Role of Fish Oil in Preventing Paternal Obesity and Improving Offspring Skeletal Muscle Health
Abstract
1. Introduction
2. Materials and Methods
3. Results
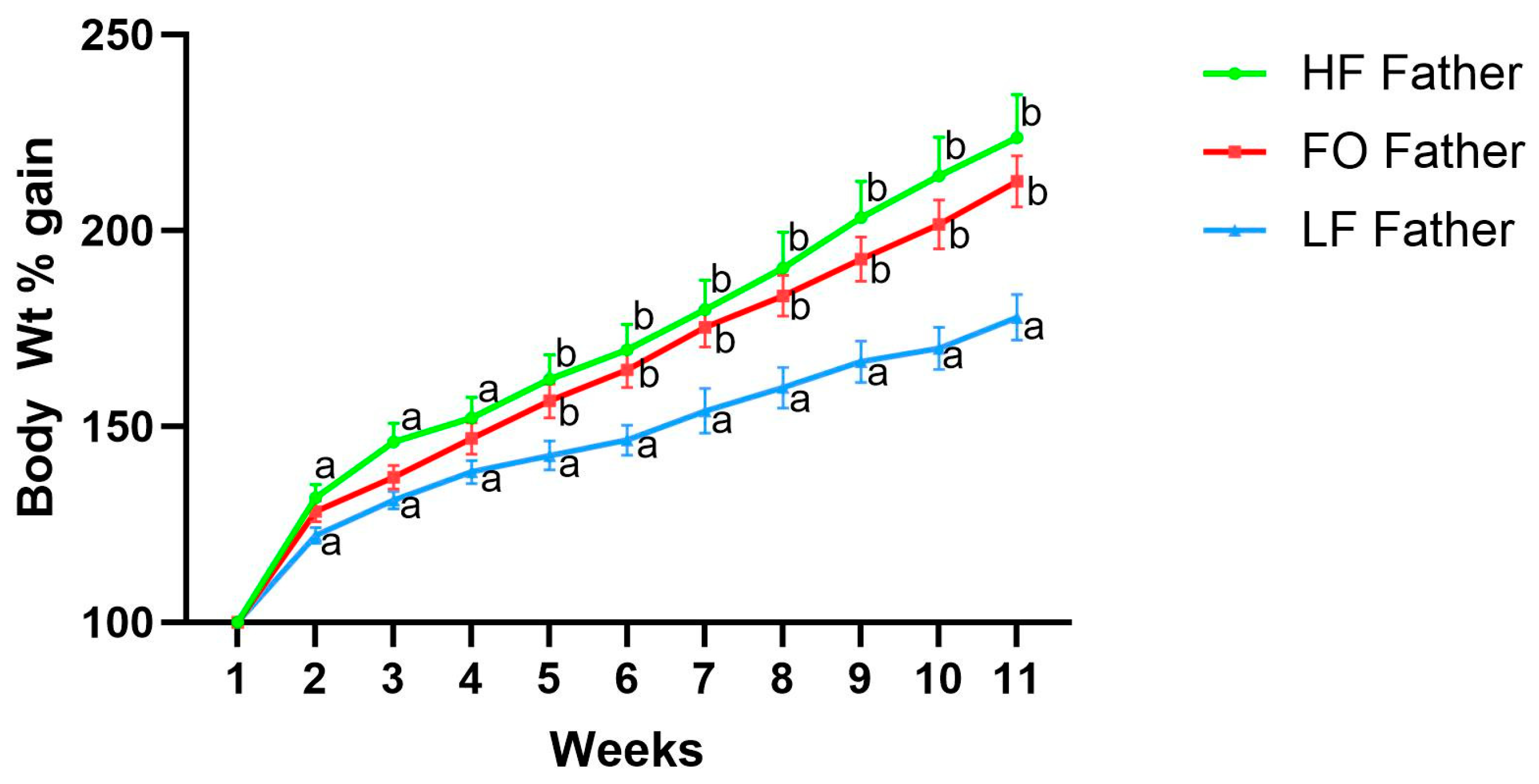
3.1. Offspring Father Body Weight
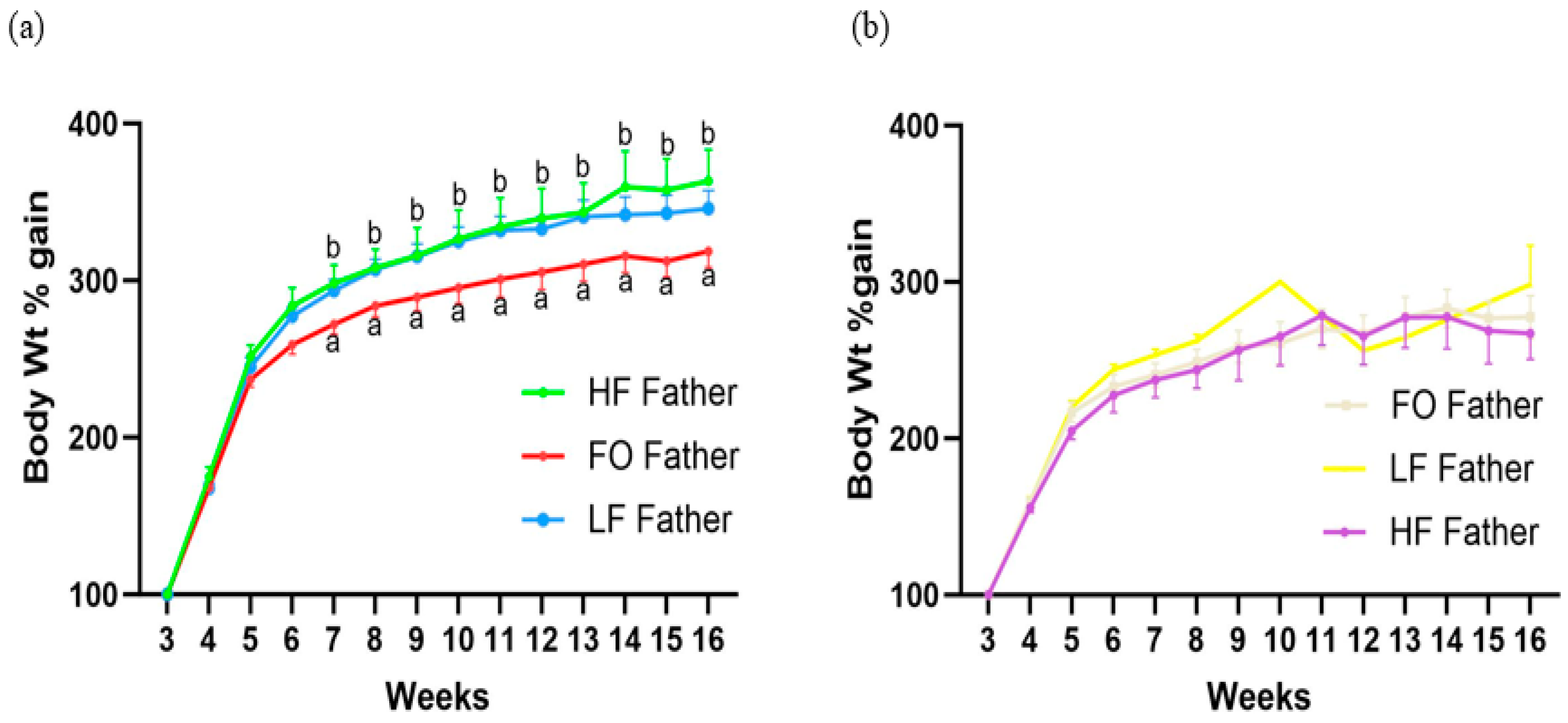
3.2. Offspring Development and Body Weight
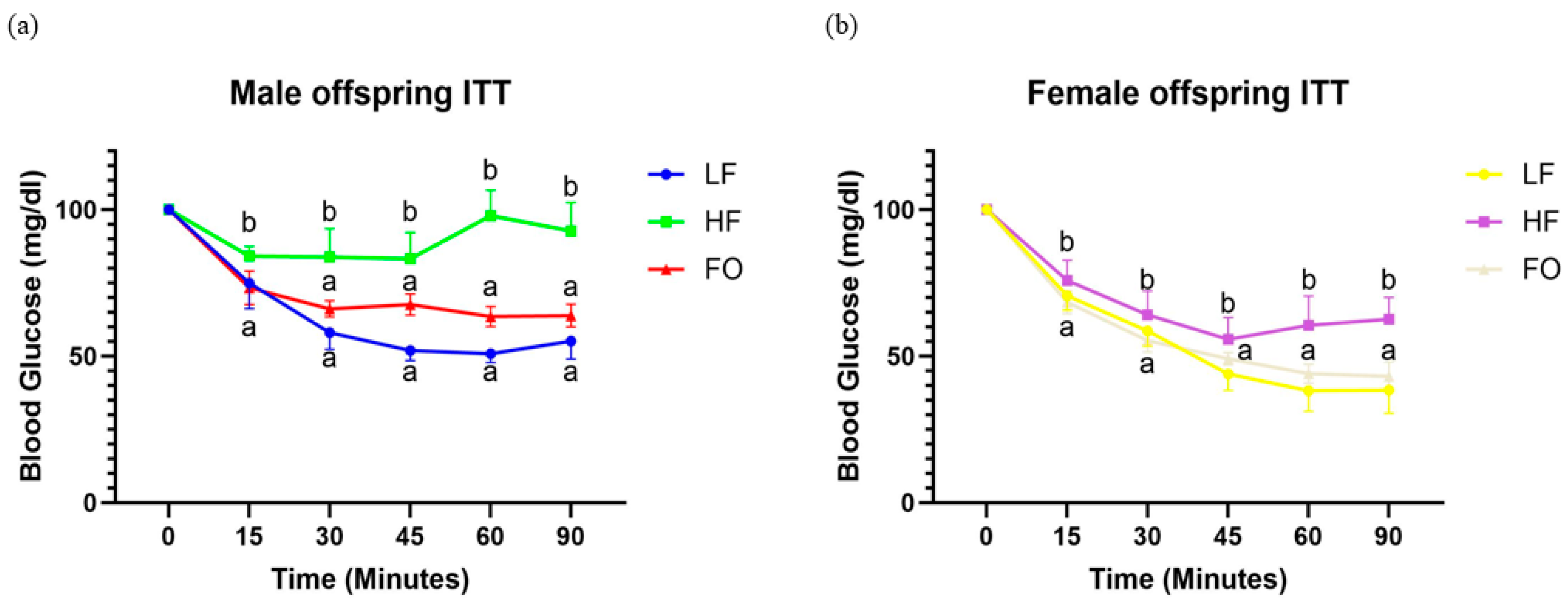
3.3. Offspring Insulin Resistance
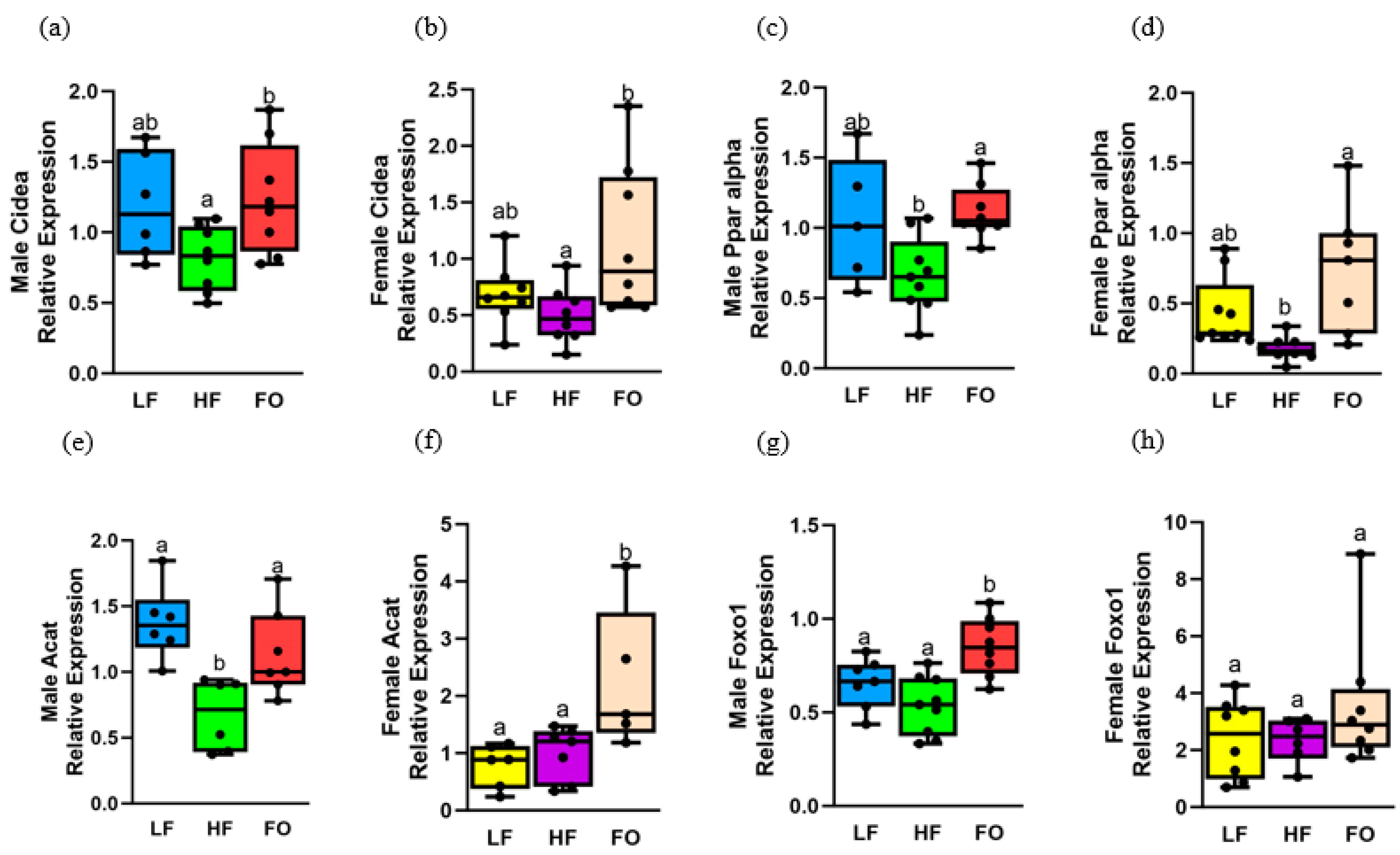
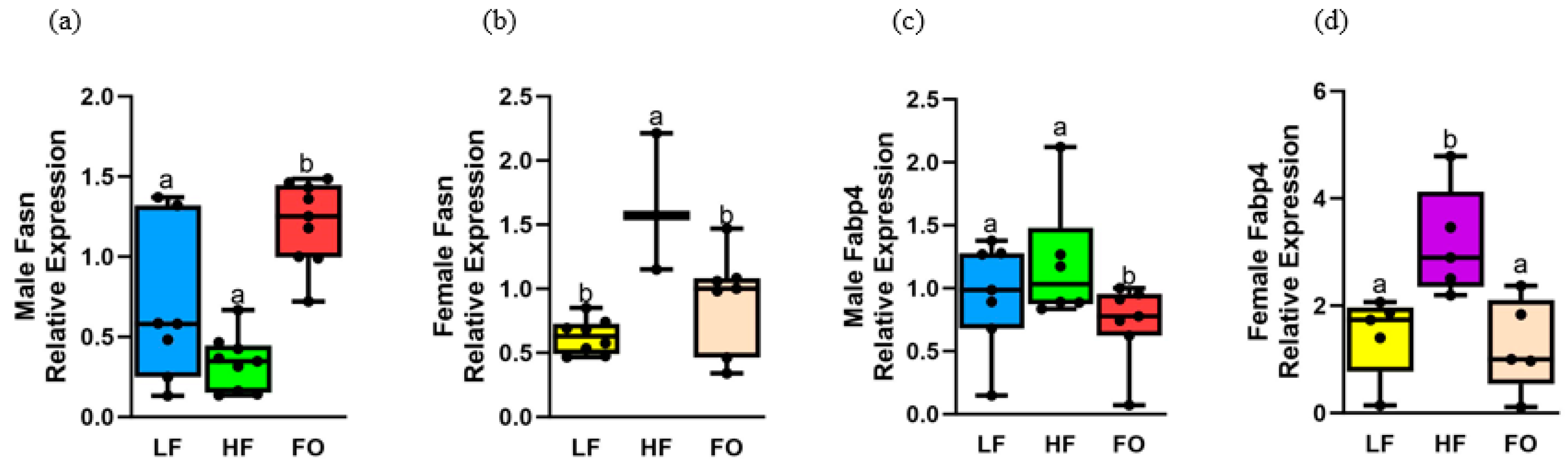
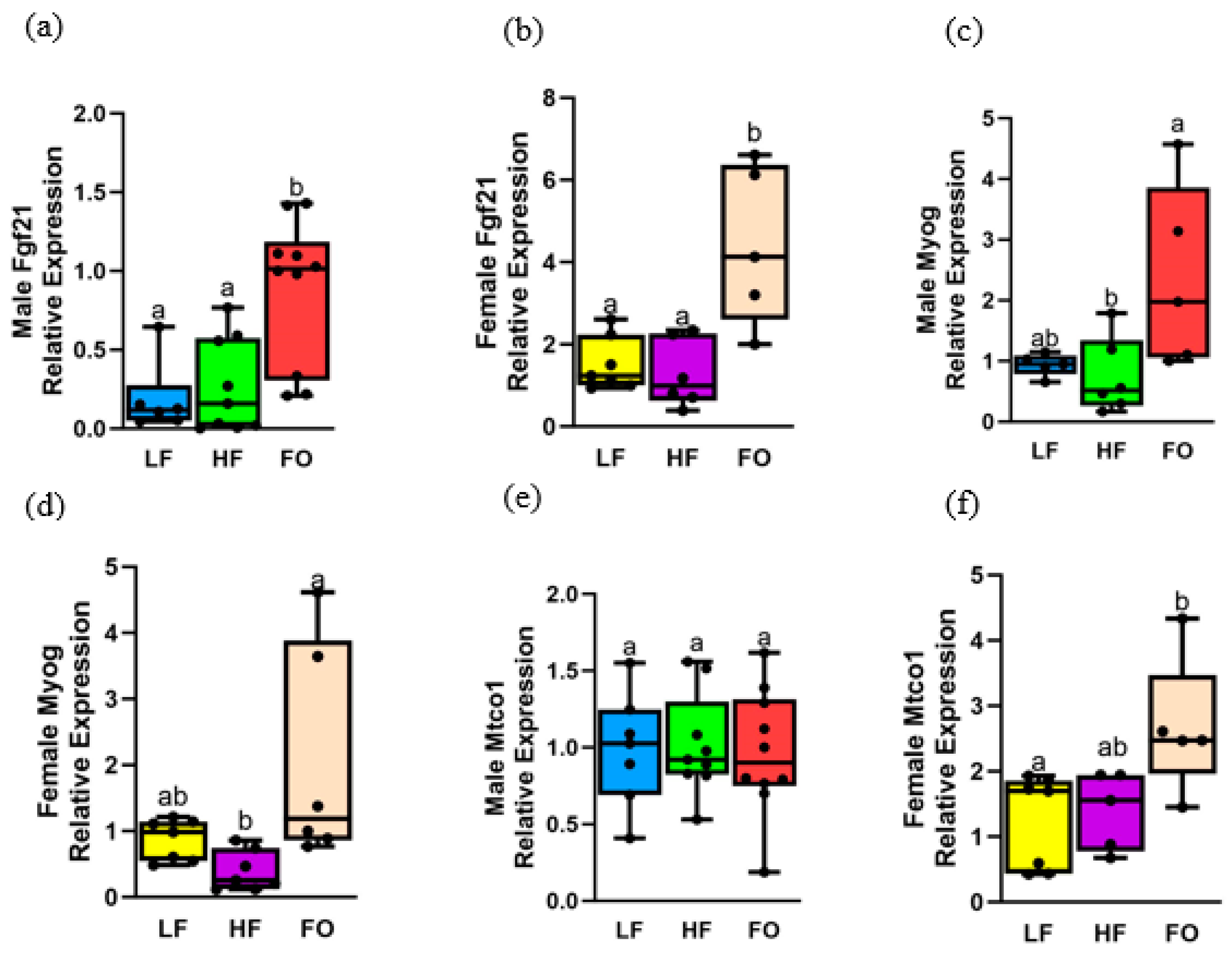
3.4. Gene Expression Related to Fatty Acid Oxidation
3.5. Gene Expression Related to Fatty Acid Synthesis
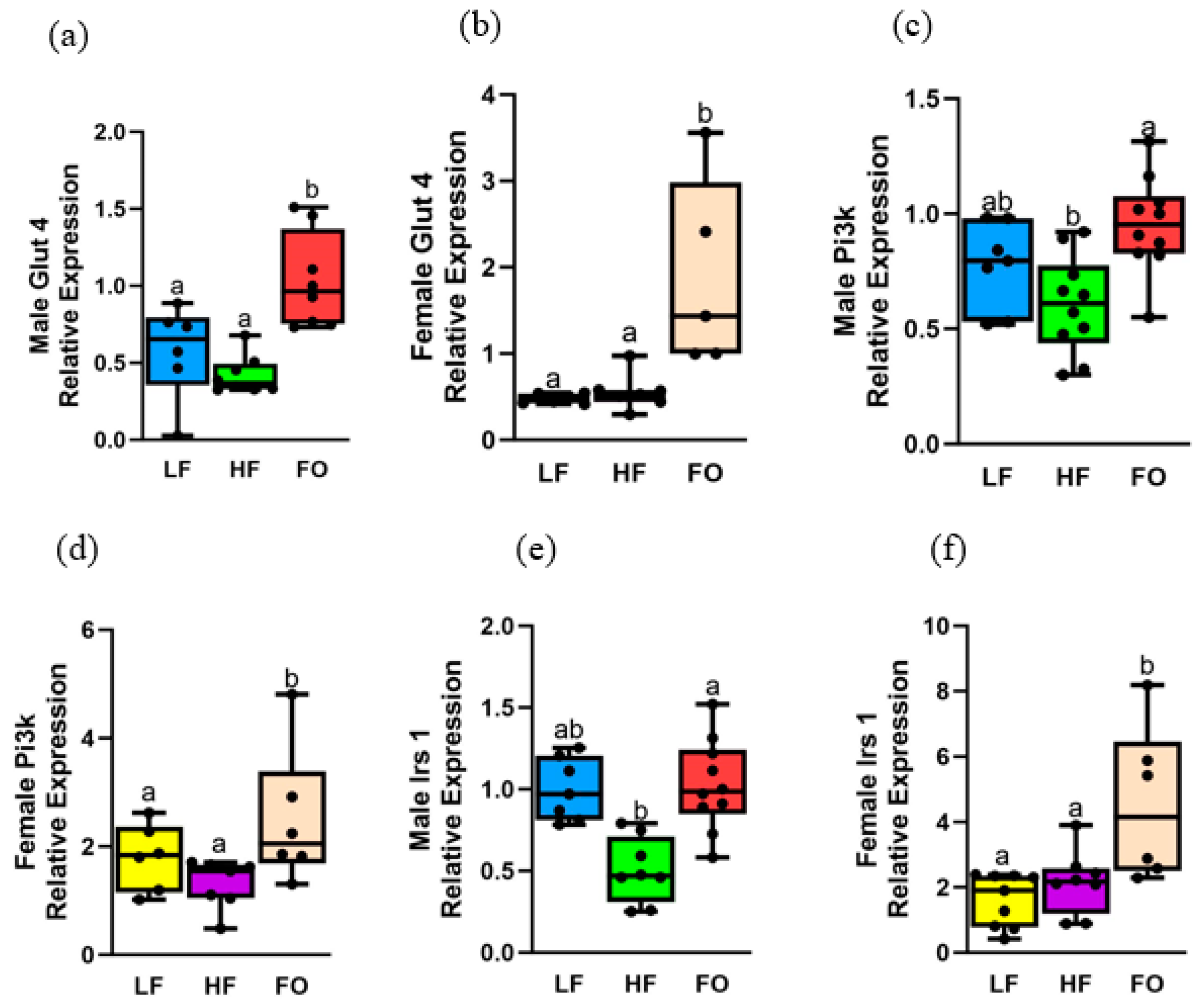
3.6. Gene Expression Relating to Insulin Signaling
3.7. Gene Expression Relating to Mitochondrial Oxidation
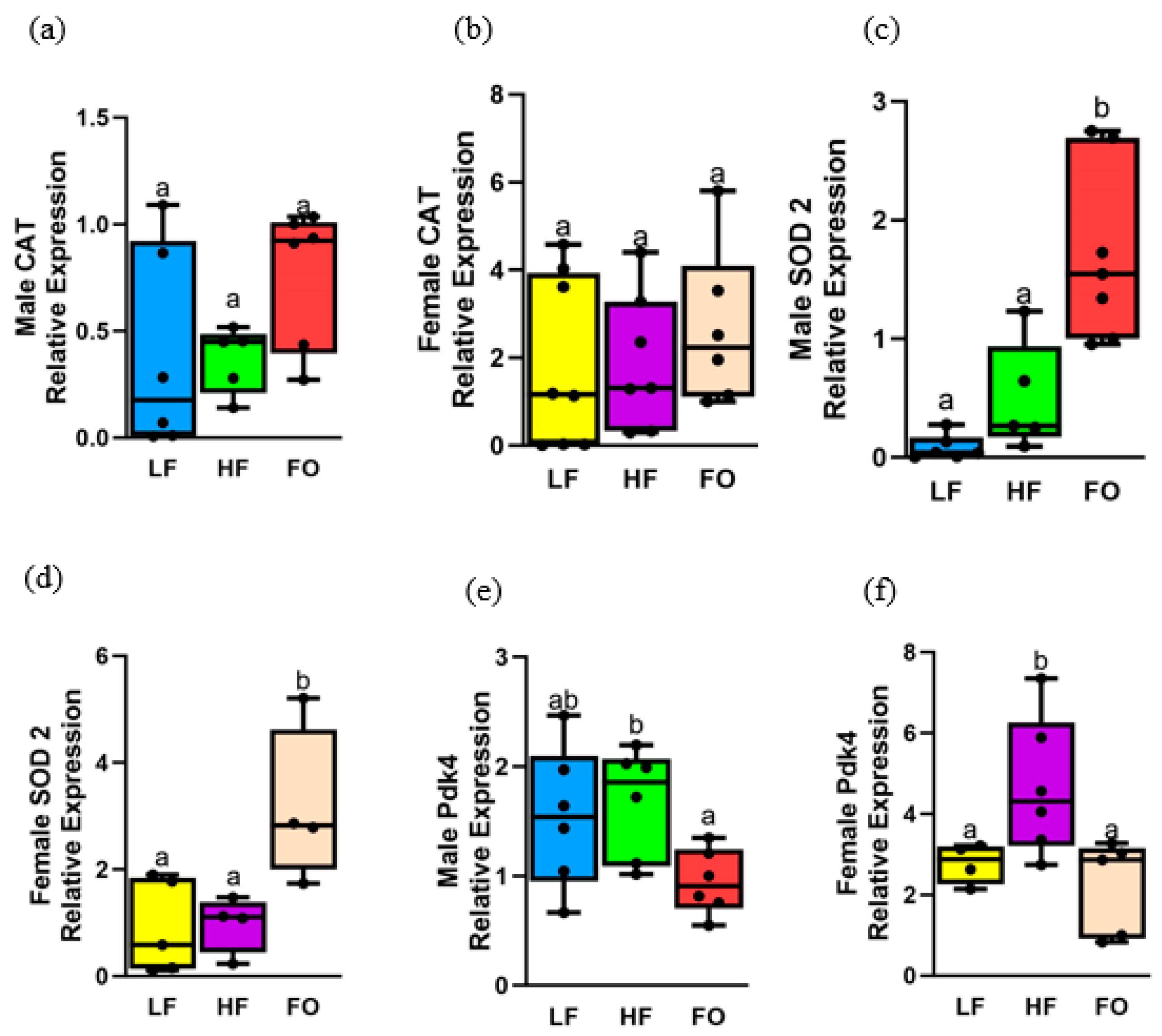
3.8. Gene Expression Related to Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hales, C.M. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018; CDC: Atlanta, GA, USA, 2020. [Google Scholar]
- Boden, G.; Homko, C.; Barrero, C.A.; Stein, T.P.; Chen, X.; Cheung, P.; Fecchio, C.; Koller, S.; Merali, S. Excessive Caloric Intake Acutely Causes Oxidative Stress, GLUT4 Carbonylation, and Insulin Resistance in Healthy Men. Sci. Transl. Med. 2015, 7, 304re7. [Google Scholar] [CrossRef] [PubMed]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2020, 10, 1638. [Google Scholar] [CrossRef]
- Shou, J.; Chen, P.-J.; Xiao, W.-H. Mechanism of Increased Risk of Insulin Resistance in Aging Skeletal Muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, B.M.; Pelosi, L.; Sica, G.; Musarò, A. The Physiopathologic Role of Oxidative Stress in Skeletal Muscle. Mech. Ageing Dev. 2018, 170, 37–44. [Google Scholar] [CrossRef]
- Steinbacher, P.; Eckl, P. Impact of Oxidative Stress on Exercising Skeletal Muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef] [PubMed]
- Pinho, R.A.; Sepa-Kishi, D.M.; Bikopoulos, G.; Wu, M.V.; Uthayakumar, A.; Mohasses, A.; Hughes, M.C.; Perry, C.G.R.; Ceddia, R.B. High-Fat Diet Induces Skeletal Muscle Oxidative Stress in a Fiber Type-Dependent Manner in Rats. Free Radic. Biol. Med. 2017, 110, 381–389. [Google Scholar] [CrossRef]
- Huang, C.-J.; McAllister, M.J.; Slusher, A.L.; Webb, H.E.; Mock, J.T.; Acevedo, E.O. Obesity-Related Oxidative Stress: The Impact of Physical Activity and Diet Manipulation. Sports Med. Open 2015, 1, 32. [Google Scholar] [CrossRef]
- Hey-Mogensen, M.; Højlund, K.; Vind, B.F.; Wang, L.; Dela, F.; Beck-Nielsen, H.; Fernström, M.; Sahlin, K. Effect of Physical Training on Mitochondrial Respiration and Reactive Oxygen Species Release in Skeletal Muscle in Patients with Obesity and Type 2 Diabetes. Diabetologia 2010, 53, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial Dysfunction in Obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Hernández-Aguilera, A.; Rull, A.; Rodríguez-Gallego, E.; Riera-Borrull, M.; Luciano-Mateo, F.; Camps, J.; Menéndez, J.A.; Joven, J. Mitochondrial Dysfunction: A Basic Mechanism in Inflammation-Related Non-Communicable Diseases and Therapeutic Opportunities. Mediat. Inflamm. 2013, 2013, 135698. [Google Scholar] [CrossRef] [PubMed]
- Jheng, H.-F.; Tsai, P.-J.; Guo, S.-M.; Kuo, L.-H.; Chang, C.-S.; Su, I.-J.; Chang, C.-R.; Tsai, Y.-S. Mitochondrial Fission Contributes to Mitochondrial Dysfunction and Insulin Resistance in Skeletal Muscle. Mol. Cell Biol. 2012, 32, 309–319. [Google Scholar] [CrossRef]
- Grattagliano, I.; de Bari, O.; Bernardo, T.C.; Oliveira, P.J.; Wang, D.Q.-H.; Portincasa, P. Role of Mitochondria in Nonalcoholic Fatty Liver Disease-from Origin to Propagation. Clin. Biochem. 2012, 45, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Bellanti, F.; Vendemiale, G.; Altomare, E. Mitochondrial Dysfunction in Nonalcoholic Steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Stephens, F.B.; Chee, C.; Wall, B.T.; Murton, A.J.; Shannon, C.E.; van Loon, L.J.C.; Tsintzas, K. Lipid-Induced Insulin Resistance Is Associated with an Impaired Skeletal Muscle Protein Synthetic Response to Amino Acid Ingestion in Healthy Young Men. Diabetes 2015, 64, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Zhu, H.; Shi, H.; Hou, Y.; Yin, J. Fish Oil Increases Muscle Protein Mass and Modulates Akt/FOXO, TLR4, and NOD Signaling in Weanling Piglets After Lipopolysaccharide Challenge. J. Nutr. 2013, 143, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Mcglory, C.; Gorissen, S.H.M.; Kamal, M.; Bahniwal, R.; Hector, A.J.; Baker, S.K.; Chabowski, A.; Phillips, S.M. Omega-3 Fatty Acid Supplementation Attenuates Skeletal Muscle Disuse Atrophy during Two Weeks of Unilateral Leg Immobilization in Healthy Young Women. FASEB J. 2019, 33, 4586–4597. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-W.; Aris, I.M.; Bernard, J.Y.; Tint, M.-T.; Colega, M.; Gluckman, P.D.; Tan, K.H.; Shek, L.P.-C.; Chong, Y.-S.; Yap, F.; et al. Associations of Maternal Macronutrient Intake during Pregnancy with Infant BMI Peak Characteristics and Childhood BMI. Am. J. Clin. Nutr. 2017, 105, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.; Otero, P.; Panadero, M.I.; Rodrigo, S.; Álvarez-Millán, J.J.; Bocos, C. Maternal Fructose Intake Induces Insulin Resistance and Oxidative Stress in Male, but Not Female, Offspring. J. Nutr. Metab. 2015, 2015, 158091. [Google Scholar] [CrossRef] [PubMed]
- Mourtakos, S.P.; Tambalis, K.D.; Panagiotakos, D.B.; Antonogeorgos, G.; Alexi, C.D.; Georgoulis, M.; Saade, G.; Sidossis, L.S. Association between Gestational Weight Gain and Risk of Obesity in Preadolescence: A Longitudinal Study (1997–2007) of 5125 Children in Greece. J. Hum. Nutr. Diet. 2017, 30, 51–58. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of Maternal Obesity on the Long-Term Health of Offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Raad, G.; Hazzouri, M.; Bottini, S.; Trabucchi, M.; Azoury, J.; Grandjean, V. Paternal Obesity: How Bad Is It for Sperm Quality and Progeny Health? Basic Clin. Androl. 2017, 27, 20. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Cheng, J.W.; Ko, E.Y. Role of Reactive Oxygen Species in Male Infertility: An Updated Review of Literature. Arab J. Urol. 2018, 16, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, V.; Maloney, C.A.; Wang, K.W.; Morris, M.J. Effects of Paternal Obesity on Growth and Adiposity of Male Rat Offspring. Am. J. Physiol.-Endocrinol. Metab. 2017, 312, E117–E125. [Google Scholar] [CrossRef] [PubMed]
- Krout, D.; Roemmich, J.N.; Bundy, A.; Garcia, R.A.; Yan, L.; Claycombe-Larson, K.J. Paternal Exercise Protects Mouse Offspring from High-Fat-Diet-Induced Type 2 Diabetes Risk by Increasing Skeletal Muscle Insulin Signaling. J. Nutr. Biochem. 2018, 57, 35–44. [Google Scholar] [CrossRef]
- Satokar, V.V.; Derraik, J.G.B.; Harwood, M.; Okesene-Gafa, K.; Beck, K.; Cameron-Smith, D.; Garg, M.L.; O’Sullivan, J.M.; Sundborn, G.; Pundir, S.; et al. Fish Oil Supplementation during Pregnancy and Postpartum in Mothers with Overweight and Obesity to Improve Body Composition and Metabolic Health during Infancy: A Double-Blind Randomized Controlled Trial. Am. J. Clin. Nutr. 2023, 117, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, L.; Menikdiwela, K.R.; Clevenger, S.; Eboh, T.; Allen, L.; Koboziev, I.; Scoggin, S.; Rashid, A.M.; Moussa, H.; Moustaid-Moussa, N. Maternal and Postnatal Supplementation of Fish Oil Improves Metabolic Health of Mouse Male Offspring. Obesity 2018, 26, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, L.; Menikdiwela, K.R.; Spainhour, S.; Eboh, T.; Moustaid-Moussa, N. Sex Differences in Early Programming by Maternal High Fat Diet Induced-Obesity and Fish Oil Supplementation in Mice. Nutrients 2021, 13, 3703. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.R.; Hoy, A.J.; Turner, N.; Watt, M.J.; Allen, T.L.; Carpenter, K.; Cooney, G.J.; Febbraio, M.A.; Kraegen, E.W. Overexpression of Carnitine Palmitoyltransferase-1 in Skeletal Muscle Is Sufficient to Enhance Fatty Acid Oxidation and Improve High-Fat Diet–Induced Insulin Resistance. Diabetes 2009, 58, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, D.; Herrero, L.; Serra, D.; Asins, G.; Hegardt, F.G. CPT I Overexpression Protects L6E9 Muscle Cells from Fatty Acid-Induced Insulin Resistance. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E677–E686. [Google Scholar] [CrossRef]
- Henique, C.; Mansouri, A.; Fumey, G.; Lenoir, V.; Girard, J.; Bouillaud, F.; Prip-Buus, C.; Cohen, I. Increased Mitochondrial Fatty Acid Oxidation Is Sufficient to Protect Skeletal Muscle Cells from Palmitate-Induced Apoptosis. J. Biol. Chem. 2010, 285, 36818–36827. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Skeletal Muscle Inflammation and Insulin Resistance in Obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Irani, B.G.; Dunn-Meynell, A.A.; Levin, B.E. Altered Hypothalamic Leptin, Insulin, and Melanocortin Binding Associated with Moderate-Fat Diet and Predisposition to Obesity. Endocrinology 2007, 148, 310–316. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.T.; Araujo, E.P.; Bordin, S.; Ashimine, R.; Zollner, R.L.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A. Consumption of a Fat-Rich Diet Activates a Proinflammatory Response and Induces Insulin Resistance in the Hypothalamus. Endocrinology 2005, 146, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- González-Périz, A.; Horrillo, R.; Ferré, N.; Gronert, K.; Dong, B.; Morán-Salvador, E.; Titos, E.; Martínez-Clemente, M.; López-Parra, M.; Arroyo, V.; et al. Obesity-induced Insulin Resistance and Hepatic Steatosis Are Alleviated by Ω-3 Fatty Acids: A Role for Resolvins and Protectins. FASEB J. 2009, 23, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Smuder, A.J.; Kwon, O.; Kavazis, A.N.; Szeto, H.H.; Powers, S.K. Mitochondrial-Targeted Antioxidants Protect Skeletal Muscle against Immobilization-Induced Muscle Atrophy. J. Appl. Physiol. 2011, 111, 1459–1466. [Google Scholar] [CrossRef]
- Martins, A.R.; Crisma, A.R.; Masi, L.N.; Amaral, C.L.; Marzuca-Nassr, G.N.; Bomfim, L.H.M.; Teodoro, B.G.; Queiroz, A.L.; Serdan, T.D.A.; Torres, R.P.; et al. Attenuation of Obesity and Insulin Resistance by Fish Oil Supplementation Is Associated with Improved Skeletal Muscle Mitochondrial Function in Mice Fed a High-Fat Diet. J. Nutr. Biochem. 2018, 55, 76–88. [Google Scholar] [CrossRef]
- Lanza, I.R.; Blachnio-Zabielska, A.; Johnson, M.L.; Schimke, J.M.; Jakaitis, D.R.; Lebrasseur, N.K.; Jensen, M.D.; Sreekumaran Nair, K.; Zabielski, P. Influence of Fish Oil on Skeletal Muscle Mitochondrial Energetics and Lipid Metabolites during High-Fat Diet. Am. J. Physiol.-Endocrinol. Metab. 2013, 304, E1391–E1403. [Google Scholar] [CrossRef]
- Reynolds, T.H.; Dalton, A.; Calzini, L.; Tuluca, A.; Hoyte, D.; Ives, S.J. The Impact of Age and Sex on Body Composition and Glucose Sensitivity in C57BL/6J Mice. Physiol. Rep. 2019, 7, e13995. [Google Scholar] [CrossRef]
- Turinsky, J.; O’Sullivan, D.M.; Bayly, B.P. 1,2-Diacylglycerol and Ceramide Levels in Insulin-Resistant Tissues of the Rat in Vivo. J. Biol. Chem. 1990, 265, 16880–16885. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Roves, P.M.; Osler, M.E.; Holmström, M.H.; Zierath, J.R. Gain-of-Function R225Q Mutation in AMP-Activated Protein Kinase Γ3 Subunit Increases Mitochondrial Biogenesis in Glycolytic Skeletal Muscle. J. Biol. Chem. 2008, 283, 35724–35734. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.-L.; Wang, C.-H.; Li, T.-L.; Chang, S.-D.; Lin, L.-C.; Chen, C.-P.; Chen, C.-T.; Liang, K.-C.; Ho, I.-K.; Yang, W.-S.; et al. Sex Differences in High-Fat Diet-Induced Obesity, Metabolic Alterations and Learning, and Synaptic Plasticity Deficits in Mice. Obesity 2010, 18, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Extier, A.; Langelier, B.; Perruchot, M.-H.; Guesnet, P.; Van Veldhoven, P.P.; Lavialle, M.; Alessandri, J.-M. Gender Affects Liver Desaturase Expression in a Rat Model of N−3 Fatty Acid Repletion. J. Nutr. Biochem. 2010, 21, 180–187. [Google Scholar] [CrossRef]
- Meadows, E.; Cho, J.-H.; Flynn, J.M.; Klein, W.H. Myogenin Regulates a Distinct Genetic Program in Adult Muscle Stem Cells. Dev. Biol. 2008, 322, 406–414. [Google Scholar] [CrossRef]
- Liu, S.-H.; Chiu, C.-Y.; Wang, L.-P.; Chiang, M.-T. Omega-3 Fatty Acids-Enriched Fish Oil Activates AMPK/PGC-1α Signaling and Prevents Obesity-Related Skeletal Muscle Wasting. Mar. Drugs 2019, 17, 380. [Google Scholar] [CrossRef] [PubMed]
- Oost, L.J.; Kustermann, M.; Armani, A.; Blaauw, B.; Romanello, V. Fibroblast Growth Factor 21 Controls Mitophagy and Muscle Mass. J. Cachexia Sarcopenia Muscle 2019, 10, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Dang, R.; Zhou, X.; Tang, M.; Xu, P.; Gong, X.; Liu, Y.; Jiao, H.; Jiang, P. Fish Oil Supplementation Attenuates Neuroinflammation and Alleviates Depressive-like Behavior in Rats Submitted to Repeated Lipopolysaccharide. Eur. J. Nutr. 2018, 57, 893–906. [Google Scholar] [CrossRef]
- Ng, S.-F.; Lin, R.C.Y.; Laybutt, D.R.; Barres, R.; Owens, J.A.; Morris, M.J. Chronic High-Fat Diet in Fathers Programs β-Cell Dysfunction in Female Rat Offspring. Nature 2010, 467, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Fullston, T.; McPherson, N.O.; Owens, J.A.; Kang, W.X.; Sandeman, L.Y.; Lane, M. Paternal Obesity Induces Metabolic and Sperm Disturbances in Male Offspring That Are Exacerbated by Their Exposure to an “Obesogenic” Diet. Physiol. Rep. 2015, 3, e12336. [Google Scholar] [CrossRef]
- Campbell, J.M.; Lane, M.; Owens, J.A.; Bakos, H.W. Paternal Obesity Negatively Affects Male Fertility and Assisted Reproduction Outcomes: A Systematic Review and Meta-Analysis. Reprod. BioMedicine Online 2015, 31, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.O.; Budu, A.; Alves-Silva, T.; Arakaki, A.M.; Gregnani, M.F.S.; Rodrigues Húngaro, T.G.; Burgos-Silva, M.; Wasinski, F.; Lanzoni, V.P.; Camara, N.O.S.; et al. Paternal Exercise Protects against Liver Steatosis in the Male Offspring of Mice Submitted to High Fat Diet. Life Sci. 2020, 263, 118583. [Google Scholar] [CrossRef] [PubMed]
- Fullston, T.; Ohlsson-Teague, E.M.C.; Print, C.G.; Sandeman, L.Y.; Lane, M. Sperm microRNA Content Is Altered in a Mouse Model of Male Obesity, but the Same Suite of microRNAs Are Not Altered in Offspring’s Sperm. PLoS ONE 2016, 11, e0166076. [Google Scholar] [CrossRef] [PubMed]
- Rando, O.J. Daddy Issues: Paternal Effects on Phenotype. Cell 2012, 151, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Batra, V.; Norman, E.; Morgan, H.L.; Watkins, A.J. Parental Programming of Offspring Health: The Intricate Interplay between Diet, Environment, Reproduction and Development. Biomolecules 2022, 12, 1289. [Google Scholar] [CrossRef] [PubMed]
- Panera, N.; Mandato, C.; Crudele, A.; Bertrando, S.; Vajro, P.; Alisi, A. Genetics, Epigenetics and Transgenerational Transmission of Obesity in Children. Front. Endocrinol. 2022, 13, 1006008. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs Contribute to Intergenerational Inheritance of an Acquired Metabolic Disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef]
- Soubry, A.; Guo, L.; Huang, Z.; Hoyo, C.; Romanus, S.; Price, T.; Murphy, S.K. Obesity-Related DNA Methylation at Imprinted Genes in Human Sperm: Results from the TIEGER Study. Clin. Epigenet. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Comas-Armangue, G.; Makharadze, L.; Gomez-Velazquez, M.; Teperino, R. The Legacy of Parental Obesity: Mechanisms of Non-Genetic Transmission and Reversibility. Biomedicines 2022, 10, 2461. [Google Scholar] [CrossRef]
- Du, S.; Jin, J.; Fang, W.; Su, Q. Does Fish Oil Have an Anti-Obesity Effect in Overweight/Obese Adults? A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2015, 10, e0142652. [Google Scholar] [CrossRef] [PubMed]
- Stubbins, R.E.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Estrogen Modulates Abdominal Adiposity and Protects Female Mice from Obesity and Impaired Glucose Tolerance. Eur. J. Nutr. 2012, 51, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Inada, A.; Fujii, N.L.; Inada, O.; Higaki, Y.; Furuichi, Y.; Nabeshima, Y. Effects of 17β-Estradiol and Androgen on Glucose Metabolism in Skeletal Muscle. Endocrinology 2016, 157, 4691–4705. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Q.; Li, R.; Chen, G.; Wang, J.; Hu, B.; Li, C.; Zhu, X.; Lu, Y. Inhibitory Effect of 17β-estradiol on Triglyceride Synthesis in Skeletal Muscle Cells Is Dependent on ESR1 and Not ESR2. Mol. Med. Rep. 2019, 19, 5087–5096. [Google Scholar] [CrossRef] [PubMed]
- Louet, J.-F.; LeMay, C.; Mauvais-Jarvis, F. Antidiabetic Actions of Estrogen: Insight from Human and Genetic Mouse Models. Curr. Atheroscler. Rep. 2004, 6, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Manrique, C.; Lastra, G.; Habibi, J.; Mugerfeld, I.; Garro, M.; Sowers, J.R. Loss of Estrogen Receptor α Signaling Leads to Insulin Resistance and Obesity in Young and Adult Female Mice. Cardiorenal. Med. 2012, 2, 200–210. [Google Scholar] [CrossRef] [PubMed]
| LF | HF | FO | Total | |
|---|---|---|---|---|
| Total offspring | 41 | 65 | 67 | 173 |
| Short term (ST; 8 weeks) | ||||
| Male | 8 | 10 | 10 | 28 |
| Female | 10 | 8 | 10 | 28 |
| Long term (LT; 16 weeks) | ||||
| Male | 8 | 14 | 14 | 36 |
| Female | 10 | 13 | 13 | 36 |
| Gene | Forward | Reverse |
|---|---|---|
| Acaca | GCAGCAGTTACACCACATAC | TCCGCCATCTTCCACAATA |
| Actin | TGAGACCTTCAACACCCCAGCCA | CGTAGATGGGCACAGTGTGGGTG |
| Cidea | TCGGCTGTCTCAATGTCAA | GGATGGCTGCTCTTCTGTA |
| Myoga | CCTTCCTGTCCACCTTCA | CACCGACACAGACTTCCT |
| Acat1 | ATATCTCACGGCAGGAACA | CACGCTTGTATTCTTCATCTTCT |
| Fabp4 | CACCGAGATTTCCTTCAAACT | TATGATGCTCTTCACCTTCCT |
| Fasn | GTCGTCTATACCACTGCTTACT | ACACCACCTGAACCTGAG |
| Glut4 | CAGTATGTTGCGGATGCTAT | TTAGGAAGGTGAAGATGAAGAAG |
| Fgf21 | CCAAGACCAAGCAGGATTC | AGAGTCAGGACGCATAGC |
| Irs1 | TCGCTAACTGAGATAGTCATACAA | TCCTGCTAACATCCACCTT |
| PI3k | GTCAGAAGGCAGGAGTCA | TAGAAGATGGCTTGGATGGAA |
| Pdk4 | TGTGATGTGGTAGCAGTAGT | TGGTGAAGGTGTGAAGGA |
| Foxo1 | GCTCTGTCCTGAAGAATCCT | CTAATCCTGCCACTGTCTGTA |
| mt-Co1 | TCCCAGATATAGCATTCCCACG | ACTGTTCATCCTGTTCCTGC |
| Cat | AGCGACCAGATGAAGCAGTG | CCGCTCTCTGTCAAAGTGTG |
| Sod2 | CAGACCTGCCTTACGACTATGG | CTCGGTGGCGTTGAGATTGTT |
| PPAR-α | TCGAGGAAGGCACTACACCT | TCTTCCCAAAGCTCCTTCAA |
| Gene Name | Sex (S) | Diet (D) | Interactions (S*D) |
|---|---|---|---|
| Acat | 0.13 | 0.0019 | 0.0031 |
| Foxo 1 | <0.01 | 0.29 | 0.94 |
| Pdk4 | <0.01 | <0.01 | 0.11 |
| CAT | <0.01 | 0.08 | 0.0328 |
| Cidea | <0.01 | <0.01 | 0.48 |
| Fabp 4 | 0.0214 | <0.01 | 0.62 |
| Fasn | <0.01 | 0.0251 | <0.01 |
| Glut4 | 0.0403 | <0.01 | 0.0274 |
| Fgf21 | <0.01 | <0.01 | <0.01 |
| Irs 1 | <0.01 | <0.01 | <0.01 |
| Myog | 0.4625 | <0.01 | 0.93 |
| Mtco-1 | <0.01 | <0.01 | <0.01 |
| Pi3k | <0.01 | <0.01 | 0.11 |
| PPAR alpha | <0.01 | <0.01 | 0.55 |
| SOD2 | <0.01 | <0.01 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, L.; Dorus, S.; Ramalingam, L. Role of Fish Oil in Preventing Paternal Obesity and Improving Offspring Skeletal Muscle Health. Biomedicines 2023, 11, 3120. https://doi.org/10.3390/biomedicines11123120
Xiong L, Dorus S, Ramalingam L. Role of Fish Oil in Preventing Paternal Obesity and Improving Offspring Skeletal Muscle Health. Biomedicines. 2023; 11(12):3120. https://doi.org/10.3390/biomedicines11123120
Chicago/Turabian StyleXiong, Ligeng, Stephen Dorus, and Latha Ramalingam. 2023. "Role of Fish Oil in Preventing Paternal Obesity and Improving Offspring Skeletal Muscle Health" Biomedicines 11, no. 12: 3120. https://doi.org/10.3390/biomedicines11123120
APA StyleXiong, L., Dorus, S., & Ramalingam, L. (2023). Role of Fish Oil in Preventing Paternal Obesity and Improving Offspring Skeletal Muscle Health. Biomedicines, 11(12), 3120. https://doi.org/10.3390/biomedicines11123120






