Artificial Neural Network (ANN)-Based Pattern Recognition Approach Illustrates a Biphasic Behavioral Effect of Ethanol in Zebrafish: A High-Throughput Method for Animal Locomotor Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Ethical Statement
2.3. Data Collection
2.4. Data Pre-Processing
2.5. Neural Networks
2.6. In Silico Experiments
2.7. Time-Resolved Analysis
3. Results
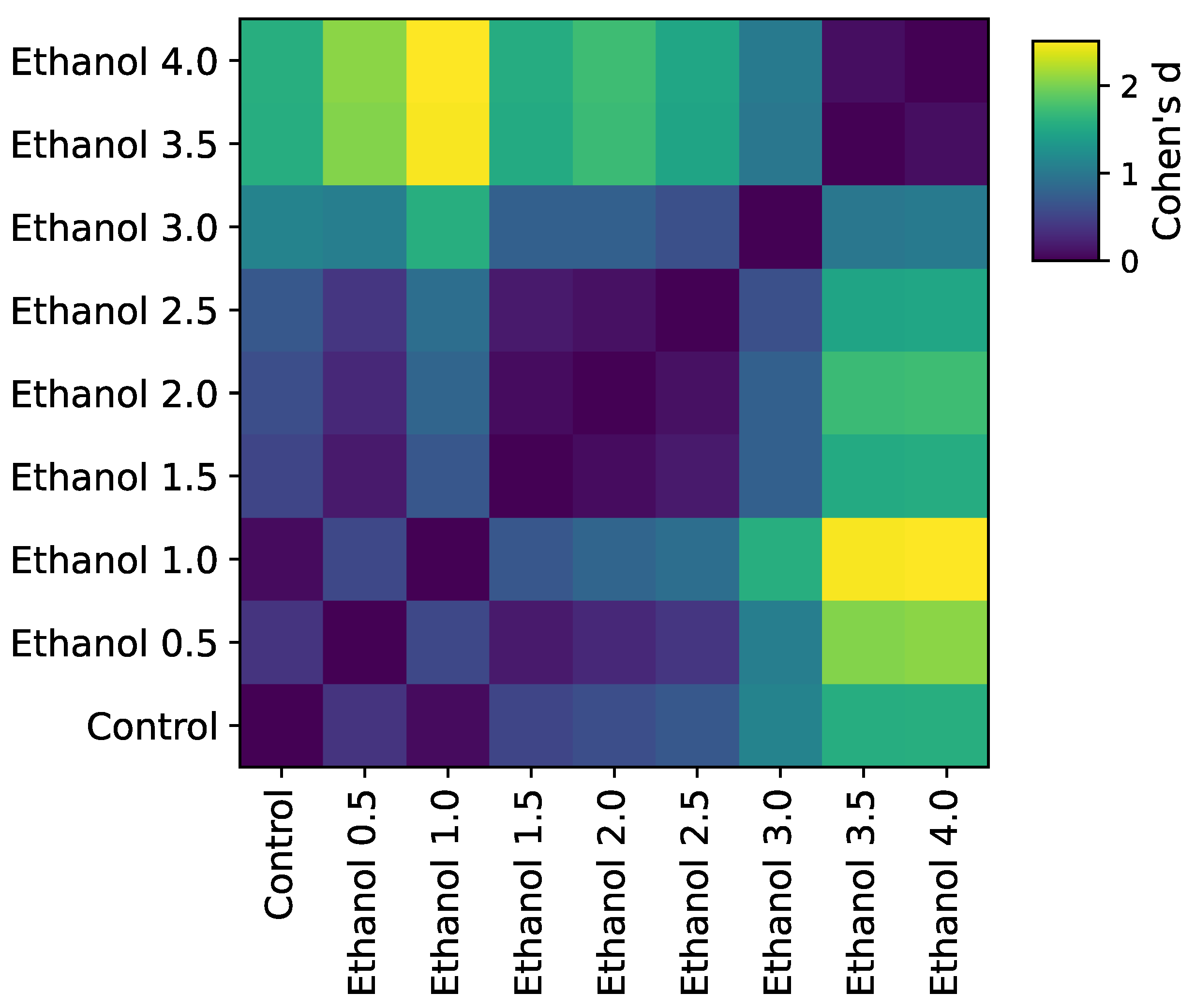
3.1. Concentration-Dependent Effect of Ethanol
3.2. Long-Lasting Effect of Ethanol
3.3. Non-Linear Nature of Locomotor Patterns
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Langova, V.; Vales, K.; Horka, P.; Horacek, J. The Role of Zebrafish and Laboratory Rodents in Schizophrenia Research. Front. Psychiatry 2020, 11, 703. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.Y.; Choi, T.I.; Lee, Y.R.; Choe, S.K.; Kim, C.H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Bailone, R.L.; Fukushima, H.C.S.; Fernandes, B.H.V.; Aguiar, L.K.D.; Corrêa, T.; Janke, H.; Setti, P.G.; Roça, R.D.O.; Borra, R.C. Zebrafish as an alternative animal model in human and animal vaccination research. Lab. Anim. Res. 2020, 36, 13. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Bradford, Y.M.; Toro, S.; Ramachandran, S.; Ruzicka, L.; Howe, D.G.; Eagle, A.; Kalita, P.; Martin, R.; Moxon, S.A.T.; Schaper, K.; et al. Zebrafish Models of Human Disease: Gaining Insight into Human Disease at ZFIN. ILAR J. 2017, 58, 4–16. [Google Scholar] [CrossRef]
- Hason, M.; Bartůněk, P. Zebrafish Models of Cancer—New Insights on Modeling Human Cancer in a Non-Mammalian Vertebrate. Genes 2019, 10, 935. [Google Scholar] [CrossRef]
- Gilbert, M.J.; Zerulla, T.C.; Tierney, K.B. Zebrafish (Danio rerio) as a model for the study of aging and exercise: Physical ability and trainability decrease with age. Exp. Gerontol. 2014, 50, 106–113. [Google Scholar] [CrossRef]
- Lüffe, T.M.; D’Orazio, A.; Bauer, M.; Gioga, Z.; Schoeffler, V.; Lesch, K.P.; Romanos, M.; Drepper, C.; Lillesaar, C. Increased locomotor activity via regulation of GABAergic signalling in foxp2 mutant zebrafish—Implications for neurodevelopmental disorders. Transl. Psychiatry 2021, 11, 529. [Google Scholar] [CrossRef]
- Kundap, U.P.; Kumari, Y.; Othman, I.; Shaikh, M.F. Zebrafish as a Model for Epilepsy-Induced Cognitive Dysfunction: A Pharmacological, Biochemical and Behavioral Approach. Front. Pharmacol. 2017, 8, 515. [Google Scholar] [CrossRef]
- Afrikanova, T.; Serruys, A.S.K.; Buenafe, O.E.M.; Clinckers, R.; Smolders, I.; de Witte, P.A.M.; Crawford, A.D.; Esguerra, C.V. Validation of the Zebrafish Pentylenetetrazol Seizure Model: Locomotor versus Electrographic Responses to Antiepileptic Drugs. PLoS ONE 2013, 8, e54166. [Google Scholar] [CrossRef]
- Irons, T.; MacPhail, R.; Hunter, D.; Padilla, S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol. Teratol. 2010, 32, 84–90. [Google Scholar] [CrossRef]
- Grossman, L.; Utterback, E.; Stewart, A.; Gaikwad, S.; Chung, K.M.; Suciu, C.; Wong, K.; Elegante, M.; Elkhayat, S.; Tan, J.; et al. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav. Brain Res. 2010, 214, 277–284. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Bruni, G.; Lakhani, P.; Kokel, D. Discovering novel neuroactive drugs through high-throughput behavior-based chemical screening in the zebrafish. Front. Pharmacol. 2014, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Grieco, F.; Tegelenbosch, R.A.; Kyzar, E.J.; Nguyen, M.; Kaluyeva, A.; Song, C.; Noldus, L.P.; Kalueff, A.V. A novel 3D method of locomotor analysis in adult zebrafish: Implications for automated detection of CNS drug-evoked phenotypes. J. Neurosci. Methods 2015, 255, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Kendall, F.; Khozin, S.; Goosen, R.; Hu, J.; Laramie, J.; Ringel, M.; Schork, N. Artificial intelligence and machine learning in clinical development: A translational perspective. Npj Digit. Med. 2019, 2, 69. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Guilbeault, N.C.; Guerguiev, J.; Martin, M.; Tate, I.; Thiele, T.R. BonZeb: Open-source, modular software tools for high-resolution zebrafish tracking and analysis. Sci. Rep. 2021, 11, 8148. [Google Scholar] [CrossRef] [PubMed]
- Marcato, D.; Alshut, R.; Breitwieser, H.; Mikut, R.; Strahle, U.; Pylatiuk, C.; Peravali, R. An automated and high-throughput Photomotor Response platform for chemical screens. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE, Milan, Italy, 25–29 August 2015. [Google Scholar] [CrossRef]
- Lin, X.; Duan, X.; Jacobs, C.; Ullmann, J.; Chan, C.Y.; Chen, S.; Cheng, S.H.; Zhao, W.N.; Poduri, A.; Wang, X.; et al. High-throughput brain activity mapping and machine learning as a foundation for systems neuropharmacology. Nat. Commun. 2018, 9, 5142. [Google Scholar] [CrossRef] [PubMed]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402. [Google Scholar] [CrossRef] [PubMed]
- Alipanahi, B.; Delong, A.; Weirauch, M.T.; Frey, B.J. Predicting the sequence specificities of DNA- and RNA-binding proteins by deep learning. Nat. Biotechnol. 2015, 33, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Rajkomar, A.; Oren, E.; Chen, K.; Dai, A.M.; Hajaj, N.; Hardt, M.; Liu, P.J.; Liu, X.; Marcus, J.; Sun, M.; et al. Scalable and accurate deep learning with electronic health records. NPJ Digit. Med. 2018, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Champagne, D.L.; Alia, A.; Richardson, M.K. Large-Scale Analysis of Acute Ethanol Exposure in Zebrafish Development: A Critical Time Window and Resilience. PLoS ONE 2011, 6, e20037. [Google Scholar] [CrossRef]
- Echevarria, D.J.; Toms, C.N.; Jouandot, D.J. Alcohol-induced behavior change in zebrafish models. Rev. Neurosci. 2011, 22, 85–93. [Google Scholar] [CrossRef]
- Bozhko, D.V.; Myrov, V.O.; Kolchanova, S.M.; Polovian, A.I.; Galumov, G.K.; Demin, K.A.; Zabegalov, K.N.; Strekalova, T.; de Abreu, M.S.; Petersen, E.V.; et al. Artificial intelligence-driven phenotyping of zebrafish psychoactive drug responses. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 112, 110405. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio); University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Drapeau, P.; Saint-Amant, L.; Buss, R.R.; Chong, M.; McDearmid, J.R.; Brustein, E. Development of the locomotor network in zebrafish. Prog. Neurobiol. 2002, 68, 85–111. [Google Scholar] [CrossRef]
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; de Polavieja, G.G. idTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743–748. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. arXiv 2015, arXiv:1512.03385. [Google Scholar] [CrossRef]
- Kingma, D.P.; Ba, J. Adam: A Method for Stochastic Optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar] [CrossRef]
- Lin, T.Y.; Goyal, P.; Girshick, R.; He, K.; Dollár, P. Focal Loss for Dense Object Detection. arXiv 2017, arXiv:1708.02002. [Google Scholar] [CrossRef]
- Ernst, M.D. Permutation Methods: A Basis for Exact Inference. Stat. Sci. 2004, 19, 676–685. [Google Scholar] [CrossRef]
- Tsang, B.; Ansari, R.; Gerlai, R. Dose dependent behavioral effects of acute alcohol administration in zebrafish fry. Pharmacol. Biochem. Behav. 2019, 179, 124–133. [Google Scholar] [CrossRef]
- Blondel, V.D.; Guillaume, J.L.; Lambiotte, R.; Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, 2008, P10008. [Google Scholar] [CrossRef]
- Soares, A.R.; Pereira, P.M.; Ferreira, V.; Reverendo, M.; Simões, J.; Bezerra, A.R.; Moura, G.R.; Santos, M.A.S. Ethanol Exposure Induces Upregulation of Specific MicroRNAs in Zebrafish Embryos. Toxicol. Sci. 2012, 127, 18–28. [Google Scholar] [CrossRef]
- Vera, L.M.; Bello, C.; Paredes, J.F.; Carmona-Antoñanzas, G.; Sánchez-Vázquez, F.J. Ethanol toxicity differs depending on the time of day. PLoS ONE 2018, 13, e0190406. [Google Scholar] [CrossRef]
- Orger, M.B.; de Polavieja, G.G. Zebrafish Behavior: Opportunities and Challenges. Annu. Rev. Neurosci. 2017, 40, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.N.; Chang, L.L.; Lai, C.H.; Lin, K.J.; Lin, M.F.; Yang, C.H.; Lin, H.H.; Chen, Y.H. Development of an Animal Model for Alcoholic Liver Disease in Zebrafish. Zebrafish 2015, 12, 271–280. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Cruchten, S.V.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2019, 33, 95–118. [Google Scholar] [CrossRef]
- Li, F.; Lin, J.; Liu, X.; Li, W.; Ding, Y.; Zhang, Y.; Zhou, S.; Guo, N.; Li, Q. Characterization of the locomotor activities of zebrafish larvae under the influence of various neuroactive drugs. Ann. Transl. Med. 2018, 6, 173. [Google Scholar] [CrossRef]
- Liu, Y.; Carmer, R.; Zhang, G.; Venkatraman, P.; Brown, S.A.; Pang, C.P.; Zhang, M.; Ma, P.; Leung, Y.F. Statistical Analysis of Zebrafish Locomotor Response. PLoS ONE 2015, 10, e0139521. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, P.; Cassidy, P.A.; Carmer, R.; Zhang, G.; Venkatraman, P.; Brown, S.A.; Pang, C.P.; Zhong, W.; Zhang, M.; et al. Statistical Analysis of Zebrafish Locomotor Behaviour by Generalized Linear Mixed Models. Sci. Rep. 2017, 7, 2937. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.C.; Lackner, S.; Félix, R.; Orger, M.B. Structure of the Zebrafish Locomotor Repertoire Revealed with Unsupervised Behavioral Clustering. Curr. Biol. 2018, 28, 181–195.e5. [Google Scholar] [CrossRef] [PubMed]
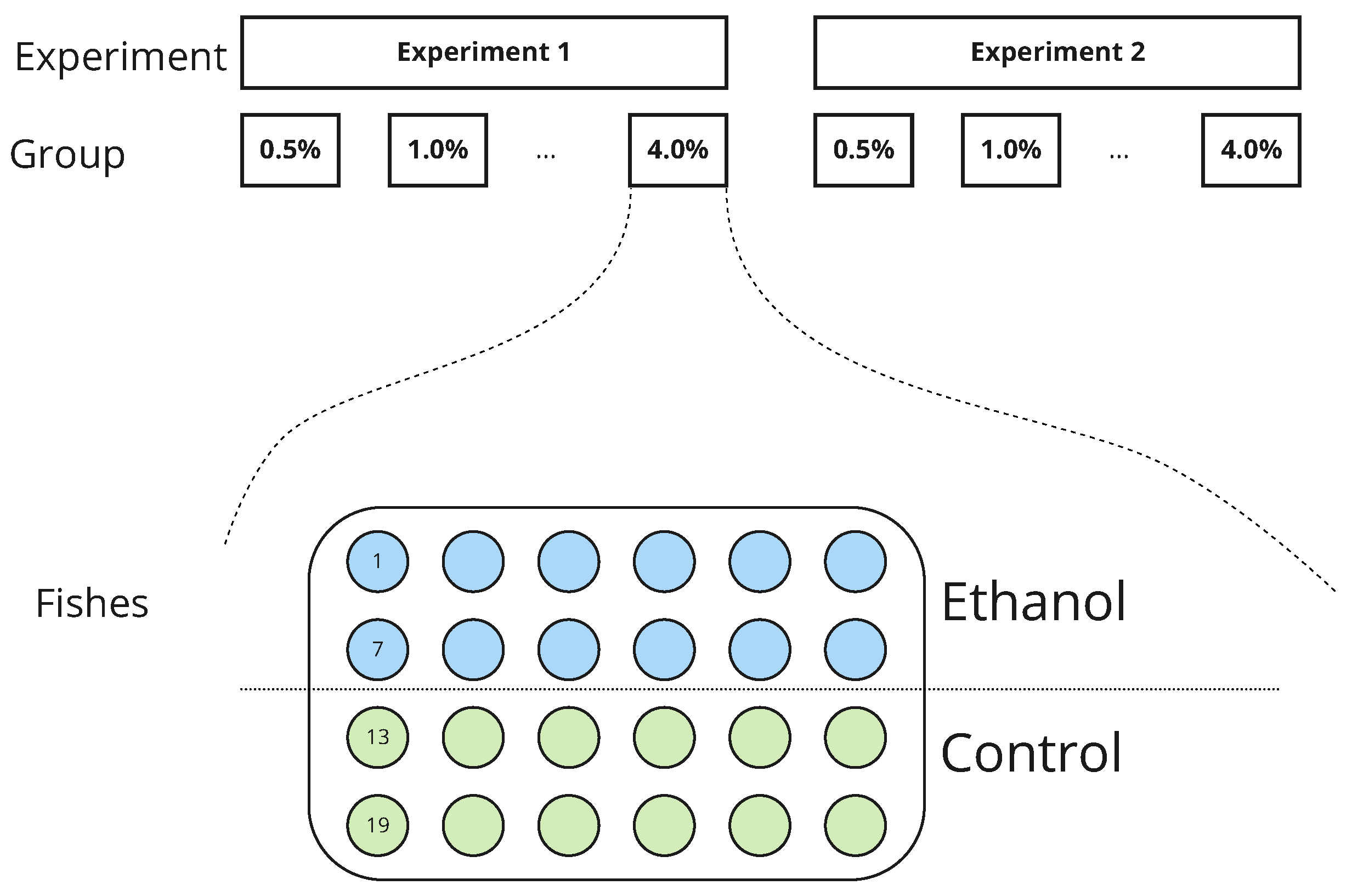
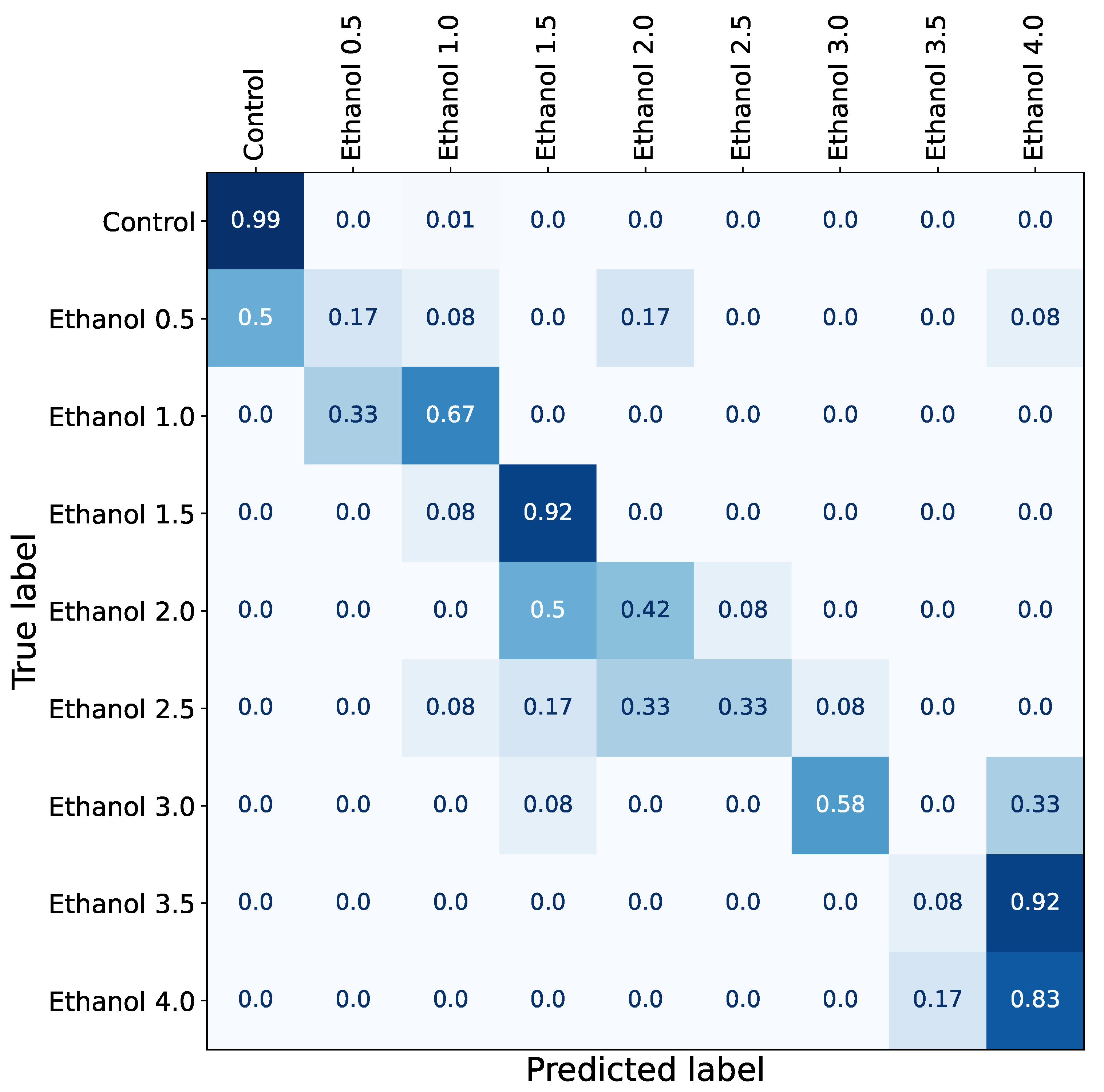
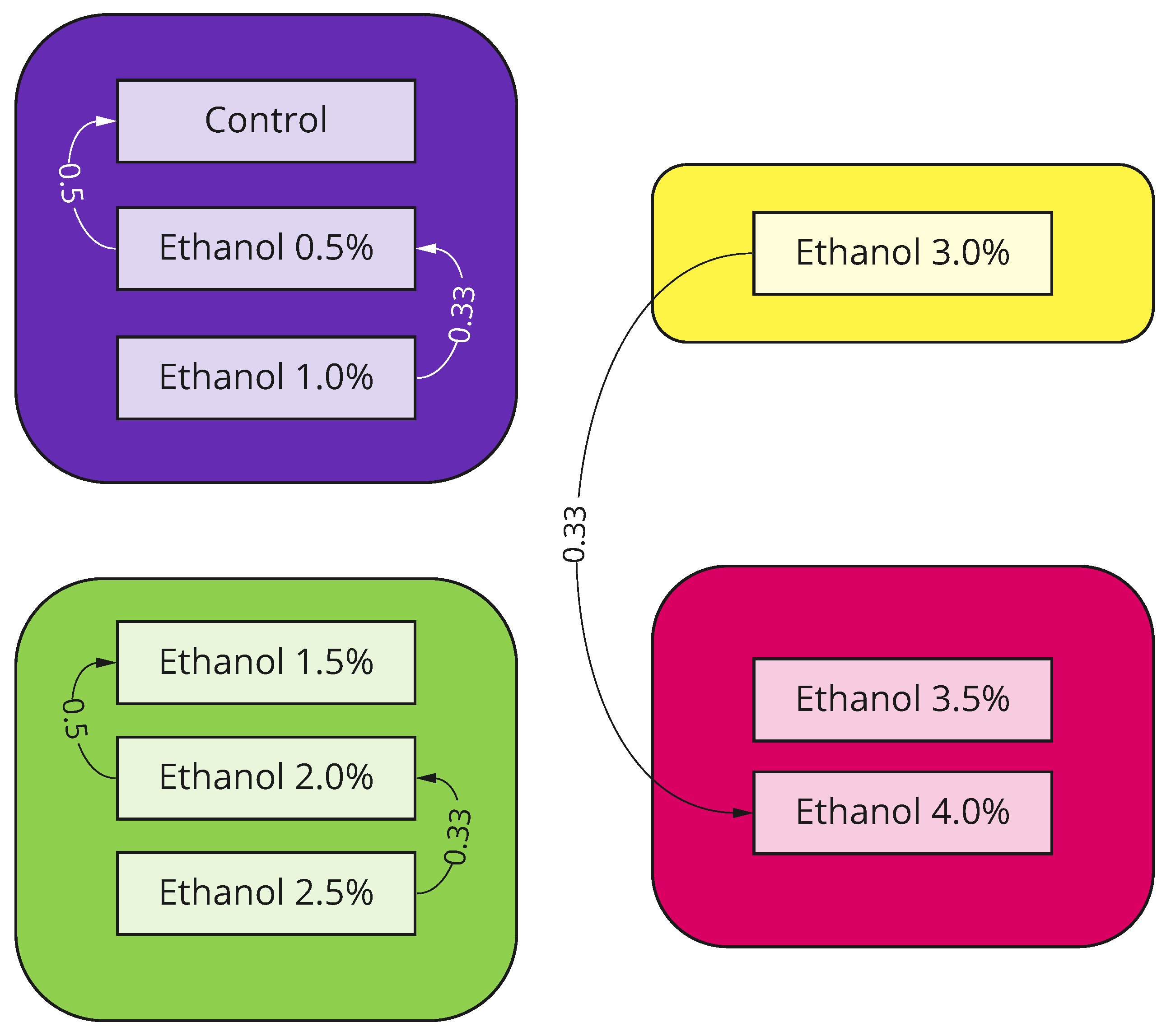

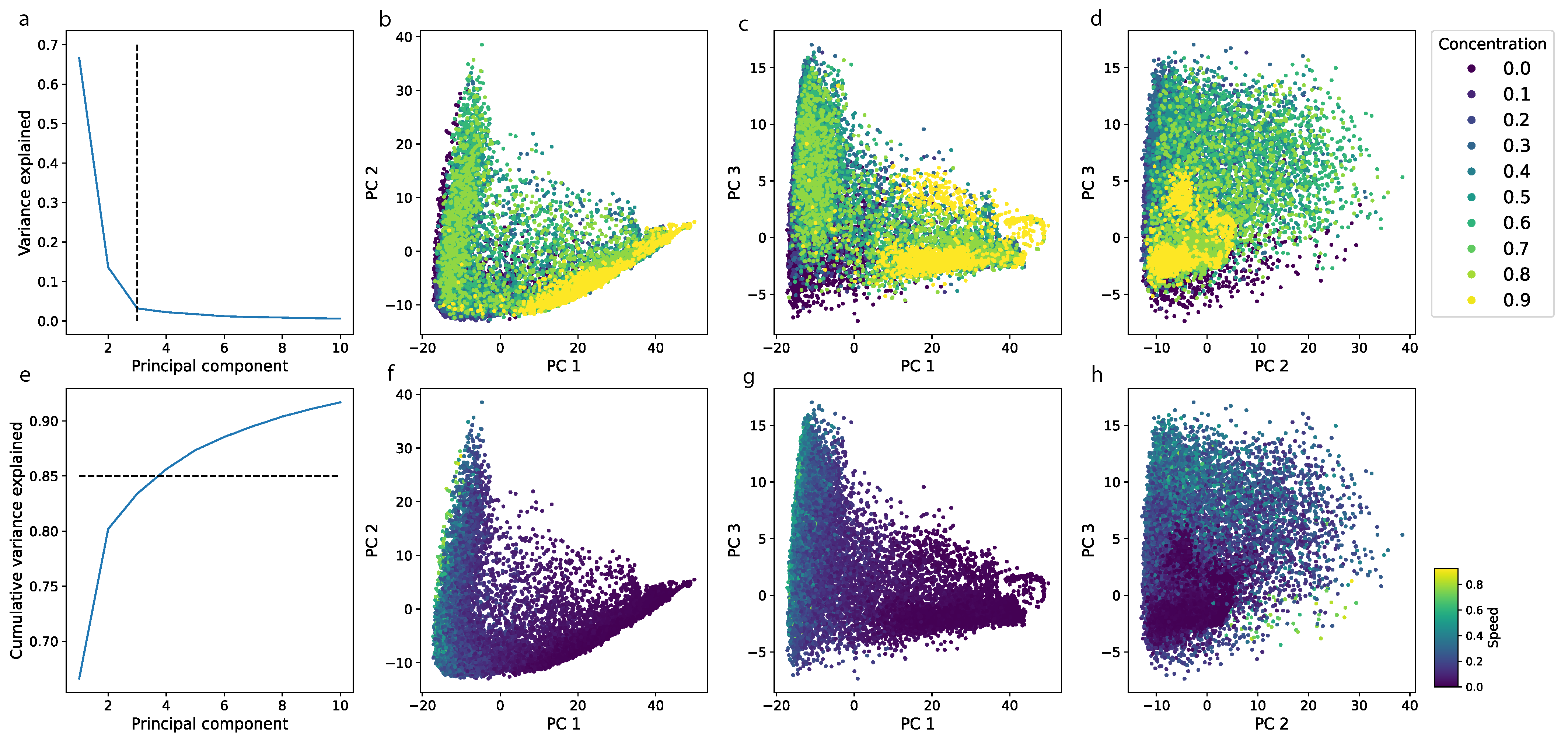
| Experiment 1 | Experiment 2 | |
|---|---|---|
| Groups 0.5–3% | 11 dpf | 12 dpf |
| Groups 3.5–4% | 12 dpf | 13 dpf |
| Ethanol Solution | Neutral Solution | Final Solution |
|---|---|---|
| 4% 1 mL | 7 mL | 0.5% |
| 4% 3 mL | 9 mL | 1.0% |
| 4% 5 mL | 8.3 mL | 1.5% |
| 4% 3 mL | 3 mL | 2.0% |
| 4% 5 mL | 3 mL | 2.5% |
| 4% 7 mL | 2.3 mL | 3.0% |
| 4% 7 mL | 1 mL | 3.5% |
| 95% 2 mL | 45.5 mL | 4.0% |
| Solution | Fraction of Dead (Non-Moving) Fish |
|---|---|
| Control | 0% |
| Ethanol 0.5–3.0% | 0% |
| Ethanol 3.5% | 9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myrov, V.O.; Polovian, A.I.; Kolchanova, S.; Galumov, G.K.; Schiöth, H.B.; Bozhko, D.V. Artificial Neural Network (ANN)-Based Pattern Recognition Approach Illustrates a Biphasic Behavioral Effect of Ethanol in Zebrafish: A High-Throughput Method for Animal Locomotor Analysis. Biomedicines 2023, 11, 3215. https://doi.org/10.3390/biomedicines11123215
Myrov VO, Polovian AI, Kolchanova S, Galumov GK, Schiöth HB, Bozhko DV. Artificial Neural Network (ANN)-Based Pattern Recognition Approach Illustrates a Biphasic Behavioral Effect of Ethanol in Zebrafish: A High-Throughput Method for Animal Locomotor Analysis. Biomedicines. 2023; 11(12):3215. https://doi.org/10.3390/biomedicines11123215
Chicago/Turabian StyleMyrov, Vladislav O., Aleksandr I. Polovian, Sofiia Kolchanova, Georgii K. Galumov, Helgi B. Schiöth, and Dmitrii V. Bozhko. 2023. "Artificial Neural Network (ANN)-Based Pattern Recognition Approach Illustrates a Biphasic Behavioral Effect of Ethanol in Zebrafish: A High-Throughput Method for Animal Locomotor Analysis" Biomedicines 11, no. 12: 3215. https://doi.org/10.3390/biomedicines11123215
APA StyleMyrov, V. O., Polovian, A. I., Kolchanova, S., Galumov, G. K., Schiöth, H. B., & Bozhko, D. V. (2023). Artificial Neural Network (ANN)-Based Pattern Recognition Approach Illustrates a Biphasic Behavioral Effect of Ethanol in Zebrafish: A High-Throughput Method for Animal Locomotor Analysis. Biomedicines, 11(12), 3215. https://doi.org/10.3390/biomedicines11123215






