The Serum ACE2, CTSL, AngII, and TNFα Levels after COVID-19 and mRNA Vaccines: The Molecular Basis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Sample Collection
2.3. Inclusion/Exclusion Criteria
2.4. Methods
2.4.1. Sample Processing and Storage
2.4.2. Enzyme Linked Immunosorbent Assay
2.4.3. Flow Cytometry
2.4.4. Turbidimetry
2.4.5. Enzyme-Linked Fluorescent Assay
2.4.6. Data Analysis
3. Results
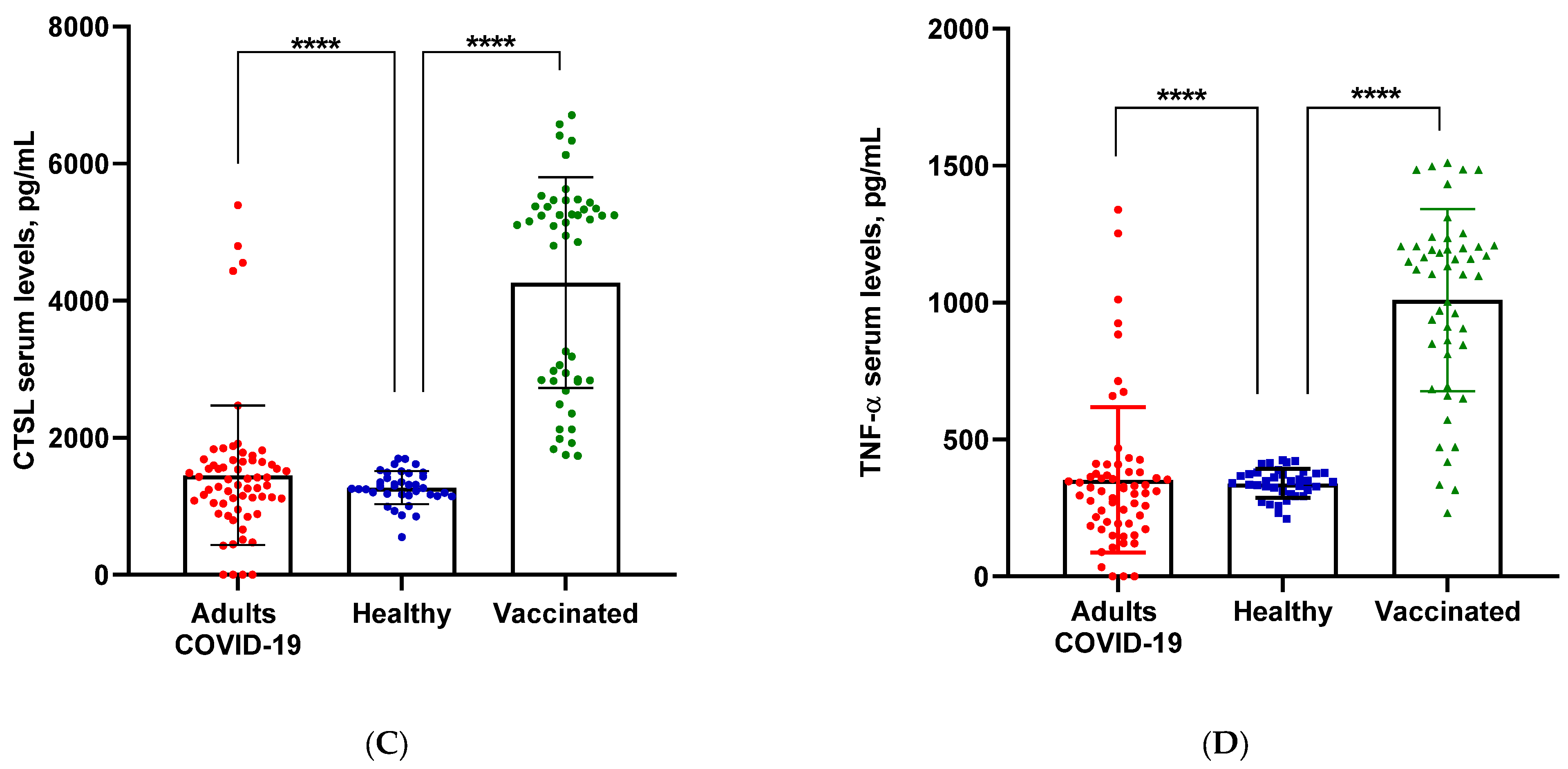
3.1. Serum Levels of ACE2, AngII, CTSL and TNFα Induced by COVID-19, BNT162b2 mRNA and mRNA-1273 Vaccines
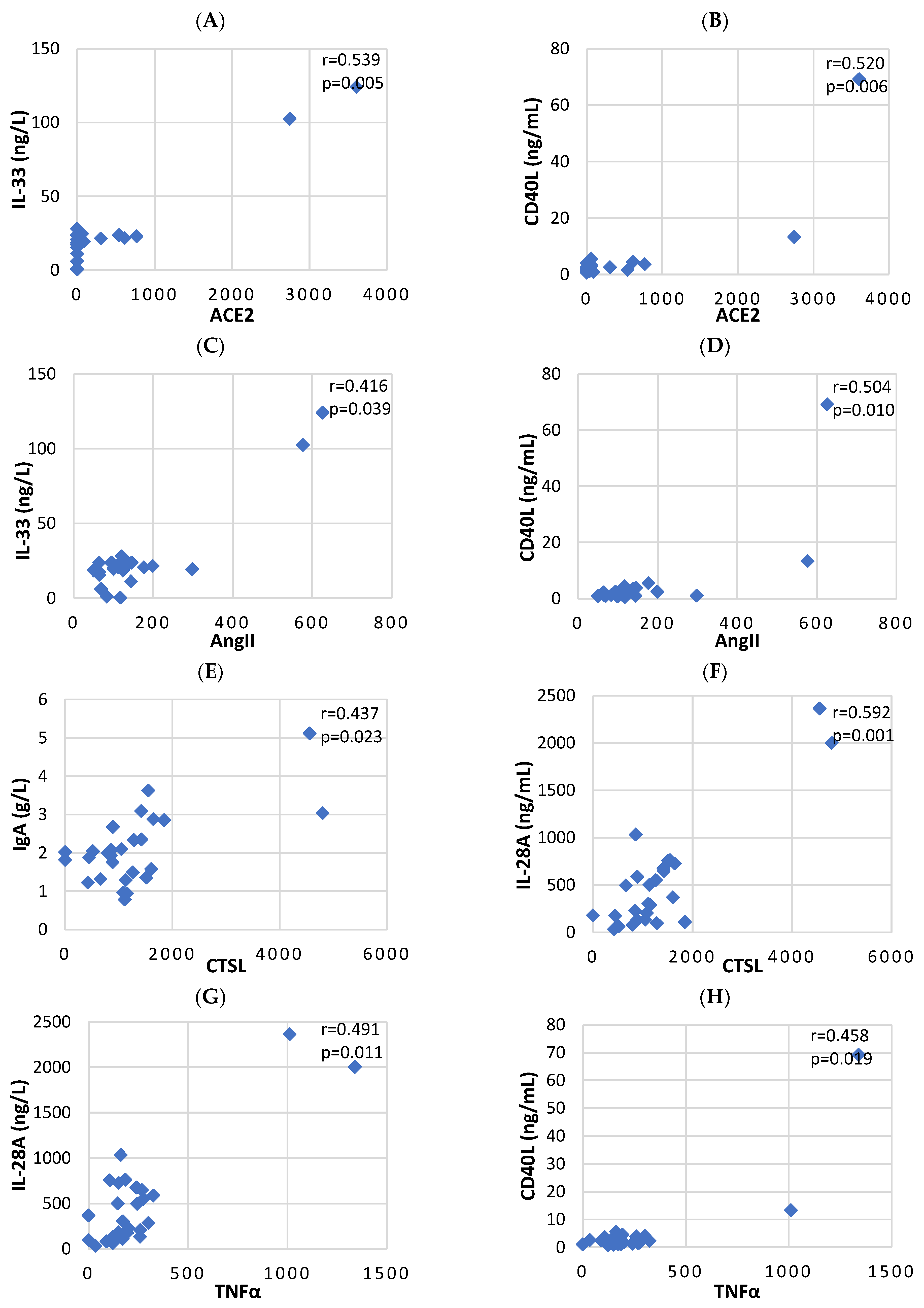
3.2. Correlation Analysis between Serum Levels of Key Biomarkers and Cytokines, Induced by COVID-19
4. Discussion
4.1. Angiotensin Converting Enzyme 2 Serum Levels
4.2. Angiotensin II Serum Levels
4.3. Cathepsin L Serum Levels
4.4. Tumor Necrosis Factor Alpha Serum Levels
4.5. mRNA Vaccines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamada, T.; Takaoka, A. Innate Immune Recognition against SARS-CoV-2. Inflamm. Regen. 2023, 43, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, L.; Cheng, G. The Battle between Host and SARS-CoV-2: Innate Immunity and Viral Evasion Strategies. Mol. Ther. 2022, 30, 1869–1884. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, Immunity and Intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef]
- Jacobs, J.L.; Bain, W.; Naqvi, A.; Staines, B.; Castanha, P.M.S.; Yang, H.; Boltz, V.F.; Barratt-Boyes, S.; Marques, E.T.A.; Mitchell, S.L.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Viremia Is Associated with Coronavirus Disease 2019 Severity and Predicts Clinical Outcomes. Clin. Infect. Dis. 2022, 74, 1525–1533. [Google Scholar] [CrossRef]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; Ekka-Zohar, A.; David, S.S.B.; Lerner, U.; Bivas-Benita, M.; et al. Long Covid Outcomes at One Year after Mild SARS-CoV-2 Infection: Nationwide Cohort Study. BMJ 2023, 380, e072529. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell Entry Mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.; Liu, K.; Guo, F.; Zhang, Y.; Zhang, G.; Jiang, C. SARS Coronavirus Entry into Host Cells through a Novel Clathrin- and Caveolae-Independent Endocytic Pathway. Cell Res. 2008, 18, 290–301. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Papola, F.; Biancofiore, V.; Angeletti, C.; Grimaldi, A.; Carucci, A.C.; Cofini, V.; Necozione, S.; Rosciano, A.; Marinangeli, F.; Cervelli, C. Anti-AT1R Autoantibodies and Prediction of the Severity of COVID-19. Hum. Immunol. 2022, 83, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Xue, J.; Ye, C.; Chen, A. Role of the Central Renin-Angiotensin System in Hypertension (Review). Int. J. Mol. Med. 2021, 47, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, J.; Hu, Z.; Han, G.; Delafontaine, P.; Garcia, G.; Mitch, W.E. IL-6 and Serum Amyloid a Synergy Mediates Angiotensin II-Induced Muscle Wasting. J. Am. Soc. Nephrol. JASN 2009, 20, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Signalling Pathways of the TNF Superfamily: A Double-Edged Sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- Berghe, T.V.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated Necrosis: The Expanding Network of Non-Apoptotic Cell Death Pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Berliner, N. Hemophagocytic Lymphohistiocytosis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 27–49. [Google Scholar] [CrossRef]
- Suntharalingam, G.; Perry, M.R.; Ward, S.; Brett, S.J.; Castello-Cortes, A.; Brunner, M.D.; Panoskaltsis, N. Cytokine Storm in a Phase 1 Trial of the Anti-CD28 Monoclonal Antibody TGN1412. N. Engl. J. Med. 2006, 355, 1018–1028. [Google Scholar] [CrossRef]
- Ronit, A.; Berg, R.M.G.; Bay, J.T.; Haugaard, A.K.; Ahlström, M.G.; Burgdorf, K.S.; Ullum, H.; Rørvig, S.B.; Tjelle, K.; Foss, N.B.; et al. Compartmental Immunophenotyping in COVID-19 ARDS: A Case Series. J. Allergy Clin. Immunol. 2021, 147, 81–91. [Google Scholar] [CrossRef]
- WHO. WHO Coronavirus (COVID-19) Dashboard. 7 April 2021. Available online: https://covid19.who.int/ (accessed on 7 April 2021).
- Daniell, H.; Nair, S.K.; Shi, Y.; Wang, P.; Montone, K.T.; Shaw, C.; Choi, G.; Abdul Ghani, D.; Weaver, J.; Rader, D.J.; et al. Decrease in Angiotensin-Converting Enzyme Activity but Not Concentration in Plasma/Lungs in COVID-19 Patients Offers Clues for Diagnosis/Treatment. Mol. Ther. Methods Clin. Dev. 2022, 26, 266–278. [Google Scholar] [CrossRef]
- Gerard, L.; Lecocq, M.; Bouzin, C.; Hoton, D.; Schmit, G.; Pereira, J.P.; Montiel, V.; Plante-Bordeneuve, T.; Laterre, P.-F.; Pilette, C. Increased Angiotensin-Converting Enzyme 2 and Loss of Alveolar Type II Cells in COVID-19–Related Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 204, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Mendonça, L.; Allen, E.R.; Howe, A.; Lee, M.; Allen, J.D.; Chawla, H.; Pulido, D.; Donnellan, F.; Davies, H.; et al. Native-like SARS-CoV-2 Spike Glycoprotein Expressed by ChAdOx1 NCoV-19/AZD1222 Vaccine. ACS Cent. Sci. 2021, 7, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.L.; Teng, J.L.L.; Jia, L.; Zhang, C.; Huang, C.; Cai, J.P.; Zhou, R.; Chan, K.H.; Zhao, H.; Zhu, L.; et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin–angiotensin system. Cell 2021, 184, 2212–2228. [Google Scholar] [CrossRef] [PubMed]
- Deshotels, M.R.; Xia, H.; Sriramula, S.; Lazartigues, E.; Filipeanu, C.M. Angiotensin II Mediates Angiotensin Converting Enzyme Type 2 Internalization and Degradation through an Angiotensin II Type I Receptor–Dependent Mechanism. Hypertension 2014, 64, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Iwata-Yoshikawa, N.; Kakizaki, M.; Shiwa-Sudo, N.; Okura, T.; Tahara, M.; Fukushi, S.; Maeda, K.; Kawase, M.; Asanuma, H.; Tomita, Y.; et al. Essential Role of TMPRSS2 in SARS-CoV-2 Infection in Murine Airways. Nat. Commun. 2022, 13, 6100. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced Isolation of SARS-CoV-2 by TMPRSS2-Expressing Cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of Spike Glycoprotein of SARS-CoV-2 on Virus Entry and Its Immune Cross-Reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Wang, P.-H.; Yang, N.; Huang, J.; Ou, J.; Xu, T.; Zhao, X.; Liu, T.; Huang, X.; et al. SARS-CoV-2 Spike Promotes Inflammation and Apoptosis through Autophagy by ROS-Suppressed PI3K/AKT/MTOR Signaling. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2021, 1867, 166260. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a Keystone Cytokine in Health and Disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Islam, H.; Chamberlain, T.C.; Mui, A.L.; Little, J.P. Elevated Interleukin-10 Levels in COVID-19: Potentiation of Pro-Inflammatory Responses or Impaired Anti-Inflammatory Action? Front. Immunol. 2021, 12, 677008. [Google Scholar] [CrossRef]
- Petrey, A.C.; Qeadan, F.; Middleton, E.A.; Pinchuk, I.V.; Campbell, R.A.; Beswick, E.J. Cytokine Release Syndrome in COVID-19: Innate Immune, Vascular, and Platelet Pathogenic Factors Differ in Severity of Disease and Sex. J. Leukoc. Biol. 2020, 109, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Runge, M.S.; Brasier, A.R. Angiotensin II Induces Interleukin-6 Transcription in Vascular Smooth Muscle Cells through Pleiotropic Activation of Nuclear Factor-ΚB Transcription Factors. Circ. Res. 1999, 84, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with COVID-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Sang, E.R.; Tian, Y.; Miller, L.C.; Sang, Y. Epigenetic Evolution of ACE2 and IL-6 Genes: Non-Canonical Interferon-Stimulated Genes Correlate to COVID-19 Susceptibility in Vertebrates. Genes 2021, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Maza, M.; Úbeda, M.; Delgado, P.; Horndler, L.; Llamas, M.A.; van Santen, H.M.; Alarcón, B.; Abia, D.; García-Bermejo, L.; Serrano-Villar, S.; et al. ACE2 Serum Levels as Predictor of Infectability and Outcome in COVID-19. Front. Immunol. 2022, 13, 836516. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Wang, T.; Zhang, B.; Luo, Y.; Mao, L.; Wang, F.; Wu, S.; Sun, Z. Detection of IgM and IgG Antibodies in Patients with Coronavirus Disease 2019. Clin. Transl. Immunol. 2020, 9, e1136. [Google Scholar] [CrossRef]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II Revisited: New Roles in Inflammation, Immunology and Aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef]
- Ozkan, S.; Cakmak, F.; Konukoglu, D.; Biberoglu, S.; Ipekci, A.; Akdeniz, Y.S.; Bolayirli, I.M.; Balkan, I.I.; Dumanli, G.Y.; Ikizceli, I. Efficacy of Serum Angiotensin II Levels in Prognosis of Patients with Coronavirus Disease 2019. Crit. Care Med. 2021, 49, e613–e623. [Google Scholar] [CrossRef]
- Serfozo, P.; Wysocki, J.; Gulua, G.; Schulze, A.; Ye, M.; Liu, P.; Jin, J.; Bader, M.; Myöhänen, T.; García-Horsman, J.A.; et al. Ang II (Angiotensin II) Conversion to Angiotensin-(1-7) in the Circulation Is POP (Prolyloligopeptidase)-Dependent and ACE2 (Angiotensin-Converting Enzyme 2)-Independent. Hypertension 2020, 75, 173–182. [Google Scholar] [CrossRef]
- Grobe, N.; Weir, N.M.; Leiva, O.; Ong, F.S.; Bernstein, K.E.; Schmaier, A.H.; Morris, M.; Elased, K.M. Identification of Prolyl Carboxypeptidase as an Alternative Enzyme for Processing of Renal Angiotensin II Using Mass Spectrometry. Am. J. Physiol.-Cell Physiol. 2013, 304, C945–C953. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Reboldi, G.; Trapasso, M.; Zappa, M.; Spanevello, A.; Verdecchia, P. COVID-19, Vaccines and Deficiency of ACE2 and Other Angiotensinases. Closing the Loop on the “Spike Effect”. Eur. J. Intern. Med. 2022, 103, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Zappa, M.; Reboldi, G.; Trapasso, M.; Cavallini, C.; Spanevello, A.; Verdecchia, P. The Pivotal Link between ACE2 Deficiency and SARS-CoV-2 Infection: One Year Later. Eur. J. Intern. Med. 2021, 93, 28–34. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Spanevello, A.; De Ponti, R.; Visca, D.; Marazzato, J.; Palmiotto, G.; Feci, D.; Reboldi, G.; Fabbri, L.M.; Verdecchia, P. Electrocardiographic Features of Patients with COVID-19 Pneumonia. Eur. J. Intern. Med. 2020, 78, 101–106. [Google Scholar] [CrossRef]
- Tukiainen, T.; Villani, A.-C.; Yen, A.; Rivas, M.A.; Marshall, J.L.; Satija, R.; Aguirre, M.; Gauthier, L.; Fleharty, M.; Kirby, A.; et al. Landscape of X Chromosome Inactivation across Human Tissues. Nature 2017, 550, 244–248. [Google Scholar] [CrossRef]
- Pal, R.; Bhansali, A. COVID-19, Diabetes Mellitus and ACE2: The Conundrum. Diabetes Res. Clin. Pract. 2020, 162, 108132. [Google Scholar] [CrossRef]
- Prieto-Fernández, E.; Egia-Mendikute, L.; Vila-Vecilla, L.; Bosch, A.; Barreira-Manrique, A.; Lee, S.Y.; García-del Río, A.; Antoñana-Vildosola, A.; Jiménez-Lasheras, B.; Moreno-Cugnon, L.; et al. Hypoxia Reduces Cell Attachment of SARS-CoV-2 Spike Protein by Modulating the Expression of ACE2, Neuropilin-1, Syndecan-1 and Cellular Heparan Sulfate. Emerg. Microbes Infect. 2021, 10, 1065–1076. [Google Scholar] [CrossRef]
- Kassiri, Z.; Zhong, J.; Guo, D.; Basu, R.; Wang, X.; Liu, P.P.; Scholey, J.W.; Penninger, J.M.; Oudit, G.Y. Loss of Angiotensin-Converting Enzyme 2 Accelerates Maladaptive Left Ventricular Remodeling in Response to Myocardial Infarction. Circ. Heart Fail. 2009, 2, 446–455. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Moudhi, A.; Mansour, F.A.; Alghamdi, J.; Alhendi, Y.; Alamro, A.; Alghamdi, A.; Alamri, H.S.; Alroqi, F.; Barhoumi, T. Angiotensin II Exaggerates SARS-CoV-2 Specific T-Cell Response in Convalescent Individuals Following COVID-19. Int. J. Mol. Sci. 2022, 23, 8669. [Google Scholar] [CrossRef]
- Guzik, T.J.; Hoch, N.E.; Brown, K.A.; McCann, L.A.; Rahman, A.; Dikalov, S.; Goronzy, J.; Weyand, C.; Harrison, D.G. Role of the T Cell in the Genesis of Angiotensin II–Induced Hypertension and Vascular Dysfunction. J. Exp. Med. 2007, 204, 2449–2460. [Google Scholar] [CrossRef]
- Su, C.M.; Wang, L.; Yoo, D. Activation of NF-ΚB and Induction of Proinflammatory Cytokine Expressions Mediated by ORF7a Protein of SARS-CoV-2. Sci. Rep. 2021, 11, 13464. [Google Scholar] [CrossRef]
- Gomes, C.P.; Fernandes, D.E.; Casimiro, F.; da Mata, G.F.; Passos, M.T.; Varela, P.; Mastroianni-Kirsztajn, G.; Pesquero, J.B. Cathepsin L in COVID-19: From Pharmacological Evidences to Genetics. Front. Cell. Infect. Microbiol. 2020, 10, 589505. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-M.; Yang, W.-L.; Yang, F.-Y.; Zhang, L.; Huang, W.-J.; Hou, W.; Fan, C.-F.; Jin, R.-H.; Feng, Y.-M.; Wang, Y.-C.; et al. Cathepsin L Plays a Key Role in SARS-CoV-2 Infection in Humans and Humanized Mice and Is a Promising Target for New Drug Development. Signal Transduct. Target. Ther. 2021, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Onishi, K. Cathepsin L Is Crucial for a Th1-Type Immune Response during Leishmania Major Infection. Microbes Infect. 2004, 6, 468–474. [Google Scholar] [CrossRef]
- Liu, C.-L.; Guo, J.; Zhang, X.; Sukhova, G.K.; Libby, P.; Shi, G.-P. Cysteine Protease Cathepsins in Cardiovascular Disease: From Basic Research to Clinical Trials. Nat. Rev. Cardiol. 2018, 15, 351–370. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, L.; Gao, K.; Zhang, M. Study on the Mechanism of Cathepsin L on the Activation of M1 Macrophages in Sepsis-induced Acute Renal Injury. Indian J. Pharm. Sci. 2020, 102–107. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, K.; Li, Y.; Lu, C.; Ling, K.; Cai, C.; Wang, W.; Ye, D. Targeting TNF-α for COVID-19: Recent Advanced and Controversies. Front. Public Health 2022, 10, 833967. [Google Scholar] [CrossRef]
- Vatansever, H.S.; Becer, E. Relationship between IL-6 and COVID-19: To Be Considered during Treatment. Future Virol. 2020, 15, 817–822. [Google Scholar] [CrossRef]
- Mahallawi, W.H.; Khabour, O.F.; Zhang, Q.; Makhdoum, H.M.; Suliman, B.A. MERS-CoV Infection in Humans Is Associated with a Pro-Inflammatory Th1 and Th17 Cytokine Profile. Cytokine 2018, 104, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Hiscott, J.; Nguyen, T.-L.A.; Arguello, M.; Nakhaei, P.; Paz, S. Manipulation of the Nuclear Factor-ΚB Pathway and the Innate Immune Response by Viruses. Oncogene 2006, 25, 6844–6867. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, P.; Veckman, V.; Sirén, J.; Klucher, K.M.; Hiscott, J.; Matikainen, S.; Julkunen, I. Gene Expression and Antiviral Activity of Alpha/Beta Interferons and Interleukin-29 in Virus-Infected Human Myeloid Dendritic Cells. J. Virol. 2005, 79, 9608–9617. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Sans, M.; Scaldaferri, F.; Sgambato, A.; Rutella, S.; Cittadini, A.; Piqué, J.M.; Panes, J.; Katz, J.A.; Gasbarrini, A.; et al. TNF-α Blockade Down-Regulates the CD40/CD40L Pathway in the Mucosal Microcirculation: A Novel Anti-Inflammatory Mechanism of Infliximab in Crohn’s Disease. J. Immunol. 2006, 176, 2617–2624. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Gu, J.X.; Kovacs, C.; Freedman, J.; Thomas, E.K.; Ostrowski, M.A. Cooperation of TNF Family Members CD40 Ligand, Receptor Activator of NF-ΚB Ligand, and TNF-α in the Activation of Dendritic Cells and the Expansion of Viral Specific CD8+ T Cell Memory Responses in HIV-1-Infected and HIV-1-Uninfected Individuals. J. Immunol. 2003, 170, 1797–1805. [Google Scholar] [CrossRef]
- Gilmore, T.D.; Wolenski, F.S. NF-ΚB: Where Did It Come from and Why? Immunol. Rev. 2012, 246, 14–35. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-ΚB by TNF Family Cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Terpos, E.; Rosati, M.; Angel, M.; Bear, J.; Stellas, D.; Karaliota, S.; Apostolakou, F.; Bagratuni, T.; Patseas, D.; et al. Systemic IL-15, IFN-γ, and IP-10/CXCL10 Signature Associated with Effective Immune Response to SARS-CoV-2 in BNT162b2 MRNA Vaccine Recipients. Cell Rep. 2021, 36, 109504. [Google Scholar] [CrossRef] [PubMed]
- Le Vu, S.; Bertrand, M.; Jabagi, M.-J.; Botton, J.; Drouin, J.; Baricault, B.; Weill, A.; Dray-Spira, R.; Zureik, M. Age and Sex-Specific Risks of Myocarditis and Pericarditis Following COVID-19 Messenger RNA Vaccines. Nat. Commun. 2022, 13, 3633. [Google Scholar] [CrossRef]
- AlGhatrif, M.; Tanaka, T.; Moore, A.Z.; Bandinelli, S.; Lakatta, E.G.; Ferrucci, L. Age-Associated Difference in Circulating ACE2, the Gateway for SARS-COV-2, in Humans: Results from the InCHIANTI Study. GeroScience 2021, 43, 619–627. [Google Scholar] [CrossRef]
- Sanchez-Ramirez, D.C.; Normand, K.; Zhaoyun, Y.; Torres-Castro, R. Long-Term Impact of COVID-19: A Systematic Review of the Literature and Meta-Analysis. Biomedicines 2021, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.; Ojha, V. Thromboembolism after COVID-19 Vaccination: A Systematic Review of Such Events in 286 Patients. Ann. Vasc. Surg. 2022, 84, 12–20. [Google Scholar] [CrossRef]
- Husby, A.; Køber, L. COVID-19 MRNA Vaccination and Myocarditis or Pericarditis. Lancet 2022, 399, 2168–2169. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Kaur, R.; Charan, J.; Bhardwaj, P.; Ambwani, S.R.; Babu, S.; Goyal, J.P.; Haque, M. Analysis of Neurological Adverse Events Reported in VigiBase from COVID-19 Vaccines. Cureus 2022, 14, e21376. [Google Scholar] [CrossRef] [PubMed]
- Babajani, F.; Kakavand, A.; Mohammadi, H.; Sharifi, A.; Zakeri, S.; Asadi, S.; Afshar, Z.M.; Rahimi, Z.; Sayad, B. COVID-19 and Renin Angiotensin Aldosterone System: Pathogenesis and Therapy. Health Sci. Rep. 2021, 4, e440. [Google Scholar] [CrossRef]
- Buso, G.; Agabiti-Rosei, C.; Muiesan, M.L. The Relationship between COVID-19 Vaccines and Increased Blood Pressure: A Word of Caution. Eur. J. Intern. Med. 2023, 111, 27–29. [Google Scholar] [CrossRef]

| ▪ | ACE2 (pg/mL) | AngII (pg/mL) | CTSL (pg/mL) | (pg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ▪ | Moderete COVID-19 | Severe COVID-19 | p | Moderete COVID-19 | Severe COVID-19 | p | Moderete COVID-19 | Severe COVID-19 | p | Moderete COVID-19 | Severe COVID-19 | p |
| ▪n | 21 | 6 | 17 | 9 | 16 | 11 | 25 | 2 | ||||
| ▪Marker mean | 326.57 ± 854.84 | 1428.75 ± 1386.70 | 155.52 ± 141.60 | 275.52 ± 191.28 | 1286.54 ± 1084.15 | 2080.73 ± 1284.15 | 253.54 ± 283.66 | 1175.24 ± 232.10 | ||||
| ▪IgG (g/L) | 9.27 ± 5.28 | 7.96 ± 2.05 | 0.798 | 9.65 ± 5.82 | 7.60 ± 1.60 | 0.312 | 8.08 ± 2.07 | 10.28 ± 7.01 | 0.645 | 9.08 ± 4.92 | 7.66 ± 1.87 | 0.627 |
| ▪IgA (g/L) | 2.02 ± 0.74 | 2.35 ± 1.51 | 0.932 | 2.01 ± 0.69 | 2.40 ± 1.27 | 0.560 | 1.68 ± 0.52 | 2.70 ± 1.09 | 0.005 * | 1.94 ± 0.71 | 4.08 ± 1.47 | 0.023 * |
| ▪IgM (g/L) | 1.55 ± 0.57 | 0.97 ± 0.51 | 0.042 * | 1.49 ± 0.45 | 1.28 ± 0.84 | 0.133 | 1.49 ± 0.40 | 1.31 ± 0.82 | 0.162 | 1.49 ± 0.56 | 0.57 ± 0.26 | 0.011 * |
| ▪IL-6 (pg/mL) | 27.94 ± 43.03 | 17.51 ± 20.31 | 0.974 | 23.29 ± 44.15 | 29.05 ± 30.64 | 0.294 | 29.73 ± 45.51 | 19.18 ± 26.80 | 0.880 | 27.22 ± 39.69 | 4.59 ± 2.67 | 0.406 |
| ▪IL-10 (pg/mL) | 319.75 ± 540.46 | 844.39 ± 1088.23 | 0.744 | 346.19 ± 584.72 | 681.27 ± 967.85 | 0.754 | 360.25 ± 621.27 | 550.69 ± 843.06 | 0.799 | 290.89 ± 495.84 | 2240.00 ± 270.11 | 0.025 * |
| ▪IL-33 (ng/L) | 17.65 ± 7.46 | 52.80 ± 47.40 | 0.009 * | 17.80 ± 7.95 | 43.29 ± 43.79 | 0.175 | 18.56 ± 7.81 | 35.57 ± 39.12 | 0.610 | 18.46 ± 7.04 | 113.33 ± 15.32 | 0.006 * |
| ▪IL-28A (ng/mL) | 381.52 ± 280.46 | 981.17 ± 973.49 | 0.295 | 352.59 ± 236.27 | 904.51 ± 868.98 | 0.157 | 296.72 ± 263.74 | 824.24 ± 717.82 | 0.013 * | 381.23 ± 281.84 | 2184.00 ± 255.97 | 0.006 * |
| ▪CD40L (ng/mL) | 1.98 ± 1.32 | 15.81 ± 26.49 | 0.006 * | 1.75 ± 0.99 | 12.56 ± 23.22 | 0.023 * | 2.57 ± 1.45 | 8.71 ± 20.39 | 0.357 | 2.16 ± 1.35 | 41.26 ± 39.52 | 0.006 * |
| ▪T-cell | 1074.99 ± 767.05 | 1472.19 ± 988.67 | 0.550 | 1291.79 ± 877.34 | 978.42 ± 725.86 | 0.339 | 986.13 ± 612.55 | 1420.90 ± 1026.18 | 0.512 | 1153.94 ± 823.77 | 1279.71 ± 1036.87 | 0.963 |
| ▪T-cytotoxic cells | 421.82 ± 359.34 | 559.16 ± 371.08 | 0.376 | 513.14 ± 389.90 | 361.44 ± 304.76 | 0.396 | 332.50 ± 200.06 | 626.67 ± 468.46 | 0.195 | 436.85 ± 351.03 | 645.87 ± 566.40 | 0.570 |
| ▪T-helper cells | 611.03 ± 488.42 | 853.01 ± 626.41 | 0.512 | 731.42 ± 562.69 | 577.66 ± 457.61 | 0.426 | 617.46 ± 404.94 | 733.67 ± 667.72 | 0.865 | 671.56 ± 532.39 | 580.30 ± 433.92 | 0.889 |
| ▪NK-cells | 169.88 ± 127.41 | 359.28 ± 202.27 | 0.049 * | 223.13 ± 156.28 | 206.00 ± 188.73 | 0.711 | 205.56 ± 148.36 | 221.29 ± 191.02 | 0.981 | 192.68 ± 148.79 | 453.07 ± 199.42 | 0.091 |
| B-cells | 216.25 ± 138.89 | 263.35 ± 149.09 | 0.589 | 233.73 ± 132.56 | 219.12 ± 166.66 | 0.491 | 201.36 ± 127.20 | 263.60 ± 154.72 | 0.342 | 233.92 ± 142.38 | 136.67 ± 57.03 | 0.410 |
| ACE2 | AngII | CTSL | TNF-α | |
|---|---|---|---|---|
| IgG (g/L) | 0.061 | −0.177 | 0.035 | −0.038 |
| IgA (g/L) | 0.008 | 0.102 | 0.437 * | 0.258 |
| IgM (g/L) | −0.273 | −0.129 | −0.273 | −0.180 |
| IL-6 (pg/mL) | −0.047 | −0.074 | −0.037 | −0.346 |
| IL-10 (pg/mL) | 0.094 | 0.196 | 0.095 | −0.168 |
| IL-33 (ng/L) | 0.539 ** | 0.416 * | 0.182 | 0.239 |
| IL-28A (ng/mL) | 0.275 | 0.096 | 0.592 ** | 0.491 * |
| CD40L (ng/mL) | 0.520 ** | 0.504 * | 0.045 | 0.458 * |
| T-cells (×109/L) | 0.006 | −0.382 | −0.122 | 0.156 |
| T-cytotoxic cells (×109/L) | 0.038 | −0.336 | −0.014 | 0.049 |
| T-helper cells (×109/L) | 0.021 | −0.313 | −0.252 | 0.178 |
| NK-cells (×109/L) | 0.261 | −0.011 | −0.176 | 0.190 |
| B-cells (×109/L) | 0.025 | −0.239 | −0.092 | 0.094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pencheva, M.; Bozhkova, M.; Kalchev, Y.; Petrov, S.; Baldzhieva, A.; Kalfova, T.; Dichev, V.; Keskinova, D.; Genova, S.; Atanasova, M.; et al. The Serum ACE2, CTSL, AngII, and TNFα Levels after COVID-19 and mRNA Vaccines: The Molecular Basis. Biomedicines 2023, 11, 3160. https://doi.org/10.3390/biomedicines11123160
Pencheva M, Bozhkova M, Kalchev Y, Petrov S, Baldzhieva A, Kalfova T, Dichev V, Keskinova D, Genova S, Atanasova M, et al. The Serum ACE2, CTSL, AngII, and TNFα Levels after COVID-19 and mRNA Vaccines: The Molecular Basis. Biomedicines. 2023; 11(12):3160. https://doi.org/10.3390/biomedicines11123160
Chicago/Turabian StylePencheva, Mina, Martina Bozhkova, Yordan Kalchev, Steliyan Petrov, Alexandra Baldzhieva, Teodora Kalfova, Valentin Dichev, Donka Keskinova, Silvia Genova, Mariya Atanasova, and et al. 2023. "The Serum ACE2, CTSL, AngII, and TNFα Levels after COVID-19 and mRNA Vaccines: The Molecular Basis" Biomedicines 11, no. 12: 3160. https://doi.org/10.3390/biomedicines11123160
APA StylePencheva, M., Bozhkova, M., Kalchev, Y., Petrov, S., Baldzhieva, A., Kalfova, T., Dichev, V., Keskinova, D., Genova, S., Atanasova, M., & Murdzheva, M. (2023). The Serum ACE2, CTSL, AngII, and TNFα Levels after COVID-19 and mRNA Vaccines: The Molecular Basis. Biomedicines, 11(12), 3160. https://doi.org/10.3390/biomedicines11123160





