Intraplaque Neovascularization, CD68+ and iNOS2+ Macrophage Infiltrate Intensity Are Associated with Atherothrombosis and Intraplaque Hemorrhage in Severe Carotid Atherosclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Fragments
2.2. Immunohistochemistry for Macrophage Density, Subtyping, and Detection of Vessel Density in the Atherosclerotic Plaque
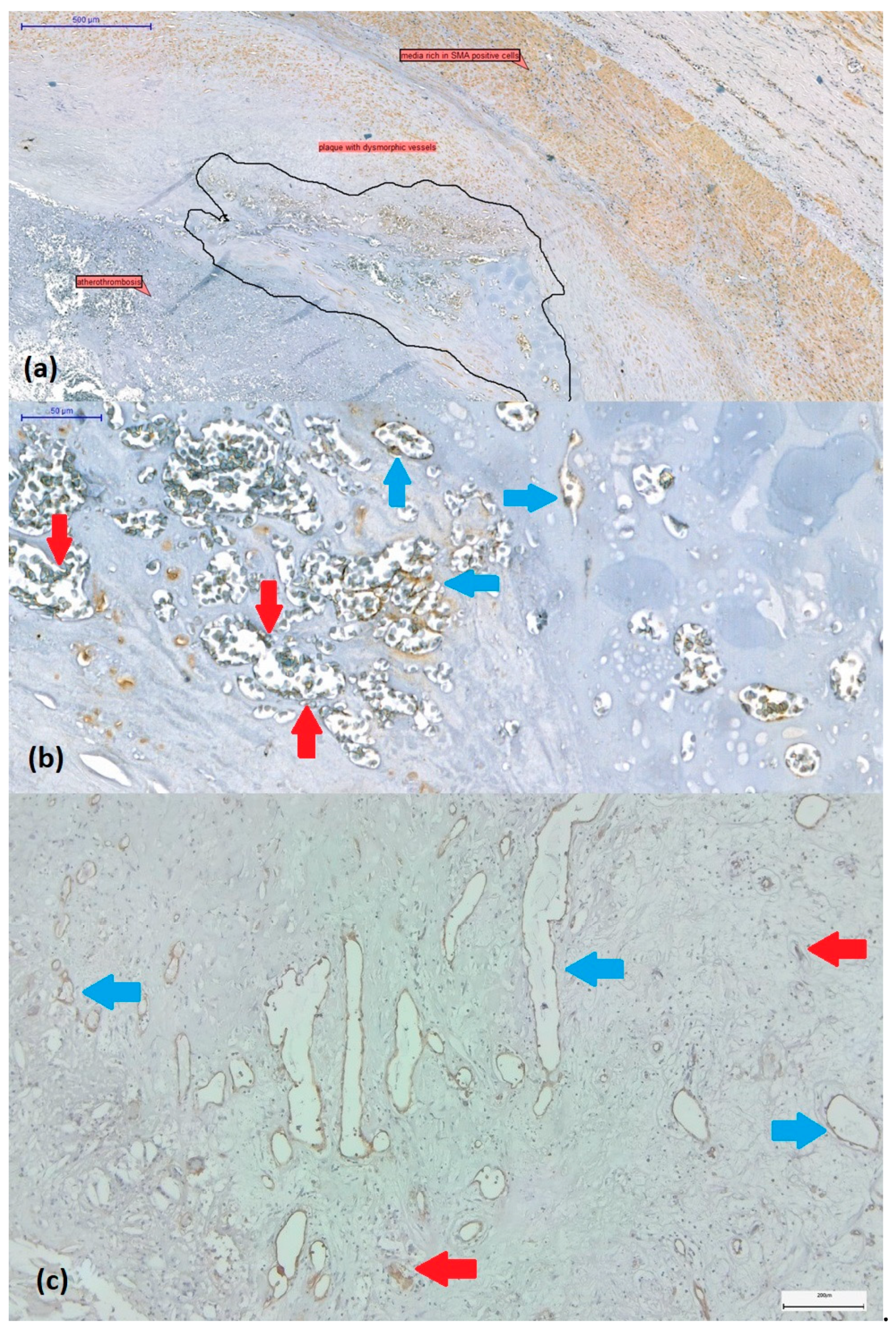
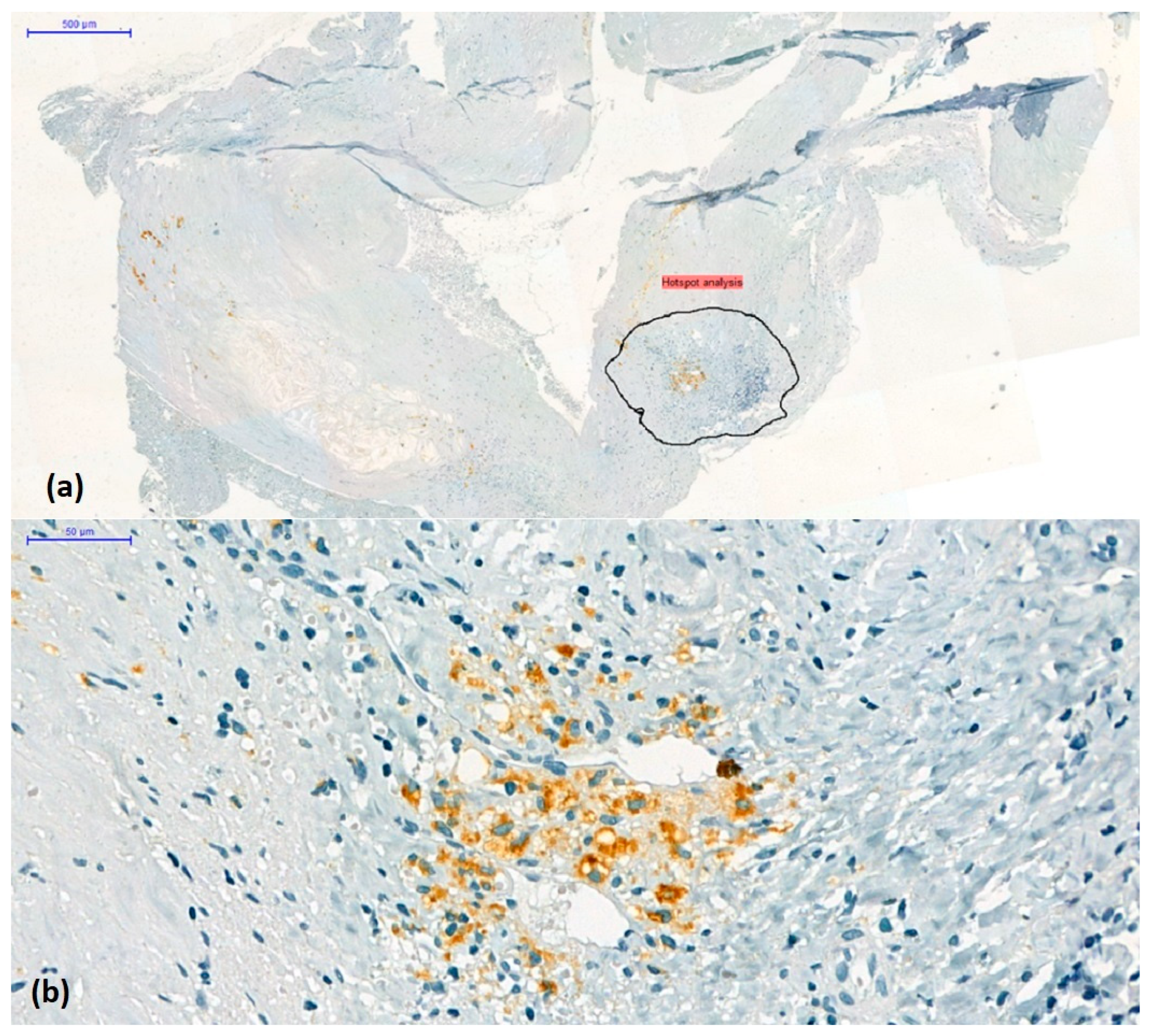
2.3. Assessment of Intraplaque Neoangiogenesis
2.4. Semi-Quantitative Scoring of the CD68+ Mononuclear Inflammatory Infiltrate
2.5. Digital Image Analysis Method to Measure Quantitative Individual Plaque Characteristics (CD68, Arg1, iNOS2, CD31)
2.6. Statistical Analysis
3. Results
3.1. Study Group Characteristics
3.2. Histological Signs of Complicated Plaque
3.3. Correlation of the CD68+ Infiltrate Grade with Signs of Plaque Complication
3.4. Comparison of the Arg1-Dominant vs. the iNOS2-Dominant Groups
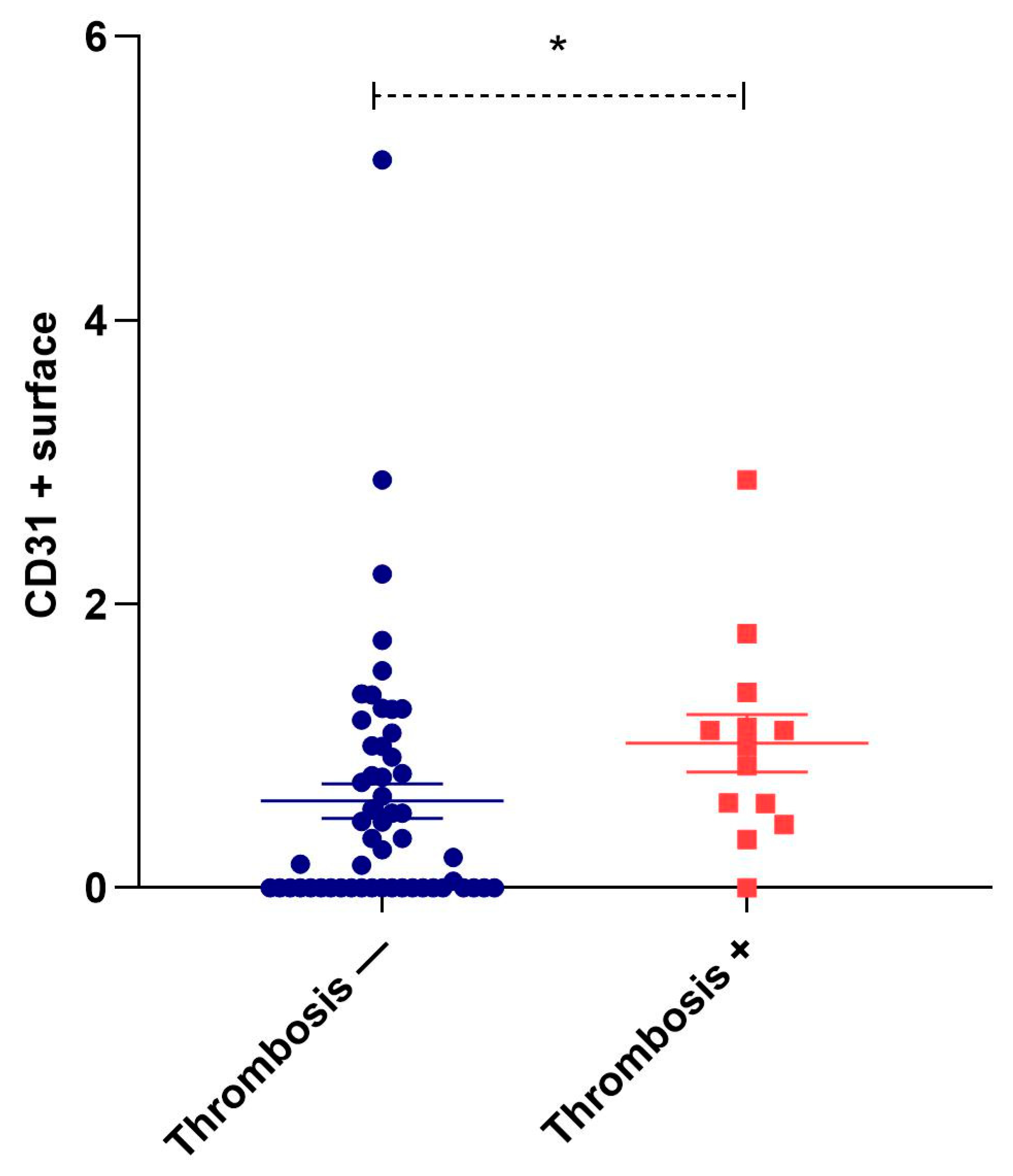
3.5. Correlation of Neovascularization with Other Histological Signs of Plaque Instability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devi, M.K.; Kuruvilla, S.; Balakrishnan, K.R. Expression of Inflammatory Markers CD68 and SMA in Endarterectomy Speci-mens. Austin J. Clin. Pathol. 2018, 5, 1057. [Google Scholar]
- Katan, M.; Luft, A. Global burden of stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Messas, E.; Goudot, G.; Halliday, A.; Sitruk, J.; Mirault, T.; Khider, L.; Saldmann, F.; Mazzolai, L.; Aboyans, V. Management of carotid stenosis for primary and secondary prevention of stroke: State-of-the-art 2020: A critical review. Eur. Heart J. Suppl. 2020, 22 (Supplement_M), M35–M42. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Finn, C.; Giambrone, A.E.; Gialdini, G.; Delgado, D.; Baradaran, H.; Kamel, H.; Gupta, A. The Association between Carotid Artery Atherosclerosis and Silent Brain Infarction: A Systematic Review and Meta-analysis. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2017, 26, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Agarwal, N.; Cau, R.; Gerosa, C.; Sanfilippo, R.; Porcu, M.; Montisci, R.; Cerrone, G.; Qi, Y.; Balestrieri, A.; et al. Review of imaging biomarkers for the vulnerable carotid plaque. JVS-Vasc. Sci. 2021, 2, 149–158. [Google Scholar] [CrossRef]
- Geiger, M.A.; Flumignan, R.L.; Sobreira, M.L.; Avelar, W.M.; Fingerhut, C.; Stein, S.; Guillaumon, A.T. Carotid plaque composition and the importance of non-invasive in imaging stroke prevention. Front. Cardiovasc. Med. 2022, 9, 885483. [Google Scholar] [CrossRef]
- Withana, N.P.; Saito, T.; Ma, X.; Garland, M.; Liu, C.; Kosuge, H.; Amsallem, M.; Verdoes, M.; Ofori, L.O.; Fischbein, M.; et al. Dual-modality activity-based probes as molecular imaging agents for vascular inflammation. J. Nucl. Med. 2016, 57, 1583–1590. [Google Scholar] [CrossRef]
- Jager, N.A.; Vries, B.M.W.; Hillebrands, J.L.; Harlaar, N.J.; Tio, R.A.; Slart, R.; Dam, G.M.; Boersma, H.H.; Zeebregts, C.J.; Westra, J. Distribution of Matrix Metalloproteinases in Human Atherosclerotic Carotid Plaques and Their Production by Smooth Muscle Cells and Macrophage Subsets. Mol. Imaging Biol. 2016, 18, 283–291. [Google Scholar] [CrossRef]
- Schultze, J.L. Transcriptional programming of human macrophages: On the way to systems immunology. J. Mol. Med.-JMM 2015, 93, 589–597. [Google Scholar] [CrossRef]
- Chen, Y.N.; Hu, M.R.; Wang, L.; Chen, W.D. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar]
- Dolfi, B.; Gallerand, A.; Haschemi, A.; Guinamard, R.R.; Ivanov, S. Macrophage metabolic regulation in atherosclerotic plaque. Atherosclerosis 2021, 334, 1–8. [Google Scholar] [CrossRef]
- Forteza, M.J.; Ketelhuth, D.F.J. Metabolism in atherosclerotic plaques: Immunoregulatory mechanisms in the arterial wall. Clin. Sci. 2022, 136, 435–454. [Google Scholar] [CrossRef]
- Moroni, F.; Ammirati, E.; Norata, G.D.; Magnoni, M.; Camici, P.G. The role of monocytes and macrophages in human atherosclerosis, plaque neoangiogenesis, and atherothrombosis. Mediat. Inflamm. 2019, 2019, 7434376. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Ming, X.F. Functions of arginase isoforms in macrophage inflammatory responses: Impact on cardiovascular diseases and metabolic disorders. Front. Immunol. 2014, 5, 533. [Google Scholar] [CrossRef] [PubMed]
- de Gaetano, M.; Crean, D.; Barry, M.; Belton, O. M1- and M2-type macrophage responses are predictive of Adverse Outcomes in human atherosclerosis. Front. Immunol. 2016, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.R.; Purushothaman, K.-R.; Sirol, M.; Levy, A.P.; Fuster, V. Neovascularization in human atherosclerosis. Circulation 2006, 113, 2245–2252. [Google Scholar] [CrossRef]
- Geiringer, E. Intimal vascularisation and atherosclerosis. J. Pathol. Bacteriol. 1951, 63, 201–211. [Google Scholar] [CrossRef]
- Sueishi, K.; Yonemitsu, Y.; Nakagawa, K.; Kaneda, Y.; Kumamoto, M.; Nakashima, Y. Atherosclerosis and angiogenesis: Its pathophysiological significance in humans as well as in an animal model induced by the gene transfer of Vascular Endothelial Growth Factor. Ann. N. Y. Acad. Sci. 1997, 811, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Camaré, C.; Pucelle, M.; Nègre-Salvayre, A.; Salvayre, R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Finn, A.V.; Gold, H.K.; Tulenko, T.N.; Wrenn, S.P.; Narula, J. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2054–2061. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.J.; Hayase, M.; Kutys, R.; et al. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 2003, 349, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Li, A.C.; Glass, C.K. The macrophage foam cell as a target for therapeutic intervention. Nat. Med. 2002, 8, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Ramel, D.; Gayral, S.; Sarthou, M.-K.; Augé, N.; Nègre-Salvayre, A.; Laffargue, M. Immune and smooth muscle cells interactions in atherosclerosis: How to target a breaking bad dialogue? Front. Pharmacol. 2019, 10, 1276. [Google Scholar] [CrossRef] [PubMed]
- Doran, A.C.; Meller, N.; McNamara, C.A. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 812–819. [Google Scholar] [CrossRef]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.H.; Sillesen, H.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2023 clinical practice guidelines on the management of atherosclerotic carotid and vertebral artery disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Saam, T.; Jäger, H.R.; Yuan, C.; Hatsukami, T.S.; Saloner, D.; Wasserman, B.A.; Bonati, L.H.; Wintermark, M. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019, 18, 559–572. [Google Scholar] [CrossRef]
- Balmos, I.A.; Horváth, E.; Brinzaniuc, K.; Muresan, A.V.; Olah, P.; Molnár, G.B.; Nagy, E.E. Inflammation, microcalcification, and increased expression of osteopontin are histological hallmarks of plaque vulnerability in patients with advanced carotid artery stenosis. Biomedicines 2023, 11, 881. [Google Scholar] [CrossRef]
- Asada, Y.; Yamashita, A.; Sato, Y.; Hatakeyama, K. Pathophysiology of atherothrombosis: Mechanisms of thrombus formation on disrupted atherosclerotic plaques. Pathol. Int. 2020, 70, 309–322. [Google Scholar] [CrossRef]
- Levy, A.P.; Moreno, P.R. Intraplaque hemorrhage. Curr. Mol. Med. 2006, 6, 479–488. [Google Scholar] [CrossRef]
- Mura, M.; Della Schiava, N.; Long, A.; Chirico, E.N.; Pialoux, V.; Millon, A. Carotid intraplaque haemorrhage: Pathogenesis, histological classification, imaging methods and clinical value. Ann. Transl. Med. 2020, 8, 1273. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Stöger, J.L.; Gijbels, M.J.J.; van der Velden, S.; Manca, M.; van der Loos, C.M.; Biessen, E.A.L.; Daemen, M.; Lutgens, E.; de Winther, M.P.J. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 2012, 225, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Brittenden, J.; Lahiri, R.; Brown, P.A.J.; Thies, F.; Wilson, H.M. Macrophage Subtypes in Symptomatic Carotid Artery and Femoral Artery Plaques. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.Y.; Miyoshi, H.; Kuroda, S.; Yasuda, H.; Kamiyama, K.; Nakagawara, J.; Takigami, M.; Kondo, T.; Atsumi, T. The Phenotype of Infiltrating Macrophages Influences Arteriosclerotic Plaque Vulnerability in the Carotid Artery. J. Stroke Cerebrovasc. Dis. 2013, 22, 910–918. [Google Scholar] [CrossRef]
- Tremble, L.F.; McCabe, M.; Walker, S.P.; McCarthy, S.; Tynan, R.F.; Beecher, S.; Werner, R.; Clover, A.J.P.; Power, X.D.G.; Forde, P.F.; et al. Differential association of CD68+ and CD163+ macrophages with macrophage enzymes, whole tumour gene expression and overall survival in advanced melanoma. Br. J. Cancer 2020, 123, 1553–1561. [Google Scholar] [CrossRef]
- Slevin, M.; Krupinski, J.; Badimon, L. Controlling the angiogenic switch in developing atherosclerotic plaques: Possible targets for therapeutic intervention. J. Angiogenes. Res. 2009, 1, 4. [Google Scholar] [CrossRef]
- Tang, J.; Lobatto, M.E.; Hassing, L.; Van Der Staay, S.; Van Rijs, S.M.; Calcagno, C.; Braza, M.S.; Baxter, S.; Fay, F.; Sanchez-Gaytan, B.L.; et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 2015, 1, e1400223. [Google Scholar] [CrossRef]
- Li, H.; Cao, Z.; Wang, L.; Liu, C.; Lin, H.; Tang, Y.; Yao, P. Macrophage Subsets and Death Are Responsible for Atherosclerotic Plaque Formation. Front. Immunol. 2022, 13, 843712. [Google Scholar] [CrossRef]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Yoo, R.J.; Kim, M.H.; Woo, S.K.; Kim, K.I.; Lee, T.S.; Choi, Y.K.; Kang, J.H.; Lim, S.M.; Lee, Y.J. Monitoring of macrophage accumulation in statin-treated atherosclerotic mouse model using sodium iodide symporter imaging system. Nucl. Med. Biol. 2017, 48, 45–51. [Google Scholar] [CrossRef]
- Parry, R.; Majeed, K.; Pixley, F.; Hillis, G.S.; Francis, R.J.; Schultz, C.J. Unravelling the role of macrophages in cardiovascular inflammation through imaging: A state-of-the-art review. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, E504–E525. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, R.; Takagi, Y.; Yoshida, K.; Miyamoto, S. Endoglin (CD105) is a more appropriate marker than CD31 for detecting microvessels in carotid artery plaques. Surg. Neurol. Int. 2013, 4, 132. [Google Scholar] [PubMed]
- Luque, A.; Slevin, M.; Turu, M.M.; Juan-Babot, O.; Badimon, L.; Krupinski, J. CD105 positive neovessels are prevalent in early stage carotid lesions, and correlate with the grade in more advanced carotid and coronary plaques. J. Angiogenes. Res. 2009, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Sedding, D.G.; Boyle, E.C.; Demandt, J.A.F.; Sluimer, J.C.; Dutzmann, J.; Haverich, A.; Bauersachs, J. Vasa Vasorum Angiogenesis: Key Player in the Initiation and Progression of Atherosclerosis and Potential Target for the Treatment of Cardiovascular Disease. Front. Immunol. 2018, 9, 706. [Google Scholar] [CrossRef]
- Krishnan, S.; Otaki, Y.; Doris, M.; Slipczuk, L.; Arnson, Y.; Rubeaux, M.; Dey, D.; Slomka, P.; Berman, D.S.; Tamarappoo, B. Molecular Imaging of Vulnerable Coronary Plaque: A Pathophysiologic Perspective. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 359–364. [Google Scholar] [CrossRef]


| Demographic Factors | |
|---|---|
| Age (years) | 65.4 ± 1.1 |
| Gender (male/female) | 47 (70.1)/20 (29.9) |
| Plaque characteristics | |
| Ulceration (yes/no) | 40 (59.7)/27 (40.3) |
| Atherothrombosis (yes/no) | 13 (19.4)/54 (80.6) |
| Intraplaque hemorrhage (yes/no) | 30 (54.5)/37 (45.5) |
| Necrotic lipid core (yes/no) | 55 (82.1)/12 (17.9) |
| Microcalcification (yes/no) | 36 (53.7)/31 (46.3) |
| Superficial/deep calcification (yes/no) | 25 (37.3)/42 (62.7) |
| Macrocalcification (yes/no) | 17 (25.3)/50 (74.7) |
| Neovascularization (yes/no) | 41 (61.2)/26 (38.8) |
| Immunohistochemistry parameters | Positive surface area |
| CD68+ surface (%) | 1.37 ± 0.14 |
| iNOS2 + surface (%) | 0.48 ± 0.08 |
| Arg1 + surface (%) | 1.79 ± 0.20 |
| CD31 + surface (%) | 0.69 ± 0.10 |
| Complete blood count parameters | |
| Neutrophils (109/L) | 5.89 ± 0.28 |
| Lymphocytes (109/L) | 2.11 ± 0.09 |
| Neutrophil/Lymphocyte ratio | 3.38 ± 0.67 |
| Monocytes (109/L) | 0.76 ± 0.08 |
| Medication | |
| Anti-hypertensive drugs (yes/no) | 65 (97)/2 (3) |
| Anticoagulants | 63 (94)/4 (6) |
| Anti-aggregants | 67 (100)/0 (0) |
| Statins | 65 (97)/2 (3) |
| CD68+ Infiltrate Density | |||
|---|---|---|---|
| Score 1 | Score 2–3 | p Value | |
| Ulceration (yes/no) | 14/12 | 26/14 | 0.318 |
| Atherothrombosis (yes/no) | 5/21 | 8/32 | 1 |
| Intraplaque hemorrhage(yes/no) | 6/20 | 24/16 | 0.003 |
| Neovascularization (yes/no) | 18/9 | 23/17 | 0.609 |
| Groups | Arg1/iNOS2 ≥ 1 (n = 55) | Arg1/iNOS2 < 1 (n = 12) | p Value |
|---|---|---|---|
| Demographic factors | |||
| Age (years) | 65.5 ± 1.2 | 64.8 ± 1.9 | |
| Gender (male/female) | 36 (65.5)/19 (34.5) | 11 (91.6)/1 (8.4) | 0.090 |
| Plaque characteristics | |||
| Ulceration (yes/no) | 33 (60)/22 (40) | 7 (58.3)/5 (41.7) | 0.990 |
| Atherothrombosis (yes/no) | 8 (14.5)/47 (85.5) | 5 (41.7)/7 (58.3) | 0.046 |
| Intraplaque hemorrhage (yes/no) | 23 (41.8)/32 (58.2) | 7 (58.3)/5 (41.7) | 0.348 |
| Necrotic lipid core (yes/no) | 45 (81.8)/10 (18.2) | 10 (83.3)/2 (16.6) | 1.000 |
| Microcalcification (yes/no) | 29 (52.7)/26 (47.3) | 7 (58.3)/5 (41.7) | 0.760 |
| Superficial/deep calcification(yes/no) | 19 (34.5)/36 (65.5) | 6 (50)/6 (50) | 0.345 |
| Macrocalcification (yes/no) | 13 (23.6)/42 (76.4) | 4 (33.3)/8 (66.6) | 0.482 |
| Neovascularization (yes/no) | 31 (56.3)/24 (43.7) | 10 (83.3)/2 (16.6) | 0.108 |
| CD68 + area (%) | 1.38 ± 0.16 | 1.33 ± 0.35 | 0.740 |
| CD31 + area (%) | 0.68 ± 0.12 | 0.75 ± 0.23 | 0.502 |
| Complete blood count parameters | |||
| Neutrophils (109/L) | 5.72 ± 0.29 | 6.65 ± 0.78 | 0.226 |
| Lymphocytes (109/L) | 2.08 ± 0.09 | 2.24 ± 0.23 | 0.491 |
| Neutrophil/Lymphocyte ratio | 3.27 ± 0.36 | 3.38 ± 0.67 | 0.692 |
| Monocytes (109/L) | 0.64 ± 0.03 | 0.76 ± 0.08 | 0.245 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balmos, I.A.; Slevin, M.; Brinzaniuc, K.; Muresan, A.V.; Suciu, H.; Molnár, G.B.; Mocian, A.; Szabó, B.; Nagy, E.E.; Horváth, E. Intraplaque Neovascularization, CD68+ and iNOS2+ Macrophage Infiltrate Intensity Are Associated with Atherothrombosis and Intraplaque Hemorrhage in Severe Carotid Atherosclerosis. Biomedicines 2023, 11, 3275. https://doi.org/10.3390/biomedicines11123275
Balmos IA, Slevin M, Brinzaniuc K, Muresan AV, Suciu H, Molnár GB, Mocian A, Szabó B, Nagy EE, Horváth E. Intraplaque Neovascularization, CD68+ and iNOS2+ Macrophage Infiltrate Intensity Are Associated with Atherothrombosis and Intraplaque Hemorrhage in Severe Carotid Atherosclerosis. Biomedicines. 2023; 11(12):3275. https://doi.org/10.3390/biomedicines11123275
Chicago/Turabian StyleBalmos, Ioan Alexandru, Mark Slevin, Klara Brinzaniuc, Adrian Vasile Muresan, Horatiu Suciu, Gyopár Beáta Molnár, Adriana Mocian, Béla Szabó, Előd Ernő Nagy, and Emőke Horváth. 2023. "Intraplaque Neovascularization, CD68+ and iNOS2+ Macrophage Infiltrate Intensity Are Associated with Atherothrombosis and Intraplaque Hemorrhage in Severe Carotid Atherosclerosis" Biomedicines 11, no. 12: 3275. https://doi.org/10.3390/biomedicines11123275
APA StyleBalmos, I. A., Slevin, M., Brinzaniuc, K., Muresan, A. V., Suciu, H., Molnár, G. B., Mocian, A., Szabó, B., Nagy, E. E., & Horváth, E. (2023). Intraplaque Neovascularization, CD68+ and iNOS2+ Macrophage Infiltrate Intensity Are Associated with Atherothrombosis and Intraplaque Hemorrhage in Severe Carotid Atherosclerosis. Biomedicines, 11(12), 3275. https://doi.org/10.3390/biomedicines11123275






