Cell Line-Based Human Bladder Organoids with Bladder-like Self-Organization—A New Standardized Approach in Bladder Cancer Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Generation of Standardized BCa Organoids
2.3. Wnt Ligand Treatment
2.4. Histology and DAB Immunostaining
2.5. Immunofluorescence
2.6. Drug and Radiation Response
2.7. Statistical Analysis
3. Results
3.1. Morphological Characterization of BCa Orgs
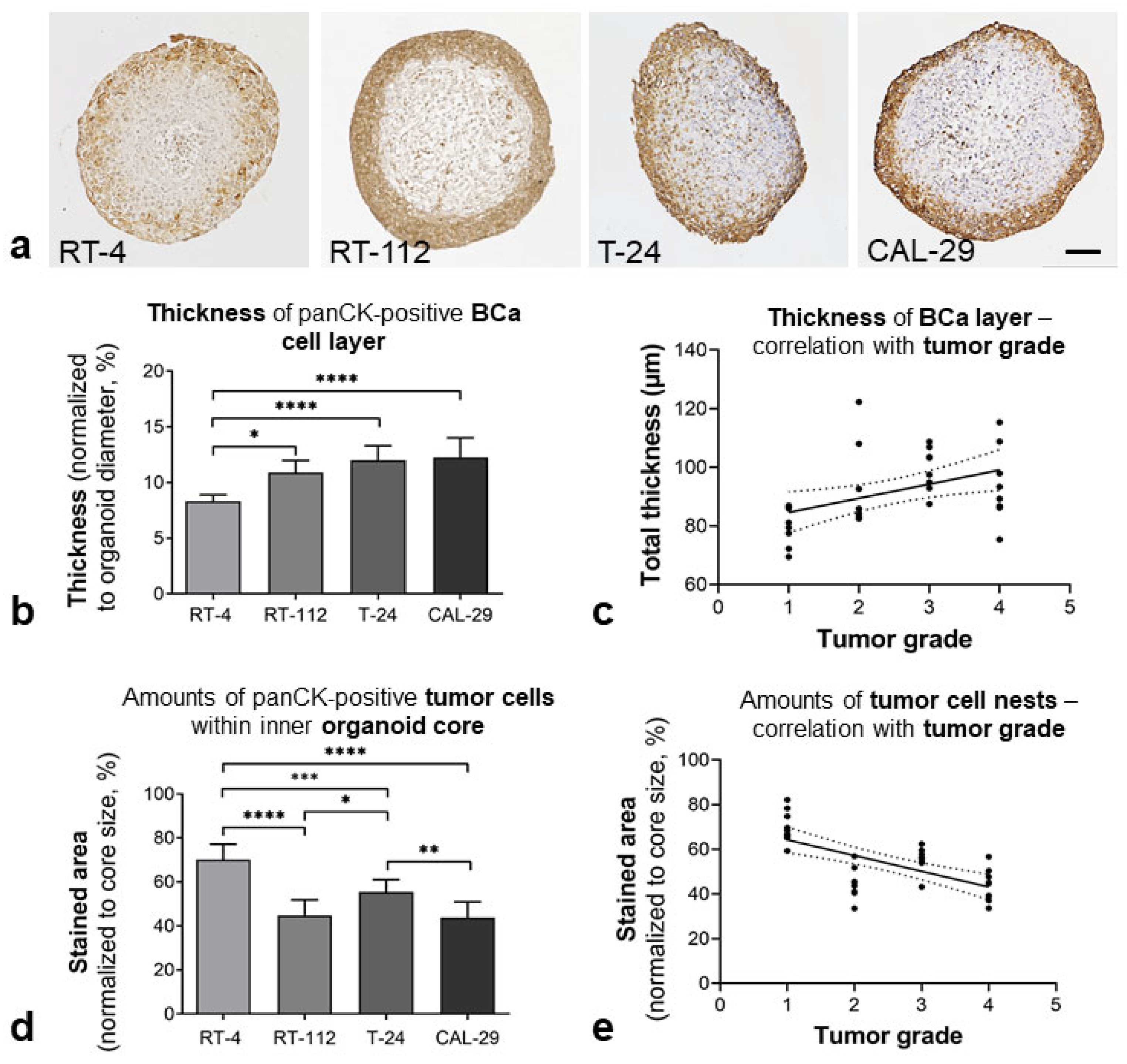
3.1.1. Bladder-like Self-Organization
3.1.2. Urothelial Differentiation
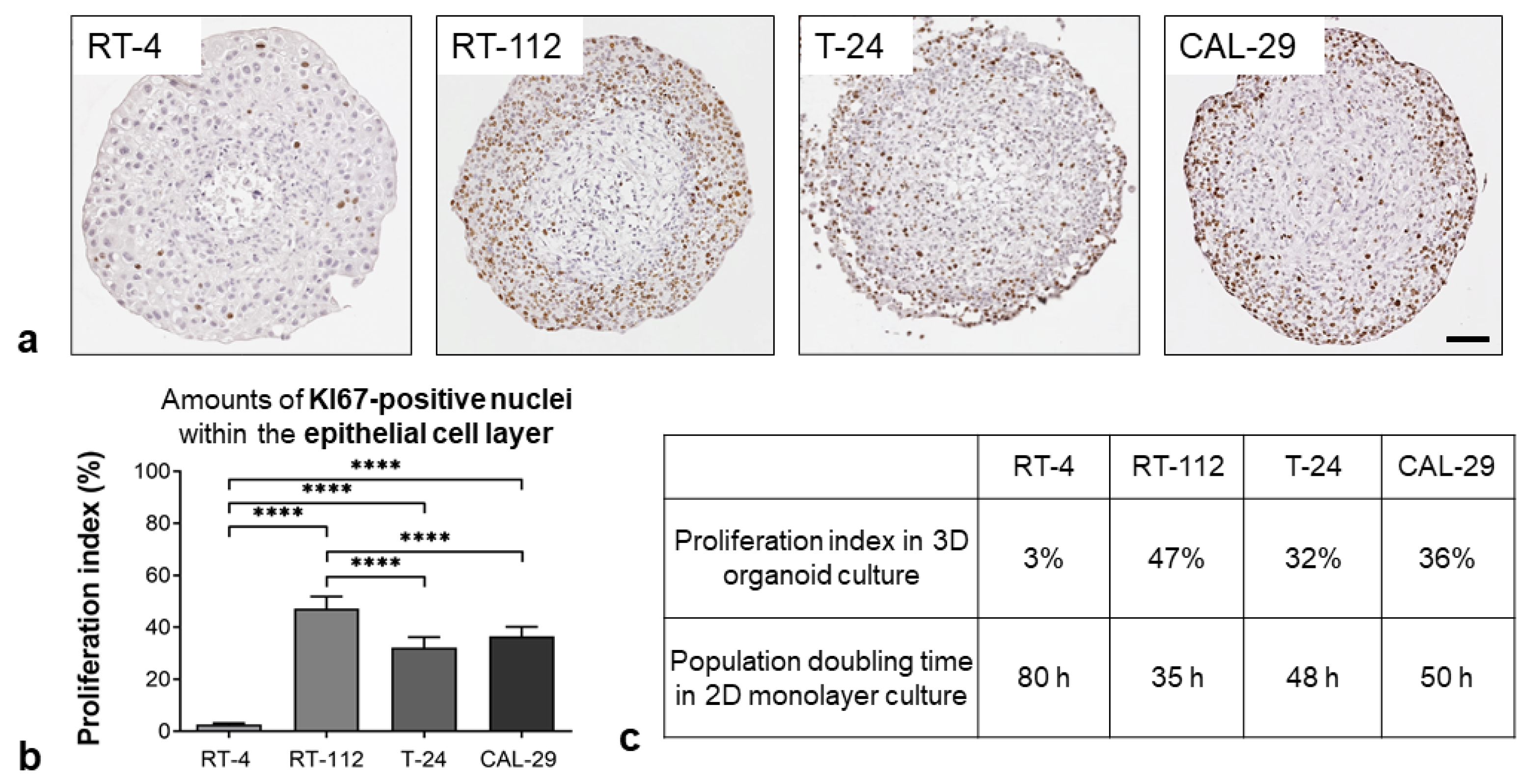
3.1.3. Proliferation Status
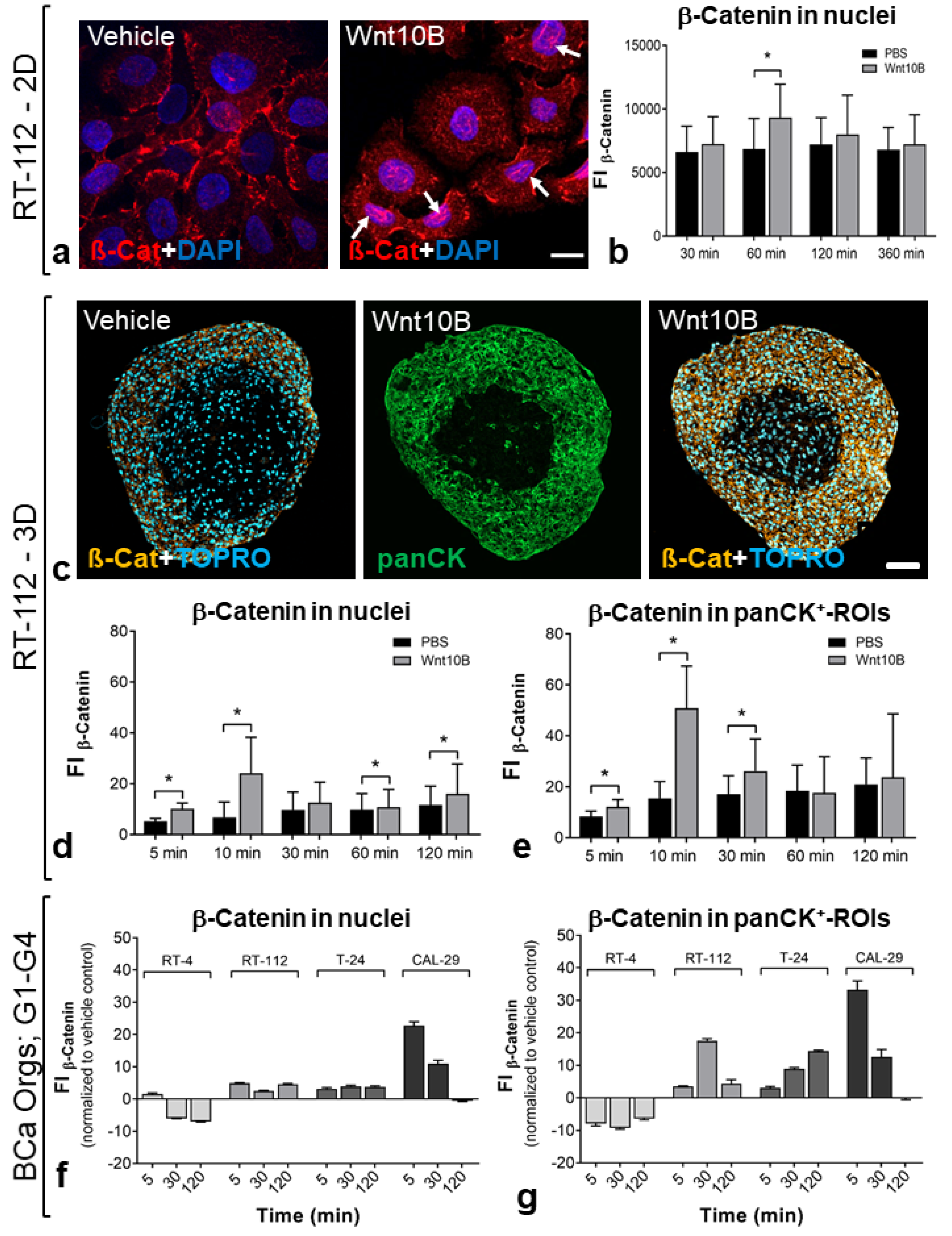
3.2. Ligand-Dependent Wnt Pathway Activation
3.3. Response to Anti-Tumor Therapies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Schutgens, F.; Clevers, H. Human Organoids: Tools for Understanding Biology and Treating Diseases. Annu. Rev. Pathol. 2020, 15, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Doctor, A.; Seifert, V.; Ullrich, M.; Hauser, S.; Pietzsch, J. Three-Dimensional Cell Culture Systems in Radiopharmaceutical Cancer Research. Cancers 2020, 12, 2765. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chintala, S.; Ciamporcero, E.; Ramakrishnan, S.; Elbanna, M.; Wang, J.; Hu, Q.; Glenn, S.T.; Murakami, M.; Liu, L.; et al. Genomic profiling is predictive of response to cisplatin treatment but not to PI3K inhibition in bladder cancer patient-derived xenografts. Oncotarget 2016, 7, 76374–76389. [Google Scholar] [CrossRef]
- Inoue, T.; Terada, N.; Kobayashi, T.; Ogawa, O. Patient-derived xenografts as in vivo models for research in urological malignancies. Nat. Rev. Urol. 2017, 14, 267–283. [Google Scholar] [CrossRef]
- Lee, S.H.; Hu, W.; Matulay, J.T.; Silva, M.V.; Owczarek, T.B.; Kim, K.; Chua, C.W.; Barlow, L.J.; Kandoth, C.; Williams, A.B.; et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 2018, 173, 515–528.e17. [Google Scholar] [CrossRef]
- Mullenders, J.; de Jongh, E.; Brousali, A.; Roosen, M.; Blom, J.P.A.; Begthel, H.; Korving, J.; Jonges, T.; Kranenburg, O.; Meijer, R.; et al. Mouse and human urothelial cancer organoids: A tool for bladder cancer research. Proc. Natl. Acad. Sci. USA 2019, 116, 4567–4574. [Google Scholar] [CrossRef]
- Yu, L.; Li, Z.; Mei, H.; Li, W.; Chen, D.; Liu, L.; Zhang, Z.; Sun, Y.; Song, F.; Chen, W.; et al. Patient-derived organoids of bladder cancer recapitulate antigen expression profiles and serve as a personal evaluation model for CAR-T cells in vitro. Clin. Transl. Immunol. 2021, 10, e1248. [Google Scholar] [CrossRef]
- Minoli, M.; Cantore, T.; Hanhart, D.; Kiener, M.; Fedrizzi, T.; La Manna, F.; Karkampouna, S.; Chouvardas, P.; Genitsch, V.; Rodriguez-Calero, A.; et al. Bladder cancer organoids as a functional system to model different disease stages and therapy response. Nat. Commun. 2023, 14, 2214. [Google Scholar] [CrossRef]
- Medle, B.; Sjödahl, G.; Eriksson, P.; Liedberg, F.; Höglund, M.; Bernardo, C. Patient-Derived Bladder Cancer Organoid Models in Tumor Biology and Drug Testing: A Systematic Review. Cancers 2022, 14, 2062. [Google Scholar] [CrossRef]
- Smith, B.A.; Kennedy, W.J.; Harnden, P.; Selby, P.J.; Trejdosiewicz, L.K.; Southgate, J. Identification of genes involved in human urothelial cell-matrix interactions: Implications for the progression pathways of malignant urothelium. Cancer Res. 2001, 61, 1678–1685. [Google Scholar]
- Smith, Y.C.; Grande, K.K.; Rasmussen, S.B.; O’Brien, A.D. Novel three-dimensional organoid model for evaluation of the interaction of uropathogenic Escherichia coli with terminally differentiated human urothelial cells. Infect. Immun. 2006, 74, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Ringuette Goulet, C.; Bernard, G.; Chabaud, S.; Couture, A.; Langlois, A.; Neveu, B.; Pouliot, F.; Bolduc, S. Tissue-engineered human 3D model of bladder cancer for invasion study and drug discovery. Biomaterials 2017, 145, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.; Zimmermann, M.; Ma, A.-H.; Zhang, H.; Swiech, K.; Pan, C.-X. A Simple Three-Dimensional In Vitro Culture Mimicking the In Vivo-Like Cell Behavior of Bladder Patient-Derived Xenograft Models. Cancers 2020, 12, 1304. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Wend, P.; Wend, K.; Krum, S.A.; Miranda-Carboni, G.A. The role of WNT10B in physiology and disease. Acta Physiol. 2012, 204, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Ingram, M.; Techy, G.B.; Ward, B.R.; Imam, S.A.; Atkinson, R.; Ho, H.; Taylor, C.R. Tissue engineered tumor models. Biotech. Histochem. 2010, 85, 213–229. [Google Scholar] [CrossRef]
- Kim, E.; Choi, S.; Kang, B.; Kong, J.; Kim, Y.; Yoon, W.H.; Lee, H.-R.; Kim, S.; Kim, H.-M.; Lee, H.; et al. Creation of bladder assembloids mimicking tissue regeneration and cancer. Nature 2020, 588, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Achtstätter, T.; Becht, E.; Balcarova-Ständer, J.; Ittensohn, M.; Franke, W.W. Cytokeratins in normal and malignant transitional epithelium. Maintenance of expression of urothelial differentiation features in transitional cell carcinomas and bladder carcinoma cell culture lines. Am. J. Pathol. 1988, 132, 123–144. [Google Scholar] [PubMed]
- Booth, C.; Harnden, P.; Trejdosiewicz, L.K.; Scriven, S.; Selby, P.J.; Southgate, J. Stromal and vascular invasion in an human in vitro bladder cancer model. Lab. Investig. 1997, 76, 843–857. [Google Scholar] [PubMed]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Amend, B.; Todenhöfer, T.; Lipke, N.; Aicher, W.K.; Fend, F.; Stenzl, A.; Harland, N. Urinary Tract Tumor Organoids Reveal Eminent Differences in Drug Sensitivities When Compared to 2-Dimensional Culture Systems. Int. J. Mol. Sci. 2022, 23, 6305. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Weng, W.; Xia, P.; Yan, S.; Zhong, C.; Xie, L.; Xie, Y.; Fan, G. Wnt signalling pathway in bladder cancer. Cell. Signal. 2021, 79, 109886. [Google Scholar] [CrossRef]
- Schmid, S.C.; Sathe, A.; Guerth, F.; Seitz, A.-K.; Heck, M.M.; Maurer, T.; Schwarzenböck, S.M.; Krause, B.J.; Schulz, W.A.; Stoehr, R.; et al. Wntless promotes bladder cancer growth and acts synergistically as a molecular target in combination with cisplatin. Urol. Oncol. 2017, 35, 544.e1–544.e10. [Google Scholar] [CrossRef]
- Yoshida, T.; Sopko, N.A.; Kates, M.; Liu, X.; Joice, G.; McConkey, D.J.; Bivalacqua, T.J. Three-dimensional organoid culture reveals involvement of Wnt/β-catenin pathway in proliferation of bladder cancer cells. Oncotarget 2018, 9, 11060–11070. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, J.; Rabien, A.; Reinhold, A.; Köhler, L.; Berndt-Paetz, M. 3D Tumor Models in Urology. Int. J. Mol. Sci. 2023, 24, 6232. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jang, J.; Cho, D.-W. Recapitulating the Cancer Microenvironment Using Bioprinting Technology for Precision Medicine. Micromachines 2021, 12, 1122. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.; Kang, S.J.; Choi, Y.W.; Choi, S.Y.; Park, J.Y.; Chang, I.H. Establishment of Three-Dimensional Bioprinted Bladder Cancer-on-a-Chip with a Microfluidic System Using Bacillus Calmette-Guérin. Int. J. Mol. Sci. 2021, 22, 8887. [Google Scholar] [CrossRef] [PubMed]




| Cell Line | Acc.-No. | Origin (Gender, Age, Stage) | Invasiveness | Grade | Subtype |
|---|---|---|---|---|---|
| RT-4 | ACC-412 | male, 63 y, T2 | NMIBC | 1 | luminal |
| RT-112 | ACC-418 | female, n.n., n.n. | NMIBC | 2 | luminal |
| T-24 | ACC-376 | female, 81 y, n.n. | MIBC | 3 | mixed |
| CAL-29 | ACC-515 | female, 80 y, T2 | MIBC | 4 | luminal |
| Antigen | Specificity | Host | Source | Cat.-No. | Dilution | |
|---|---|---|---|---|---|---|
| Cytokeratin Pan | panCK | Epithelial cells | Ms | Sigma-Aldrich, Munich, Germany | C2931 | 1:400 |
| Vimentin | VIM | VIM-positive cells, e.g., fibroblasts | Ms | Sigma-Aldrich, Munich, Germany | V6389 | 1:400 |
| Alpha Smooth muscle cell actin | αSMCA | αSMCA-positive cells, e.g., detrusor myocytes | Ms | Sigma-Aldrich, Munich, Germany | A2547 | 1:1000 |
| Cytokeratin 7 | CK7 | Poorly differentiated epithelial cells | Ms | Abcam, Cambridge, UK | ab9021 | 1:400 |
| Cytokeratin 13 | CK13 | Moderately differentiated epithelial cells | Ms | Abcam, Cambridge, UK | ab101001 | 1:50 |
| Cytokeratin 20 | CK20 | Well-differentiated epithelial cells | Rb | Abcam, Cambridge, UK | Ab53120 | 1:100 |
| Uroplakin-II | UPL-II | Terminally differentiated urothelial cells | Rb | Novus Biologicals, Abingdon, UK | NBP2-33389 | 1:50 |
| Uroplakin-III | UPL-III | Terminally differentiated urothelial cells | Ms | Abcam, Cambridge, UK | ab78196 | 1:30 |
| KI67 | Nuclei of proliferating cells | Ms | Dako, Glostrup, Denmark | M0722 | 1:50 | |
| Claudin 4 | CL4 | Tight junction protein CL4 | Rb | Abcam, Cambridge, UK | ab15104 | 1:400 |
| Zonula occludens-1 | ZO-1 | Tight junction protein ZO-1 | Ms | BD Biosciences, Franklin Lakes, NJ, USA | 610966 | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berndt-Paetz, M.; Han, S.; Weimann, A.; Reinhold, A.; Nürnberger, S.; Neuhaus, J. Cell Line-Based Human Bladder Organoids with Bladder-like Self-Organization—A New Standardized Approach in Bladder Cancer Research. Biomedicines 2023, 11, 2958. https://doi.org/10.3390/biomedicines11112958
Berndt-Paetz M, Han S, Weimann A, Reinhold A, Nürnberger S, Neuhaus J. Cell Line-Based Human Bladder Organoids with Bladder-like Self-Organization—A New Standardized Approach in Bladder Cancer Research. Biomedicines. 2023; 11(11):2958. https://doi.org/10.3390/biomedicines11112958
Chicago/Turabian StyleBerndt-Paetz, Mandy, Shanfu Han, Annett Weimann, Annabell Reinhold, Sandra Nürnberger, and Jochen Neuhaus. 2023. "Cell Line-Based Human Bladder Organoids with Bladder-like Self-Organization—A New Standardized Approach in Bladder Cancer Research" Biomedicines 11, no. 11: 2958. https://doi.org/10.3390/biomedicines11112958
APA StyleBerndt-Paetz, M., Han, S., Weimann, A., Reinhold, A., Nürnberger, S., & Neuhaus, J. (2023). Cell Line-Based Human Bladder Organoids with Bladder-like Self-Organization—A New Standardized Approach in Bladder Cancer Research. Biomedicines, 11(11), 2958. https://doi.org/10.3390/biomedicines11112958







