Oxidative Stress in Health and Disease
Abstract
:1. Introduction
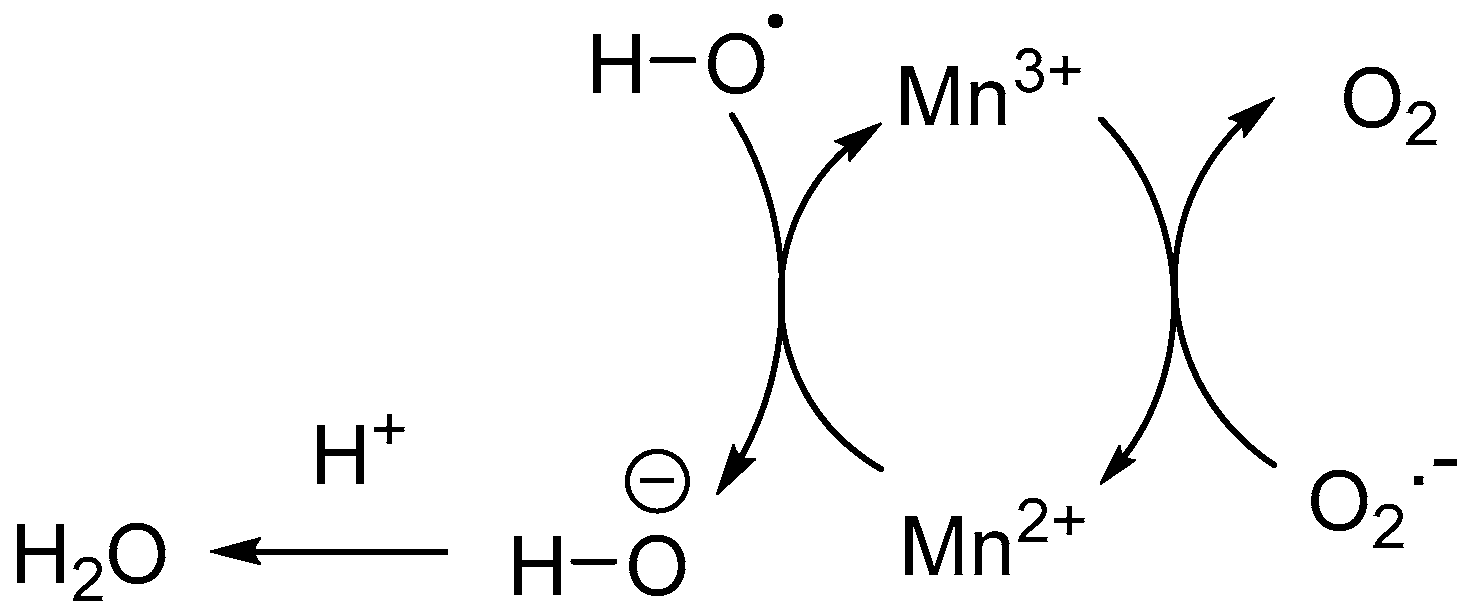
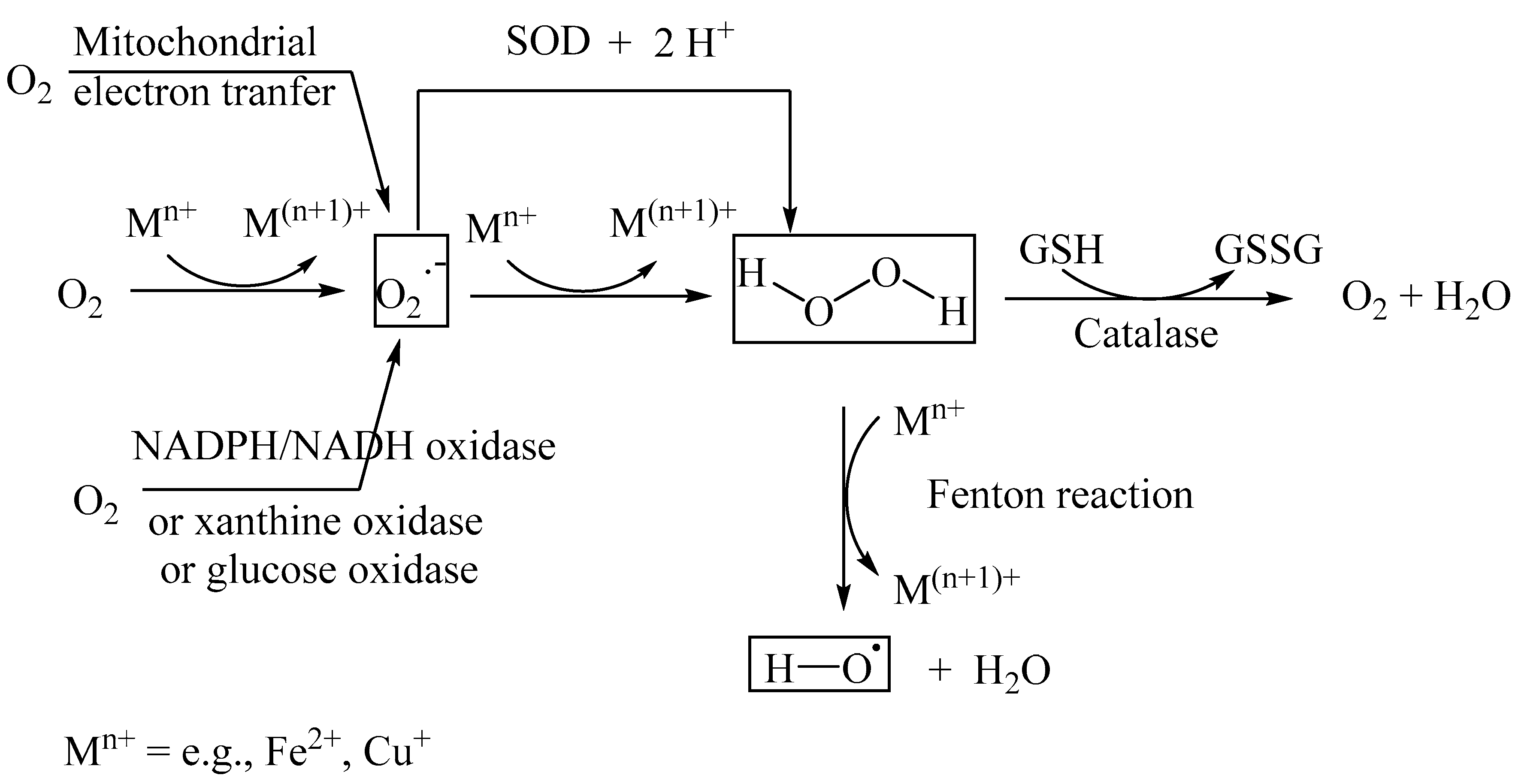
2. Reactive Oxygen Species (ROS)
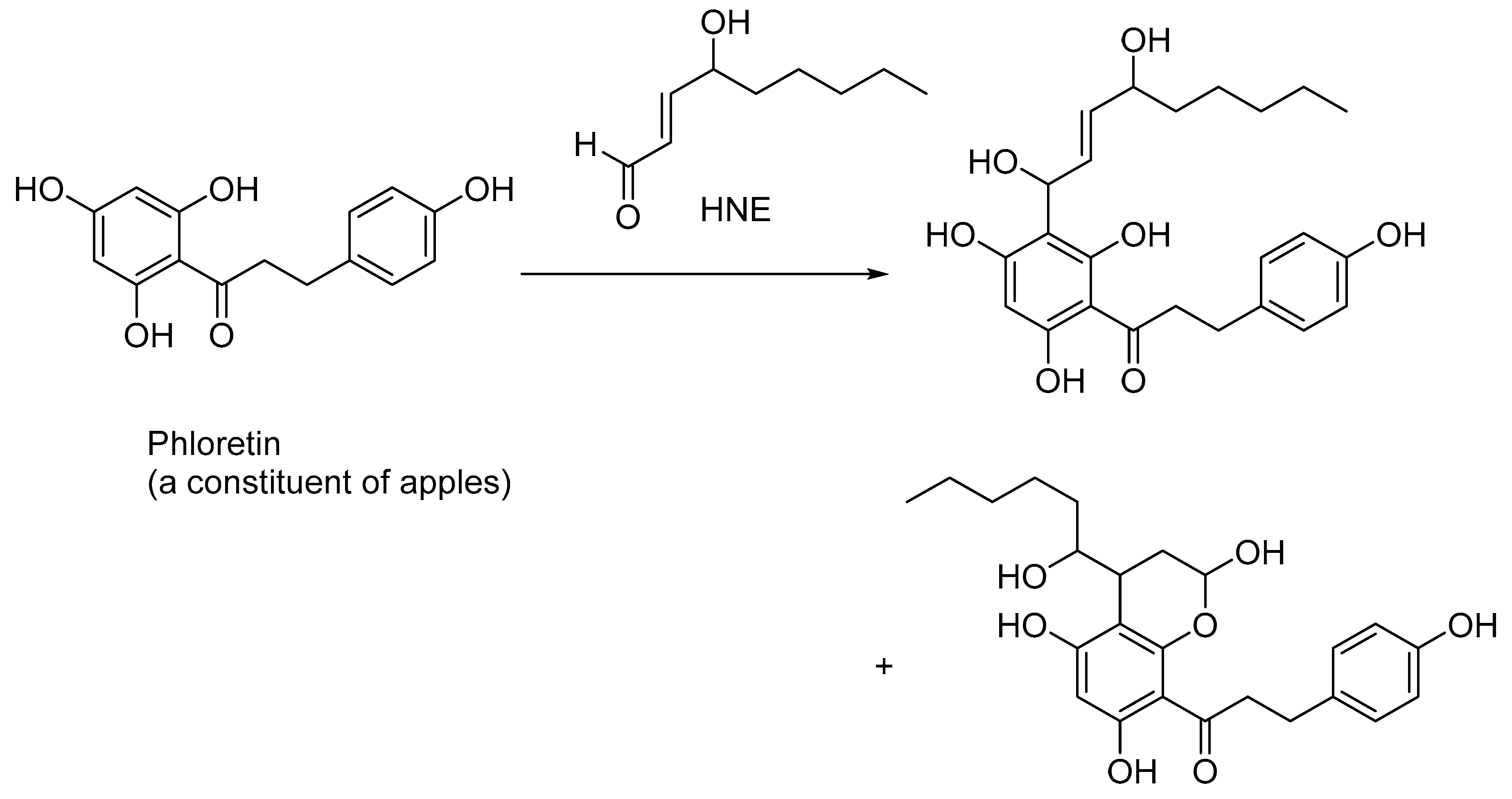
3. Lipid Peroxidation
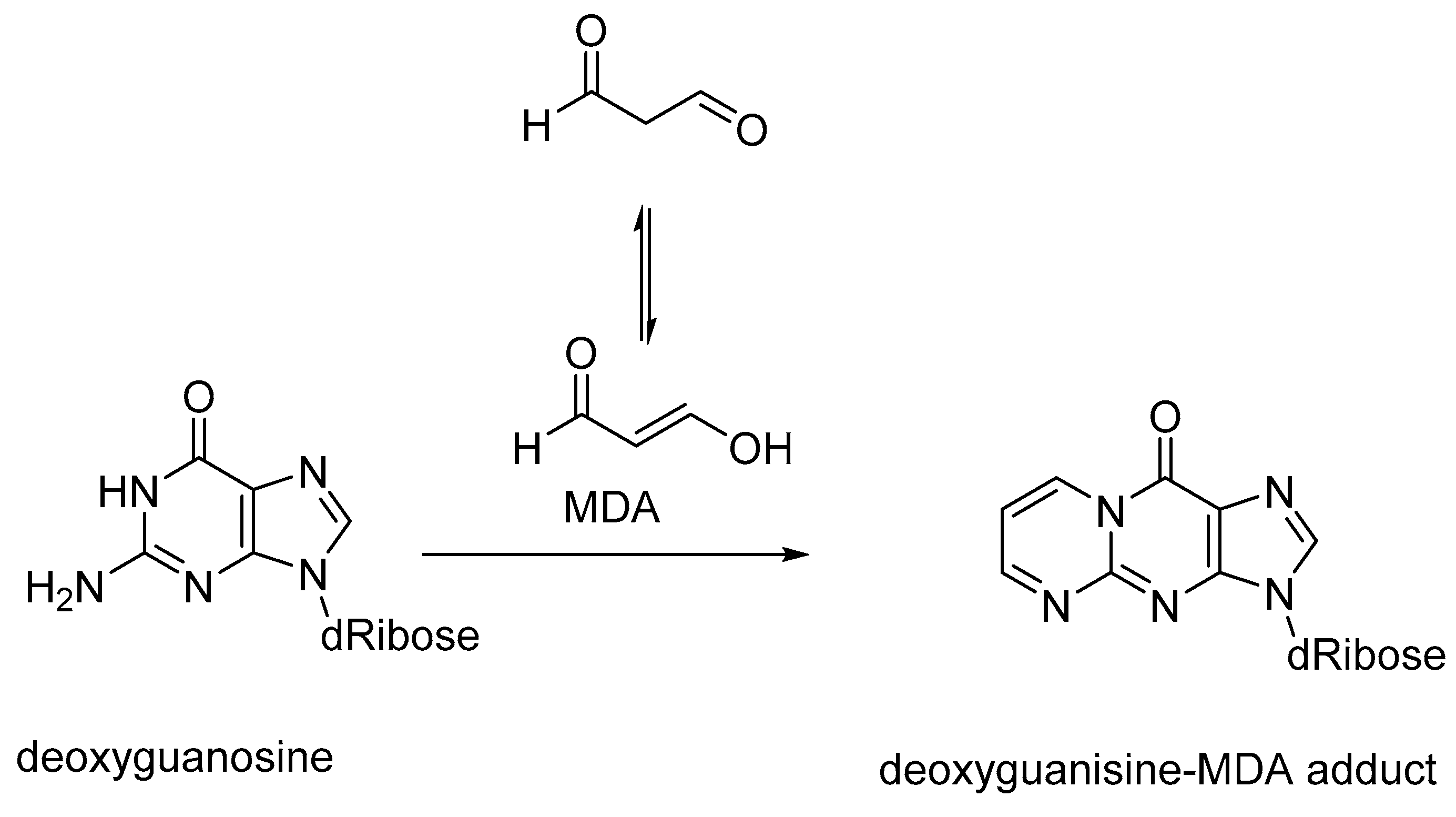
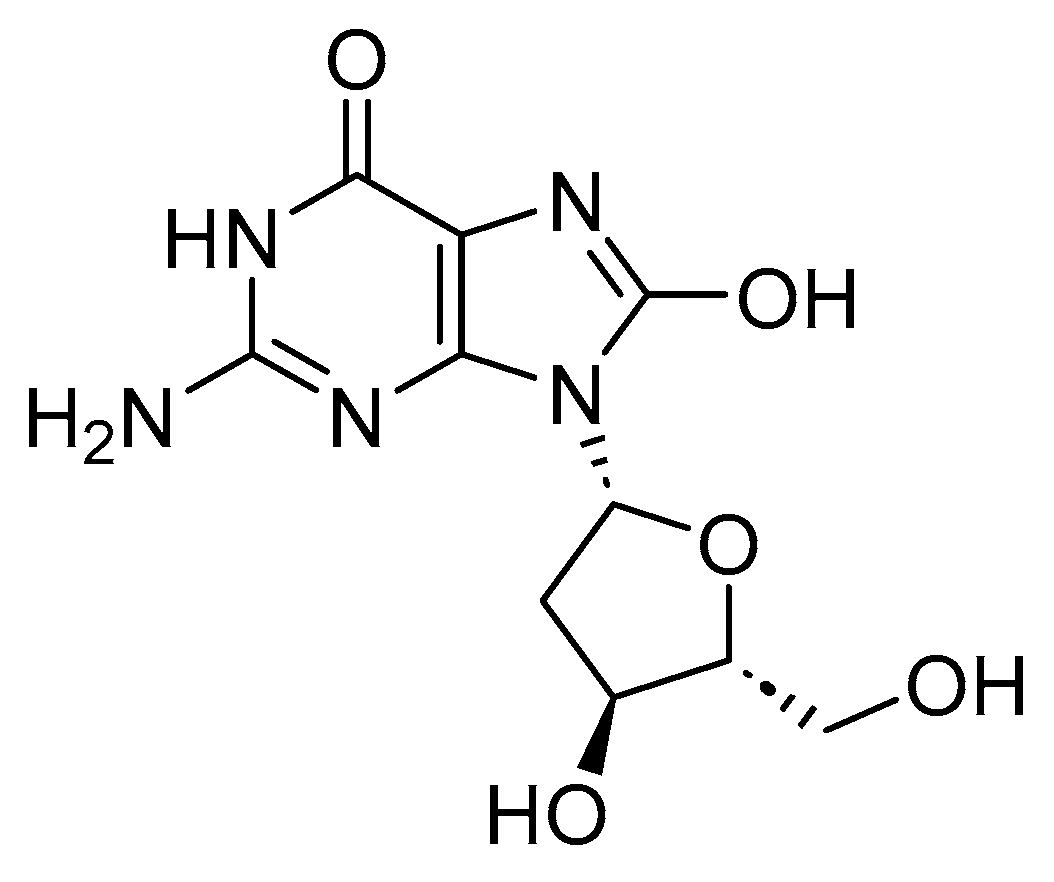
4. ROS and DNA Damage
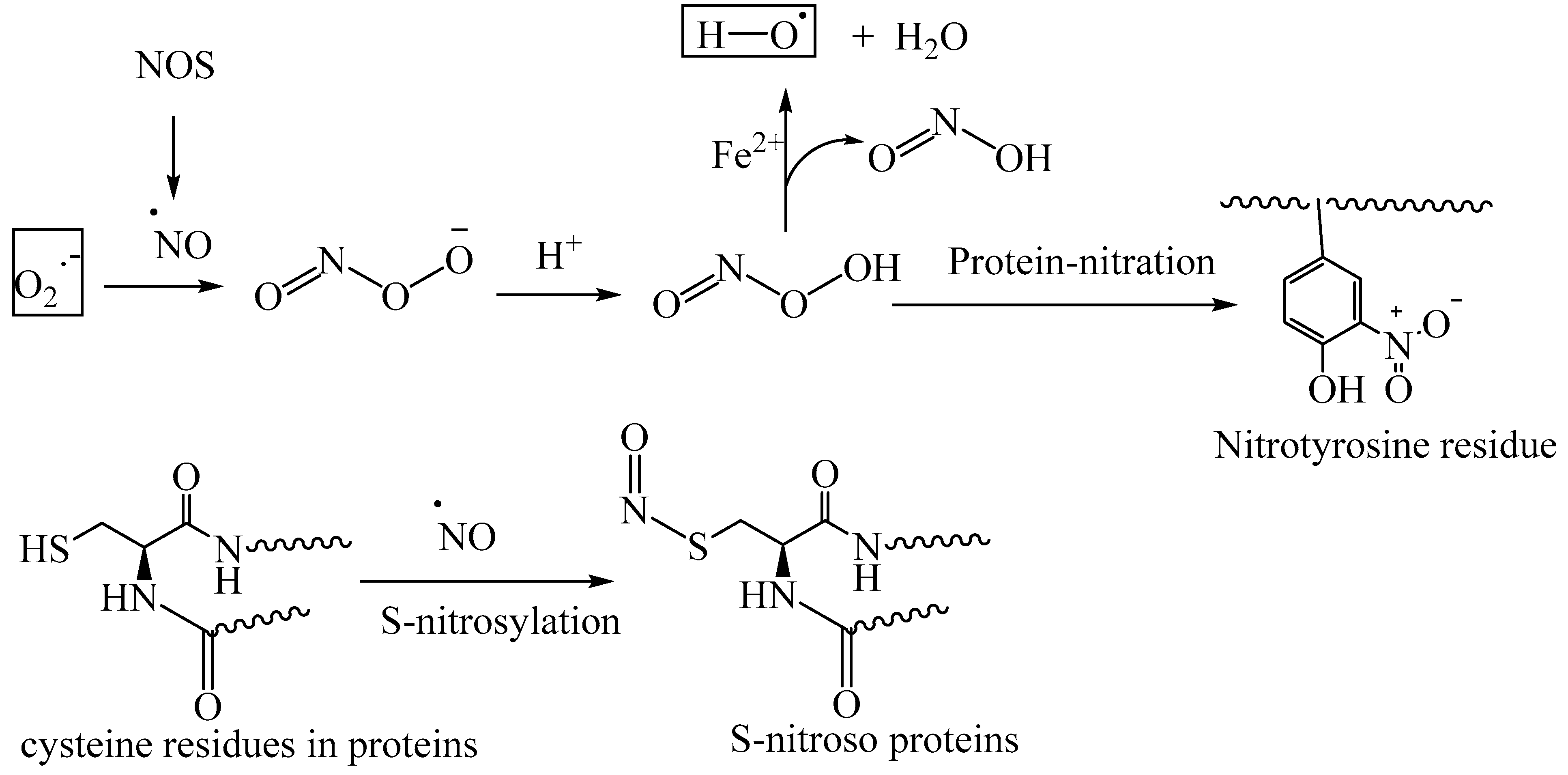
5. Reactive Nitrogen Species (RNS)
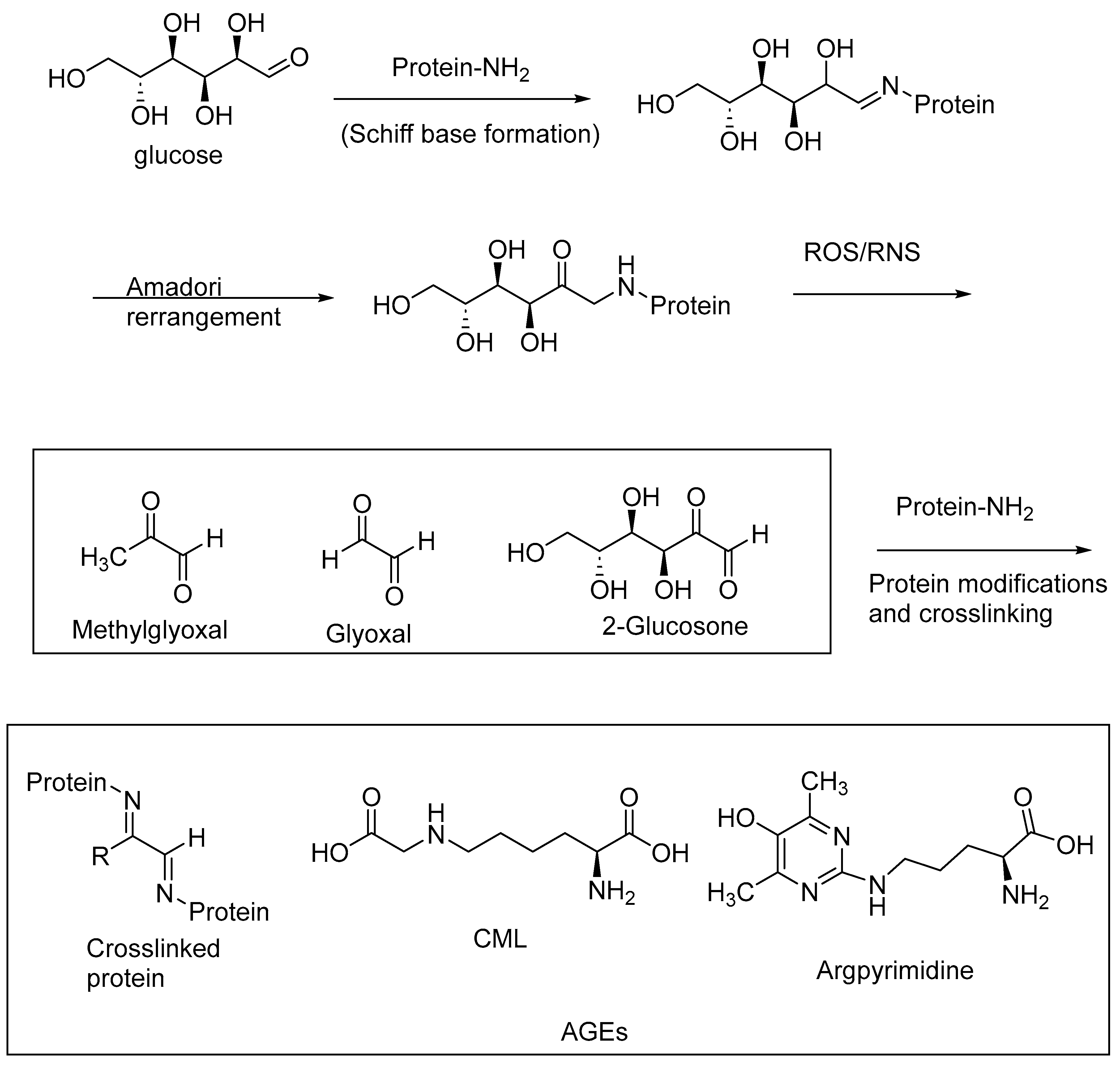
6. Oxidative Stress and Advanced Glycation End Products (AGEs)
7. AGE Inhibitors as Therapeutic Targets
8. AGE–RAGE Interactions and Oxidative Stress
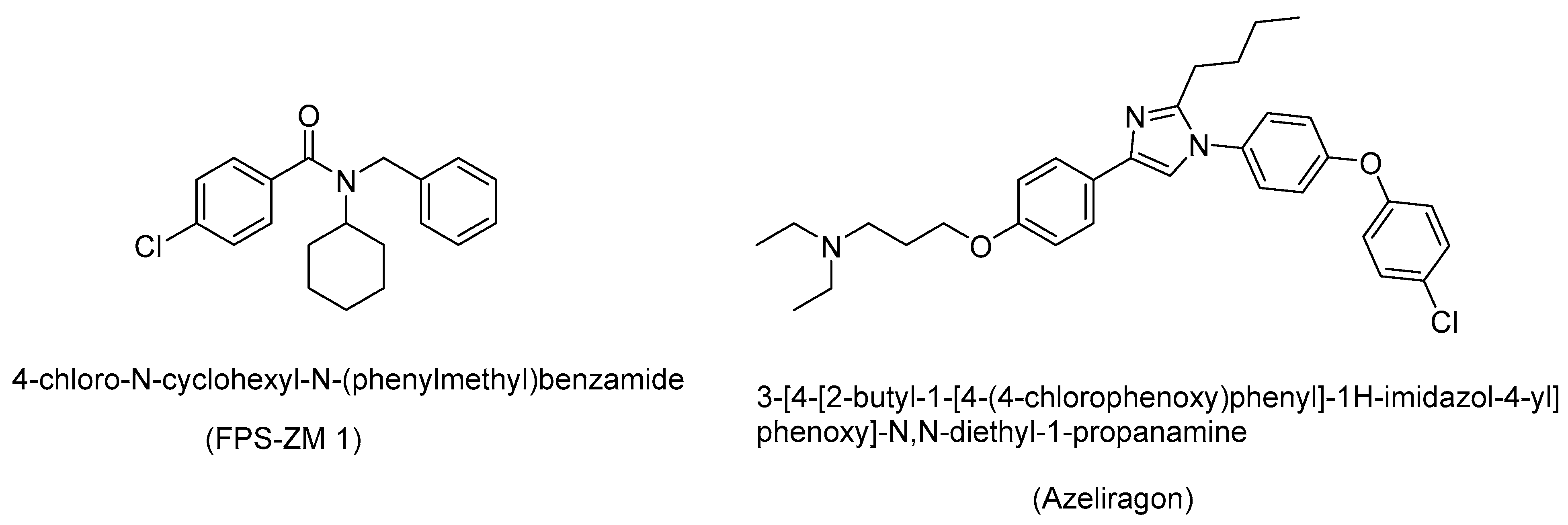
9. RAGE Inhibitors as Therapeutic Candidates
10. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Reddy, V.P.; Zhu, X.; Perry, G.; Smith, M.A. Oxidative Stress in Diabetes and Alzheimer’s Disease. J. Alzheimer’s Dis. 2009, 16, 763–774. [Google Scholar] [CrossRef]
- Reddy, V.P.; Beyaz, A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov. Today 2006, 11, 646–654. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, Y.; Weng, Z.; Xu, H.; Zhang, W.; Li, Q.; Liu, H.; Liu, L.; Wang, Y.; Liu, X.; et al. Microenvironment-Responsive Metal-Phenolic Nanozyme Release Platform with Antibacterial, ROS Scavenging, and Osteogenesis for Periodontitis. ACS Nano 2023, 17, 18732–18746. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, N.; Kumar, V.; Sharma, D.; Devi, B.; Kapil, L.; Singh, C.; Singh, A. The Role of Mitophagy in Various Neurological Diseases as a Therapeutic Approach. Cell. Mol. Neurobiol. 2023, 43, 1849–1865. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; You, L.; Zhang, J.; Chang, Y.-Z.; Yu, P. Brain Iron Metabolism, Redox Balance and Neurological Diseases. Antioxidants 2023, 12, 1289. [Google Scholar] [CrossRef]
- Waseem, R.; Shamsi, A.; Kazim, S.N.; Islam, A. An Insight Into Mitochondrial Dysfunction and its Implications in Neurological Diseases. Curr. Drug Targets 2021, 22, 1585–1595. [Google Scholar] [CrossRef]
- Mates, J.M.; Perez-Gomez, C.; De Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Zheng, L.; Wu, J.; Duan, M.; Liu, Q.; Liu, P.; Shen, C.; Liu, J.; Ye, Q.; Wen, J.; et al. The thioesterase APT1 is a bidirectional-adjustment redox sensor. Nat. Commun. 2023, 14, 2807. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2006, 39, 44–84. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Sang, W.; Wang, J.; Yan, Q. Peroxynitrite (ONOO−) Redox Signaling Molecule-Responsive Polymersomes. ACS Macro Lett. 2016, 5, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Fialkow, L.; Wang, Y.; Downey, G.P. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free. Radic. Biol. Med. 2007, 42, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Aryal, P.; Soni, P. RAGE Inhibitors in Neurodegenerative Diseases. Biomedicines 2023, 11, 1131. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Aryal, P.; Darkwah, E.K. Advanced Glycation End Products in Health and Disease. Microorganisms 2022, 10, 1848. [Google Scholar] [CrossRef]
- Vitek, M.P.; Bhattacharya, K.; Glendening, J.M.; Stopa, E.; Vlassara, H.; Bucala, R.; Manogue, K.; Cerami, A. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1994, 91, 4766. [Google Scholar] [CrossRef]
- Deane, R.; Singh, I.; Sagare, A.P.; Bell, R.D.; Ross, N.T.; LaRue, B.; Love, R.; Perry, S.; Paquette, N.; Deane, R.J.; et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Investig. 2012, 122, 1377–1392. [Google Scholar] [CrossRef]
- Shoeb, M.; Ansari, N.H.; Srivastava, S.K.; Ramana, K.V. 4-Hydroxynonenal in the Pathogenesis and Progression of Human Diseases. Curr. Med. Chem. 2014, 21, 230–237. [Google Scholar] [CrossRef]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; De La Monte, S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—Is this type 3 diabetes? J. Alzheimer’s Dis. 2005, 7, 63–80. [Google Scholar] [CrossRef]
- Ott, A.; Stolk, R.P.; van Harskamp, F.; Pols, H.A.; Hofman, A.; Breteler, M.M. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999, 53, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Kroner, Z. The relationship between Alzheimer’s disease and diabetes: Type 3 diabetes? Altern. Med. Rev. 2009, 14, 373–379. [Google Scholar]
- Stanciu, G.D.; Bild, V.; Ababei, D.C.; Rusu, R.N.; Cobzaru, A.; Paduraru, L.; Bulea, D. Link between diabetes and alzheimer’s disease due to the shared amyloid aggregation and deposition involving both neurodegenerative changes and neurovascular damages. J. Clin. Med. 2020, 9, 1713. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Calfío, C.; Churruca, M.; Maccioni, R.B. Glucose metabolism and AD: Evidence for a potential diabetes type 3. Alzheimers Res. Ther. 2022, 14, 56. [Google Scholar] [CrossRef]
- Faiq, M.A.; Dada, R.; Saluja, D.; Dada, T. Glaucoma—Diabetes of the brain: A radical hypothesis about its nature and pathogenesis. Med. Hypotheses 2014, 82, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s disease a type 3 diabetes? A critical appraisal. Biochim. Biophys. Acta, Mol. Basis Dis. 2017, 1863, 1078–1089. [Google Scholar] [CrossRef]
- Ruze, R.; Song, J.; Yin, X.; Chen, Y.; Xu, R.; Wang, C.; Zhao, Y. Mechanisms of obesity- and diabetes mellitus-related pancreatic carcinogenesis: A comprehensive and systematic review. Signal Transduct. Target. Ther. 2023, 8, 139. [Google Scholar] [CrossRef]
- Wei, X.; Yin, H. Covalent modification of DNA by α, β-unsaturated aldehydes derived from lipid peroxidation: Recent progress and challenges. Free. Radic. Res. 2015, 49, 905–917. [Google Scholar] [CrossRef]
- Priester, A.; Waters, R.; Abbott, A.; Hilmas, K.; Woelk, K.; Miller, H.A.; Tarudji, A.W.; Gee, C.C.; McDonald, B.; Kievit, F.M.; et al. Theranostic Copolymers Neutralize Reactive Oxygen Species and Lipid Peroxidation Products for the Combined Treatment of Traumatic Brain Injury. Biomacromolecules 2022, 23, 1703–1712. [Google Scholar] [CrossRef]
- Magna, M.; Hwang, G.H.; McIntosh, A.; Drews-Elger, K.; Takabatake, M.; Ikeda, A.; Mera, B.J.; Kwak, T.; Miller, P.; Lippman, M.E.; et al. RAGE inhibitor TTP488 (Azeliragon) suppresses metastasis in triple-negative breast cancer. Npj Breast Cancer 2023, 9, 59. [Google Scholar] [CrossRef]
- Zhang, F.; Kent, K.C.; Yamanouchi, D.; Zhang, Y.; Kato, K.; Tsai, S.; Nowygrod, R.; Schmidt, A.M.; Liu, B. Anti-receptor for advanced glycation end products therapies as novel treatment for abdominal aortic aneurysm. Ann. Surg. 2009, 250, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Tanuma, S.-I.; Yoshimori, A.; Inoue, S.; Abe, T. Inhibitors of Receptor for Advanced Glycation End Products (RAGE). WO2016158810, 2016. [Google Scholar]
- Banner, W.K.; Gaddam, B.; Polisetti, D.R.; Andrews, R.C.; Victory, S. Preparation of Metabolites of RAGE Antagonist Azeliragon. WO2020076668, 2020. [Google Scholar]
- Wang, X.; Chen, Q.; Zhu, Y.; Wang, K.; Chang, Y.; Wu, X.; Bao, W.; Cao, T.; Chen, H.; Zhang, Y.; et al. Destroying pathogen-tumor symbionts synergizing with catalytic therapy of colorectal cancer by biomimetic protein-supported single-atom nanozyme. Signal Transduct. Target. Ther. 2023, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Mandakhbayar, N.; Ji, Y.; El-Fiqi, A.; Patel, K.D.; Yoon, D.S.; Dashnyam, K.; Bayaraa, O.; Jin, G.; Tsogtbaatar, K.; Kim, T.-H.; et al. Double hits with bioactive nanozyme based on cobalt-doped nanoglass for acute and diabetic wound therapies through anti-inflammatory and pro-angiogenic functions. Bioact. Mater. 2023, 31, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Wang, M.; Li, P. The applications of nanozymes in neurological diseases: From mechanism to design. Theranostics 2023, 13, 2492–2514. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Sun, S.; Wang, J.; Zhang, X.-D. Catalytic nanozymes for central nervous system disease. Coord. Chem. Rev. 2021, 432, 213751. [Google Scholar] [CrossRef]
- Liu, F.; Huang, B.; Tang, T.; Wang, F.; Cui, R.; Zhang, M.; Sun, T. Near-infrared-IIb fluorescent nanozymes for imaging-guided treatment of traumatic brain injury. Chem. Eng. J. 2023, 471, 144697. [Google Scholar] [CrossRef]
- Huang, B.; Tang, T.; Chen, S.-H.; Li, H.; Sun, Z.-J.; Zhang, Z.-L.; Zhang, M.; Cui, R. Near-infrared-IIb emitting single-atom catalyst for imaging-guided therapy of blood-brain barrier breakdown after traumatic brain injury. Nat. Commun. 2023, 14, 197. [Google Scholar] [CrossRef]
- Ceriello, A. Oxidative stress and diabetes-associated complications. Endocr. Pract. 2006, 12 (Suppl. 1), 60–62. [Google Scholar] [CrossRef]
- Teixeira, R.B.; Pfeiffer, M.; Zhang, P.; Shafique, E.; Rayta, B.; Karbasiafshar, C.; Ahsan, N.; Sellke, F.W.; Abid, M.R. Reduction in mitochondrial ROS improves oxidative phosphorylation and provides resilience to coronary endothelium in non-reperfused myocardial infarction. Basic Res. Cardiol. 2023, 118, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Wang, J.; He, L.; Lai, H.; Zhang, T.; Wang, X.; Li, W. Mitochondrial oxidative stress in brain microvascular endothelial cells: Triggering blood-brain barrier disruption. Mitochondrion 2023, 69, 71–82. [Google Scholar] [CrossRef]
- Li, B.; Bai, Y.; Yion, C.; Wang, H.; Su, X.; Feng, G.; Guo, M.; Peng, W.; Shen, B.; Zheng, B. Single-Atom Nanocatalytic Therapy for Suppression of Neuroinflammation by Inducing Autophagy of Abnormal Mitochondria. ACS Nano 2023, 17, 7511–7529. [Google Scholar] [CrossRef]
- Xia, X.; Li, H.; Xu, X.; Zhao, G.; Du, M. Facilitating Pro-survival Mitophagy for Alleviating Parkinson′s Disease via Sequence-Targeted Lycopene Nanodots. ACS Nano 2023, 17, 17979–17995. [Google Scholar] [CrossRef] [PubMed]
- Mohazzab-H, K.M.; Kaminski, P.M.; Wolin, M.S. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am. J. Physiol. 1994, 266, H2568. [Google Scholar]
- Zheng, X.; Li, X.; Meng, S.; Shi, G.; Li, H.; Du, H.; Dai, L.; Yang, H. Cascade amplification of tumor chemodynamic therapy and starvation with re-educated TAMs via Fe-MOF based functional nanosystem. J. Nanobiotechnol. 2023, 21, 127. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-H.; Chen, C.; Fang, S.-Y.; Lin, S.-C.; Chen, J.-W.; Chang, T.-T. Xanthine oxidase/NADPH oxidase inhibition by hydralazine attenuates acute kidney injury and prevents the transition of acute kidney injury to chronic kidney disease. Life Sci. 2023, 327, 121863. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Han, J.S. Scopoletin protects INS-1 pancreatic β cells from glucotoxicity by reducing oxidative stress and apoptosis. Toxicol. Vitr. 2023, 93, 105665. [Google Scholar] [CrossRef]
- Vijayraghavan, S.; Saini, N. Aldehyde-Associated Mutagenesis-Current State of Knowledge. Chem. Res. Toxicol. 2023, 36, 983–1001. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Reiter, R.J.; Asemi, Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Dham, D.; Roy, B.; Gowda, A.; Pan, G.; Sridhar, A.; Zeng, X.; Thandavarayan, R.A.; Palaniyandi, S.S. 4-Hydroxy-2-nonenal, a lipid peroxidation product, as a biomarker in diabetes and its complications: Challenges and opportunities. Free. Radic. Res. 2021, 55, 547–561. [Google Scholar] [CrossRef]
- Renuka Sanotra, M.; Huang, W.-C.; Silver, S.; Lin, C.-Y.; Chang, T.-C.; Nguyen, D.P.Q.; Lee, C.-K.; Kao, S.-H.; Chang-Cheng Shieh, J.; Lin, Y.-F. Serum levels of 4-hydroxynonenal adducts and responding autoantibodies correlate with the pathogenesis from hyperglycemia to Alzheimer’s disease. Clin. Biochem. 2022, 101, 26–34. [Google Scholar] [CrossRef]
- Jaganjac, M.; Zarkovic, N. Lipid Peroxidation Linking Diabetes and Cancer: The Importance of 4-Hydroxynonenal. Antioxid. Redox Signal. 2022, 37, 1222–1233. [Google Scholar] [CrossRef]
- Reyes-Jimenez, E.; Ramirez-Hernandez, A.A.; Santos-Alvarez, J.C.; Velazquez-Enriquez, J.M.; Pina-Canseco, S.; Baltierrez-Hoyos, R.; Vasquez-Garzon, V.R. Involvement of 4-hydroxy-2-nonenal in the pathogenesis of pulmonary fibrosis. Mol. Cell. Biochem. 2021, 476, 4405–4419. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, L.; Guo, Z.; Wang, G.; Xu, J.; Zheng, Z.; Sun, K.; Guo, F. Lipid peroxidation in osteoarthritis: Focusing on 4-hydroxynonenal, malondialdehyde, and ferroptosis. Cell Death Discov. 2023, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Morquette, B.; Shi, Q.; Lavigne, P.; Ranger, P.; Fernandes, J.C.; Benderdour, M. Production of lipid peroxidation products in osteoarthritic tissues: New evidence linking 4-hydroxynonenal to cartilage degradation. Arthritis Rheum. 2006, 54, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Djorgbenoo, R.; Wang, W.; Zhu, Y.; Sang, S. Detoxification of the Lipid Peroxidation Aldehyde, 4-Hydroxynonenal, by Apple Phloretin In Vitro and in Mice. J. Agric. Food Chem. 2023, 71, 10629–10637. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, B.; Zeng, Y.; Yang, J.; Li, Z.; Ng, J.P.L.; Xu, X.; Su, L.; Yun, X.; Qu, L.; et al. N-Acetylcysteine overcomes epalrestat-mediated increase of toxic 4-hydroxy-2-nonenal and potentiates the anti-arthritic effect of epalrestat in AIA model. Int. J. Biol. Sci. 2023, 19, 4082–4102. [Google Scholar] [CrossRef] [PubMed]
- Linetsky, M.; Mondal, A.; Liu, S.-Y.; Hite, A.M.; Enduri, S.; Cheng, Y.-S.; Feijo, B.; Kang, G.; Arhin, N.; Zeng, H.; et al. Cysteinyl leukotriene-like metabolites are generated in retinal pigment epithelial cells through glutathionylation/reduction of an oxidatively truncated fragment of arachidonate. Results Chem. 2023, 6, 100995. [Google Scholar] [CrossRef]
- Alary, J.; Fernandez, Y.; Debrauwer, L.; Perdu, E.; Gueraud, F. Identification of Intermediate Pathways of 4-Hydroxynonenal Metabolism in the Rat. Chem. Res. Toxicol. 2003, 16, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, H.; Kozekova, A.; Rizzo, C.J.; Stone, M.P. Formation of a N2-dG:N2-dG Carbinolamine DNA Cross-link by the trans-4-Hydroxynonenal-Derived (6S,8R,11S) 1,N2-dG Adduct. J. Am. Chem. Soc. 2011, 133, 16101–16110. [Google Scholar] [CrossRef]
- Keasler, K.T.; Zick, M.E.; Stacy, E.E.; Kim, J.; Lee, J.-H.; Aeindartehran, L.; Runčevski, T.; Milner, P.J. Handling fluorinated gases as solid reagents using metal-organic frameworks. Science 2023, 381, 1455–1461. [Google Scholar] [CrossRef]
- Nair, J.; Barbin, A.; Velic, I.; Bartsch, H. Etheno DNA-base adducts from endogenous reactive species. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1999, 424, 59–69. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Su, P.-L.; Huang, T.-H.; Lin, C.-C.; Chen, C.-W.; Tsai, J.-S.; Liao, X.-M.; Chan, T.-Y.; Shieh, C.-C. Cigarette smoke increases susceptibility of alveolar macrophages to SARS-CoV-2 infection through inducing reactive oxygen species-upregulated angiotensin-converting enzyme 2 expression. Sci. Rep. 2023, 13, 7894. [Google Scholar] [CrossRef] [PubMed]
- Hirao, Y.; Kobayashi, H.; Mori, Y.; Kato, S.; Kawanishi, S.; Murata, M.; Oikawa, S. Myricetin causes site-specific DNA damage via reactive oxygen species generation by redox interactions with copper ions. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2023, 891, 503694. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Xiao, Y.; Ghaemnezhad, A. Diosgenin promotes cisplatin-induced apoptosis through oxidative DNA damage in A549 non-small cell lung cells. Cell Biol. Int. 2022, 46, 1571–1576. [Google Scholar] [CrossRef]
- Sanchez-Fernandez, R.; Sanchez-Temprano, A.; Esteban-Gomez, D.; Pazos, E. Probing Tyrosine Nitration with a Small TbIII-Metallopeptide. ChemBioChem 2023, 24, e202300072. [Google Scholar] [CrossRef]
- Stomberski, C.T.; Hess, D.T.; Stamler, J.S. Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling. Antioxid. Redox Signal. 2019, 30, 1331–1351. [Google Scholar] [CrossRef]
- Nakamura, T.; Prikhodko, O.A.; Pirie, E.; Nagar, S.; Akhtar, M.W.; Oh, C.-K.; McKercher, S.R.; Ambasudhan, R.; Okamoto, S.-i.; Lipton, S.A. Aberrant protein S-nitrosylation contributes to the pathophysiology of neurodegenerative diseases. Neurobiol. Dis. 2015, 84, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M.T.; Benhar, M.; Stamler, J.S. Nitrosative stress in the ER: A new role for S-nitrosylation in neurodegenerative diseases. ACS Chem. Biol. 2006, 1, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Ischiropoulos, H.; Beckman, J.S. Oxidative stress and nitration in neurodegeneration: Cause, effect or association? J. Clin. Investig. 2003, 111, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, M.; Li, Y.; Shen, J. Reactive nitrogen species as therapeutic targets for autophagy/mitophagy modulation to relieve neurodegeneration in multiple sclerosis: Potential application for drug discovery. Free. Radic. Biol. Med. 2023, 208, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, L.; Giri, S.; Chatterjee, S. Inhibitors of Nitric Oxide Synthase: What’s up and What’s Next? Curr. Enzym. Inhib. 2016, 12, 81–107. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Baynes, J.W.; Thorpe, S.R. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 1999, 48, 1–9. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Chen, Q.; Liu, Y.; Wu, Z.; Pan, D.; Yan, N.; Liu, L. Cereal polyphenols inhibition mechanisms on advanced glycation end products and regulation on type 2 diabetes. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef]
- Shu, M.; Cheng, W.; Jia, X.; Bai, X.; Zhao, Y.; Lu, Y.; Zhu, L.; Zhu, Y.; Wang, L.; Shu, Y.; et al. AGEs promote atherosclerosis by increasing LDL transcytosis across endothelial cells via RAGE/NF-κB/Caveolin-1 pathway. Mol. Med. 2023, 29, 113. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Tikellis, C.; Pickering, R.J.; Dragoljevic, D.; Lee, M.K.S.; Block, T.; Scheijen, J.L.J.M.; Wouters, K.; Miyata, T.; Cooper, M.E.; et al. Pyridoxamine prevents increased atherosclerosis by intermittent methylglyoxal spikes in the aortic arches of ApoE−/− mice. Biomed. Pharmacother. 2023, 158, 114211. [Google Scholar] [CrossRef]
- Kare, P.K.; Sdarth, M.; Ghosh, R.; Banerjee, B.D.; Kalra, O.P.; Tripathi, A.K. Advanced Glycation End Product (AGE)-mediated reactive oxygen species production in cultured endothelial cells is dependent on NADPH oxidase activation. J. Clin. Diagn. Res. 2019, 13, 4–7. [Google Scholar] [CrossRef]
- Jahromi, M.K.; Tehrani, A.N.; Teymoori, F.; Daftari, G.; Ahmadirad, H.; Saber, N.; Salehi-Sahlabadi, A.; Farhadnejad, H.; Mirmiran, P. Dietary advanced glycation end products are associated with an increased risk of non-alcoholic fatty liver disease in Iranian adults. BMC Endocr. Disord. 2023, 23, 111. [Google Scholar] [CrossRef]
- Coppola, S.; Paparo, L.; Trinchese, G.; Rivieri, A.M.; Masino, A.; De Giovanni Di Santa Severina, A.F.; Cerulo, M.; Escolino, M.; Turco, A.; Esposito, C.; et al. Increased dietary intake of ultraprocessed foods and mitochondrial metabolism alterations in pediatric obesity. Sci. Rep. 2023, 13, 12609. [Google Scholar] [CrossRef]
- Wada, K.; Nakashima, Y.; Yamakawa, M.; Hori, A.; Seishima, M.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; Nagata, C. Dietary advanced glycation end products and cancer risk in Japan: From the Takayama study. Cancer Sci. 2022, 113, 2839–2848. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, F.; Liu, D.; Li, X.; Ma, Z.; Zhang, Y.; Ma, A.; Qin, L.-Q.; Chen, G.-C.; Wan, Z. Higher dietary advanced glycation products intake is associated with increased risk of dementia, independent from genetic predisposition. Clin. Nutr. 2023, 42, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zong, M.; Wu, H.; He, D.; Li, L.; Zhang, X.; Zhao, D.; Li, B. Dietary Advanced Glycation End-Products Affects the Progression of Early Diabetes by Intervening in Carbohydrate and Lipid Metabolism. Mol. Nutr. Food Res. 2022, 66, 2200046. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Bank, A.J.; Kass, D.A.; Neutel, J.M.; Preston, R.A.; Oparil, S. Advanced glycation end-product cross-link breakers: A novel approach to cardiovascular pathologies related to the aging process. Am. J. Hypertens. 2004, 17, 23S. [Google Scholar] [CrossRef]
- Little, W.C.; Zile, M.R.; Kitzman, D.W.; Hundley, W.G.; O’Brien, T.X.; deGroof, R.C. The Effect of Alagebrium Chloride (ALT-711), a Novel Glucose Cross-Link Breaker, in the Treatment of Elderly Patients With Diastolic Heart Failure. J. Card. Failure 2005, 11, 191–195. [Google Scholar] [CrossRef]
- Engelen, L.; Stehouwer, C.D.A.; Schalkwijk, C.G. Current therapeutic interventions in the glycation pathway: Evidence from clinical studies. Diabetes Obes. Metab. 2013, 15, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.I.; Wuerth, J.P.; Cartwright, K.; Bain, R.P.; Dippe, S.; Hershon, K.; Mooradian, A.D.; Spinowitz, B.S. Design and baseline characteristics for the aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II). Control. Clin. Trials 1999, 20, 493–510. [Google Scholar] [CrossRef]
- Yan, S.D.; Schmidt, A.M.; Anderson, G.M.; Zhang, J.; Brett, J.; Zou, Y.S.; Pinsky, D.; Stern, D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J. Biol. Chem. 1994, 269, 9889. [Google Scholar] [CrossRef]
- Naz, S.; Mahmood, T.; Gupta, R.; Siddiqui, M.H.; Ahsan, F.; Ansari, V.A.; Shamim, A.; Rizvi, A.A. Clinical Manifestation of AGE-RAGE Axis in Neurodegenerative and Cognitive Impairment Disorders. Drug Res. 2023, 73, 309–317. [Google Scholar] [CrossRef]
- Prasad, K. AGE-RAGE stress play a role in aortic aneurysm: A comprehensive review and novel potential therapeutic target. Rev. Cardiovasc. Med. 2019, 20, 201–208. [Google Scholar]
- Farrag, E.A.E.; Hammad, M.O.; Safwat, S.M.; Hamed, S.; Hellal, D. Artemisinin attenuates type 2 diabetic cardiomyopathy in rats through modulation of AGE-RAGE/HMGB-1 signaling pathway. Sci. Rep. 2023, 13, 11043. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Y.; Shen, Y.; Zhang, Y.; Liu, L.; Yang, X. Dietary polyphenols: Regulate the advanced glycation end products-RAGE axis and the microbiota-gut-brain axis to prevent neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2022, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Aryal, P.; Robinson, S.; Rafiu, R.; Obrenovich, M.; Perry, G. Polyphenols in Alzheimer’s disease and in the gut-brain axis. Microorganisms 2020, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Ma, Y.; Zeng, Z.; Yin, Q.; Hong, Y.; Hou, X.; Liu, X. RAGE-Specific Inhibitor FPS-ZM1 Attenuates AGEs-Induced Neuroinflammation and Oxidative Stress in Rat Primary Microglia. Neurochem. Res. 2017, 42, 2902–2911. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.; Lue, L.F.; Paul, G.; Patel, A.; Sabbagh, M.N. Receptor for advanced glycation endproduct modulators: A new therapeutic target in Alzheimer’s disease. Expert Opin. Investig. Drugs 2015, 24, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Ihara, M. New therapeutic approaches for Alzheimer’s disease and cerebral amyloid angiopathy. Front. Aging Neurosci. 2014, 6, 290. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Wang, Y.; Li, J.; Liu, N. Azeliragon ameliorates Alzheimer’s disease via the Janus tyrosine kinase and signal transducer and activator of transcription signaling pathway. Clinics 2021, 76, e2348. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. https://doi.org/10.3390/biomedicines11112925
Reddy VP. Oxidative Stress in Health and Disease. Biomedicines. 2023; 11(11):2925. https://doi.org/10.3390/biomedicines11112925
Chicago/Turabian StyleReddy, V. Prakash. 2023. "Oxidative Stress in Health and Disease" Biomedicines 11, no. 11: 2925. https://doi.org/10.3390/biomedicines11112925
APA StyleReddy, V. P. (2023). Oxidative Stress in Health and Disease. Biomedicines, 11(11), 2925. https://doi.org/10.3390/biomedicines11112925







