Coenzyme Q10-Loaded Albumin Nanoparticles Protect against Redox Imbalance and Inflammatory, Apoptotic, and Histopathological Alterations in Mercuric Chloride-Induced Hepatorenal Toxicity in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
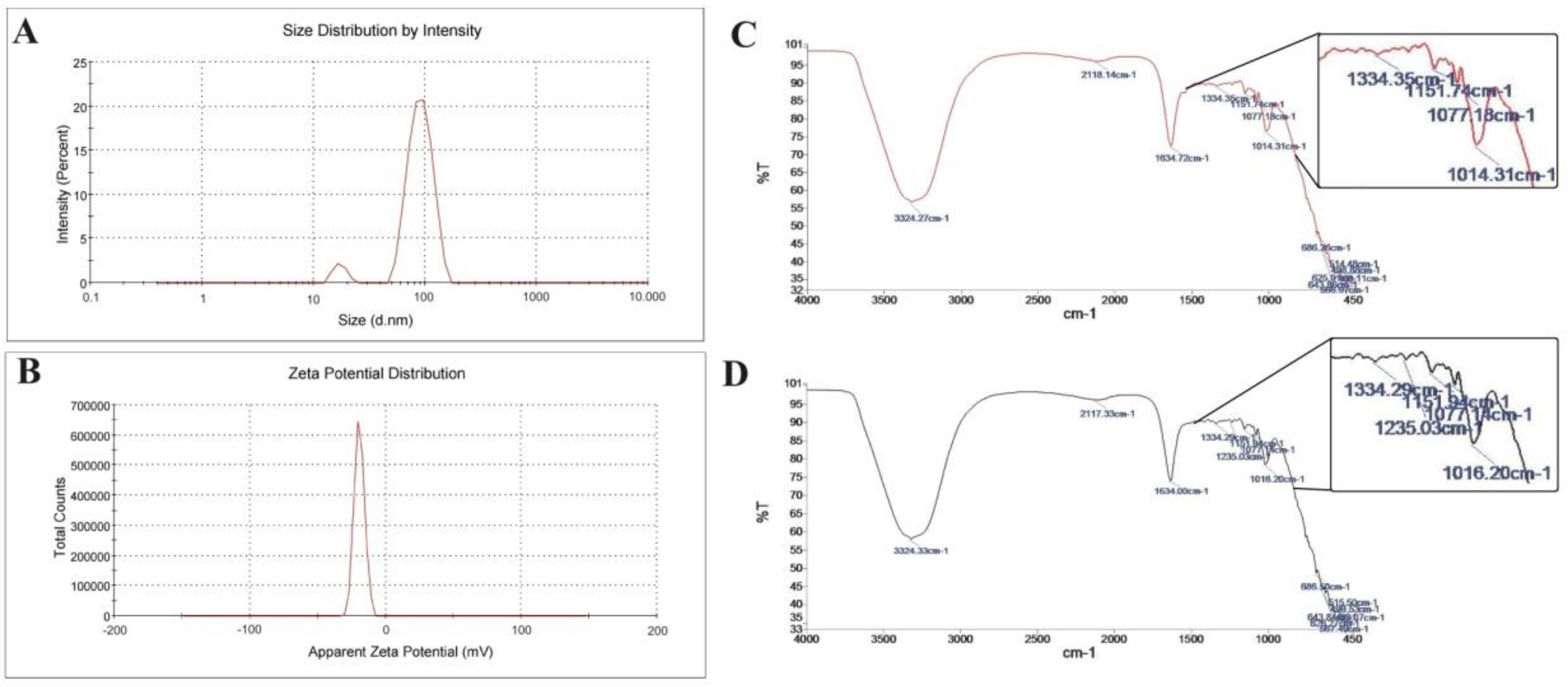
2.2. Preparation and Characterization of CoQ10NPs
2.3. Animals, Treatment Protocol, and Sampling
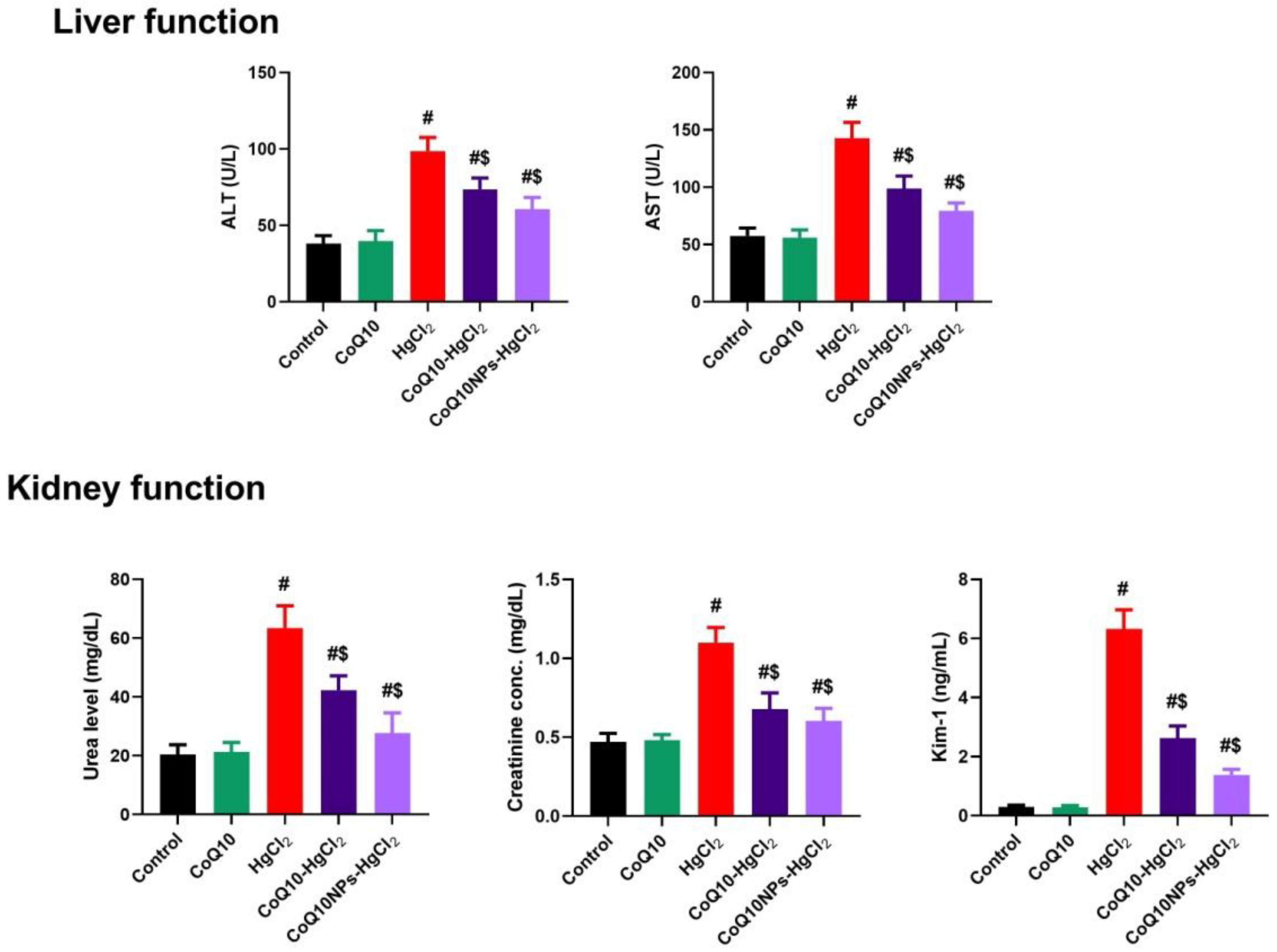
2.4. Serum Liver and Renal Markers
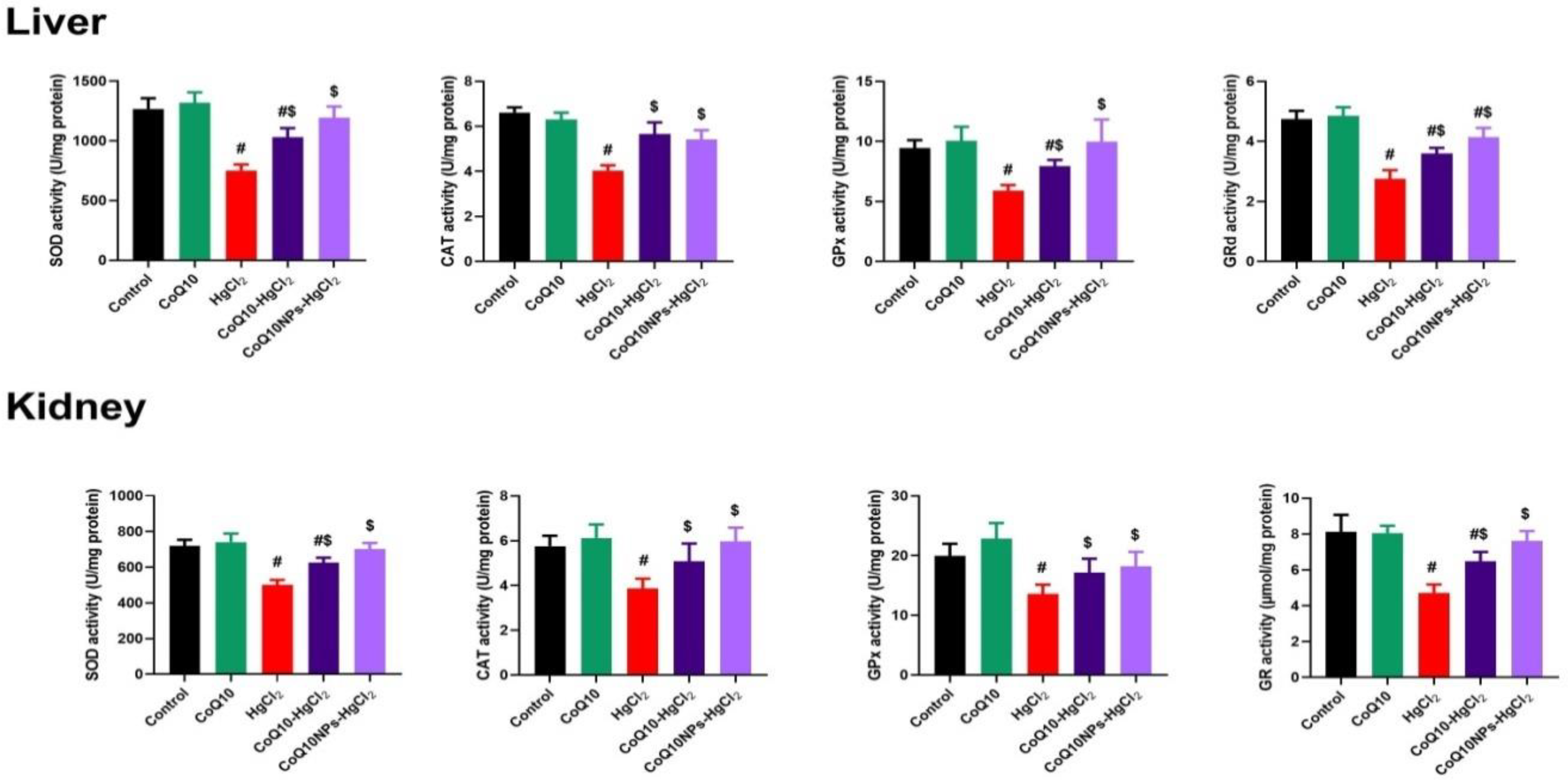
2.5. Hepatorenal Oxidative/Antioxidant Status
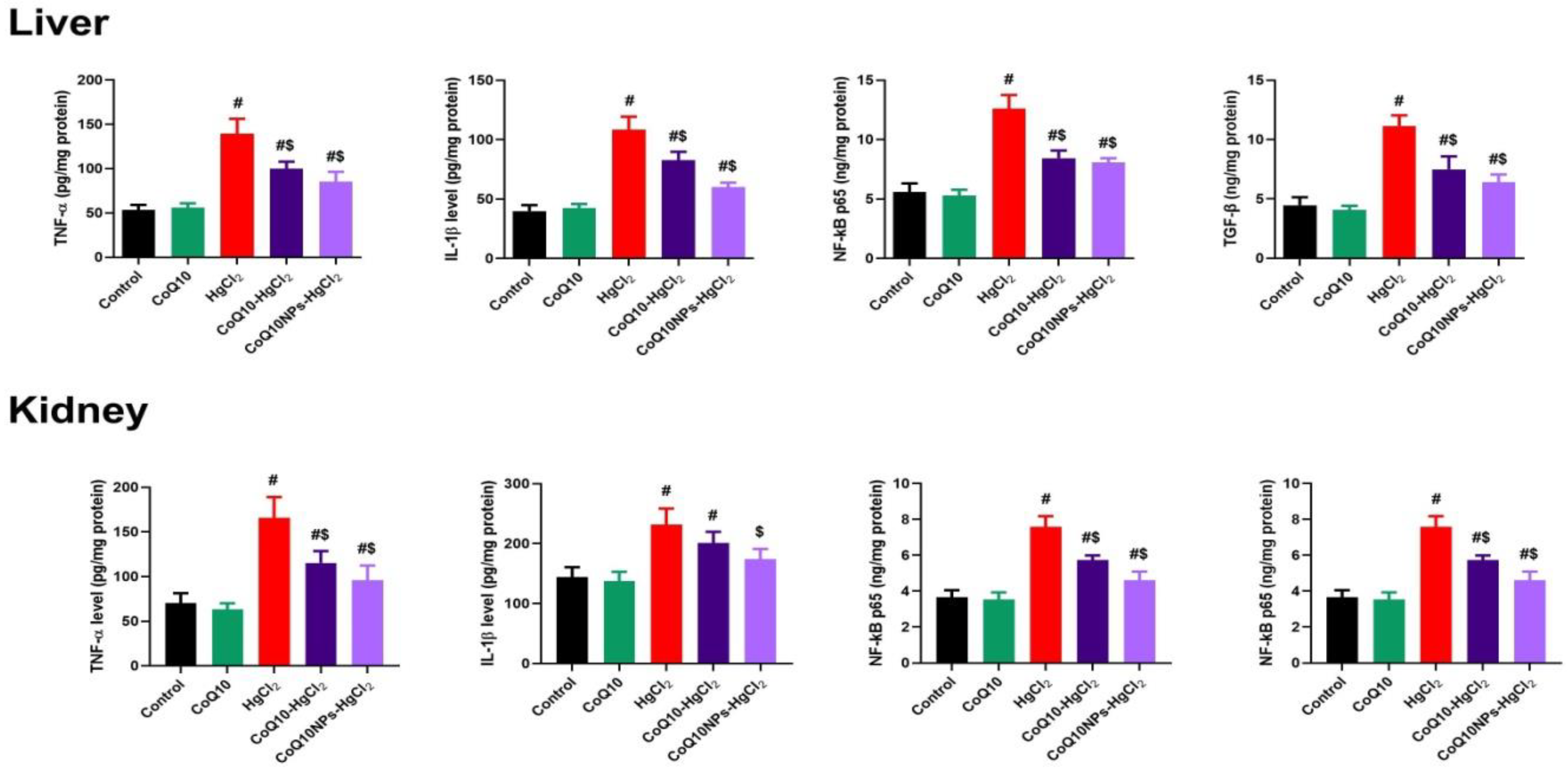
2.6. Hepatorenal Inflammatory and Fibrotic Mediators
2.7. Hepatorenal Apoptotic Markers
2.8. Histopathology of Liver and Kidney
2.9. Statistical Evaluation
3. Results
3.1. Coenzyme Q10-Loaded Albumin Nanoparticle (CoQ10NP) Characterization
3.2. Effect of CoQ10NPs on Serum Hepatic and Renal Markers upon HgCl2 Exposure
3.3. Effect of CoQ10NPs on Hepatorenal Oxidative Stress Markers upon HgCl2 Exposure
3.4. Effect of CoQ10NPs on Hepatorenal Inflammatory Mediators upon HgCl2 Exposure
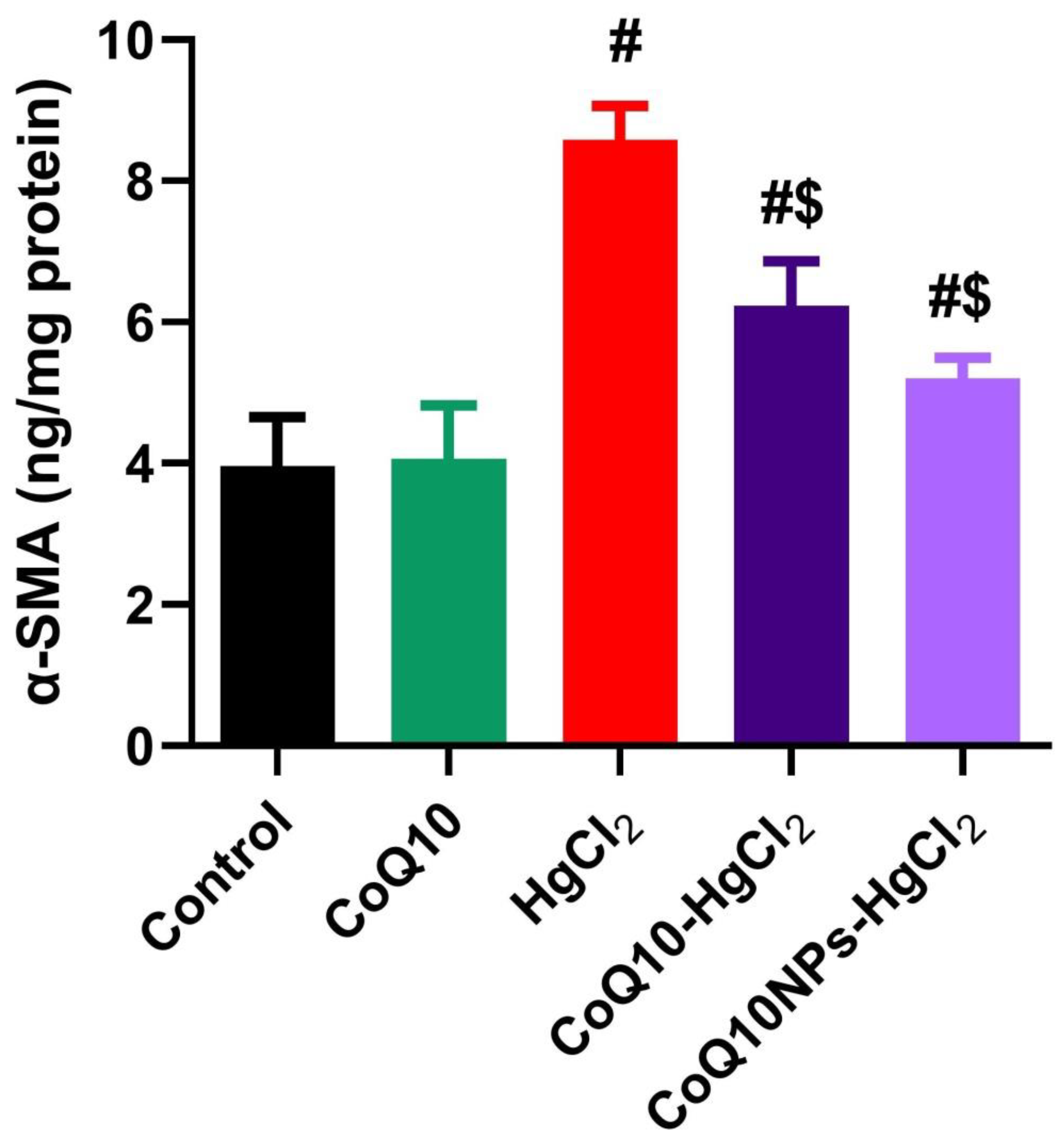
3.5. Effect of CoQ10NPs on Hepatic Alpha-Smooth Muscle Actin (α-SMA) Level upon HgCl2 Exposure
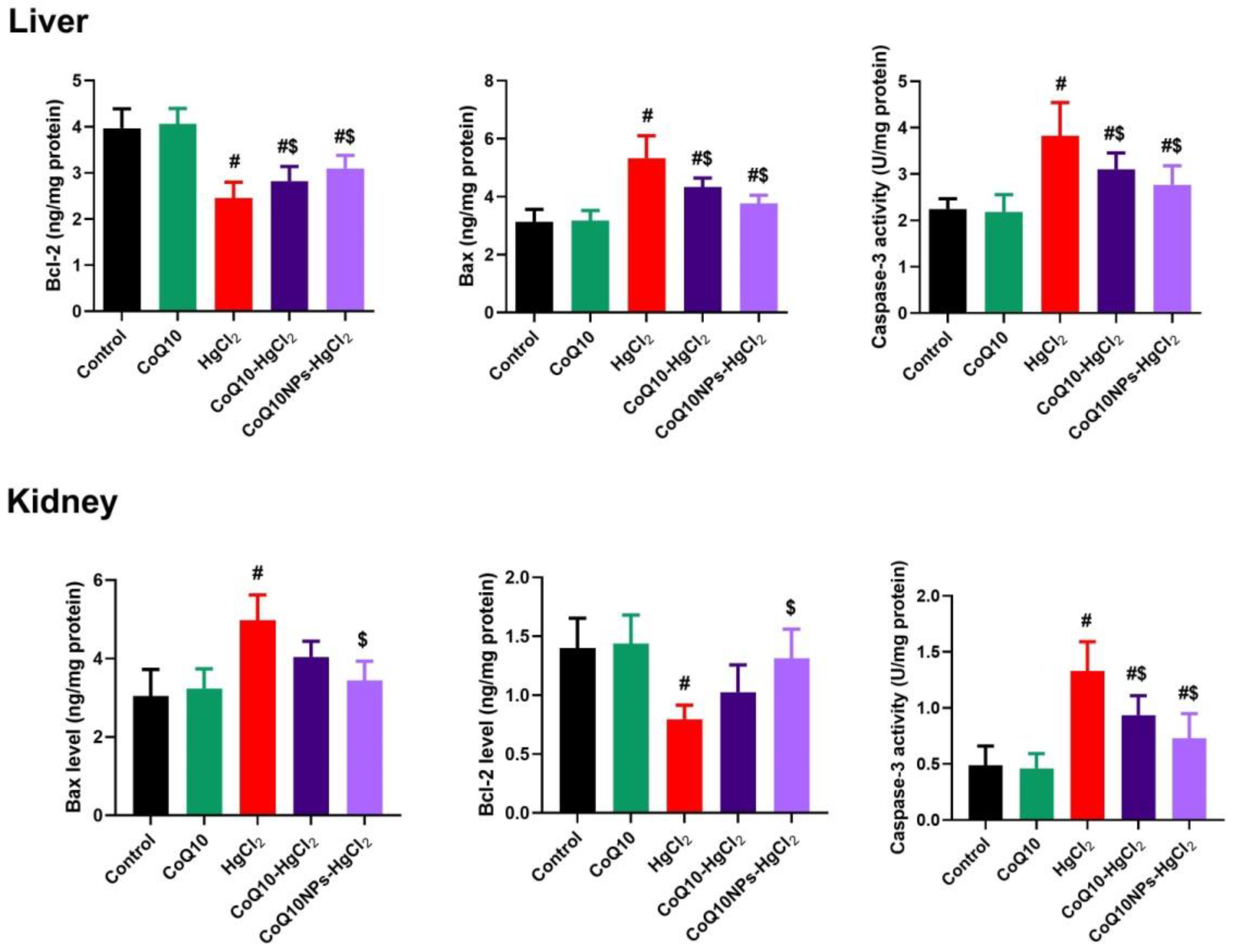
3.6. Effect of CoQ10NPs on Hepatorenal Apoptotic Markers upon HgCl2 Exposure
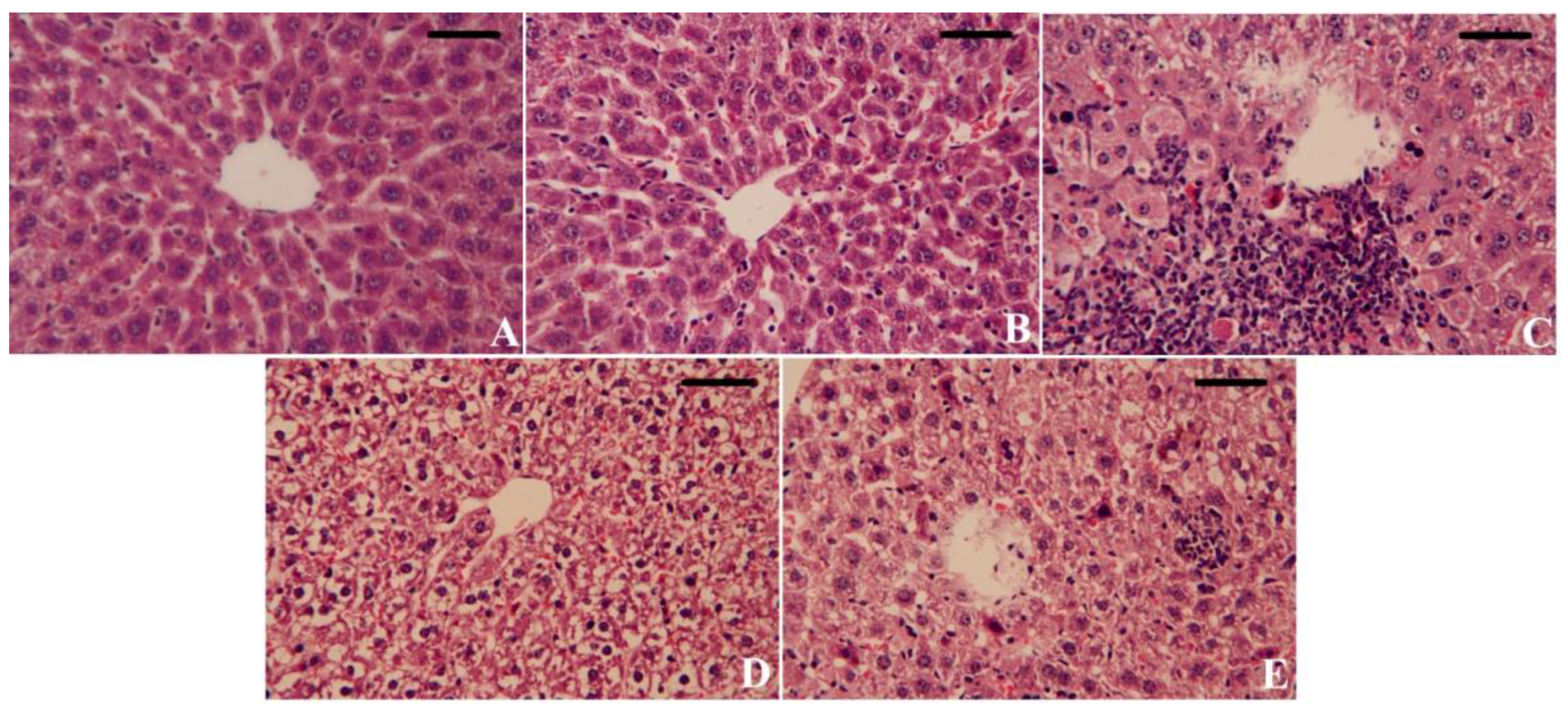
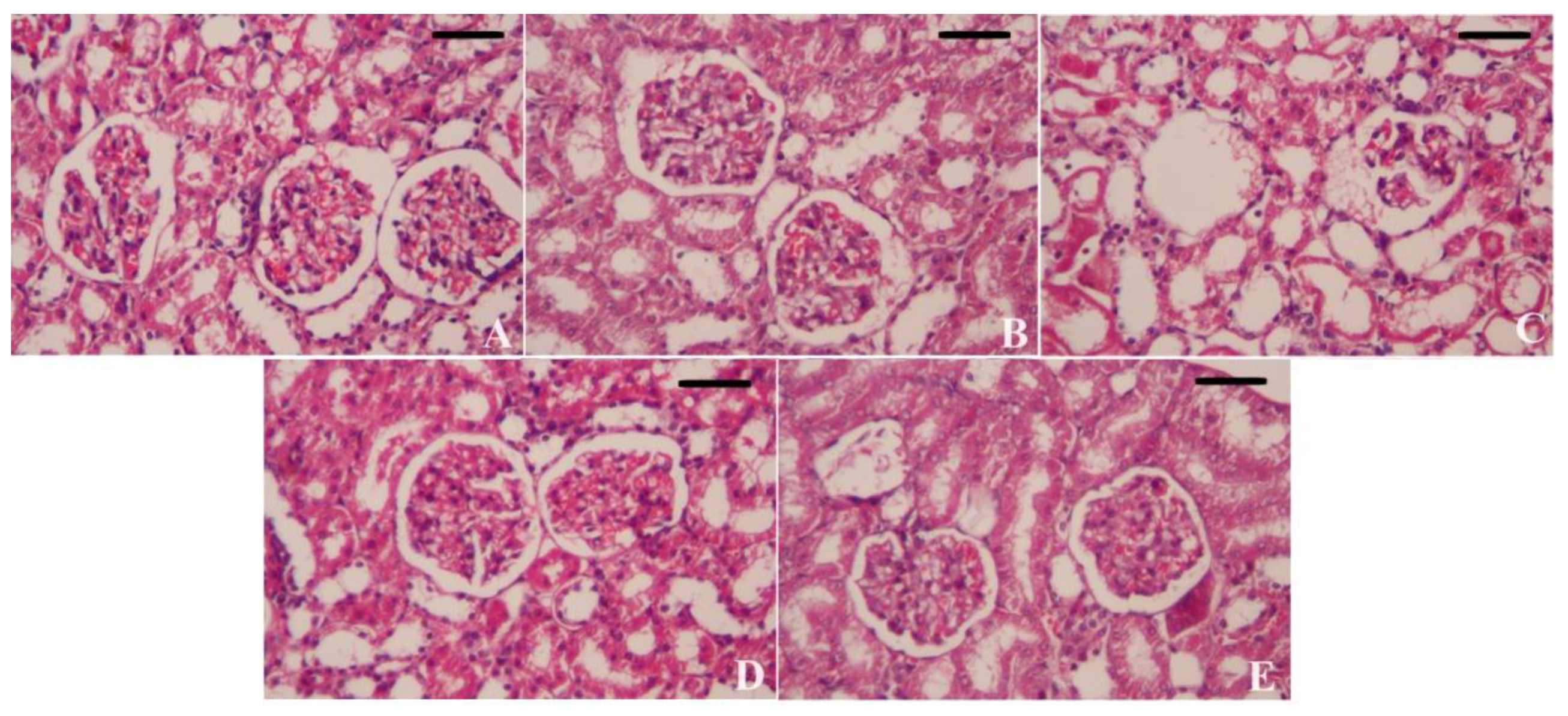
3.7. Effect of CoQ10NPs on Histological Alterations in Liver and Kidney upon HgCl2 Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Olayan, E.M.; Aloufi, A.S.; AlAmri, O.D.; El-Habit, O.H.; Abdel Moneim, A.E. Protocatechuic acid mitigates cadmium-induced neurotoxicity in rats: Role of oxidative stress, inflammation and apoptosis. Sci. Total Environ. 2020, 723, 137969. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.S.; Safwat, G.; Aboulkhair, M.; Abdel Moneim, A.E. The potential effect of berberine in mercury-induced hepatorenal toxicity in albino rats. Food Chem. Toxicol. 2014, 69, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.; Kalantar, M.; Kalantar, H. The hepatoprotective effect of gallic acid on mercuric chloride-induced liver damage in rats. Jundishapur J. Nat. Pharm. Prod. 2017, 12, e12345. [Google Scholar] [CrossRef]
- Almeer, R.S.; Albasher, G.; Kassab, R.B.; Ibrahim, S.R.; Alotibi, F.; Alarifi, S.; Ali, D.; Alkahtani, S.; Abdel Moneim, A.E. Ziziphus spina-christi leaf extract attenuates mercury chloride-induced testicular dysfunction in rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 3401–3412. [Google Scholar] [CrossRef] [PubMed]
- Boujbiha, M.A.; Hamden, K.; Guermazi, F.; Bouslama, A.; Omezzine, A.; Kammoun, A.; El Feki, A. Testicular toxicity in mercuric chloride treated rats: Association with oxidative stress. Reprod. Toxicol. 2009, 28, 81–89. [Google Scholar] [CrossRef]
- Moneim, A.E.A. The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metab. Brain Dis. 2015, 30, 935–942. [Google Scholar] [CrossRef]
- Rozgaj, R.; Kašuba, V.; Blanuša, M. Mercury chloride genotoxicity in rats following oral exposure, evaluated by comet assay and micronucleus test. Arh. Za Hig. Rada I Toksikol. 2005, 56, 9–15. [Google Scholar]
- Sharma, M.K.; Sharma, A.; Kumar, A.; Kumar, M. Evaluation of protective efficacy of Spirulina fusiformis against mercury induced nephrotoxicity in Swiss albino mice. Food Chem. Toxicol. 2007, 45, 879–887. [Google Scholar] [CrossRef]
- Durak, D.; Kalender, S.; Uzun, F.G.; Kalender, Y. Mercury chloride-induced oxidative stress in human erythrocytes and the effect of vitamins C and E in vitro. Afr. J. Biotechnol. 2010, 9, 488–495. [Google Scholar]
- Boujbiha, M.A.M.; Hamden, K.; Guermazi, F.; Bouslama, A.; Omezzine, A.; El Feki, A. Impairment of spermatogenesis in rats by mercuric chloride: Involvement of low 17β-estradiol level in induction of acute oxidative stress. Biol. Trace Elem. Res. 2011, 142, 598–610. [Google Scholar] [CrossRef]
- Vijayaprakash, S.; Langeswaran, K.; Kumar, S.G.; Revathy, R.; Balasubramanian, M.P. Nephro-protective significance of kaempferol on mercuric chloride induced toxicity in Wistar albino rats. Biomed. Aging Pathol. 2013, 3, 119–124. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, X.; Yang, D.; Lu, J.; Liu, B.; Baiyun, R.; Zhang, Z. Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-κB/P53 signaling pathway in rats. Oncotarget 2017, 8, 40982. [Google Scholar] [CrossRef]
- Ansar, S.; Iqbal, M. Protective effect of diallylsulphide against mercuric chloride-induced hepatic injury in rats. Hum. Exp. Toxicol. 2016, 35, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Nabil, A.; Elshemy, M.M.; Asem, M.; Gomaa, H.F. Protective effect of DPPD on mercury chloride-induced Hepatorenal toxicity in rats. J. Toxicol. 2020, 2020, 4127284. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q10 analogues: Benefits and challenges for therapeutics. Antioxidants 2021, 10, 236. [Google Scholar] [CrossRef]
- Parmar, S.S.; Jaiwal, A.; Dhankher, O.P.; Jaiwal, P.K. Coenzyme Q10 production in plants: Current status and future prospects. Crit. Rev. Biotechnol. 2015, 35, 152–164. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; Soliman, D.; Kassab, R.B.; Metwally, D.M.; Moneim, A.E.A.; El-Khadragy, M.F. Coenzyme Q10 activates the antioxidant machinery and inhibits the inflammatory and apoptotic cascades against lead acetate-induced renal injury in rats. Front. Physiol. 2020, 11, 64. [Google Scholar] [CrossRef]
- Yousef, S.; Omar, A.; Fahad, A.A.; Abdel Moneim, A.E.; Metwally, D.M.; El-khadragy, M.F.; Kassab, R.B. The neuroprotective role of coenzyme Q10 against lead acetate-induced neurotoxicity is mediated by antioxidant, anti-inflammatory and anti-apoptotic activities. Int. J. Environ. Res. Public Health 2019, 16, 2895. [Google Scholar] [CrossRef]
- Sohet, F.M.; Delzenne, N.M. Is there a place for coenzyme Q in the management of metabolic disorders associated with obesity? Nutr. Rev. 2012, 70, 631–641. [Google Scholar] [CrossRef]
- Noh, Y.; Kim, K.; Shim, M.; Choi, S.; Choi, S.; Ellisman, M.; Weinreb, R.; Perkins, G.; Ju, W. Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death Dis. 2013, 4, e820. [Google Scholar] [CrossRef]
- Blatt, T.; Littarru, G.P. Biochemical rationale and experimental data on the antiaging properties of CoQ10 at skin level. Biofactors 2011, 37, 381–385. [Google Scholar] [CrossRef]
- Patil, T.S.; Deshpande, A.S. Nanostructured lipid carriers-based drug delivery for treating various lung diseases: A state-of-the-art review. Int. J. Pharm. 2018, 547, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F.; Fichtner, I.; Beyer, U.; Schumacher, P.; Roth, T.; Fiebig, H.; Unger, C. Antitumour activity of acid labile transferrin and albumin doxorubicin conjugates in in vitro and in vivo human tumour xenograft models. Eur. J. Cancer 1997, 33, S175. [Google Scholar] [CrossRef]
- Mariam, J.; Sivakami, S.; Dongre, P.M. Albumin corona on nanoparticles—A strategic approach in drug delivery. Drug Deliv. 2016, 23, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Kunde, S.S.; Wairkar, S. Targeted delivery of albumin nanoparticles for breast cancer: A review. Colloids Surf. B Biointerfaces 2022, 213, 112422. [Google Scholar] [CrossRef]
- Pinto, S.; Gaspar, M.M.; Ascensão, L.; Faísca, P.; Reis, C.P.; Pacheco, R. Nanoformulation of Seaweed Eisenia bicyclis in Albumin Nanoparticles Targeting Cardiovascular Diseases: In Vitro and In Vivo Evaluation. Mar. Drugs 2022, 20, 608. [Google Scholar] [CrossRef]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
- Fatima, S.; Suhail, N.; Alrashed, M.; Wasi, S.; Aljaser, F.S.; AlSubki, R.A.; Alsharidah, A.S.; Banu, N. Epigallocatechin gallate and coenzyme Q10 attenuate cisplatin-induced hepatotoxicity in rats via targeting mitochondrial stress and apoptosis. J. Biochem. Mol. Toxicol. 2021, 35, e22701. [Google Scholar] [CrossRef]
- El-Desoky, G.E.; Bashandy, S.A.; Alhazza, I.M.; Al-Othman, Z.A.; Aboul-Soud, M.A.; Yusuf, K. Improvement of mercuric chloride-induced testis injuries and sperm quality deteriorations by Spirulina platensis in rats. PLoS ONE 2013, 8, e59177. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.A.; Morsy, M.A.; Mahmoud, M.M.; Rifaai, R.A.; Abdelrahman, A.M. Effect of coenzyme-Q10 on doxorubicin-induced nephrotoxicity in rats. Adv. Pharmacol. Pharm. Sci. 2012, 2012, 981461. [Google Scholar] [CrossRef]
- Jithan, A.; Madhavi, K.; Madhavi, M.; Prabhakar, K. Preparation and characterization of albumin nanoparticles encapsulating curcumin intended for the treatment of breast cancer. Int. J. Pharm. Investig. 2011, 1, 119. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis (2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kalender, S.; Uzun, F.G.; Demir, F.; Uzunhisarcıklı, M.; Aslanturk, A. Mercuric chloride-induced testicular toxicity in rats and the protective role of sodium selenite and vitamin E. Food Chem. Toxicol. 2013, 55, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Wang, M.; Yin, S.-T.; Wang, H.-L.; Chen, L.; Sun, L.-G.; Ruan, D.-Y. The interaction of selenium and mercury in the accumulations and oxidative stress of rat tissues. Ecotoxicol. Environ. Saf. 2008, 70, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.N.; Sharma, U.S.; Singh, S.; Gupta, Y.K. Effect of Tribulus terrestris in mercuric chloride-induced renal accumulation of mercury and nephrotoxicity in rat. J. Adv. Pharm. Technol. Res. 2019, 10, 132. [Google Scholar] [CrossRef]
- Joshi, D.; Mittal, D.K.; Shukla, S.; Srivastav, S.K.; Dixit, V.A. Curcuma longa Linn. extract and curcumin protect CYP 2E1 enzymatic activity against mercuric chloride-induced hepatotoxicity and oxidative stress: A protective approach. Exp. Toxicol. Pathol. 2017, 69, 373–382. [Google Scholar] [CrossRef]
- Elblehi, S.S.; Hafez, M.H.; El-Sayed, Y.S. L-α-Phosphatidylcholine attenuates mercury-induced hepato-renal damage through suppressing oxidative stress and inflammation. Environ. Sci. Pollut. Res. 2019, 26, 9333–9342. [Google Scholar] [CrossRef]
- Uzunhisarcikli, M.; Aslanturk, A.; Kalender, S.; Apaydin, F.G.; Bas, H. Mercuric chloride induced hepatotoxic and hematologic changes in rats: The protective effects of sodium selenite and vitamin E. Toxicol. Ind. Health 2016, 32, 1651–1662. [Google Scholar] [CrossRef]
- Salman, M.M.; Kotb, A.M.; Haridy, M.A.; Hammad, S. Hepato-and nephroprotective effects of bradykinin potentiating factor from scorpion (Buthus occitanus) venom on mercuric chloride-treated rats. EXCLI J. 2016, 15, 807. [Google Scholar]
- Oriquat, G.A.; Saleem, T.H.; Naik, R.R.; Moussa, S.Z.; Al-Gindy, R.M. A Sub-Chronic Toxicity Study of Mercuric Chloride in the Rat. Jordan J. Biol. Sci. 2012, 5, 141–146. [Google Scholar]
- Al-Rekabi, B.K.K.; Al-Diwan, M.A.; Sawad, A.A. The protective role of CoQ10 and DHEA and their combination on CCl4 induced liver injury in adult male rats (Rattus norvegicus). J. Biosci. Appl. Res. 2019, 5, 375–389. [Google Scholar] [CrossRef]
- Ali, S.A.; Faddah, L.; Abdel-Baky, A.; Bayoumi, A. Protective effect of L-carnitine and coenzyme Q10 on CCl4-induced liver injury in rats. Sci. Pharm. 2010, 78, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Ustuner, M.A.; Kaman, D.; Colakoglu, N. Effects of benfotiamine and coenzyme Q10 on kidney damage induced gentamicin. Tissue Cell 2017, 49, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Ki, Y.; Kim, W.; Kim, Y.H.; Kim, D.; Bae, J.S.; Park, D.; Jeon, H.; Lee, J.H.; Lee, J.; Nam, J. Effect of coenzyme Q10 on radiation nephropathy in rats. J. Korean Med. Sci. 2017, 32, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.; Abdelkader, A.; Elgazzar, D.; Aboubakr, M.; Abdulah, O.A.; Shoghy, K.; Abdel-Daim, M.; El-Serehy, H.A.; Najda, A.; El-Mleeh, A. Coenzyme Q10 supplementation mitigates piroxicam-induced oxidative injury and apoptotic pathways in the stomach, liver, and kidney. Biomed. Pharmacother. 2020, 130, 110627. [Google Scholar] [CrossRef]
- Mwaeni, V.K.; Nyariki, J.N.; Jillani, N.; Omwenga, G.; Ngugi, M.; Isaac, A.O. Coenzyme Q10 protected against arsenite and enhanced the capacity of 2,3-dimercaptosuccinic acid to ameliorate arsenite-induced toxicity in mice. BMC Pharmacol. Toxicol. 2021, 22, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Özyurt, H.; Söğüt, S.; Yıldırım, Z.; Kart, L.; Iraz, M.; Armutçu, F.; Temel, İ.; Özen, S.; Uzun, A.; Akyol, Ö. Inhibitory effect of caffeic acid phenethyl ester on bleomycine-induced lung fibrosis in rats. Clin. Chim. Acta 2004, 339, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Aslanturk, A.; Uzunhisarcikli, M.; Kalender, S.; Demir, F. Sodium selenite and vitamin E in preventing mercuric chloride induced renal toxicity in rats. Food Chem. Toxicol. 2014, 70, 185–190. [Google Scholar] [CrossRef]
- Rao, M.V.; Chhunchha, B. Protective role of melatonin against the mercury induced oxidative stress in the rat thyroid. Food Chem. Toxicol. 2010, 48, 7–10. [Google Scholar] [CrossRef]
- Hosseini, A.; Rajabian, A.; Fanoudi, S.; Farzadnia, M.; Boroushaki, M.T. Protective effect of Rheum turkestanicum root against mercuric chloride-induced hepatorenal toxicity in rats. Avicenna J. Phytomed. 2018, 8, 488. [Google Scholar]
- Mustafa, H.N.; Hegazy, G.A.; El Awdan, S.A.; AbdelBaset, M. Protective role of CoQ10 or L-carnitine on the integrity of the myocardium in doxorubicin induced toxicity. Tissue Cell 2017, 49, 410–426. [Google Scholar] [CrossRef]
- Caglayan, C.; Kandemir, F.M.; Darendelioğlu, E.; Yıldırım, S.; Kucukler, S.; Dortbudak, M.B. Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J. Trace Elem. Med. Biol. 2019, 56, 60–68. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Xiao, Y.; Wang, Y.; Wan, Y.; Li, X.; Li, Q.; Tang, X.; Cai, D.; Ran, B. Curcumin ameliorates mercuric chloride-induced liver injury via modulating cytochrome P450 signaling and Nrf2/HO-1 pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111426. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Tan, X.; Lv, Z.; Liu, B.; Baiyun, R.; Lu, J.; Zhang, Z. Regulation of Sirt1/Nrf2/TNF-α signaling pathway by luteolin is critical to attenuate acute mercuric chloride exposure induced hepatotoxicity. Sci. Rep. 2016, 6, 37157. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-H.; Chen, C.-L.; Jawan, B.; Chung, Y.-H.; Sun, C.-K.; Kuo, S.-M.; Hu, T.-H.; Lin, Y.-C.; Chan, H.-H.; Cheng, K.-H. Upregulation of hepatoma-derived growth factor is involved in murine hepatic fibrogenesis. J. Hepatol. 2010, 52, 96–105. [Google Scholar] [CrossRef]
- Suzuki, K.; Wang, R.; Kubota, H.; Shibuya, H.; Saegusa, J.; Sato, T. Kinetics of biglycan, decorin and thrombospondin-1 in mercuric chloride-induced renal tubulointerstitial fibrosis. Exp. Mol. Pathol. 2005, 79, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, N.; Lehmann, I.; Wichmann, G.; Lehmann, J.; Emmrich, F.; Sack, U. Immunomodulation by mercuric chloride in vitro: Application of different cell activation pathways. Clin. Exp. Immunol. 2007, 148, 325–337. [Google Scholar] [CrossRef]
- Karabulut, D.; Akin, A.T.; Unsal, M.; Lekesizcan, A.; Ozyazgan, T.M.; Keti, D.B.; Yakan, B.; Ekebas, G. L-Carnitine ameliorates the liver by regulating alpha-SMA, iNOS, HSP90, HIF-1alpha, and RIP1 expressions of CCL4-toxic rats. Iran. J. Basic Med. Sci. 2021, 24, 184. [Google Scholar]
- Elshemy, M.M.; AbdEl-Mejied, A.E.; Zahran, F.; Omran, M.M.; Nabil, A. DPPD ameliorates renal fibrosis induced by HgCl2 in rats. Biosci. Res. 2018, 15, 2416–2425. [Google Scholar]
- Tao, Y.; Wang, Q.; Yuan, J.; Shen, L.; Liu, C. Effects of vitamin E on mercuric chloride-induced renal interstitial fibrosis in rats and the antioxidative mechanism. J. Chin. Integr. Med. 2011, 9, 201–208. [Google Scholar] [CrossRef]
- Lavinya, B.U.; Bardhan, I.; Prince, S.E. Efficacy of CoenzymeQ10 in inhibiting monosodium urate crystal-induced inflammation in rats. Eur. J. Pharmacol. 2016, 791, 589–594. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Said, R.S. Coenzyme Q10 attenuates inflammation and fibrosis implicated in radiation enteropathy through suppression of NF-kB/TGF-β/MMP-9 pathways. Int. Immunopharmacol. 2021, 92, 107347. [Google Scholar] [CrossRef] [PubMed]
- Abdel Moneim, A.E. Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS ONE 2016, 11, e0158965. [Google Scholar] [CrossRef] [PubMed]
- Izuta, H.; Shimazawa, M.; Tazawa, S.; Araki, Y.; Mishima, S.; Hara, H. Protective effects of Chinese propolis and its component, chrysin, against neuronal cell death via inhibition of mitochondrial apoptosis pathway in SH-SY5Y cells. J. Agric. Food Chem. 2008, 56, 8944–8953. [Google Scholar] [CrossRef] [PubMed]
- Darendelioglu, E.; Aykutoglu, G.; Tartik, M.; Baydas, G. Turkish propolis protects human endothelial cells in vitro from homocysteine-induced apoptosis. Acta Histochem. 2016, 118, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Sumi, K.; Okura, T.; Fujioka, Y.; Kato, M.; Imamura, T.; Taniguchi, S.-i.; Yamamoto, K. Coenzyme Q10 suppresses apoptosis of mouse pancreatic β-cell line MIN6. Diabetol. Metab. Syndr. 2018, 10, 1–6. [Google Scholar] [CrossRef]
- Mo, Y.; Barnett, M.E.; Takemoto, D.; Davidson, H.; Kompella, U.B. Human serum albumin nanoparticles for efficient delivery of Cu, Zn superoxide dismutase gene. Mol. Vis. 2007, 13, 746. [Google Scholar]
- Zaher, S.; Soliman, M.E.; Elsabahy, M.; Hathout, R.M. Sesamol loaded albumin nanoparticles: A boosted protective property in animal models of oxidative stress. Pharmaceuticals 2022, 15, 733. [Google Scholar] [CrossRef]
- Arroyo, V.; García-Martinez, R.; Salvatella, X. Human serum albumin, systemic inflammation, and cirrhosis. J. Hepatol. 2014, 61, 396–407. [Google Scholar] [CrossRef]
- Gholijani, N.; Abolmaali, S.-S.; Kalantar, K.; Ravanrooy, M.-H. Therapeutic effect of carvacrol-loaded albumin nanoparticles on arthritic rats. Iran. J. Pharm. Res. IJPR 2020, 19, 312. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadan, S.S.; El Zaiat, F.A.; Habashy, E.A.; Montaser, M.M.; Hassan, H.E.; Tharwat, S.S.; El-khadragy, M.; Abdel Moneim, A.E.; Elshopakey, G.E.; Akabawy, A.M.A. Coenzyme Q10-Loaded Albumin Nanoparticles Protect against Redox Imbalance and Inflammatory, Apoptotic, and Histopathological Alterations in Mercuric Chloride-Induced Hepatorenal Toxicity in Rats. Biomedicines 2023, 11, 3054. https://doi.org/10.3390/biomedicines11113054
Ramadan SS, El Zaiat FA, Habashy EA, Montaser MM, Hassan HE, Tharwat SS, El-khadragy M, Abdel Moneim AE, Elshopakey GE, Akabawy AMA. Coenzyme Q10-Loaded Albumin Nanoparticles Protect against Redox Imbalance and Inflammatory, Apoptotic, and Histopathological Alterations in Mercuric Chloride-Induced Hepatorenal Toxicity in Rats. Biomedicines. 2023; 11(11):3054. https://doi.org/10.3390/biomedicines11113054
Chicago/Turabian StyleRamadan, Shimaa S., Farah A. El Zaiat, Engy A. Habashy, Mostafa M. Montaser, Habeba E. Hassan, Shahinaz S. Tharwat, Manal El-khadragy, Ahmed E. Abdel Moneim, Gehad E. Elshopakey, and Ahmed M. A. Akabawy. 2023. "Coenzyme Q10-Loaded Albumin Nanoparticles Protect against Redox Imbalance and Inflammatory, Apoptotic, and Histopathological Alterations in Mercuric Chloride-Induced Hepatorenal Toxicity in Rats" Biomedicines 11, no. 11: 3054. https://doi.org/10.3390/biomedicines11113054
APA StyleRamadan, S. S., El Zaiat, F. A., Habashy, E. A., Montaser, M. M., Hassan, H. E., Tharwat, S. S., El-khadragy, M., Abdel Moneim, A. E., Elshopakey, G. E., & Akabawy, A. M. A. (2023). Coenzyme Q10-Loaded Albumin Nanoparticles Protect against Redox Imbalance and Inflammatory, Apoptotic, and Histopathological Alterations in Mercuric Chloride-Induced Hepatorenal Toxicity in Rats. Biomedicines, 11(11), 3054. https://doi.org/10.3390/biomedicines11113054






