SARS-CoV-2 Impact on Red Blood Cell Morphology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Blood Sample Preparation
2.3. Low-Voltage Scanning Electron Microscopy for Erythrocyte Size Evaluation Technique Description
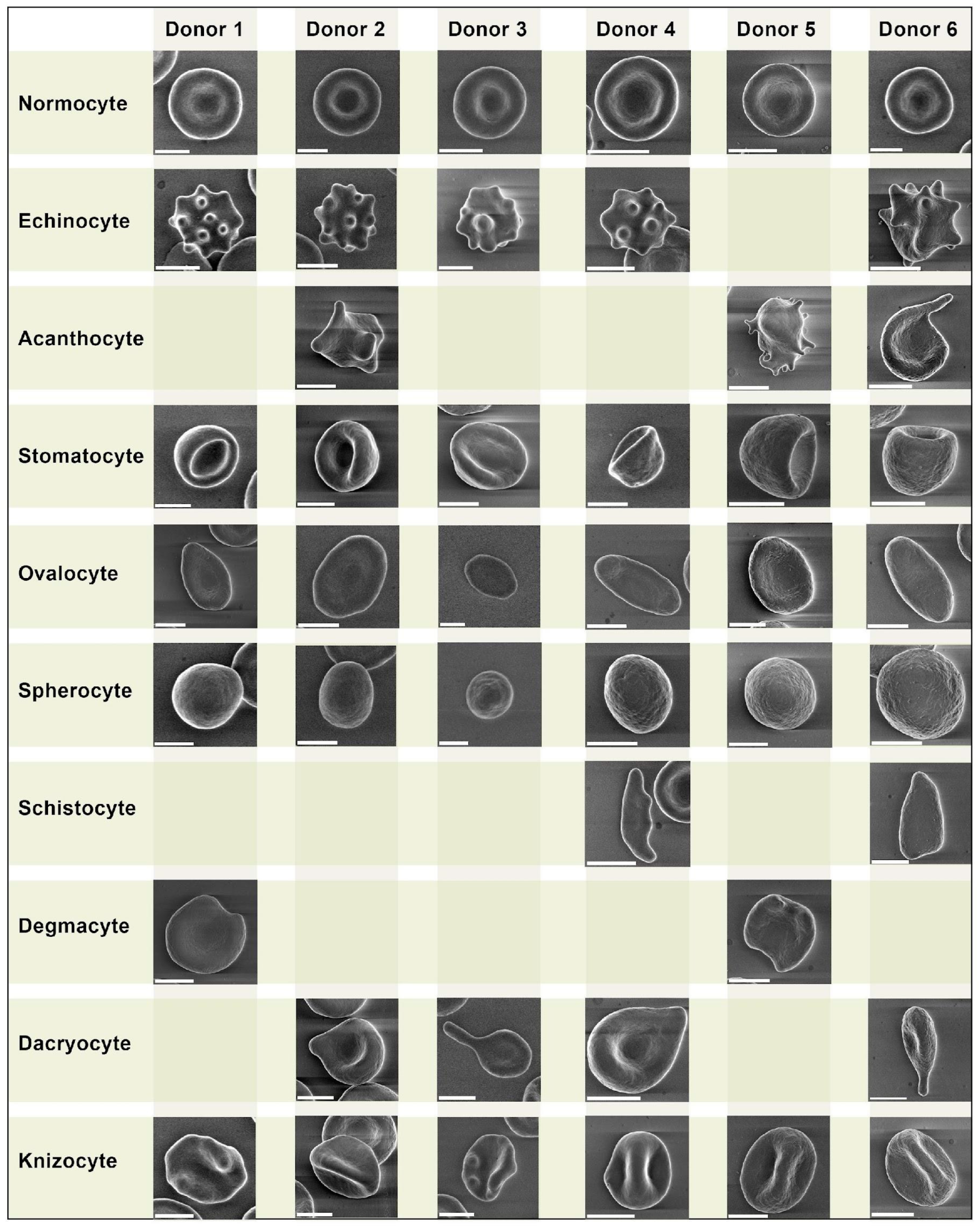
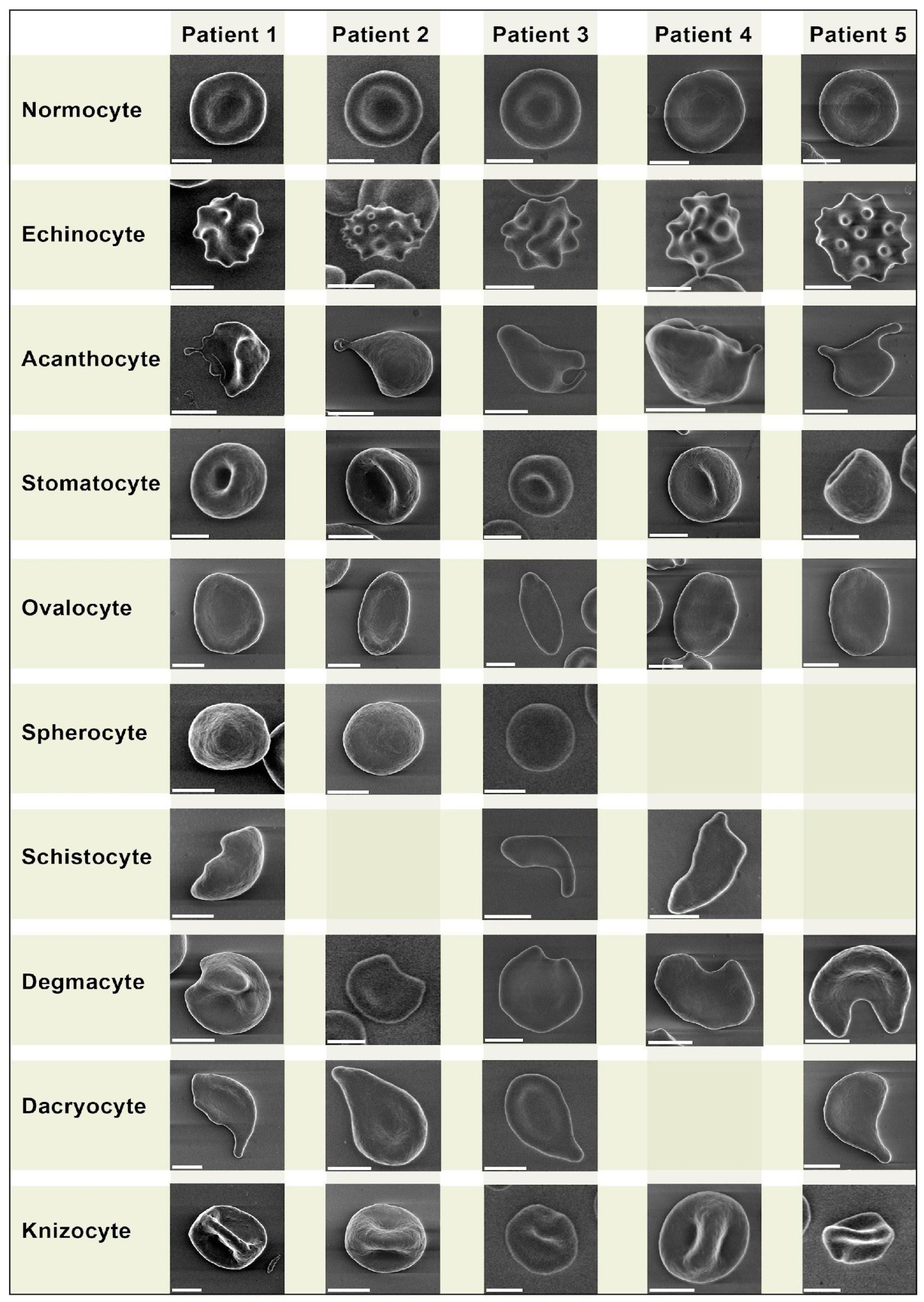
2.4. Erythrocyte Morphology Study
- −
- Echinocytes—presence of three or more evenly spread spike-like protrusions of plasmalemma on membrane surface with length varying from 0.5 to 2 µm with wide base; the angle between the apical part of the spike and the surface of the echinocyte membrane is usually in the range from 100 to 130 degrees. The end sections of the spikes form an acute angle.
- −
- Acanthocytes—presence of irregularly distributed plasmalemma protrusions in the form of spines, including single ones, from 2 µm in length. The end sections of the spines end with a club-shaped extension at the apical end. The size and shape of the spines on a single acanthocyte may vary and have no strict pattern of distribution on the membrane surface.
- −
- Stomatocytes—increased volume compared to normocytes by 20–30% and deep slit-shaped central lumen, which on the opposite side forms a semi-oval convexity with a smooth surface. The size of the central lumen depends on the degree of crenulation and can range from wide funnel-shaped to slit-shaped. Because of the strong roundness of one of the sides, they lie on their sides and are usually easily detected.
- −
- Ovalocytes are oval or elongated erythrocytes from ovoid to bacilliform or pencil shape. The central lumen is flattened; may not be defined. The end sections of the cells are blunt, and the membrane is smooth.
- −
- Spherocytes are erythrocytes that have lost their biconcave shape. Spherocytes are globular in shape and lack a central lumen or depression, which is most clearly visible under light microscopy.
- −
- Schistocytes—erythrocytes are separated into fragments 2 to 3 µm in diameter. The usual round shape is absent; instead, they have a triangular or other angular morphology. Schistocytes are also classified as any degenerately altered irregularly shaped cells not conforming to other known shapes. The central lumen zone is often absent.
- −
- Degmacytes—a bitten cell—the cell looks as if it has been bitten; has a semicircular depression on the outer side of the membrane.
- −
2.5. Statistical Analysis
3. Results
3.1. Erythrocyte Morphology Study
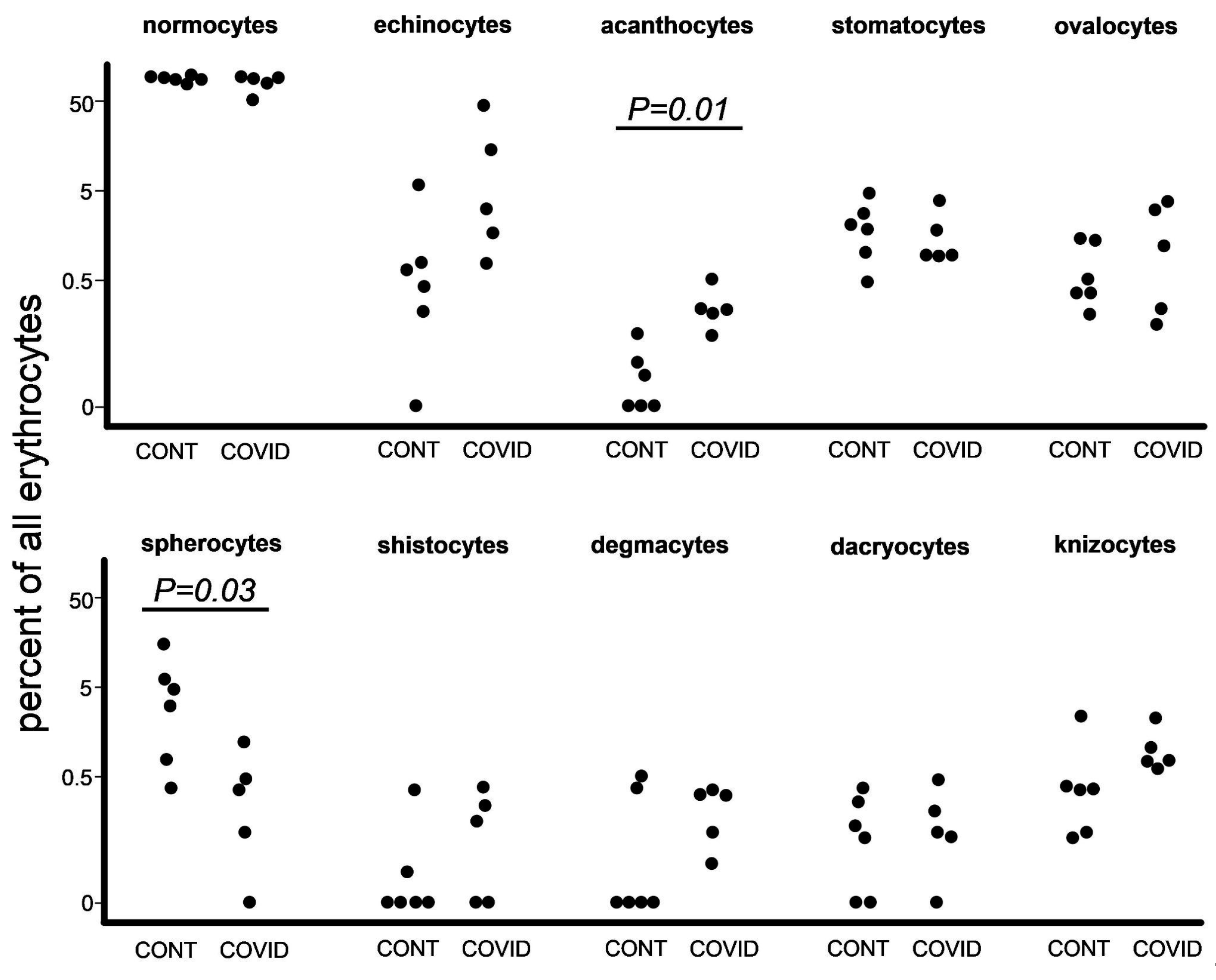
3.2. Poikilocyte Percentage Count
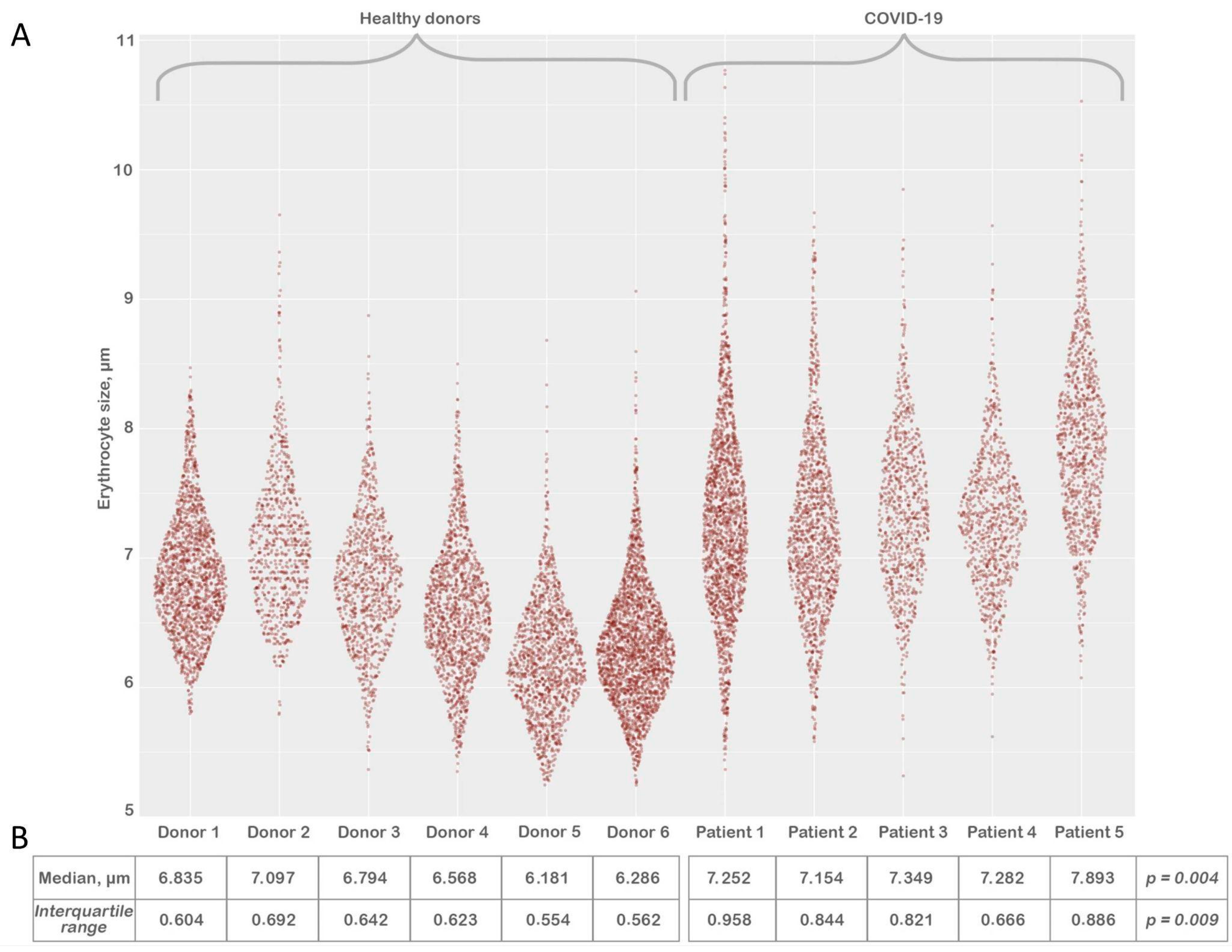
3.3. Erythrocyte Size Evaluation
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boechat, J.L.; Chora, I.; Morais, A.; Delgado, L. The Immune Response to SARS-CoV-2 and COVID-19 Immunopathology—Current Perspectives. Pulmonology 2021, 27, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal Analyses Reveal Immunological Misfiring in Severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, Y.V.; Aleksandrova, E.V.; Krivoruchko, A.B.; Meshkova, M.E.; Minaeva, L.V.; Zhdanov, K.V.; Artamonov, A.A.; Kozlov, K.V.; Ivanov, A.M.; Maltsev, O.V.; et al. Interrelations between viral load and cellular immunity in patients with COVID-19 of varying severity. Med. Immunol. 2023, 25, 167–180. [Google Scholar] [CrossRef]
- Lee, C.-T.; Teo, W.Z.Y. Peripheral Blood Smear Demonstration of Lymphocyte Changes in Severe COVID-19. Am. J. Trop. Med. Hyg. 2020, 103, 1350–1351. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, B.; Vesal, A.; Edalatifard, M. Coronavirus and Its Effect on the Respiratory System: Is There Any Association between Pneumonia and Immune Cells. J. Fam. Med. Prim. Care 2020, 9, 4729–4735. [Google Scholar] [CrossRef]
- Nitsure, M.; Sarangi, B.; Shankar, G.H.; Reddy, V.S.; Walimbe, A.; Sharma, V.; Prayag, S. Mechanisms of Hypoxia in COVID-19 Patients: A Pathophysiologic Reflection. Indian J. Crit. Care Med. 2020, 24, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, V.; Diederich, L.; Keller, T.C.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal 2017, 26, 718–742. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Kondratov, K.; Kishenko, V.; Mikhailovskii, V.; Kudryavtsev, I.; Belyakova, M.; Sidorkevich, S.; Vavilova, T.; Kostareva, A.; Sirotkina, O.; et al. Application of High-Sensitivity Flow Cytometry in Combination with Low-Voltage Scanning Electron Microscopy for Characterization of Nanosized Objects during Platelet Concentrate Storage. Platelets 2020, 31, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Bessis, M. Red Cell Shapes. An Illustrated Classification and Its Rationale. Nouv. Rev. Fr. Hematol. 1972, 12, 721–745. [Google Scholar] [PubMed]
- Karandeniya, D.M.W.; Holmes, D.W.; Sauret, E.; Gu, Y.T. A New Membrane Formulation for Modelling the Flow of Stomatocyte, Discocyte, and Echinocyte Red Blood Cells. Biomech. Model. Mechanobiol. 2022, 21, 899–917. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Peng, C. Automated Quantification and Analysis of Cell Counting Procedures Using ImageJ Plugins. JoVE 2016, 117, 54719. [Google Scholar] [CrossRef]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The Immunology and Immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- Gérard, D.; Ben Brahim, S.; Lesesve, J.F.; Perrin, J. Are Mushroom-shaped Erythrocytes an Indicator of COVID-19? Br. J. Haematol. 2021, 192, 230. [Google Scholar] [CrossRef]
- Sileshi, B.; Urgessa, F.; Wordofa, M. A Comparative Study of Hematological Parameters between Hypertensive and Normotensive Individuals in Harar, Eastern Ethiopia. PLoS ONE 2021, 16, e0260751. [Google Scholar] [CrossRef] [PubMed]
- Fois, A.G.; Scano, V.; Baglieri, A.; Masotto, E.; Zinellu, E.; Sotgiu, E.; Mellino, S.; Mangoni, A.A.; Carru, C.; Pirina, P.; et al. The red blood cell distribution width is independently associated with idiopathic pulmonary fibrosis. Minerva Respir. Med. 2021, 60, 23–28. [Google Scholar] [CrossRef]
- Boltshauser, E.; Weber, K.P. Laboratory Investigations. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 154, pp. 287–298. ISBN 978-0-444-63956-1. [Google Scholar]
- Naeim, F.; Nagesh Rao, P.; Song, S.X.; Grody, W.W. Disorders of Red Blood Cells—Anemias. In Atlas of Hematopathology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 675–704. ISBN 978-0-12-385183-3. [Google Scholar]
- Pacheco, J.M.; Yilmaz, M.; Rice, L. How Low Is Too Low: Statin Induced Hemolysis. Am. J. Hematol. 2016, 91, 267. [Google Scholar] [CrossRef] [PubMed]
- Lausch, E.; Yoshimi, A. Acanthocytosis: A Key Feature for the Diagnosis of Abetalipoproteinemia. Blood 2023, 141, 3231. [Google Scholar] [CrossRef]
- Loosen, S.H.; Jensen, B.-E.O.; Tanislav, C.; Luedde, T.; Roderburg, C.; Kostev, K. Obesity and Lipid Metabolism Disorders Determine the Risk for Development of Long COVID Syndrome: A Cross-Sectional Study from 50,402 COVID-19 Patients. Infection 2022, 50, 1165–1170. [Google Scholar] [CrossRef]
- Jin, H.; He, J.; Dong, C.; Li, B.; Ma, Z.; Li, B.; Huang, T.; Fan, J.; He, G.; Zhao, X. Altered Lipid Profile Is a Risk Factor for the Poor Progression of COVID-19: From Two Retrospective Cohorts. Front. Cell. Infect. Microbiol. 2021, 11, 712530. [Google Scholar] [CrossRef]
- Klei, T.R.L.; Meinderts, S.M.; Van Den Berg, T.K.; Van Bruggen, R. From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Front. Immunol. 2017, 8, 73. [Google Scholar] [CrossRef]
- Thiagarajan, P.; Parker, C.J.; Prchal, J.T. How Do Red Blood Cells Die? Front. Physiol. 2021, 12, 655393. [Google Scholar] [CrossRef]
- Lippi, G.; Henry, B.M.; Sanchis-Gomar, F. Red Blood Cell Distribution Is a Significant Predictor of Severe Illness in Coronavirus Disease 2019. Acta Haematol. 2021, 144, 360–364. [Google Scholar] [CrossRef]
- Marchi, G.; Bozzini, C.; Bertolone, L.; Dima, F.; Busti, F.; Castagna, A.; Stranieri, C.; Fratta Pasini, A.M.; Friso, S.; Lippi, G.; et al. Red Blood Cell Morphologic Abnormalities in Patients Hospitalized for COVID-19. Front. Physiol. 2022, 13, 932013. [Google Scholar] [CrossRef] [PubMed]
- Karampitsakos, T.; Akinosoglou, K.; Papaioannou, O.; Panou, V.; Koromilias, A.; Bakakos, P.; Loukides, S.; Bouros, D.; Gogos, C.; Tzouvelekis, A. Increased Red Cell Distribution Width Is Associated With Disease Severity in Hospitalized Adults With SARS-CoV-2 Infection: An Observational Multicentric Study. Front. Med. 2020, 7, 616292. [Google Scholar] [CrossRef]
- Kim, H.S.; Ko, H.H.; Lee, D.H. The Measurement of Red Cell Size in Peripheral Blood Smear: Comparison of Mean Corpuscular Area and Mean Corpuscular Volume. Korean J. Clin. Pathol. 2001, 21, 13–17. [Google Scholar]
- Hoffmann, J.J.M.L.; Nabbe, K.C.A.M.; van den Broek, N.M.A. Effect of Age and Gender on Reference Intervals of Red Blood Cell Distribution Width (RDW) and Mean Red Cell Volume (MCV). Clin. Chem. Lab. Med. 2015, 53, 2015–2019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, T.; Wang, X.; Chen, Q.; Shen, H.; Xiong, B. Measurement for the Area of Red Blood Cells From Microscopic Images Based on Image Processing Technology and Its Applications in Aplastic Anemia, Megaloblastic Anemia, and Myelodysplastic Syndrome. Front. Med. 2021, 8, 796920. [Google Scholar] [CrossRef]
- Bouchla, A.; Kriebardis, A.G.; Georgatzakou, H.T.; Fortis, S.P.; Thomopoulos, T.P.; Lekkakou, L.; Markakis, K.; Gkotzias, D.; Panagiotou, A.; Papageorgiou, E.G.; et al. Red Blood Cell Abnormalities as the Mirror of SARS-CoV-2 Disease Severity: A Pilot Study. Front. Physiol. 2022, 12, 825055. [Google Scholar] [CrossRef]
- Ballas, S.K. Lactate Dehydrogenase and Hemolysis in Sickle Cell Disease. Blood 2013, 121, 243–244. [Google Scholar] [CrossRef]
- Berlin, N.; Berk, P. Quantitative Aspects of Bilirubin Metabolism for Hematologists. Blood 1981, 57, 983–999. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Wagner-Britz, L.; Maia, S.; Steffen, P.; Wagner, C.; Kaestner, L.; Bernhardt, I. Regulation of Phosphatidylserine Exposure in Red Blood Cells. Cell. Physiol. Biochem. 2011, 28, 847–856. [Google Scholar] [CrossRef]
- Hoffman, J.F.; Joiner, W.; Nehrke, K.; Potapova, O.; Foye, K.; Wickrema, A. The HSK4 (KCNN4) Isoform Is the Ca2+-Activated K+ Channel (Gardos Channel) in Human Red Blood Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 7366–7371. [Google Scholar] [CrossRef]
- Qadri, S.M.; Bissinger, R.; Solh, Z.; Oldenborg, P.-A. Eryptosis in Health and Disease: A Paradigm Shift towards Understanding the (Patho)Physiological Implications of Programmed Cell Death of Erythrocytes. Blood Rev. 2017, 31, 349–361. [Google Scholar] [CrossRef]
- Föller, M.; Braun, M.; Qadri, S.M.; Lang, E.; Mahmud, H.; Lang, F. Temperature Sensitivity of Suicidal Erythrocyte Death. Eur. J. Clin. Invest. 2010, 40, 534–540. [Google Scholar] [CrossRef]
- Abassi, Z.; Higazi, A.A.R.; Kinaneh, S.; Armaly, Z.; Skorecki, K.; Heyman, S.N. ACE2, COVID-19 Infection, Inflammation, and Coagulopathy: Missing Pieces in the Puzzle. Front. Physiol. 2020, 11, 574753. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.M.; Mackman, N.; Warren, R.Q.; Wolberg, A.S.; Mosnier, L.O.; Campbell, R.A.; Gralinski, L.E.; Rondina, M.T.; Van De Veerdonk, F.L.; Hoffmeister, K.M.; et al. Understanding COVID-19-Associated Coagulopathy. Nat. Rev. Immunol. 2022, 22, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Berzuini, A.; Bianco, C.; Migliorini, A.C.; Maggioni, M.; Valenti, L.; Prati, D. Red Blood Cell Morphology in Patients with COVID-19-Related Anaemia. Blood Transfus. 2020, 19, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Plassmeyer, M.; Alpan, O.; Corley, M.J.; Premeaux, T.A.; Lillard, K.; Coatney, P.; Vaziri, T.; Michalsky, S.; Pang, A.P.S.; Bukhari, Z.; et al. Caspases and Therapeutic Potential of Caspase Inhibitors in Moderate–Severe SARS-CoV-2 Infection and Long COVID. Allergy 2022, 77, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.P.; Engels, I.H.; Rothbart, A.; Lauber, K.; Renz, A.; Schlosser, S.F.; Schulze-Osthoff, K.; Wesselborg, S. Human Mature Red Blood Cells Express Caspase-3 and Caspase-8, but Are Devoid of Mitochondrial Regulators of Apoptosis. Cell Death Differ. 2001, 8, 1197–1206. [Google Scholar] [CrossRef]
- Gregoli, P.A.; Bondurant, M.C. Function of Caspases in Regulating Apoptosis Caused by Erythropoietin Deprivation in Erythroid Progenitors. J. Cell Physiol. 1999, 178, 133–143. [Google Scholar] [CrossRef]
- Carlile, G.W.; Smith, D.H.; Wiedmann, M. Caspase-3 Has a Nonapoptotic Function in Erythroid Maturation. Blood 2004, 103, 4310–4316. [Google Scholar] [CrossRef] [PubMed]
- Zermati, Y.; Garrido, C.; Amsellem, S.; Fishelson, S.; Bouscary, D.; Valensi, F.; Varet, B.; Solary, E.; Hermine, O. Caspase Activation Is Required for Terminal Erythroid Differentiation. J. Exp. Med. 2001, 193, 247–254. [Google Scholar] [CrossRef]
- Kronstein-Wiedemann, R.; Stadtmüller, M.; Traikov, S.; Georgi, M.; Teichert, M.; Yosef, H.; Wallenborn, J.; Karl, A.; Schütze, K.; Wagner, M.; et al. SARS-CoV-2 Infects Red Blood Cell Progenitors and Dysregulates Hemoglobin and Iron Metabolism. Stem Cell Rev. Rep. 2022, 18, 1809–1821. [Google Scholar] [CrossRef] [PubMed]
- Schoener, B.; Borger, J. Erythropoietin Stimulating Agents. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bapat, A.; Schippel, N.; Shi, X.; Jasbi, P.; Gu, H.; Kala, M.; Sertil, A.; Sharma, S. Hypoxia Promotes Erythroid Differentiation through the Development of Progenitors and Proerythroblasts. Exp. Hematol. 2021, 97, 32–46.e35. [Google Scholar] [CrossRef]
- Vlaski, M.; Lafarge, X.; Chevaleyre, J.; Duchez, P.; Boiron, J.-M.; Ivanovic, Z. Low Oxygen Concentration as a General Physiologic Regulator of Erythropoiesis beyond the EPO-Related Downstream Tuning and a Tool for the Optimization of Red Blood Cell Production Ex Vivo. Exp. Hematol. 2009, 37, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.M.; Yu, X.; Wen, J.; Smith, R.; Fibach, E.; Noguchi, C.T. Hypoxia Alters Progression of the Erythroid Program. Exp. Hematol. 2008, 36, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, V.G.; Ludwig, L.S.; Sicinska, E.; Xu, J.; Bauer, D.E.; Eng, J.C.; Patterson, H.C.; Metcalf, R.A.; Natkunam, Y.; Orkin, S.H.; et al. Cyclin D3 Coordinates the Cell Cycle during Differentiation to Regulate Erythrocyte Size and Number. Genes Dev. 2012, 26, 2075–2087. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W. Regulation of Erythropoietin Production. J. Physiol. 2011, 589, 1251–1258. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondratov, K.A.; Artamonov, A.A.; Mikhailovskii, V.Y.; Velmiskina, A.A.; Mosenko, S.V.; Grigoryev, E.A.; Anisenkova, A.Y.; Nikitin, Y.V.; Apalko, S.V.; Sushentseva, N.N.; et al. SARS-CoV-2 Impact on Red Blood Cell Morphology. Biomedicines 2023, 11, 2902. https://doi.org/10.3390/biomedicines11112902
Kondratov KA, Artamonov AA, Mikhailovskii VY, Velmiskina AA, Mosenko SV, Grigoryev EA, Anisenkova AY, Nikitin YV, Apalko SV, Sushentseva NN, et al. SARS-CoV-2 Impact on Red Blood Cell Morphology. Biomedicines. 2023; 11(11):2902. https://doi.org/10.3390/biomedicines11112902
Chicago/Turabian StyleKondratov, Kirill A., Alexander A. Artamonov, Vladimir Yu. Mikhailovskii, Anastasiya A. Velmiskina, Sergey V. Mosenko, Evgeniy A. Grigoryev, Anna Yu. Anisenkova, Yuri V. Nikitin, Svetlana V. Apalko, Natalya N. Sushentseva, and et al. 2023. "SARS-CoV-2 Impact on Red Blood Cell Morphology" Biomedicines 11, no. 11: 2902. https://doi.org/10.3390/biomedicines11112902
APA StyleKondratov, K. A., Artamonov, A. A., Mikhailovskii, V. Y., Velmiskina, A. A., Mosenko, S. V., Grigoryev, E. A., Anisenkova, A. Y., Nikitin, Y. V., Apalko, S. V., Sushentseva, N. N., Ivanov, A. M., & Scherbak, S. G. (2023). SARS-CoV-2 Impact on Red Blood Cell Morphology. Biomedicines, 11(11), 2902. https://doi.org/10.3390/biomedicines11112902








