visual field defects | no | n (%) | 49 | 38 (77.55) | 59 | 54 (91.53) | 0.060 |
| small | 5 (10.20) | 4 (6.78) | |||||
| quadrantanopia | 6 (12.24) | 1 (1.69) | |||||
| Variable | Unit or Category | Statistics | Sparsely Granulated | Densely Granulated | p | ||
|---|---|---|---|---|---|---|---|
| N | Results | N | Results | ||||
| Tumor imaging in MRI | microadenoma | n (%) | 48 | 4 (8.33) | 57 | 21 (36.84) | 0.001 * |
| macroadenoma | 44 (91.67) | 36 (63.16) | |||||
| Maximal tumor diameter | mm | Me (IQR) | 41 | 23 (20–34) | 59 | 13 (9–20) | <0.001 * |
| Extrasellar expansion | yes | n (%) | 48 | 36 (75.00) | 57 | 20 (35.09) | <0.001 * |
| Compression of the optic chiasm | yes | n (%) | 48 | 22 (45.83) | 57 | 2 (3.51) | <0.001 * |
| Degree of cavernous sinus invasion | no | n (%) | 41 | 12 (29.27) | 49 | 30 (61.22) | 0.002 * |
| unilateral | 19 (46.34) | 17 (34.69) | |||||
| bilateral | 10 (24.39) | 2 (4.08) | |||||
| Immunohistochemical evaluation of tumor | pure GH(+) | n (%) | 49 | 30 (61.22) | 57 | 29 (50.88) | 0.562 |
| GH(+)/PRL(+) | 17 (34.69) | 24 (42.11) | |||||
| plurihormonal | 2 (4.08) | 4 (7.02) | |||||
| alpha-SU | (−) | n (%) | 48 | 36 (75.00) | 57 | 20 (35.09) | <0.001 * |
| (+) | 12 (25.00) | 37 (64.91) | |||||
| Ki-67 Index | Ki67 < 1% | n (%) | 49 | 29 (59.18) | 56 | 53 (94.64) | <0.001 * |
| 1 ≤ Ki67 < 3% | 10 (20.41) | 3 (5.36) | |||||
| Ki67 ≥ 3% | 10 (20.41) | 0 (0.00) | |||||
| Variable | Unit or Category | Statistics | Pure GH(+) | GH(+) PRL(+) | Plurihormonal | p | |||
|---|---|---|---|---|---|---|---|---|---|
| N | Results | N | Results | N | Results | ||||
| Age | years | Me (IQR) | 63 | 56 (45–64) | 46 | 48 (40–61) | 9 | 48 (46–62) | 0.231 |
| Age at diagnosis | years | Me (IQR) | 63 | 46 (36–58) | 46 | 40 (33–50) | 9 | 43 (36–51) | 0.234 |
| Sex | male | n (%) | 63 | 26 (41.27) | 46 | 23 (50.00) | 9 | 4 (44.44) | 0.656 |
| female | 37 (58.73) | 23 (50.00) | 5 (55.56) | ||||||
| Diagnostic delay | years | Me (IQR) | 61 | 5 (3–10) | 41 | 5 (3–10) | 9 | 5 (1–5) | 0.249 |
| Fasting GH at diagnosis | µg/L | Me (IQR) | 58 | 15.60 (4.10–33.60) | 40 | 11.40 (4.38–20.55) | 7 | 4.20 (2.80–14.50) | 0.261 |
| IGF-1 at diagnosis | ng/mL | Me (IQR) | 56 | 861 (639–1102) | 39 | 786 (617–990) | 6 | 618 (420–917) | 0.242 |
| x ULN | Me (IQR) | 56 | 3.34 (2.76–4.63) | 39 | 3.42 (2.44–4.20) | 6 | 2.84 (1.59–3.46) | 0.434 | |
| PRL at diagnosis | ng/mL | Me (IQR) | 47 | 10.6 (6.3–20.2) | 36 | 21.4 (11.2–39.4) | 5 | 19.3 (14.8–102.0) | 0.004 * |
| Hyperprolactinemia at diagnosis | yes | n (%) | 47 | 11 (23.40) | 36 | 15 (41.67) | 5 | 3 (60.00) | 0.087 |
| Pituitary axis dysfunction at diagnosis | at least one | n (%) | 63 | 15 (23.81) | 46 | 18 (39.13) | 9 | 5 (55.56) | 0.070 |
| FSH/LH | 9 (14.29) | 17 (36.96) | 2 (22.22) | 0.022 * | |||||
| ACTH | 4 (6.35) | 3 (6.52) | 2 (22.22) | 0.234 | |||||
| TSH | 4 (6.35) | 4 (8.70) | 1 (11.11) | 0.661 | |||||
| Clinical diagnosis of genetic syndrome associated with acromegaly | at least one | n (%) | 63 | 6 (9.52) | 46 | 5 (10.87) | 9 | 1 (11.11) | 0.999 |
| Visual field defects | no | n (%) | 63 | 54 (85.71) | 46 | 39 (84.78) | 9 | 7 (77.78) | 0.880 |
| small | 5 (7.94) | 4 (8.70) | 1 (11.11) | ||||||
| quadrantanopia | 4 (6.35) | 3 (6.52) | 1 (11.11) | ||||||
| Variable | Unit or Category | Statistics | Pure GH(+) | GH(+) PRL(+) | Plurihormonal | p | |||
|---|---|---|---|---|---|---|---|---|---|
| N | Results | N | Results | N | Results | ||||
| Tumor imaging in MRI | microadenoma | n (%) | 63 | 12 (19.05) | 44 | 13 (29.55) | 7 | 1 (14.29) | 0.510 |
| macroadenoma | 51 (80.95) | 31 (70.45) | 6 (85.71) | ||||||
| Maximal tumor diameter | mm | Me (IQR) | 55 | 20 (13–25) | 38 | 15 (10–24) | 7 | 20 (16–34) | 0.229 |
| Extrasellar expansion | yes | n (%) | 63 | 32 (50.79) | 44 | 21 (47.73) | 7 | 5 (71.43) | 0.570 |
| Compression of the optic chiasm | yes | n (%) | 63 | 14 (22.22) | 44 | 9 (20.45) | 7 | 2 (28.57) | 0.818 |
| Degree of cavernous sinus invasion | no | n (%) | 53 | 25 (47.17) | 39 | 22 (56.41) | 6 | 2 (33.33) | 0.584 |
| unilateral | 21 (39.62) | 12 (30.77) | 4 (66.67) | ||||||
| bilateral | 7 (13.21) | 5 (12.82) | 0 (0.00) | ||||||
| Granulation subtype of tumor | densely | n (%) | 59 | 29 (49.15) | 41 | 24 (58.54) | 6 | 4 (66.67) | 0.557 |
| sparsely | 30 (50.85) | 17 (41.46) | 2 (33.33) | ||||||
| alpha-SU | (−) | n (%) | 63 | 35 (55.56) | 45 | 22 (48.89) | 9 | 5 (55.56) | 0.807 |
| (+) | 28 (44.44) | 23 (51.11) | 4 (44.44) | ||||||
| Ki-67 Index | Ki67 < 1% | n (%) | 62 | 47 (75.81) | 45 | 37 (82.22) | 9 | 5 (55.56) | 0.165 |
| 1 ≤ Ki67 < 3% | 9 (14.52) | 6 (13.33) | 1 (11.11) | ||||||
| Ki67 ≥ 3% | 6 (9.68) | 2 (4.44) | 3 (33.33) | ||||||
| Variable | Unit or Category | Statistics | alpha-SU (+) | alpha-SU (−) | p | ||
|---|---|---|---|---|---|---|---|
| N | Results | N | Results | ||||
| Age | years | Me (IQR) | 55 | 49 (42–61) | 62 | 52 (45–64) | 0.308 |
| Age at diagnosis | years | Me (IQR) | 55 | 45 (36–58) | 62 | 43 (34–53) | 0.236 |
| Sex | male | n (%) | 55 | 27 (49.09) | 62 | 26 (41.94) | 0.438 |
| female | 28 (50.91) | 36 (58.06) | |||||
| Diagnostic delay | years | Me (IQR) | 53 | 5 (3–10) | 57 | 5 (3–9) | 0.131 |
| Fasting GH at diagnosis | µg/L | Me (IQR) | 51 | 11.70 (3.36–20.30) | 54 | 11.90 (4.80–27.90) | 0.211 |
| IGF-1 at diagnosis | ng/mL | Me (IQR) | 50 | 777 (625–981) | 51 | 877 (614–1162) | 0.113 |
| x ULN | Me (IQR) | 50 | 3.34 (2.58–4.00) | 51 | 3.42 (2.39–4.42) | 0.750 | |
| PRL at diagnosis | ng/mL | Me (IQR) | 48 | 14.7 (9.0–29.9) | 40 | 12.9 (8.0–27.9) | 0.750 |
| Hyperprolactinemia at diagnosis | yes | n (%) | 48 | 14 (29.17) | 40 | 15 (37.50) | 0.408 |
| Pituitary axis dysfunction at diagnosis | at least one | n (%) | 55 | 21 (38.18) | 62 | 17 (27.42) | 0.215 |
| FSH/LH | 15 (27.27) | 13 (20.97) | 0.425 | ||||
| ACTH | 5 (9.09) | 4 (6.45) | 0.733 | ||||
| TSH | 5 (9.09) | 4 (6.45) | 0.733 | ||||
| Clinical diagnosis of genetic syndrome associated with acromegaly | at least one | n (%) | 55 | 6 (10.91) | 62 | 6 (9.68) | 0.827 |
| Visual field defects | no | n (%) | 55 | 47 (85.45) | 62 | 52 (83.87) | 0.358 |
| small | 6 (10.91) | 4 (6.45) | |||||
| quadrantanopia | 2 (3.64) | 6 (9.68) | |||||
| Variable | Unit or Category | Statistics | alpha-SU (+) | alpha-SU (−) | p | ||
|---|---|---|---|---|---|---|---|
| N | Results | N | Results | ||||
| Tumor imaging in MRI | microadenoma | n (%) | 55 | 16 (29.09) | 59 | 10 (16.95) | 0.123 |
| macroadenoma | 39 (70.91) | 49 (83.05) | |||||
| Maximal tumor diameter | mm | Me (IQR) | 51 | 16 (10–21) | 49 | 20 (14–31) | 0.013 * |
| Extrasellar expansion | yes | n (%) | 55 | 22 (40.00) | 59 | 36 (61.02) | 0.039 * |
| Compression of the optic chiasm | yes | n (%) | 55 | 8 (14.55) | 59 | 17 (28.81) | 0.066 |
| Degree of cavernous sinus invasion | no | n (%) | 47 | 29 (61.70) | 51 | 20 (39.22) | 0.081 |
| unilateral | 14 (29.79) | 23 (45.10) | |||||
| bilateral | 4 (8.51) | 8 (15.69) | |||||
| Granulation subtype of tumor | densely | n (%) | 49 | 37 (75.51) | 56 | 20 (35.71) | <0.001 * |
| sparsely | 12 (24.49) | 36 (64.29) | |||||
| Immunohistochemical evaluation of tumor | pure GH(+) | n (%) | 55 | 28 (50.91) | 62 | 35 (56.45) | 0.807 |
| GH(+)/PRL(+) | 23 (41.82) | 22 (35.48) | |||||
| plurihormonal | 4 (7.27) | 5 (8.06) | |||||
| Ki-67 Index | Ki67 < 1% | n (%) | 54 | 45 (83.33) | 61 | 43 (70.49) | 0.234 |
| 1 ≤ Ki67 < 3% | 6 (11.11) | 10 (16.39) | |||||
| Ki67 ≥ 3% | 3 (5.56) | 8 (13.11) | |||||
| Variable | Unit or Category | Statistics | Ki-67 < 1% | 1% ≤ Ki-67 < 3% | Ki-67 ≥ 3% | p | |||
|---|---|---|---|---|---|---|---|---|---|
| N | Results | N | Results | N | Results | ||||
| Age | years | Me (IQR) | 89 | 55 (45–64) | 16 | 47 (41–57) | 11 | 39 (31–46) | 0.001 * |
| Age at diagnosis | years | Me (IQR) | 89 | 46 (38–58) | 16 | 38 (33–47) | 11 | 27 (22–39) | <0.001 * |
| Sex | male | n (%) | 89 | 44 (49.44) | 16 | 7 (43.75) | 11 | 2 (18.18) | 0.159 |
| female | 45 (50.56) | 9 (56.25) | 9 (81.82) | ||||||
| Diagnostic delay | years | Me (IQR) | 84 | 5 (3–10) | 15 | 4 (2–12) | 10 | 3 (1–7) | 0.096 |
| Fasting GH at diagnosis | µg/L | Me (IQR) | 82 | 9.84 (4.07–19.40) | 13 | 20.30 (10.80–34.00) | 8 | 53.00 (19.73–78.45) | 0.007 * |
| IGF-1 at diagnosis | ng/mL | Me (IQR) | 80 | 804 (616–1049) | 13 | 940 (760–1075) | 7 | 811 (621–1260) | 0.549 |
| x ULN | Me (IQR) | 80 | 3.42 (2.53–4.44) | 13 | 3.45 (2.80–3.90) | 7 | 2.75 (2.73–3.42) | 0.572 | |
| PRL at diagnosis | ng/mL | Me (IQR) | 71 | 12.1 (8.5–27.7) | 9 | 20.2 (13.7–29.0) | 6 | 21.7 (4.5–37.8) | 0.559 |
| Hyperprolactinemia at diagnosis | yes | n (%) | 71 | 20 (28.17) | 9 | 5 (55.56) | 6 | 3 (50.00) | 0.127 |
| Pituitary axis dysfunction at diagnosis | at least one | n (%) | 89 | 26 (29.21) | 16 | 6 (37.50) | 11 | 6 (54.55) | 0.233 |
| FSH/LH | 20 (22.47) | 5 (31.25) | 3 (27.27) | 0.696 | |||||
| ACTH | 7 (7.87) | 0 (0.00) | 2 (18.18) | 0.227 | |||||
| TSH | 5 (5.62) | 1 (6.25) | 3 (27.27) | 0.069 | |||||
| Clinical diagnosis of genetic syndrome associated with acromegaly | at least one | n (%) | 89 | 6 (6.74) | 16 | 1 (6.25) | 11 | 4 (36.36) | 0.020 * |
| Visual field defects | no | n (%) | 89 | 77 (86.52) | 16 | 13 (81.25) | 11 | 8 (72.73) | 0.372 |
| small | 7 (7.87) | 2 (12.50) | 1 (9.09) | ||||||
| quadrantanopia | 5 (5.62) | 1 (6.25) | 2 (18.18) | ||||||
| Variable | Unit or Category | Statistics | Ki-67 < 1 | 1 ≤ Ki-67 < 3 | Ki-67 ≥ 3 | p | |||
|---|---|---|---|---|---|---|---|---|---|
| N | Results | N | Results | N | Results | ||||
| Tumor imaging in MRI | microadenoma | n (%) | 86 | 24 (27.91) | 15 | 2 (13.33) | 11 | 0 (0.00) | 0.076 |
| macroadenoma | 62 (72.09) | 13 (86.67) | 11 (100.00) | ||||||
| Maximal tumor diameter | mm | Me (IQR) | 80 | 17 (10–25) | 11 | 20 (16–21) | 8 | 32 (26–36) | 0.006 * |
| Extrasellar expansion | yes | n (%) | 86 | 39 (45.35) | 15 | 9 (60.00) | 11 | 9 (81.82) | 0.054 |
| Compression of the optic chiasm | yes | n (%) | 86 | 16 (18.60) | 15 | 4 (26.67) | 11 | 5 (45.45) | 0.107 |
| Degree of cavernous sinus invasion | no | n (%) | 75 | 43 (57.33) | 12 | 4 (33.33) | 9 | 1 (11.11) | 0.022 * |
| unilateral | 24 (32.00) | 5 (41.67) | 7 (77.78) | ||||||
| bilateral | 8 (10.67) | 3 (25.00) | 1 (11.11) | ||||||
| Granulation subtype of tumor | densely | n (%) | 82 | 53 (64.63) | 13 | 3 (23.08) | 10 | 0 (0.00) | <0.001 * |
| sparsely | 29 (35.37) | 10 (76.92) | 10 (100.00) | ||||||
| Immunohistochemical evaluation of tumor | pure GH(+) | n (%) | 89 | 47 (52.81) | 16 | 9 (56.25) | 11 | 6 (54.55) | 0.165 |
| GH(+)/PRL(+) | 37 (41.57) | 6 (37.50) | 2 (18.18) | ||||||
| plurihormonal | 5 (5.62) | 1 (6.25) | 3 (27.27) | ||||||
| alpha-SU | (−) | n (%) | 88 | 43 (48.86) | 16 | 10 (62.50) | 11 | 8 (72.73) | 0.234 |
| (+) | 45 (51.14) | 6 (37.50) | 3 (27.27) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomasik, A.; Stelmachowska-Banaś, M.; Maksymowicz, M.; Czajka-Oraniec, I.; Raczkiewicz, D.; Zieliński, G.; Kunicki, J.; Zgliczyński, W. Pathologic Characteristics of Somatotroph Pituitary Tumors—An Observational Single-Center Study. Biomedicines 2023, 11, 3315. https://doi.org/10.3390/biomedicines11123315
Tomasik A, Stelmachowska-Banaś M, Maksymowicz M, Czajka-Oraniec I, Raczkiewicz D, Zieliński G, Kunicki J, Zgliczyński W. Pathologic Characteristics of Somatotroph Pituitary Tumors—An Observational Single-Center Study. Biomedicines. 2023; 11(12):3315. https://doi.org/10.3390/biomedicines11123315
Chicago/Turabian StyleTomasik, Agnieszka, Maria Stelmachowska-Banaś, Maria Maksymowicz, Izabella Czajka-Oraniec, Dorota Raczkiewicz, Grzegorz Zieliński, Jacek Kunicki, and Wojciech Zgliczyński. 2023. "Pathologic Characteristics of Somatotroph Pituitary Tumors—An Observational Single-Center Study" Biomedicines 11, no. 12: 3315. https://doi.org/10.3390/biomedicines11123315
APA StyleTomasik, A., Stelmachowska-Banaś, M., Maksymowicz, M., Czajka-Oraniec, I., Raczkiewicz, D., Zieliński, G., Kunicki, J., & Zgliczyński, W. (2023). Pathologic Characteristics of Somatotroph Pituitary Tumors—An Observational Single-Center Study. Biomedicines, 11(12), 3315. https://doi.org/10.3390/biomedicines11123315