Accuracy of the END-PAC Model in Predicting the Risk of Developing Pancreatic Cancer in Patients with New-Onset Diabetes: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Search Methods
2.3. Selection of Studies and Data Extraction
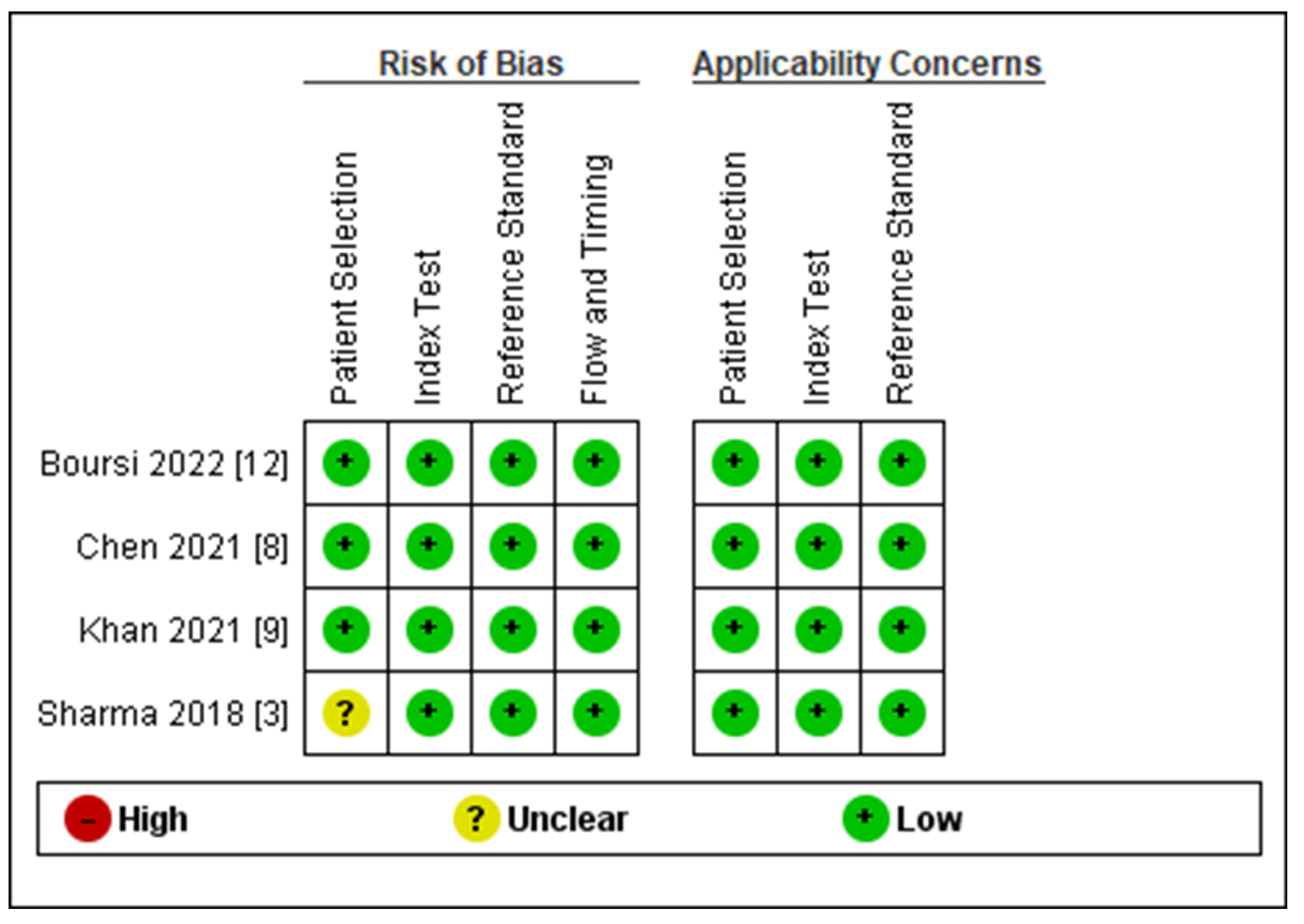
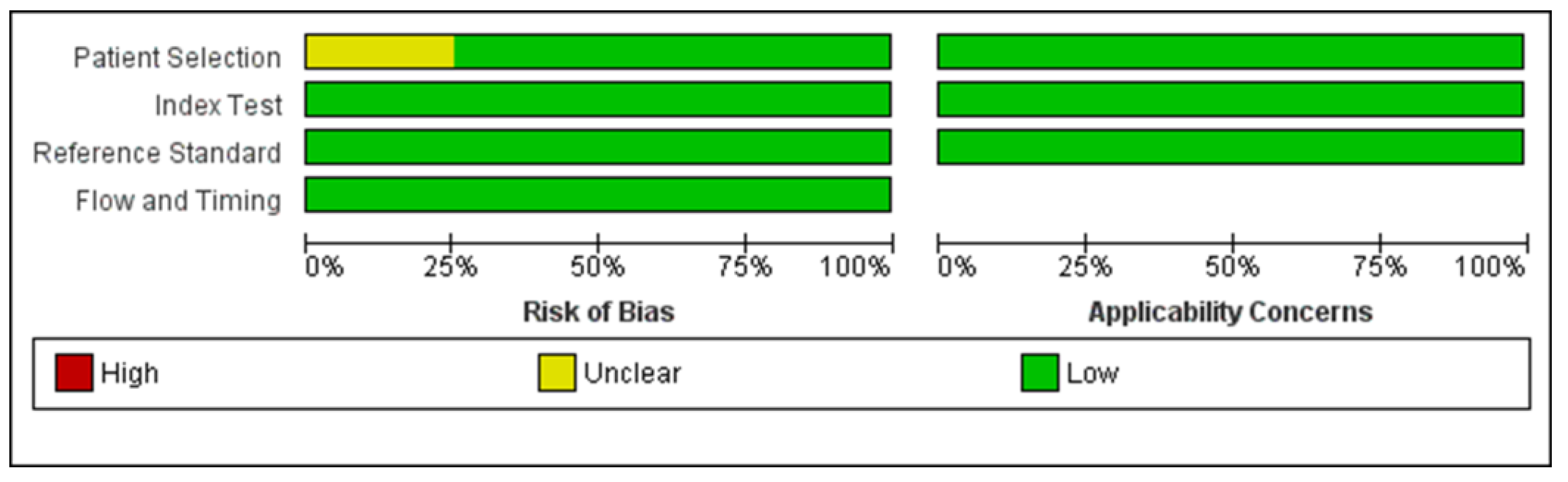
2.4. Risk of Bias Assessment
2.5. Statistical Analyses
2.6. Subgroup Analysis
2.7. Sensitivity Analyses
3. Results
3.1. Results of the Search
3.2. Risk of Bias Assessment
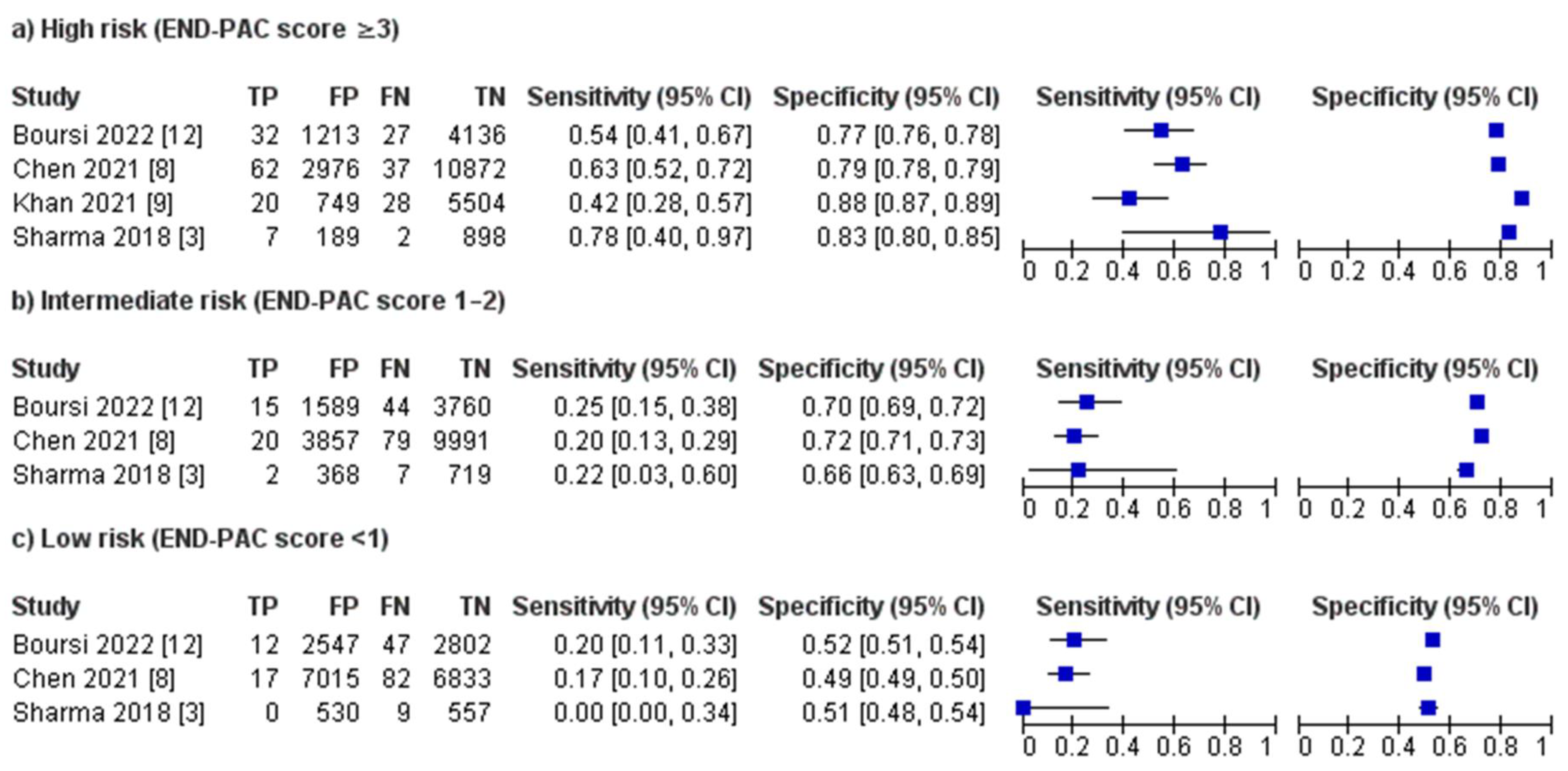
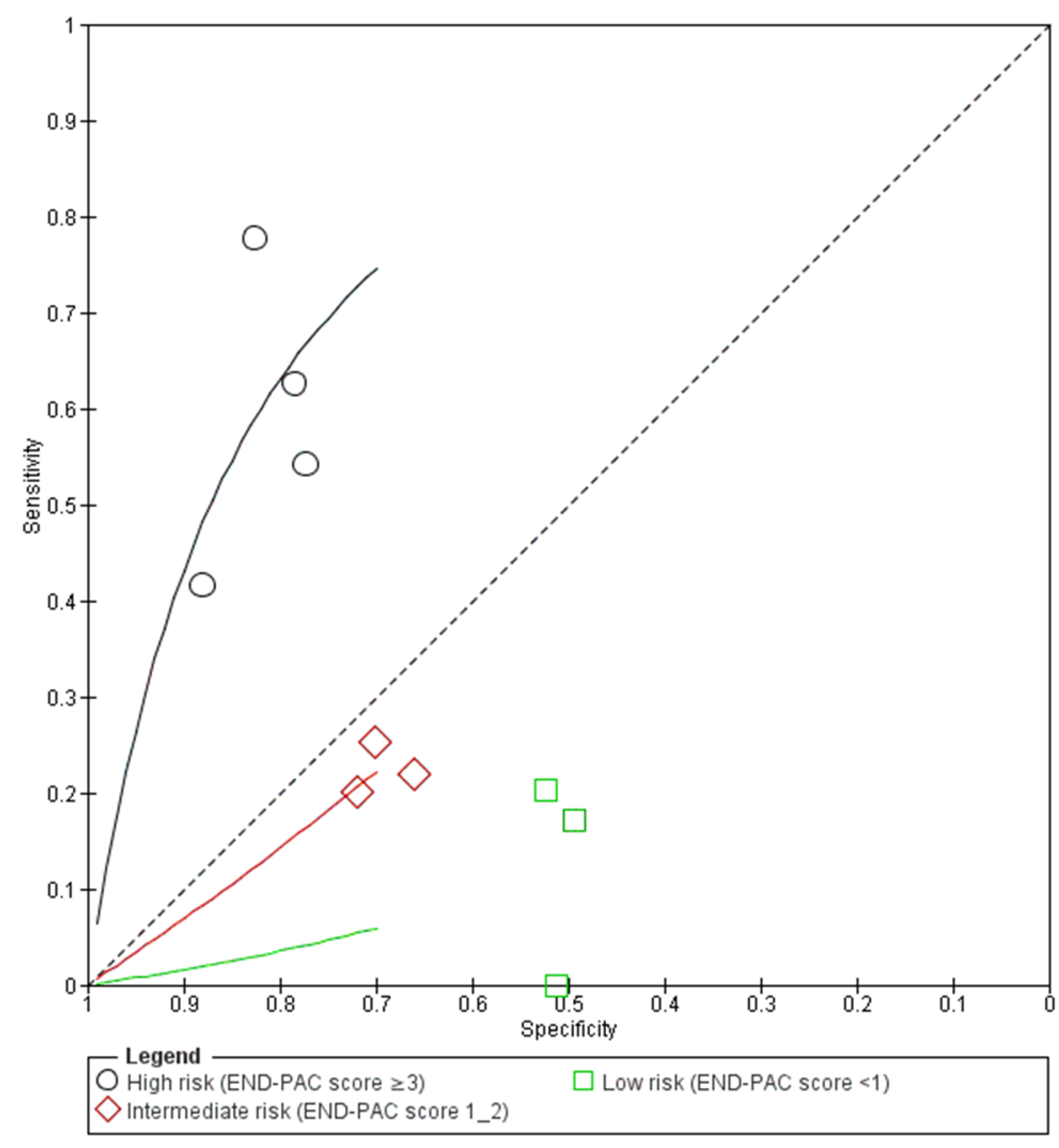
3.3. Performance of the END-PAC Model
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Search No | Search Strategy † |
| #1 | new-onset near 2 diabetes: TI, AB, KW |
| #2 | MeSH descriptor: [pancreatic cancer] explode all trees |
| #3 | pancreatic cancer: TI, AB, KW |
| #4 | #2 OR #3 |
| #5 | ENDPAC: TI, AB, KW |
| #6 | END-PAC: TI, AB, KW |
| #7 | model: TI, AB, KW |
| #8 | #5 OR #6 OR #7 |
| #9 | #1 AND #4 AND #8 |
References
- Barros, A.G.; Pulido, C.F.; Machado, M.; Brito, M.J.; Couto, N.; Sousa, O.; Melo, S.A.; Mansinho, H. Treatment optimization of locally advanced and metastatic pancreatic cancer. Int. J. Oncol. 2021, 59, 110. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kandlakunta, H.; Nagpal, S.J.; Feng, Z.; Hoos, W.; Petersen, G.M.; Chari, S.T. Model to determine risk of pancreatic cancer in patients with new-onset diabetes. Gastroenterology 2018, 155, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.T.; Leibson, C.L.; Rabe, K.G.; Ransom, J.; de Andrade, M.; Petersen, G.M. Probability of pancreatic cancer following diabetes: A populationbased study. Gastroenterology 2005, 129, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Jin, J. Screening for pancreatic cancer. J. Am. Med. Assoc. 2019, 322, 478. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.P.; Nagpal, S.J.; Mukhopadhyay, D.; Chari, S.T. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Poruk, K.E.; Firpo, M.A.; Adler, D.G.; Mulvihill, S.J. Screening for pancreatic cancer: Why, how, and who? Ann. Surg. 2013, 257, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Butler, R.K.; Lustigova, E.; Chari, S.T.; Wu, B.U. Validation of the enriching new-onset diabetes for pancreatic cancer model in a diverse and integrated healthcare setting. Dig. Dis. Sci. 2021, 66, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Safarudin, R.F.; Kupec, J.T. Validation of the ENDPAC model: Identifying new-onset diabetics at risk of pancreatic cancer. Pancreatology 2021, 21, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Patalon, T.; Webb, M.; Margalit, O.; Beller, T.; Yang, Y.X.; Chodick, G. Validation of the Enriching New-Onset Diabetes for Pancreatic Cancer Model: A Retrospective Cohort Study Using Real-World Data. Pancreas 2022, 51, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Finkelman, B.; Giantonio, B.J.; Haynes, K.; Rustgi, A.K.; Rhim, A.D.; Mamtani, R.; Yang, Y.-X. A clinical prediction model to assess risk for pancreatic cancer among patients with new-onset diabetes. Gastroenterology 2017, 152, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Sahoo, J.; Kamalanathan, S.; Naik, D.; Mohan, P.; Kalayarasan, R. Diabetes and pancreatic cancer: Exploring the two-way traffic. World J. Gastroenterol. 2021, 27, 4939. [Google Scholar] [CrossRef] [PubMed]
- Illés, D.; Ivány, E.; Holzinger, G.; Kosár, K.; Adam, M.G.; Kamlage, B.; Zsóri, G.; Tajti, M.; Svébis, M.M.; Horváth, V.; et al. New Onset of DiabetEs in aSsociation with pancreatic ductal adenocarcinoma (NODES Trial): Protocol of a prospective, multicentre observational trial. BMJ Open 2020, 10, e037267. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.T.; Maitra, A.; Matrisian, L.M.; Shrader, E.E.; Wu, B.U.; Kambadakone, A.; Zhao, Y.-Q.; Kenner, B.; Rinaudo, J.A.S.; Srivastava, S.; et al. Early detection initiative: A randomized controlled trial of algorithm-based screening in patients with new onset hyperglycemia and diabetes for early detection of pancreatic ductal adenocarcinoma. Contemp. Clin. Trials 2022, 113, 106659. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.R.; Matrisian, L.M.; Shrader, E.E.; Feng, Z.; Chari, S.; Roth, J.A. Potential cost-effectiveness of risk-based pancreatic cancer screening in patients with new-onset diabetes. J. Natl. Compr. Cancer Netw. 2021, 20, 451–459. [Google Scholar] [CrossRef] [PubMed]



| Study | Year | Country | Journal | Study Design | Length of Follow-Up | Description of Included Population | Model Evaluated | Model Variables | Sample Size |
|---|---|---|---|---|---|---|---|---|---|
| Boursi et al. [12] | 2022 | Israel | Pancreas | Retrospective cohort | 3 years | Patients aged over 50 with new-onset diabetes | END-PAC | Age at diagnosis, change in body weight (kg), and change in fasting plasma glucose | 5408 |
| Khan et al. [9] | 2021 | USA | Pancreatology | Retrospective cohort | 4 years | Patients aged over 50 with new-onset diabetes | END-PAC | Age at diagnosis, change in body weight (kg), and change in fasting plasma glucose | 6301 |
| Chen et al. [8] | 2021 | USA | Digestive Diseases and Sciences | Retrospective cohort | 3 years | Patients aged between 50–85 with new-onset diabetes | END-PAC | Age at diagnosis, change in body weight (kg), and change in fasting plasma glucose | 13,947 |
| Sharma et al. [3] | 2018 | USA | Gastroenterology | Retrospective cohort | 3 years | Patients aged over 50 with new-onset diabetes | END-PAC | Age at diagnosis, change in body weight (kg), and change in fasting plasma glucose | 1096 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajibandeh, S.; Intrator, C.; Carrington-Windo, E.; James, R.; Hughes, I.; Hajibandeh, S.; Satyadas, T. Accuracy of the END-PAC Model in Predicting the Risk of Developing Pancreatic Cancer in Patients with New-Onset Diabetes: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 3040. https://doi.org/10.3390/biomedicines11113040
Hajibandeh S, Intrator C, Carrington-Windo E, James R, Hughes I, Hajibandeh S, Satyadas T. Accuracy of the END-PAC Model in Predicting the Risk of Developing Pancreatic Cancer in Patients with New-Onset Diabetes: A Systematic Review and Meta-Analysis. Biomedicines. 2023; 11(11):3040. https://doi.org/10.3390/biomedicines11113040
Chicago/Turabian StyleHajibandeh, Shahab, Christina Intrator, Eliot Carrington-Windo, Rhodri James, Ioan Hughes, Shahin Hajibandeh, and Thomas Satyadas. 2023. "Accuracy of the END-PAC Model in Predicting the Risk of Developing Pancreatic Cancer in Patients with New-Onset Diabetes: A Systematic Review and Meta-Analysis" Biomedicines 11, no. 11: 3040. https://doi.org/10.3390/biomedicines11113040
APA StyleHajibandeh, S., Intrator, C., Carrington-Windo, E., James, R., Hughes, I., Hajibandeh, S., & Satyadas, T. (2023). Accuracy of the END-PAC Model in Predicting the Risk of Developing Pancreatic Cancer in Patients with New-Onset Diabetes: A Systematic Review and Meta-Analysis. Biomedicines, 11(11), 3040. https://doi.org/10.3390/biomedicines11113040






