Identification and Preliminary Clinical Validation of Key Extracellular Proteins as the Potential Biomarkers in Hashimoto’s Thyroiditis by Comprehensive Analysis
Abstract
:1. Introduction
2. Materials and Methods
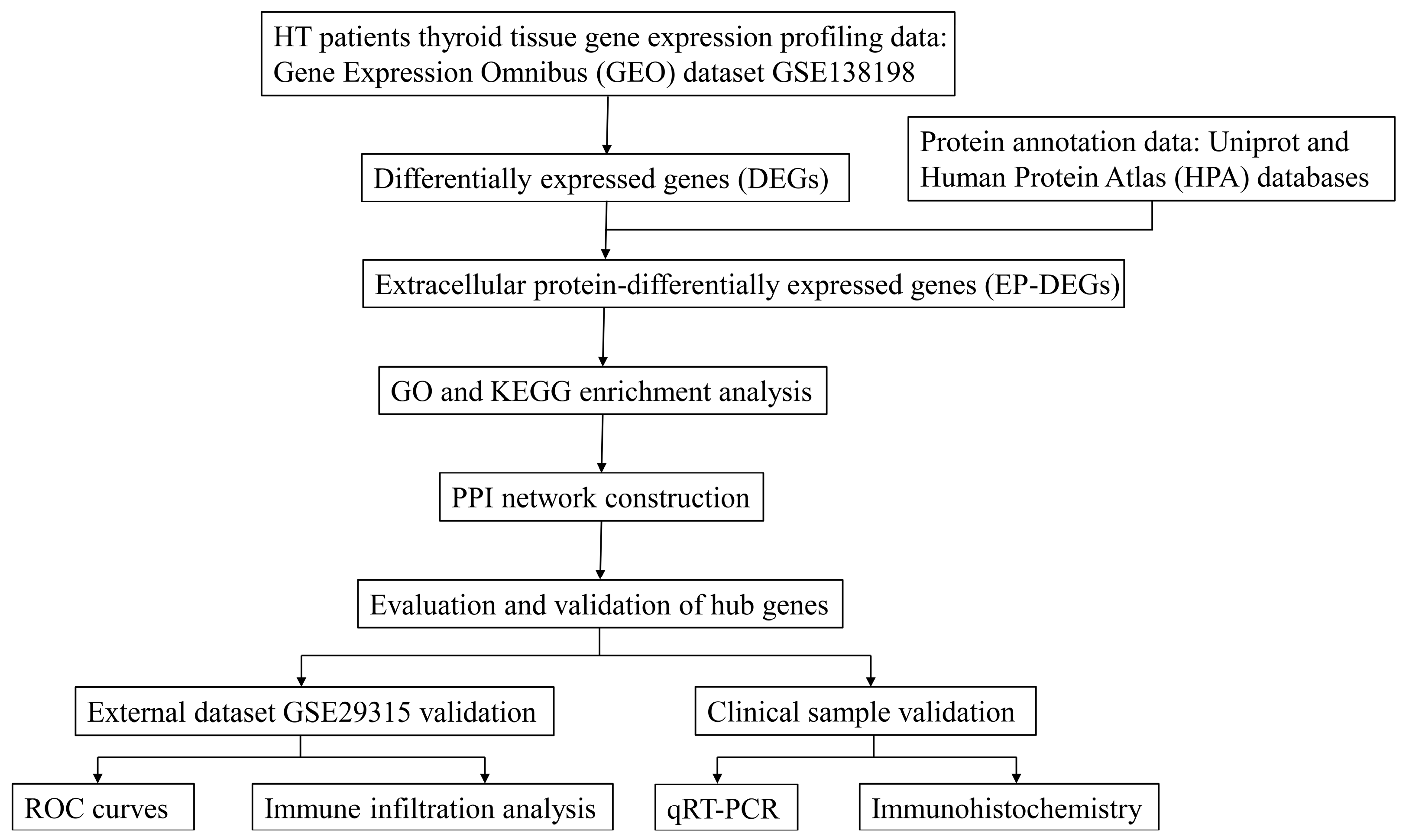
2.1. Study Design and Data Acquisition
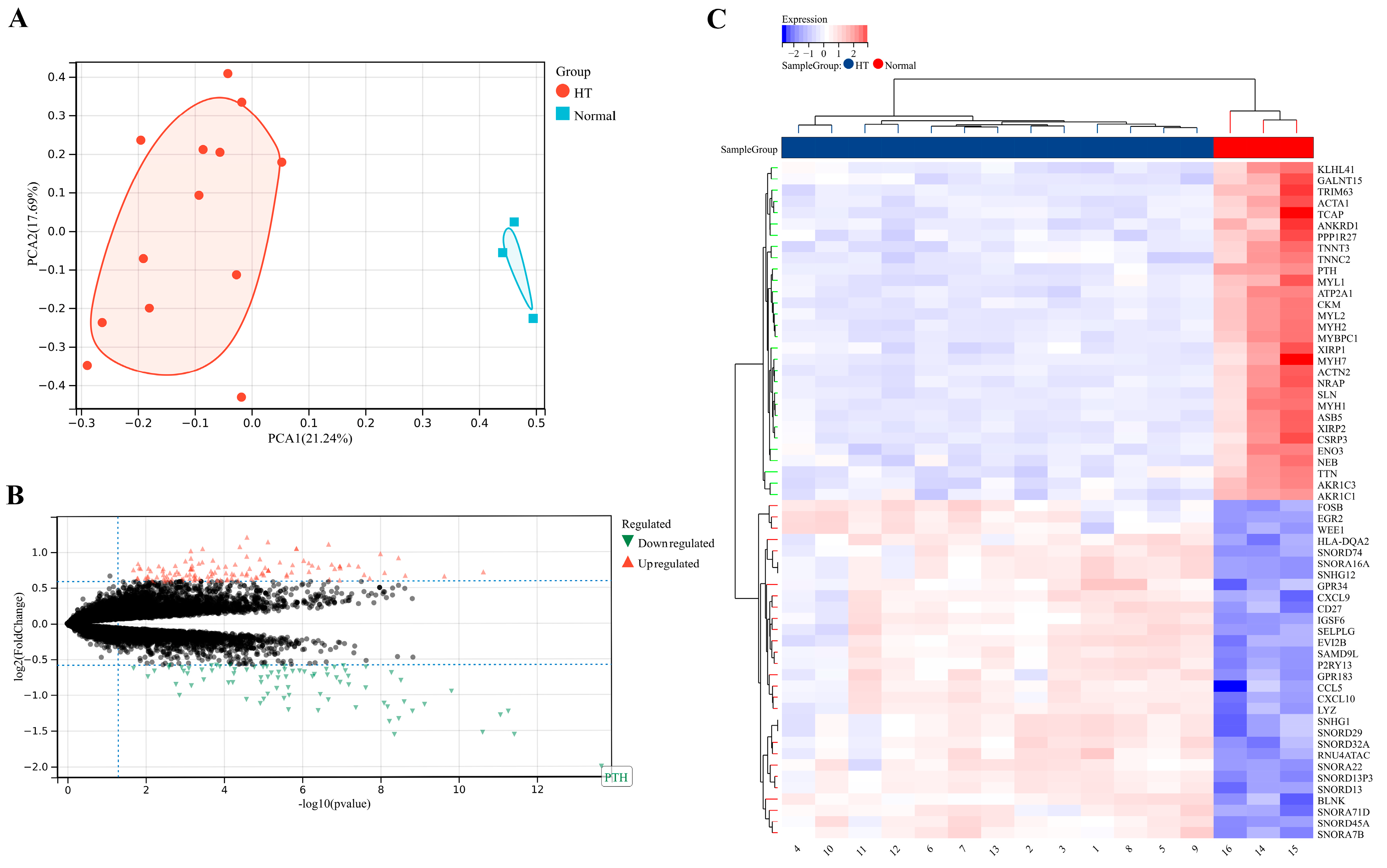
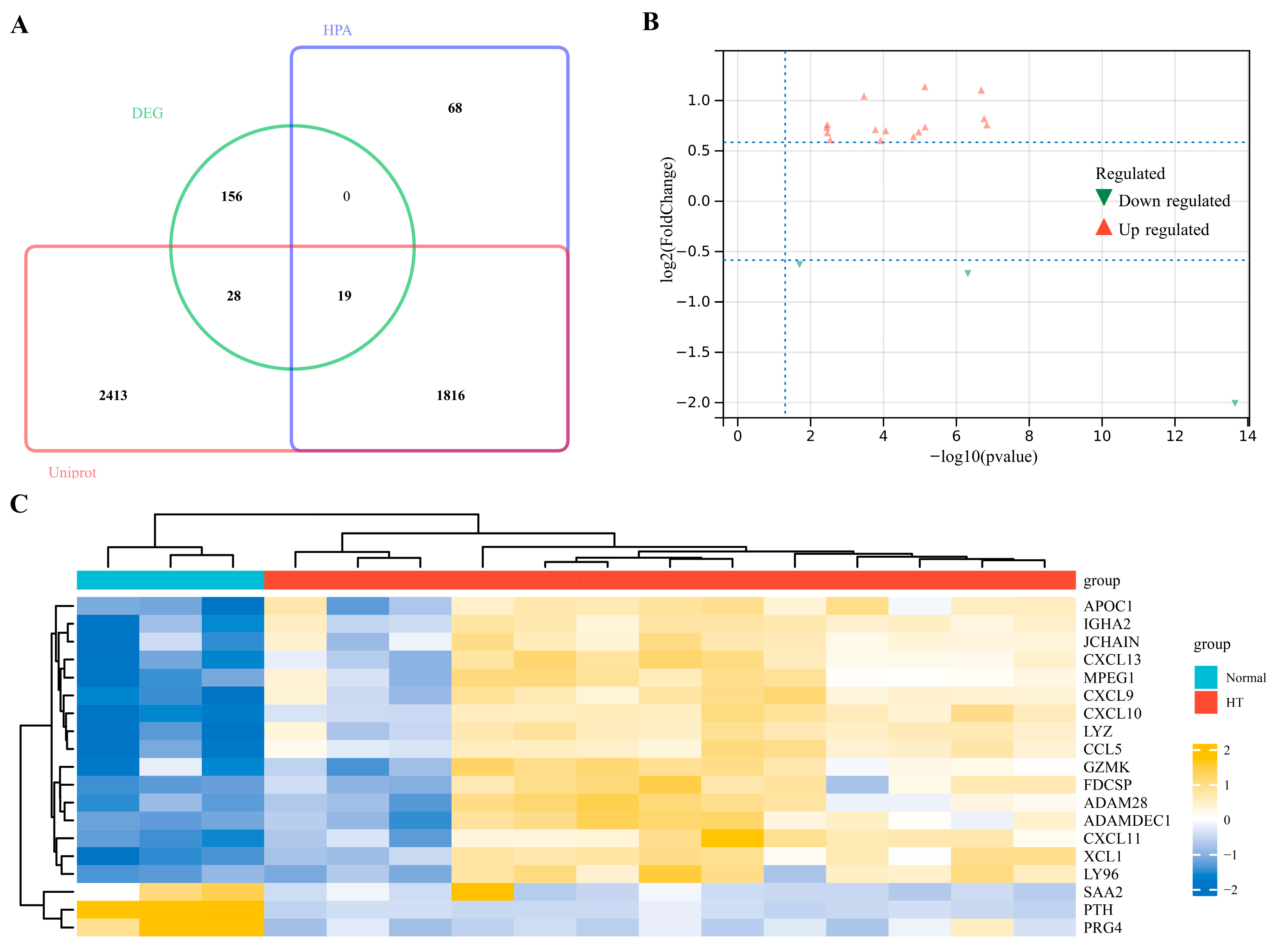
2.2. Identification of DEGs and EP-DEGs
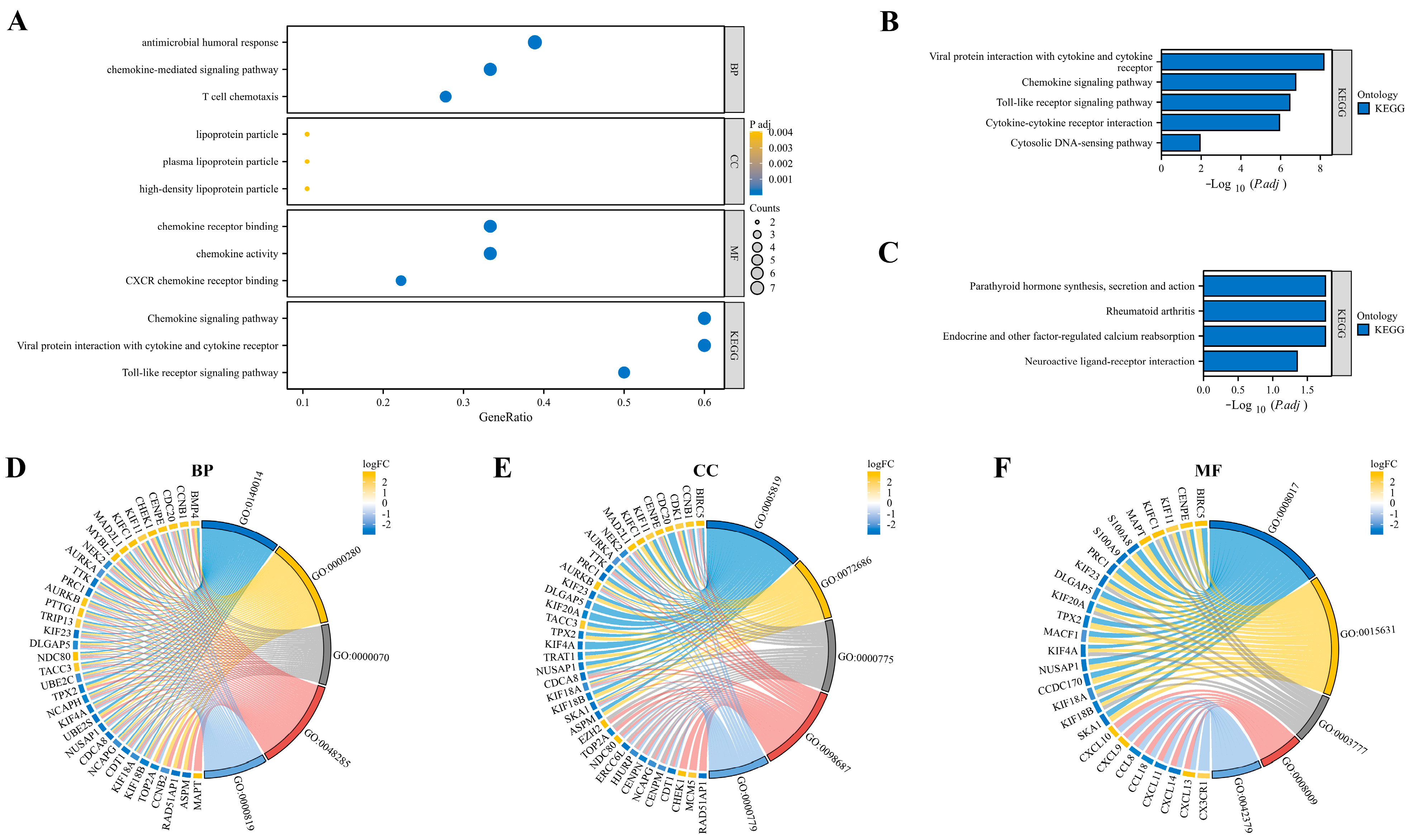
2.3. Function and Pathway Enrichment Analysis
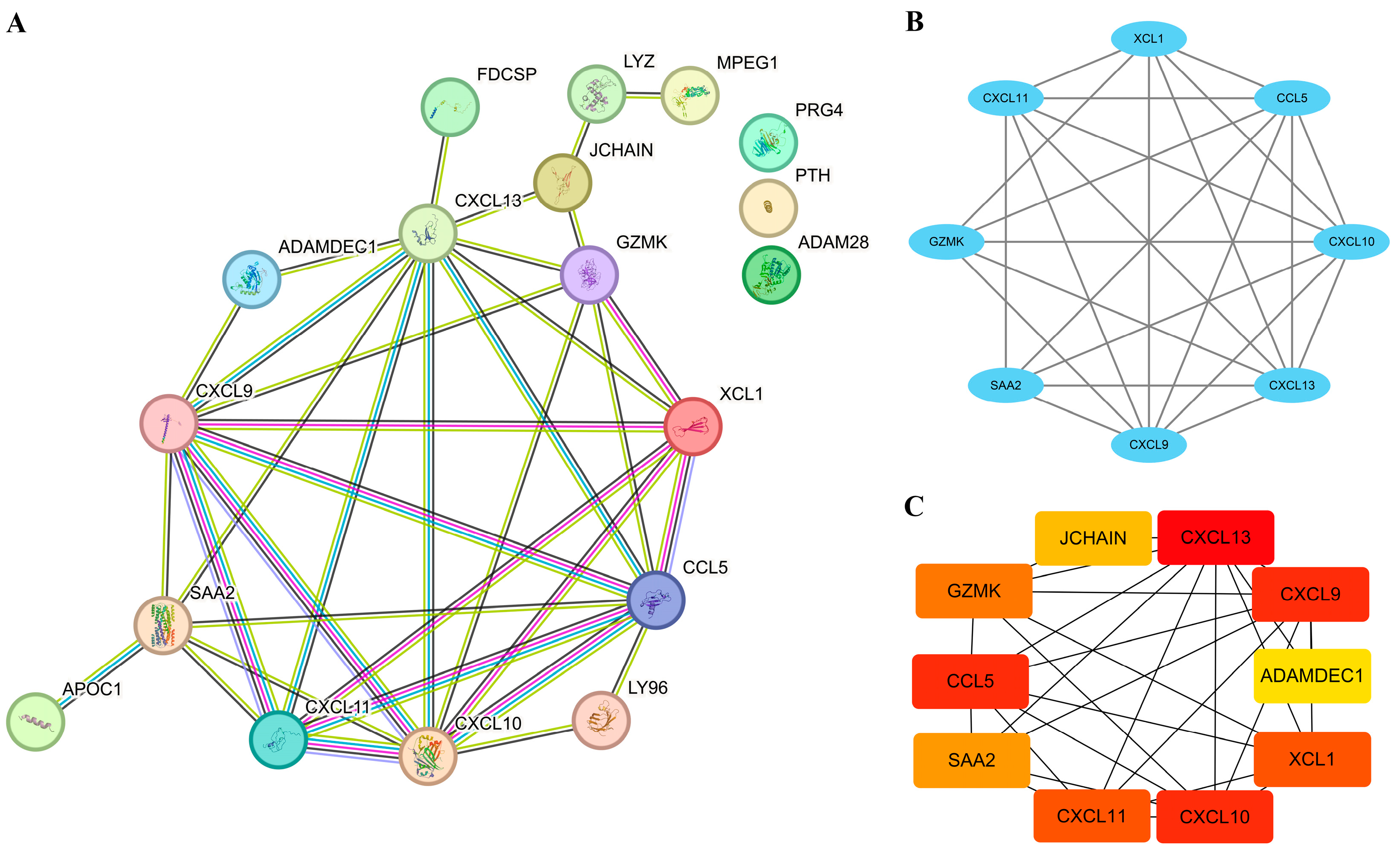
2.4. Construction of PPI Network
2.5. Evaluation and Validation of the Key EP-DEGs
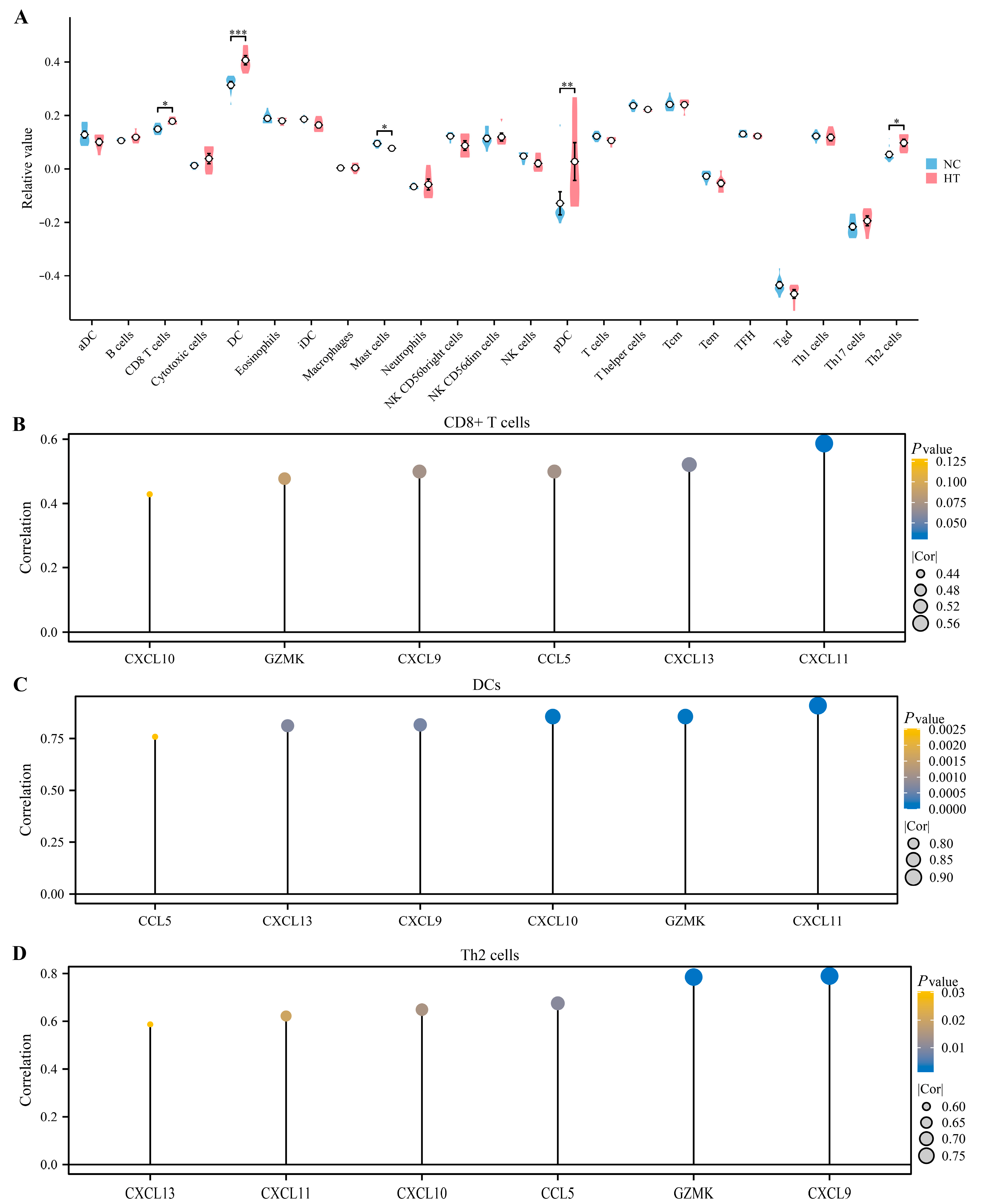
2.6. Immune Infiltration Analysis
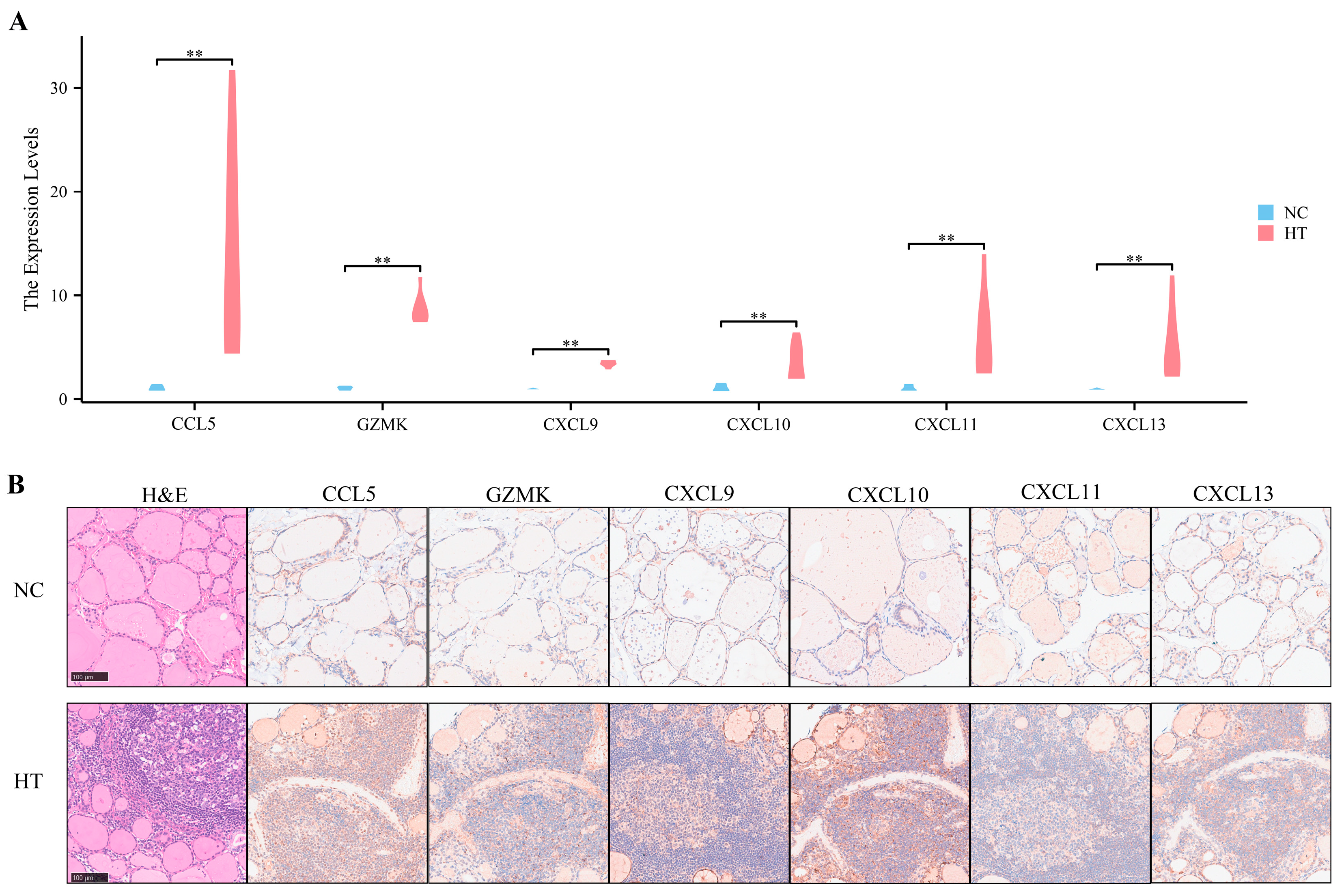
2.7. Validation of Clinical Samples
2.8. RNA Extraction and RT-PCR
2.9. H&E Staining and IHC Staining
2.10. Statistical Methods
3. Results
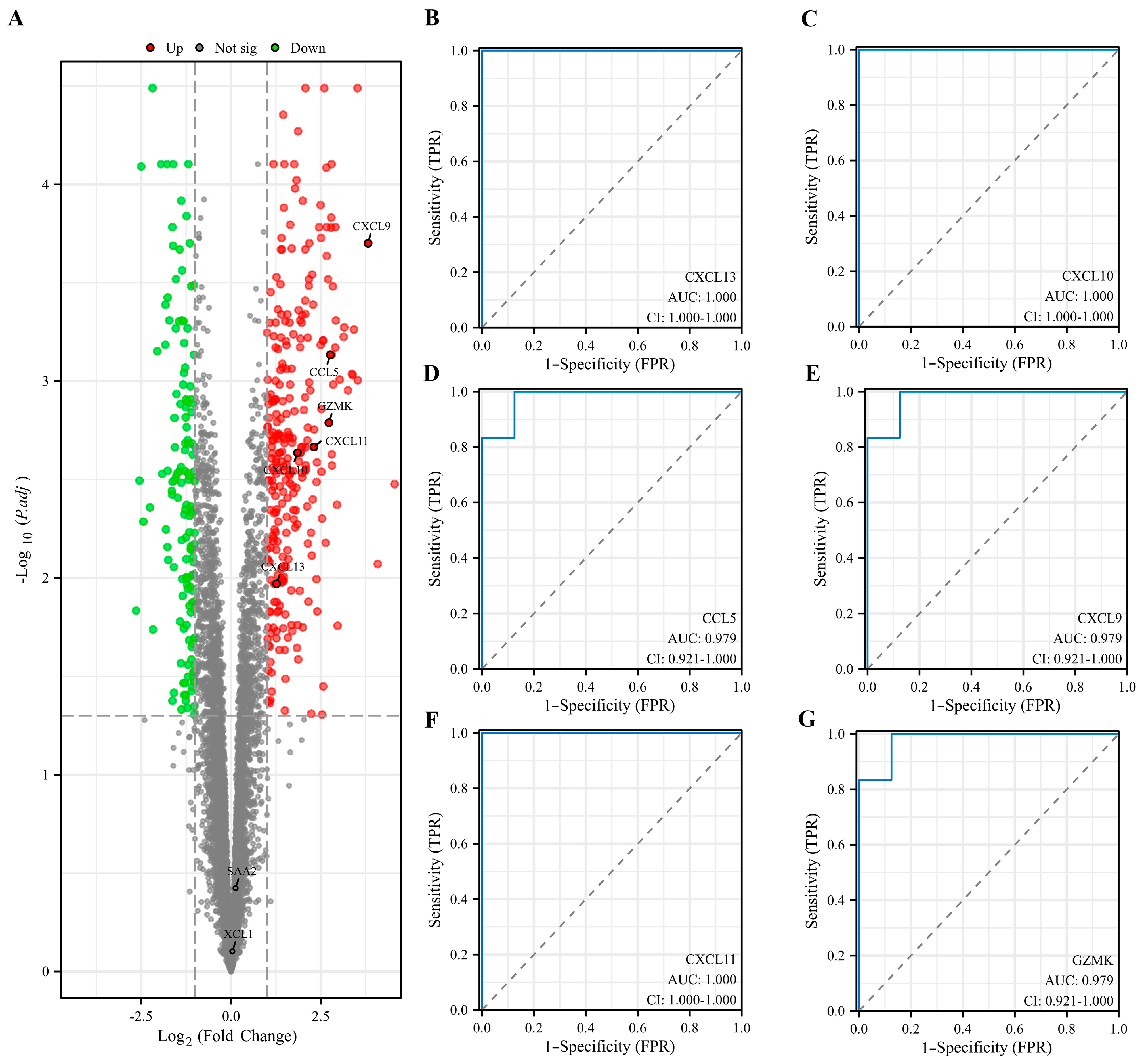
3.1. Identification of DEGs
3.2. Identification of EP-DEGs
3.3. Function and Pathway Enrichment Analysis
3.4. Establishment of PPI Network and Identification of Hub EP-DEGs
3.5. Evaluation and Validation of Key EP-DEGs
3.6. Immune Infiltration Analysis
3.7. Preliminary Validation of Clinical Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hiromatsu, Y.; Satoh, H.; Amino, N. Hashimoto’s thyroiditis: History and future outlook. Hormones 2013, 12, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-K.; Lin, C.-L.; Cheng, F.T.-F.; Sung, F.-C.; Kao, C.-H. Cancer risk in patients with Hashimoto’s thyroiditis: A nationwide cohort study. Br. J. Cancer 2013, 109, 2496–2501. [Google Scholar] [CrossRef]
- McLeod, D.S.; Cooper, D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine 2012, 42, 252–265. [Google Scholar] [CrossRef]
- Ott, J.; Meusel, M.; Schultheis, A.; Promberger, R.; Pallikunnel, S.J.; Neuhold, N.; Hermann, M. The incidence of lymphocytic thyroid infiltration and Hashimoto’s thyroiditis increased in patients operated for benign goiter over a 31-year period. Virchows Arch. 2011, 459, 277–281. [Google Scholar] [CrossRef]
- Tunbridge, W.M.; Brewis, M.; French, J.M.; Appleton, D.; Bird, T.; Clark, F.; Evered, D.C.; Evans, J.G.; Hall, R.; Smith, P. Natural history of autoimmune thyroiditis. Br. Med. J. (Clin. Res. Ed.) 1981, 282, 258–262. [Google Scholar] [CrossRef]
- McLeod, D.S.; Caturegli, P.; Cooper, D.S.; Matos, P.G.; Hutfless, S. Variation in rates of autoimmune thyroid disease by race/ethnicity in US military personnel. JAMA 2014, 311, 1563–1565. [Google Scholar] [CrossRef]
- Kust, D.; Matesa, N. The impact of familial predisposition on the development of Hashimoto’s thyroiditis. Acta Clin. Belg. 2020, 75, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Oryoji, D.; Yamamoto, K.; Noh, J.Y.; Okamura, K.; Noda, M.; Kashiwase, K.; Kosuga, Y.; Sekiya, K.; Inoue, K.; et al. Identification of independent susceptible and protective HLA alleles in Japanese autoimmune thyroid disease and their epistasis. J. Clin. Endocrinol. Metab. 2014, 99, E379–E383. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chang, T.-Y.; Chu, C.-C.; Lo, F.-S.; Ting, W.-H.; Wu, Y.-L.; Chu, S.-Y.; Chang, S.-C.; Chen, W.-F.; Lin, C.-L.; et al. The HLA-B gene and Hashimoto disease in Han Chinese children: A case-control and family-based study. Tissue Antigens 2012, 80, 431–436. [Google Scholar] [CrossRef]
- Wiersinga, W.M. Clinical Relevance of Environmental Factors in the Pathogenesis of Autoimmune Thyroid Disease. Endocrinol. Metab. 2016, 31, 213–222. [Google Scholar] [CrossRef]
- Hu, S.; Rayman, M.P. Multiple Nutritional Factors and the Risk of Hashimoto’s Thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef]
- McLachlan, S.M.; Rapoport, B. Breaking tolerance to thyroid antigens: Changing concepts in thyroid autoimmunity. Endocr. Rev. 2014, 35, 59–105. [Google Scholar] [CrossRef] [PubMed]
- Stassi, G.; De Maria, R. Autoimmune thyroid disease: New models of cell death in autoimmunity. Nat. Rev. Immunol. 2002, 2, 195–204. [Google Scholar] [CrossRef]
- Ehlers, M.; Thiel, A.; Bernecker, C.; Porwol, D.; Papewalis, C.; Willenberg, H.S.; Schinner, S.; Hautzel, H.; Scherbaum, W.A.; Schott, M. Evidence of a combined cytotoxic thyroglobulin and thyroperoxidase epitope-specific cellular immunity in Hashimoto’s thyroiditis. J. Clin. Endocrinol. Metab. 2012, 97, 1347–1354. [Google Scholar] [CrossRef]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, H.; Yin, P.; Song, J.; Jiang, F.; Tang, Z.; Fan, X.; Xu, C.; Wang, Y.; Xue, Y.; et al. Identification and Clinical Validation of Key Extracellular Proteins as the Potential Biomarkers in Relapsing-Remitting Multiple Sclerosis. Front. Immunol. 2021, 12, 753929. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; Di Domenicantonio, A.; Fallahi, P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015, 14, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Ye, X.-P.; Zhou, Z.; Zhu, C.-F.; Li, R.; Fang, Y.; Zhang, R.-J.; Li, L.; Liu, W.; Wang, Z.; et al. Lymphocyte infiltration and thyrocyte destruction are driven by stromal and immune cell components in Hashimoto’s thyroiditis. Nat. Commun. 2022, 13, 775. [Google Scholar] [CrossRef]
- Armengol, M.-P.; Cardoso-Schmidt, C.B.; Fernández, M.; Ferrer, X.; Pujol-Borrell, R.; Juan, M. Chemokines determine local lymphoneogenesis and a reduction of circulating CXCR4+ T and CCR7 B and T lymphocytes in thyroid autoimmune diseases. J. Immunol. 2003, 170, 6320–6328. [Google Scholar] [CrossRef]
- Raghu, H.; Lepus, C.M.; Wang, Q.; Wong, H.H.; Lingampalli, N.; Oliviero, F.; Punzi, L.; Giori, N.J.; Goodman, S.B.; Chu, C.R.; et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 914–922. [Google Scholar] [CrossRef]
- Liu, C.; Pan, Y.; Li, Q.; Zhang, Y. Bioinformatics analysis identified shared differentially expressed genes as potential biomarkers for Hashimoto’s thyroiditis-related papillary thyroid cancer. Int. J. Med. Sci. 2021, 18, 3478–3487. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Li, K.; Zeng, T.; Liao, Y.; Min, J.; Zhang, N.; Peng, M.; Kong, W.; Chen, L.-L. Integrative Analyses of Genes Associated with Hashimoto’s Thyroiditis. J. Immunol. Res. 2021, 2021, 8263829. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Dou, X.J.; Song, H.J.; Wang, P.W.; Qu, W.; Zheng, X.H. Bioinformatics analysis of key genes and pathways in Hashimoto thyroiditis tissues. Biosci. Rep. 2020, 40, BSR20200759. [Google Scholar] [CrossRef] [PubMed]
- Ben-Skowronek, I.; Szewczyk, L.; Kulik-Rechberger, B.; Korobowicz, E. The differences in T and B cell subsets in thyroid of children with Graves’ disease and Hashimoto’s thyroiditis. World J. Pediatr. 2013, 9, 245–250. [Google Scholar] [CrossRef]
- Weetman, A.P. Autoimmune thyroid disease: Propagation and progression. Eur. J. Endocrinol. 2003, 148, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Paparo, S.R.; Ragusa, F.; Elia, G.; Mazzi, V.; Patrizio, A.; Ghionzoli, M.; Varricchi, G.; Centanni, M.; Ulisse, S.; et al. Chemokines in thyroid autoimmunity. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101773. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, H.; Zhang, Y.; Zhang, T.; Gong, J.; Wei, Y.; Duan, Y.G.; Shu, M.; Yang, Y.; Wu, D.; et al. Dimensionality reduction by UMAP reinforces sample heterogeneity analysis in bulk transcriptomic data. Cell Rep. 2021, 36, 109442. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Ito, K.; Murphy, D. Application of ggplot2 to Pharmacometric Graphics. CPT Pharmacomet. Syst Pharmacol. 2013, 2, e79. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Fabris, F.; Palmer, D.; De Magalhães, J.P.; Freitas, A.A. Comparing enrichment analysis and machine learning for identifying gene properties that discriminate between gene classes. Brief. Bioinform. 2020, 21, 803–814. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res. 2017, 45, D331–D338. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Bader, G.D.; Hogue, C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Y.; Yan, F.; Zhao, Y.; Zhao, H.; Guo, Y. A Novel Immune-Related Gene Signature to Identify the Tumor Microenvironment and Prognose Disease among Patients with Oral Squamous Cell Carcinoma Patients Using ssGSEA: A Bioinformatics and Biological Validation Study. Front. Immunol. 2022, 13, 922195. [Google Scholar] [CrossRef]
- Song, Y.; Westerhuis, J.A.; Aben, N.; Michaut, M.; Wessels, L.F.A.; Smilde, A.K. Principal component analysis of binary genomics data. Brief. Bioinform. 2019, 20, 317–329. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef]
- Pan, Z.; Zhu, T.; Liu, Y.; Zhang, N. Role of the CXCL13/CXCR5 Axis in Autoimmune Diseases. Front. Immunol. 2022, 13, 850998. [Google Scholar] [CrossRef]
- Zeng, Z.; Lan, T.; Wei, Y.; Wei, X. CCL5/CCR5 axis in human diseases and related treatments. Genes Dis. 2022, 9, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, A.C.; van Daalen, K.R.; Crnko, S.; Ten Broeke, T.; Bovenschen, N. Intracellular and Extracellular Roles of Granzyme K. Front. Immunol. 2021, 12, 677707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, Y. Comprehensive analysis of lncRNA-mediated ceRNA regulatory networks and key genes associated with papillary thyroid cancer coexistent with Hashimoto’s thyroiditis. BMC Endocr. Disord. 2022, 22, 252. [Google Scholar] [CrossRef] [PubMed]
- Aust, G.; Sittig, D.; Becherer, L.; Anderegg, U.; Schutz, A.; Lamesch, P.; Schmucking, E. The role of CXCR5 and its ligand CXCL13 in the compartmentalization of lymphocytes in thyroids affected by autoimmune thyroid diseases. Eur. J. Endocrinol. 2004, 150, 225–234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, Z.; Yang, T.; Huang, T.; Zhou, J.; Yang, P. Identification and Preliminary Clinical Validation of Key Extracellular Proteins as the Potential Biomarkers in Hashimoto’s Thyroiditis by Comprehensive Analysis. Biomedicines 2023, 11, 3127. https://doi.org/10.3390/biomedicines11123127
Xi Z, Yang T, Huang T, Zhou J, Yang P. Identification and Preliminary Clinical Validation of Key Extracellular Proteins as the Potential Biomarkers in Hashimoto’s Thyroiditis by Comprehensive Analysis. Biomedicines. 2023; 11(12):3127. https://doi.org/10.3390/biomedicines11123127
Chicago/Turabian StyleXi, Zihan, Tinglin Yang, Tao Huang, Jun Zhou, and Peng Yang. 2023. "Identification and Preliminary Clinical Validation of Key Extracellular Proteins as the Potential Biomarkers in Hashimoto’s Thyroiditis by Comprehensive Analysis" Biomedicines 11, no. 12: 3127. https://doi.org/10.3390/biomedicines11123127
APA StyleXi, Z., Yang, T., Huang, T., Zhou, J., & Yang, P. (2023). Identification and Preliminary Clinical Validation of Key Extracellular Proteins as the Potential Biomarkers in Hashimoto’s Thyroiditis by Comprehensive Analysis. Biomedicines, 11(12), 3127. https://doi.org/10.3390/biomedicines11123127





