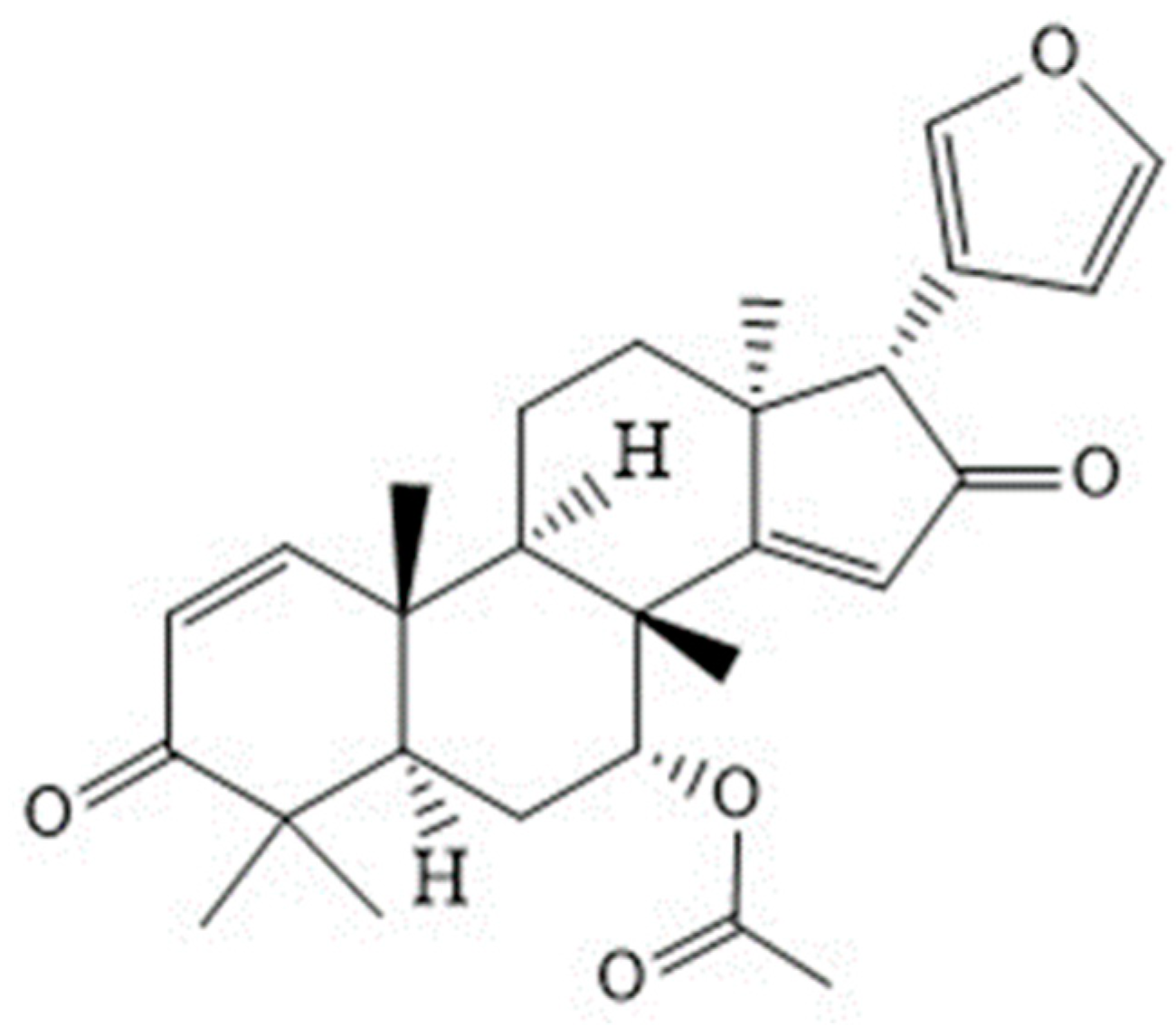
Azadiradione, a Component of Neem Oil, Behaves as a Superoxide Dismutase Mimic When Scavenging the Superoxide Radical, as Shown Using DFT and Hydrodynamic Voltammetry
Abstract
:1. Introduction
2. Materials and Methods
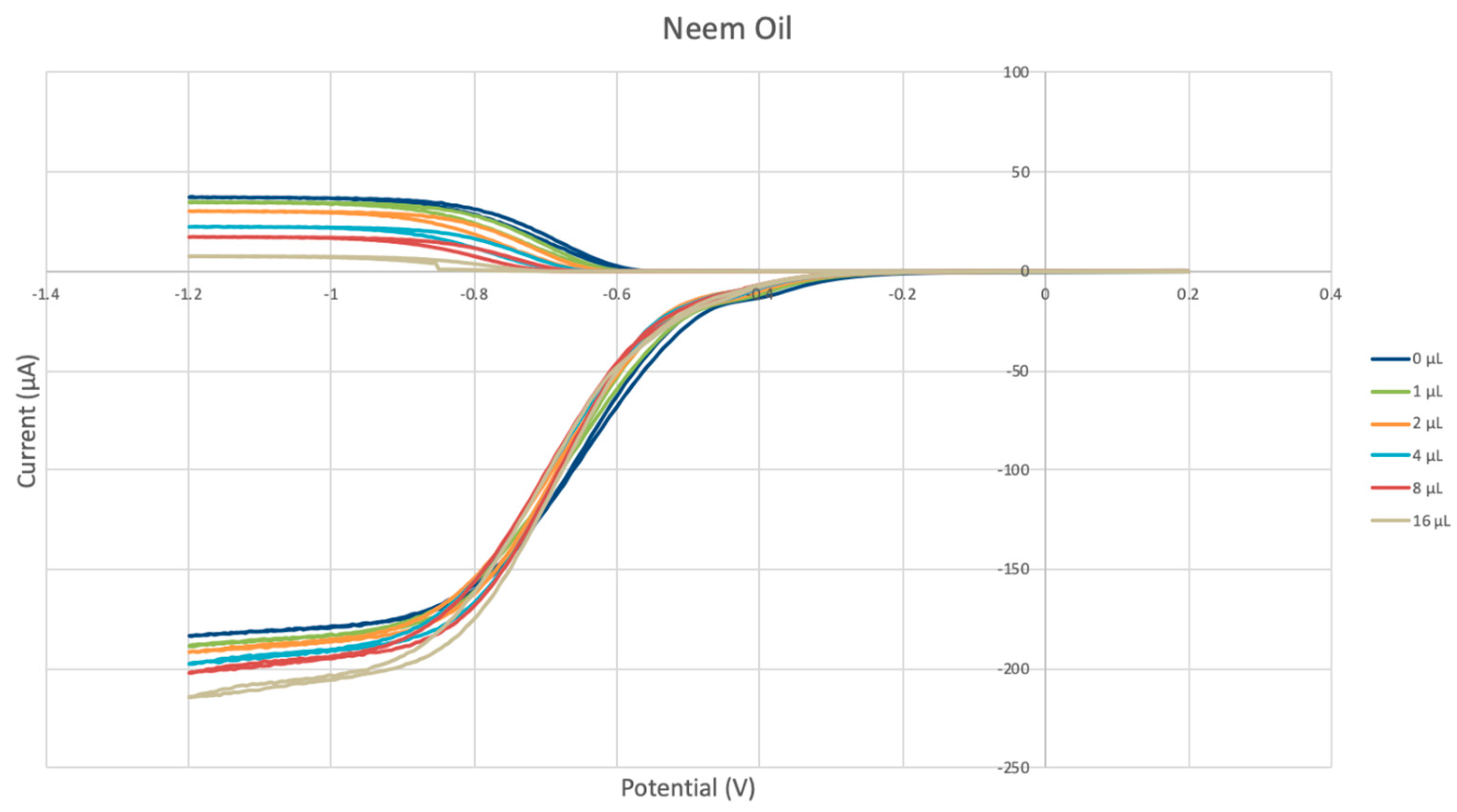
2.1. Hydrodynamic Voltammetry (RRDE)
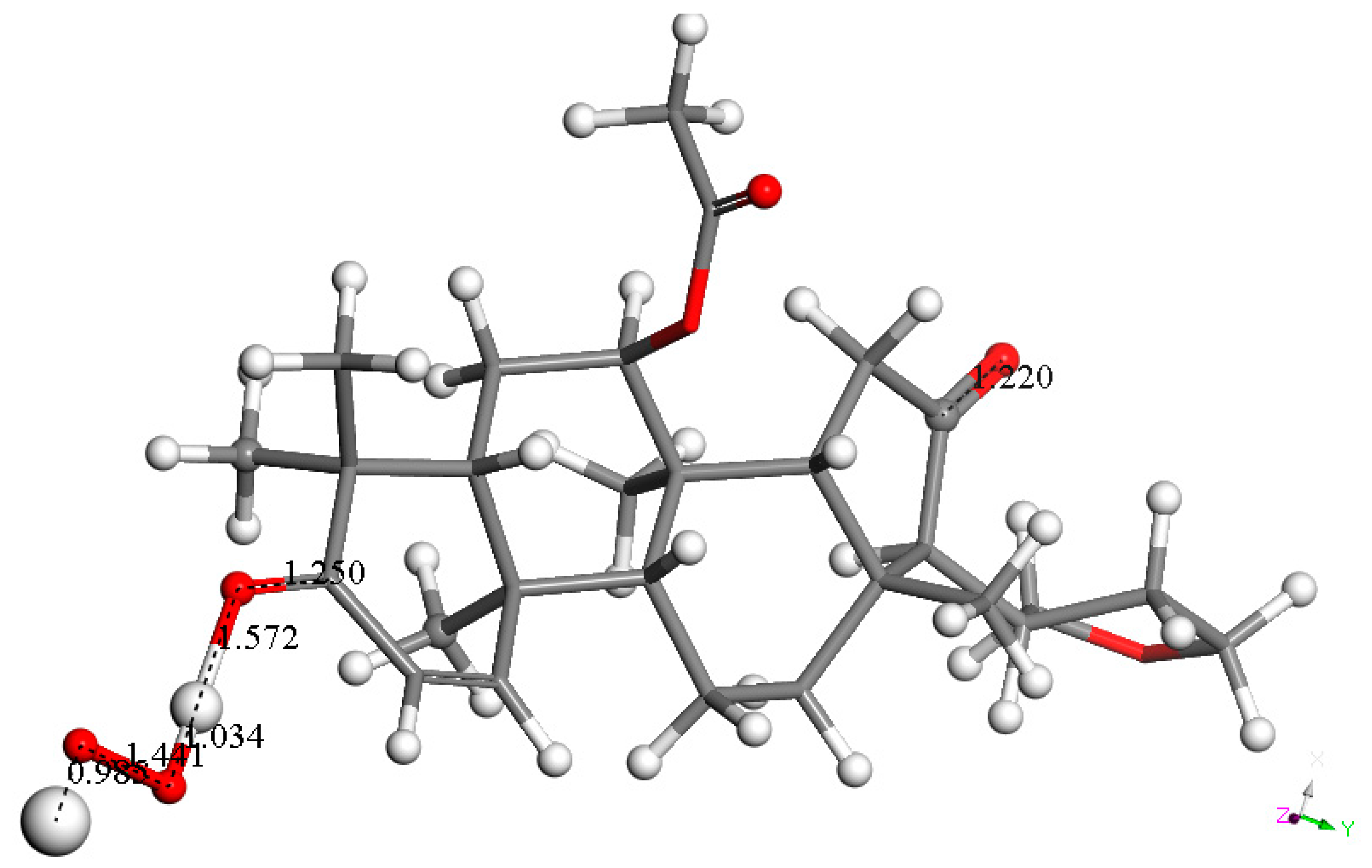
2.2. Computational Study
3. Results and Discussion
3.1. Electrochemical Study
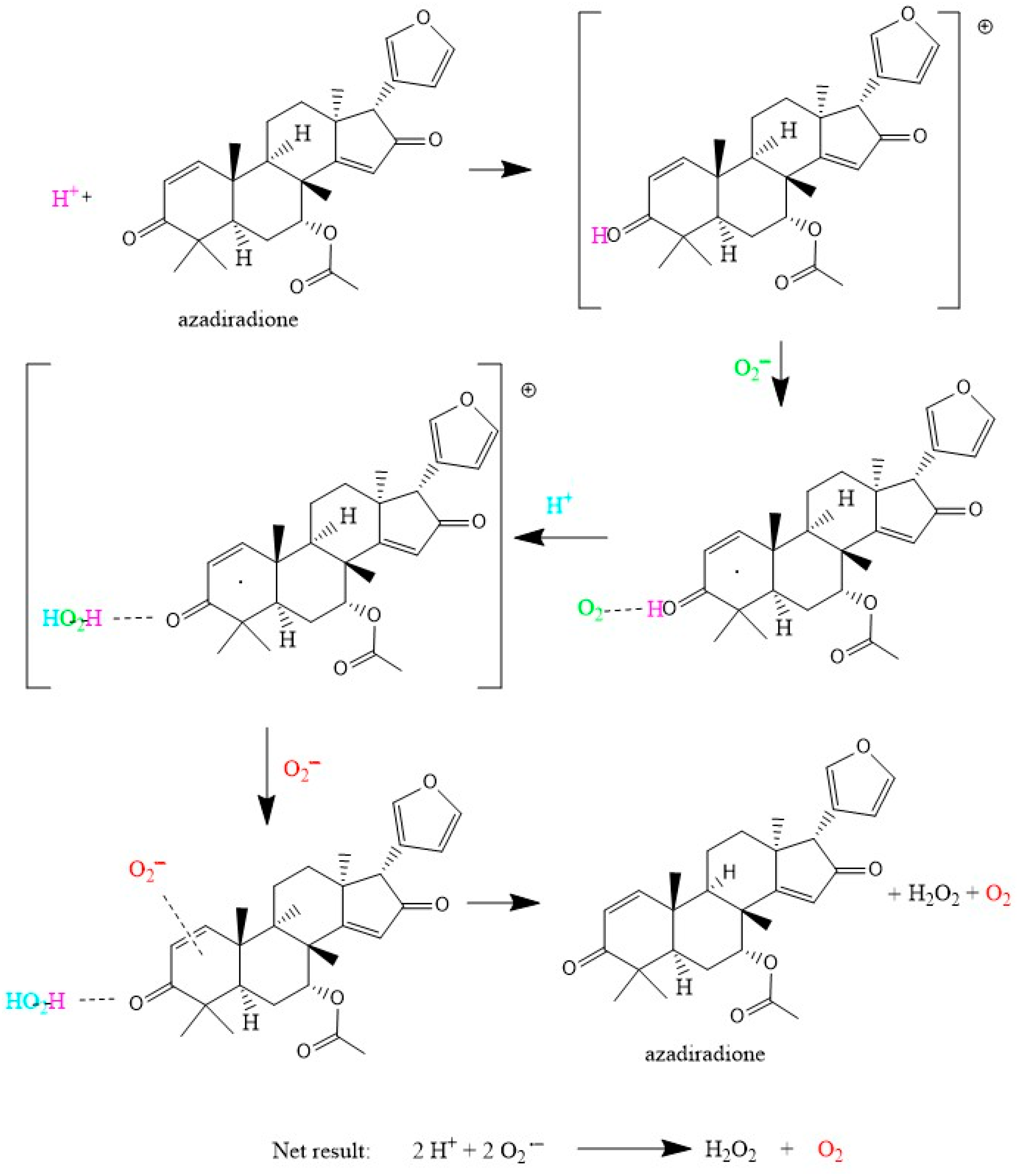
3.2. DFT Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siddiqui, S. Siddha medicine. Curr. Sci. 1942, 11, 278–279. [Google Scholar]
- Sarkar, S.; Singh, R.P.; Bhattacharya, G. Exploring the role of Azadirachta indica (neem) and its active compounds in the regulation of biological pathways: An update on molecular approach. 3 Biotech. 2021, 11, 178. [Google Scholar] [CrossRef]
- National Research Council (US) Panel on Neem. Neem: A Tree For Solving Global Problems; National Academies Press: Washington, DC, USA, 1992; Volume 3, The Tree. Available online: https://www.ncbi.nlm.nih.gov/books/NBK234651/ (accessed on 20 June 2023).
- Biswas, K.; Chattopadhyay, I.; Banerjee, R.K.; Bandyopadhyay, U. Biological activities and medicinal properties of neem (Azadirachta indica). Curr. Sci. 2002, 82, 1336–1345. [Google Scholar]
- Subapriya, R.; Nagini, S. Medicinal properties of neem leaves: A review. Curr. Med. Chem. Anti-Cancer Agents 2005, 5, 149–156. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Tyagi, A.K.; Kunnumakkara, A.B.; Aggarwal, B.B. Neem (Azadirachta indica): An indian traditional panacea with modern molecular basis. Phytomedicine 2017, 34, 14–20. [Google Scholar] [CrossRef]
- Alzohairy, M.A. Therapeutics Role of Azadirachta indica (Neem) and Their Active Constituents in Diseases Prevention and Treatment. Evid. Based Complement Altern. Med. 2016, 2016, 7382506. [Google Scholar] [CrossRef]
- Islas, J.F.; Acosta, E.; G-Buentello, Z.; Delgado-Gallegos, J.L.; Moreno-Treviño, M.G.; Escalante, B.A.; Moreno-Cuevas, J.E. An overview of Neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods 2020, 74, 104171. [Google Scholar] [CrossRef]
- Patel, S.M.; Nagulapalli Venkata, K.C.; Bhattacharyya, P.; Sethi, G.; Bishayee, A. Potential of neem (Azadirachta indica L.) for prevention and treatment of oncologic diseases. Semin. Cancer Biol. 2016, 40–41, 100–115. [Google Scholar] [CrossRef]
- Deng, Y.X.; Cao, M.; Shi, D.X.; Yin, Z.Q.; Jia, R.Y.; Xu, J.; Wang, C.; Lv, C.; Liang, X.X.; He, C.L.; et al. Toxicological evaluation of neem (Azadirachta indica) oil: Acute and subacute toxicity. Environ. Toxicol. Pharmacol. 2013, 35, 240–246. [Google Scholar] [CrossRef]
- Kadu, T.B.; Dighade, S.J.; Dongare, P.N.; Dod, S.R. An overview on Azadirachta indica plant parts benefits and marketed preparation. World J. Pharm. Pharmaceut. Sci. 2022, 11, 659–678. [Google Scholar] [CrossRef]
- Proshkina, E.; Plyusnin, S.; Babak, T.; Lashmanova, E.; Maganova, F.; Koval, L.; Platonova, E.; Shaposhnikov, M.; Moskalev, A. Terpenoids as Potential Geroprotectors. Antioxidants 2020, 9, 529. [Google Scholar] [CrossRef]
- Setzer, W.N.; Setzer, M.C. Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev. Med. Chem. 2003, 3, 540–556. [Google Scholar] [CrossRef]
- Barrek, S.; Paisse, O.; Grenier-Loustalot, M.F. Analysis of neem oils by LC-MS and degradation kinetics of azadirachtin-A in a controlled environment. Characterization of degradation products by HPLC-MS-MS. Anal. Bioanal. Chem. 2004, 378, 753–763. [Google Scholar] [CrossRef]
- Tan, Q.G.; Luo, X.D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011, 111, 7437–7522, Erratum in Chem. Rev. 2012, 112, 2591. [Google Scholar] [CrossRef]
- Lin, M.; Yang, S.; Huang, J.; Zhou, L. Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules and Activities: Part I (Aphanamixis-Chukrasia). Int. J. Mol. Sci. 2021, 22, 13262. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, R.V.; Manikandan, P.; Kumar, G.H.; Nagini, S. The neem limonoids azadirachtin and nimbolide inhibit hamster cheek pouch carcinogenesis by modulating xenobiotic-metabolizing enzymes, DNA damage, antioxidants, invasion and angiogenesis. Free Rad. Res. 2009, 43, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Harish Kumar, G.; Vidya Priyadarsini, R.; Vinothini, G.; Vidjaya Letchoumy, P.; Nagini, S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Investig. New Drugs 2010, 28, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Nagini, S. Neem limonoids as anticancer agents: Modulation of cancer hallmarks and oncogenic signaling. Enzymes 2014, 36, 131–147. [Google Scholar] [CrossRef]
- Jaiswara, P.K.; Kumar, A. Nimbolide retards T cell lymphoma progression by altering apoptosis, glucose metabolism, pH regulation, and ROS homeostasis. Environ. Toxicol. 2022, 37, 1445–1457. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, S.; Meng, J.; Li, L. Protective effect of nimbolide against streptozotocin induced gestational diabetes mellitus in rats via alteration of inflammatory reaction, oxidative stress, and gut microbiota. Environ. Toxicol. 2022, 37, 1382–1393. [Google Scholar] [CrossRef]
- Smirnova, N.A.; Haskew-Layton, R.E.; Basso, M.; Hushpulian, D.M.; Payappilly, J.B.; Speer, R.E.; Ahn, Y.-H.; Rakhman, I.; Cole, P.A.; Pinto, J.T.; et al. Development of Neh2-Luciferase Reporter and Its Application for High Throughput Screening and Real-Time Monitoring of Nrf2 Activators. Chem. Biol. 2011, 18, 752–765. [Google Scholar] [CrossRef]
- Mazumdar, S.; Marar, T.; Devarajan, S.; Patki, J. Functional relevance of Gedunin as a bona fide ligand of NADPH oxidase 5 and ROS scavenger: An in silico and in vitro assessment in a hyperglycemic RBC model. Biochem. Biophys. Rep. 2021, 25, 100904. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.A.; Duddeck, H.; Gonzalez-Sierra, M. Isolation and characterization of an antimalarial agent of the neem tree Azadirachta indica. J. Nat. Prod. 1989, 52, 922–926. [Google Scholar] [CrossRef]
- Al Akeel, R.; Mateen, A.; Janardhan, K.; Gupta, V.C. Analysis of anti-bacterial and anti-oxidative activity of Azadirachta indica bark using various solvents extracts. Saudi J. Biol. Sci. 2017, 24, 11–14. [Google Scholar] [CrossRef]
- Basir, S.; Shailey, S. Strengthening of antioxidant defense by Azadirachta indica in alloxan-diabetic rat tissues. J. Ayurveda Integr. Med. 2012, 3, 130. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. Antioxidant activity and inhibition of key enzymes linked to type-2 diabetes and hypertension by Azadirachta indica-yogurt. J. Saudi Chem. Soc. 2013, 17, 295–301. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, P.; Bains, A.; Chawla, P.; Sridhar, K.; Sharma, M.; Inbaraj, B.S. Antimicrobial and Anti-Inflammatory Activity of Low-Energy Assisted Nanohydrogel of Azadirachta indica Oil. Gels 2022, 8, 434. [Google Scholar] [CrossRef]
- Nikolova, G.; Ananiev, J.; Ivanov, V.; Petkova-Parlapanska, K.; Georgieva, E.; Karamalakova, Y. The Azadirachta indica (Neem) Seed Oil Reduced Chronic Redox-Homeostasis Imbalance in a Mice Experimental Model on Ochratoxine A-Induced Hepatotoxicity. Antioxidants 2022, 11, 1678. [Google Scholar] [CrossRef]
- Manikandan, P.; Anandan, R.; Nagini, S. Evaluation of Azadirachta indica Leaf Fractions for in Vitro Antioxidant Potential and Protective Effects against H2O2-Induced Oxidative Damage to pBR322 DNA and Red Blood Cells. J. Agric. Food Chem. 2009, 57, 6990–6996. [Google Scholar] [CrossRef] [PubMed]
- Gahukar, R.T. Factors affecting content and bioefficacy of neem (Azadirachta indica A. Juss.) phytochemicals used in agricultural pest control: A review. Crop Prot. 2014, 62, 93–99. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Ali, S.K.; Ali, S.T.; Naqvi, S.N.; Tariq, R.M. Variation of major limonoids in Azadirachta indica fruits at different ripening stages and toxicity against Aedes aegypti. Nat. Prod. Commun. 2009, 4, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Ilango, K.; Maharajan, G.; Narasimhan, S. Anti-nociceptive and anti-inflammatory activities of Azadirachta indica fruit skin extract and its isolated constituent azadiradione. Nat. Prod. Res. 2013, 27, 1463–1467. [Google Scholar] [CrossRef]
- Jin, T.; Cao, X.; Gao, Z.; Yan, X.-Q. Azadiradione exerts anti-inflammatory and anti-oxidant effects, alleviates dopaminergic neurodegeneration and reduces α-synuclein levels in MPTP-induced mouse model of Parkinson’s disease. Trop. J. Pharm. Res. 2019, 18, 2332. [Google Scholar] [CrossRef]
- Nelson, V.K.; Ali, A.; Dutta, N.; Ghosh, S.; Jana, M.; Ganguli, A.; Komarov, A.; Paul, S.; Dwivedi, V.; Chatterjee, S.; et al. Azadiradione ameliorates polyglutamine expansion disease in Drosophila by potentiating DNA binding activity of heat shock factor 1. Oncotarget 2016, 29, 78281–78296. [Google Scholar] [CrossRef]
- Murugan, R.; Rajesh, R.; Guru, A.; Haridevamuthu, B.; Almutairi, B.O.; Almutairi, M.H.; Juliet, A.; Renganayagi, S.; Gopinath, P.; Arockiaraj, J. Deacetylepoxyazadiradione Derived from Epoxyazadiradione of Neem (Azadirachta indica A. Juss) Fruits Mitigates LPS-Induced Oxidative Stress and Inflammation in Zebrafish Larvae. Chem. Biodivers. 2022, 19, e202200041. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Singh, M.; Belli, S.; Berinato, M.; Rossi, M. Interrelated Mechanism by Which the Methide Quinone Celastrol, Obtained from the Roots of Tripterygium wilfordii, Inhibits Main Protease 3CLpro of COVID-19 and Acts as Superoxide Radical Scavenger. Int. J. Mol. Sci. 2020, 21, 9266. [Google Scholar] [CrossRef] [PubMed]
- Belli, S.; Rossi, M.; Molasky, N.; Middleton, L.; Caldwell, C.; Bartow-McKenney, C.; Duong, M.; Chiu, J.; Gibbs, E.; Caldwell, A.; et al. Effective and novel application of superoxide radical scavenging by natural phenolic antioxidants. Antioxidants 2019, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Delley, B.J. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Rossi, M.; Caruso, F.; Kwok, L.; Lee, G.; Caruso, A.; Gionfra, F.; Candelotti, E.; Belli, S.; Molasky, N.; Raley-Susman, K.M.; et al. Protection by extra virgin olive oil against oxidative stress in vitro and in vivo. Chemical and biological studies on the health benefits due to a major component of the Mediterranean diet. PLoS ONE 2017, 12, e0189341. [Google Scholar] [CrossRef] [PubMed]
- Sakib, R.; Caruso, F.; Aktar, S.; Belli, S.; Kaur, S.; Hernandez, M.; Rossi, M. Antioxidant Properties of Thymoquinone, Thymohydroquinone and Black Cumin (Nigella sativa L.) Seed Oil: Scavenging of Superoxide Radical Studied Using Cyclic Voltammetry, DFT and Single Crystal X-ray Diffraction. Antioxidants 2023, 12, 607. [Google Scholar] [CrossRef]
- Caruso, F.; Berinato, M.; Hernandez, M.; Belli, S.; Smart, C.; Rossi, M. Antioxidant properties of bee propolis and an important component, galangin, described by X-ray crystal structure, DFT-D and hydrodynamic voltammetry. PLoS ONE 2022, 17, e0267624. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Bilton, J.N.; Broughton, H.B.; Jones, P.S.; Ley, S.V.; Lidert, Z.; Morgan, E.D.; Rzepa, H.Z.; Sheppard, R.N.; Slawin, A.M.Z.; Williams, D.J. An X-ray crystallographic, mass spectroscopic, and NMR-study of the limonoid insect antifeedant azadirachtin and related derivatives. Tetrahedron 1987, 43, 2805–2815. [Google Scholar] [CrossRef]
- Alshammari, G.M.; Balakrishnan, A.; Chinnasamy, T. Nimbolide attenuates the lipid accumulation, oxidative stress and antioxidant in primary hepatocytes. Mol. Biol. Rep. 2017, 44, 463–474. [Google Scholar] [CrossRef]
- Kumbar, S.B.; Jadaramkunti, U.C.; Aladakatti, R.H. In-vitro effect of nimbolide, an isoprenoid of neem leaf, on antioxidant system of rat cauda epididymal spermatozoa: A dose dependent study. J. Appl. Pharm. Sci. 2012, 2, 84–93. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.F.; Teixeira, M.; Valentine, J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef]
- Fan, M.-X.; Chen, G.-L.; Guo, M.-Q. Potential Antioxidative Components in Azadirachta indica Revealed by Bio-Affinity Ultrafiltration with SOD and XOD. Antioxidants 2022, 11, 658. [Google Scholar] [CrossRef]
- Policar, C.; Bouvet, J.; Bertrand, H.C.; Delsuc, N. SOD mimics: From the tool box of the chemists to cellular studies. Curr. Opin. Chem. Biol. 2022, 67, 102109. [Google Scholar] [CrossRef]
- Yu, S.; Caruso, F.; Belli, S.; Rossi, M. Scavenging of Superoxide in Aprotic Solvents of Four Isoflavones That Mimic Superoxide Dismutase. Int. J. Mol. Sci. 2023, 24, 3815. [Google Scholar] [CrossRef]
- Caruso, F.; Incerpi, S.; Pedersen, J.; Belli, S.; Kaur, S.; Rossi, M. Aromatic Polyphenol π-π Interactions with Superoxide Radicals Contribute to Radical Scavenging and Can Make Polyphenols Mimic Superoxide Dismutase Activity. Curr. Issues Mol. Biol. 2022, 44, 5209–5220. [Google Scholar] [CrossRef] [PubMed]
- Zarepour, M.; Kaspari, K.; Stagge, S.; Rethmeier, R.; Mendel, R.R.; Bittner, F. Xanthine dehydrogenase AtXDH1 from Arabidopsis thaliana is a potent producer of superoxide anions via its NADH oxidase activity. Plant. Mol. Biol. 2010, 72, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Barthomeuf, C.M.; Debiton, E.; Barbakade, V.V.; Kemertelidze, E.P. Evaluation of the dietetic and therapeutic potential of a high molecular weight hydroxycinnamate-derived polymer from Symphytum asperum Lepech. Regarding its antioxidant, antilipoperoxidant, antiinflammatory, and cytotoxic properties. J. Agric. Food Chem. 2001, 49, 3942–3946. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakib, R.; Caruso, F.; Belli, S.; Rossi, M. Azadiradione, a Component of Neem Oil, Behaves as a Superoxide Dismutase Mimic When Scavenging the Superoxide Radical, as Shown Using DFT and Hydrodynamic Voltammetry. Biomedicines 2023, 11, 3091. https://doi.org/10.3390/biomedicines11113091
Sakib R, Caruso F, Belli S, Rossi M. Azadiradione, a Component of Neem Oil, Behaves as a Superoxide Dismutase Mimic When Scavenging the Superoxide Radical, as Shown Using DFT and Hydrodynamic Voltammetry. Biomedicines. 2023; 11(11):3091. https://doi.org/10.3390/biomedicines11113091
Chicago/Turabian StyleSakib, Raiyan, Francesco Caruso, Stuart Belli, and Miriam Rossi. 2023. "Azadiradione, a Component of Neem Oil, Behaves as a Superoxide Dismutase Mimic When Scavenging the Superoxide Radical, as Shown Using DFT and Hydrodynamic Voltammetry" Biomedicines 11, no. 11: 3091. https://doi.org/10.3390/biomedicines11113091
APA StyleSakib, R., Caruso, F., Belli, S., & Rossi, M. (2023). Azadiradione, a Component of Neem Oil, Behaves as a Superoxide Dismutase Mimic When Scavenging the Superoxide Radical, as Shown Using DFT and Hydrodynamic Voltammetry. Biomedicines, 11(11), 3091. https://doi.org/10.3390/biomedicines11113091







