Early Saphenous Vein Graft Aneurysm Rupture: A Not So-Late Complication. Case Report and Comprehensive Literature Review
Abstract
1. Introduction
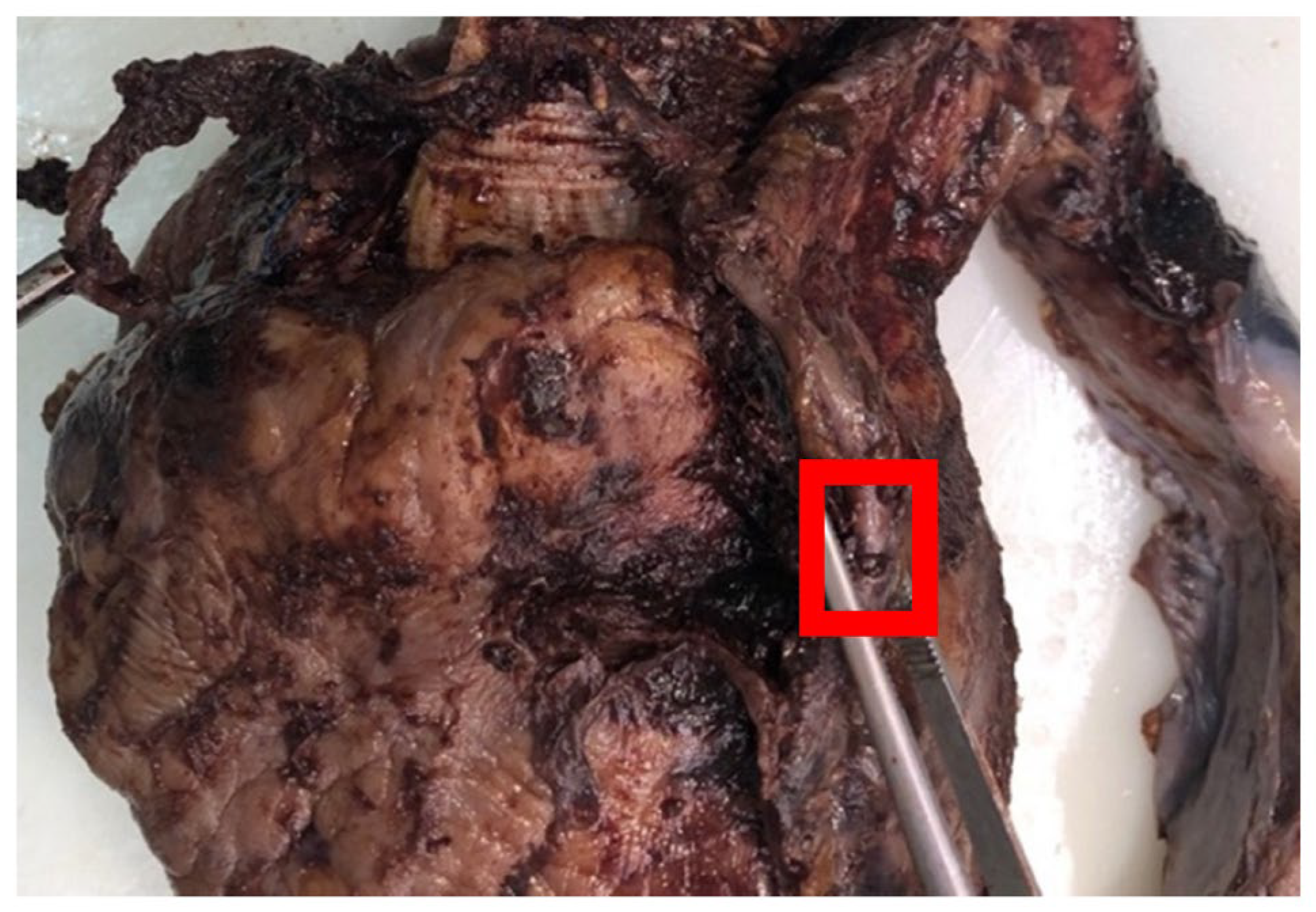
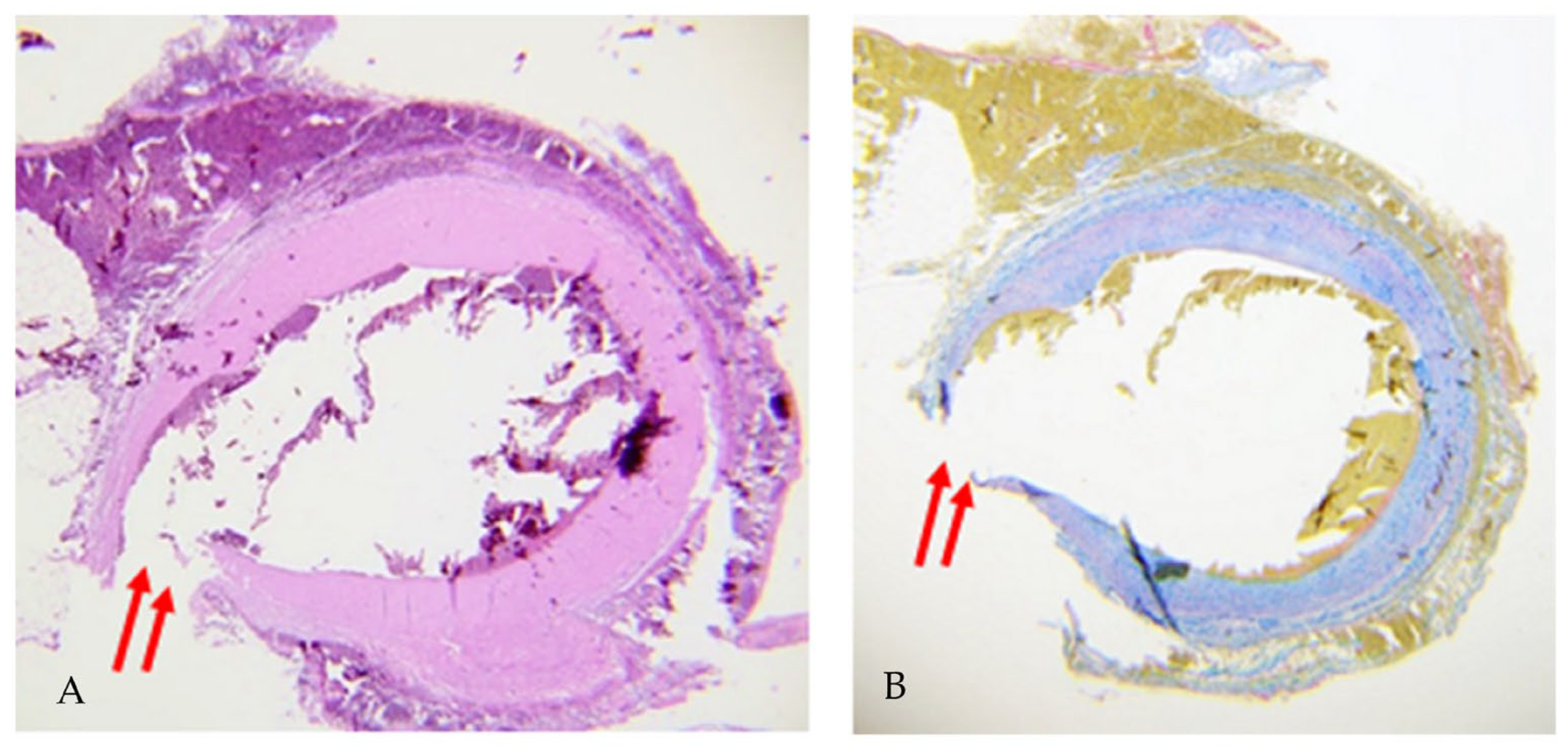
2. Case Report
3. Materials and Methods
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzgibbon, G.M.; Kafka, H.P.; Leach, A.J.; Keon, W.J.; Hooper, G.D.; Burton, J.R. Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5065 grafts related to survival and reoperation in 1388 patients during 25 years. J. Am. Coll. Cardiol. 1996, 28, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Hillis, L.D.; Smith, P.K.; Anderson, J.L.; Bittl, J.A.; Bridges, C.R.; Byrne, J.G.; Cigarroa, J.E.; Disesa, V.J.; Hiratzka, L.F.; Hutter, A.M., Jr.; et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011, 6, 652–735. [Google Scholar]
- Nathaniel, C.; Missri, J.C. Coronary artery bypass graft pseudoaneurysm communicating with the right atrium: A case report and review. Catheter. Cardiovasc. Diagn. 1996, 38, 80–82. [Google Scholar] [CrossRef]
- Ramirez, F.D.; Hibbert, B.; Simard, T.; Pourdjabbar, A.; Wilson, K.R.; Hibbert, R.; Kazmi, M.; Hawken, S.; Ruel, M.; Labinaz, M.; et al. Natural history and management of aortocoronary saphenous vein graft aneurysms: A systematic review of published cases. Circulation 2012, 126, 2248–2256. [Google Scholar] [CrossRef]
- Xenogiannis, I.; Zenati, M.; Bhatt, D.L.; Rao, S.V.; Rodés-Cabau, J.; Goldman, S.; Shunk, K.A.; Mavromatis, K.; Banerjee, S.; Alaswad, K.; et al. Saphenous Vein Graft Failure: From Pathophysiology to Prevention and Treatment Strategies. Circulation 2021, 144, 728–745. [Google Scholar] [CrossRef]
- Riahi, M.; Vasu, C.M.; Tomatis, L.A.; Schlosser, R.J.; Zimmerman, G. Aneurysm of saphenous vein bypass graft to coronary artery. J. Thorac. Cardiovasc. Surg. 1975, 70, 358–359. [Google Scholar] [CrossRef]
- Benchimol, A.; Harris, C.L.; Desser, K.B.; Fleming, H. Aneurysms of an aorto-coronary artery saphenous vein bypass graft--a case report. Vasc. Surg. 1975, 9, 261–264. [Google Scholar] [CrossRef]
- Douglas, B.P.; Bulkley, B.H.; Hutchins, G.M. Infected saphenous vein coronary artery bypass graft with mycotic aneurysm. Fatal dehiscence of the proximal anastomosis. Chest 1979, 75, 76–77. [Google Scholar] [CrossRef]
- Forster, D.A.; Haupert, M.S. Large mediastinal mass secondary to an aortocoronary saphenous vein bypass graft aneurysm. Ann. Thorac. Surg. 1991, 52, 547–548. [Google Scholar] [CrossRef]
- Bramlet, D.A.; Behar, V.S.; Ideker, R.E. Aneurysm of a saphenous vein bypass graft associated with aneurysms of native coronary arteries. Catheter. Cardiovasc. Diagn. 1982, 8, 489–494. [Google Scholar] [CrossRef]
- Shapeero, L.G.; Guthaner, D.F.; Swerdlow, C.D.; Wexler, L. Rupture of a coronary bypass graft aneurysm: CT evaluation and coil occlusion therapy. AJR Am. J. Roentgenol. 1983, 141, 1060–1062. [Google Scholar] [CrossRef]
- Murphy, J.P., Jr.; Shabb, B.; Nishikawa, A.; Adams, P.R.; Walker, W.E. Rupture of an aortocoronary saphenous vein graft aneurysm. Am. J. Cardiol. 1986, 58, 555–557. [Google Scholar] [CrossRef]
- Yousem, D.; Scott, W., Jr.; Fishman, E.K.; Watson, A.J.; Traill, T.; Gimenez, L. Saphenous vein graft aneurysms demonstrated by computed tomography. J. Comput. Assist. Tomogr. 1986, 10, 526–528. [Google Scholar]
- Steg, P.G.; Benacerraf, M.; Chatel, D.; Laissy, J.P. Images in cardiovascular medicine. False aortic aneurysm due to rupture of an aortocoronary saphenous vein bypass graft. Circulation 1997, 96, 3778. [Google Scholar] [CrossRef]
- Távora, F.R.; Jeudy, J.; Burke, A.P. Multiple aneurysms of aortocoronary saphenous vein grafts with fatal rupture. Arq. Bras. Cardiol. 2007, 88, 10. [Google Scholar]
- Taguchi, E.; Sawamura, T.; Kamio, T.; Fukunaga, T.; Oe, Y.; Miyamoto, S.; Koyama, J.; Tayama, S.; Sakamoto, T.; Nishigami, K.; et al. An autopsy case of the rupture of a giant aneurysm in a saphenous vein graft: 18 years after CABG. J. Cardiol. Cases 2010, 2, 88–91. [Google Scholar] [CrossRef]
- Salcedo, J.D.; Bhakta, M.D.; Kern, M.J. Aortocoronary saphenous vein graft rupture during diagnostic angiography. Catheter. Cardiovasc. Interv. 2013, 82, 230–234. [Google Scholar] [CrossRef]
- Koshy, G.B.; Hale, A.; Srouji, D.; Kim, S.; Wildes, D. Late Spontaneous Rupture of a Saphenous Vein Graft. Methodist. Debakey Cardiovasc. J. 2020, 16, 318–322. [Google Scholar] [CrossRef]
- Montgomery, R.; Javorski, M.J.; Bakaeen, F.; Tong, M.Z.; Pettersson, G.B.; Weiss, A.J. Cardiogenic Shock From an Acute Rupture of an Infectious Saphenous Vein Graft Aneurysm. Ann. Thorac. Surg. 2021, 111, 419–420. [Google Scholar] [CrossRef]
- Rosin, M.D.; Ridley, P.D.; Maxwell, P.H. Rupture of a pseudoaneurysm of a saphenous vein coronary arterial bypass graft presenting with superior caval venous obstruction. Int. J. Cardiol. 1989, 25, 121–123. [Google Scholar]
- Karwande, S.V.; Sharp, S.D. Saphenous vein graft pseudoaneurysm presenting as a mediastinal mass. Tex. Heart Inst. J. 1990, 17, 129–132. [Google Scholar] [PubMed]
- Werthman, P.E.; Sutter, F.P.; Flicker, S.; Goldman, S.M. Spontaneous, late rupture of an aortocoronary saphenous vein graft. Ann. Thorac. Surg. 1991, 51, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Dimitri, W.R.; Reid, A.W.; Dunn, F.G. Leaking false aneurysm of right coronary saphenous vein graft; successful treatment by percutaneous coil embolisation. Br. Heart J. 1992, 68, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Kallis, P.; Keogh, B.E.; Davies, M.J. Pseudoaneurysm of aortocoronary vein graft secondary to late venous rupture: Case report and literature review. Br. Heart J. 1993, 70, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Mohara, J.; Konishi, H.; Kato, M.; Misawa, Y.; Kamisawa, O.; Fuse, K. Saphenous vein graft pseudoaneurysm rupture after coronary artery bypass grafting. Ann. Thorac. Surg. 1998, 65, 831–832. [Google Scholar] [CrossRef]
- Puri, R.; Dundon, B.K.; Leong, D.P.; Worthley, S.G.; Worthley, M.I. Giant saphenous vein graft pseudoaneurysm rupture presenting with cardiac tamponade. Heart Lung Circ. 2009, 18, 52–54. [Google Scholar] [CrossRef]
- Smer, A.; Alla, V.; Chandraprakasam, S.; Abuzaid, A.; Saurav, A.; Holmberg, J. Saphenous venous graft pseudoaneurysm: A review of the literature. J. Card. Surg. 2015, 30, 70–73. [Google Scholar] [CrossRef]
- Desai, M.; Seifalian, A.M.; Hamilton, G. Role of prosthetic conduits in coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2011, 40, 394–398. [Google Scholar] [CrossRef]
- Murray, G. Blood vessel surgery. Can. Med. Assoc. J. 1953, 69, 296–299. [Google Scholar]
- Smith, S.; Beasley, M.; Hodes, R.; Hall, H.; Biel, E.; Huth, E.W. Auxiliary myocardial vascularization by prosthetic graft implantation. Surg. Gynecol. Obstet. 1957, 104, 263–268. [Google Scholar]
- Ferrari, E.R.; von Segesser, L.K. Arterial grafting for myocardial revascularization: How better is it? Curr. Opin. Cardiol. 2006, 21, 584–588. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. SC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart. J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Aldea, G.S.; Bakaeen, F.G.; Pal, J.; Fremes, S.; Head, S.J.; Sabik, J.; Rosengart, T.; Kappetein, A.P.; Thourani, V.H.; Firestone, S.; et al. The Society of Thoracic Surgeons Clinical Practice Guidelines on Arterial Conduits for Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2016, 101, 801–809. [Google Scholar] [CrossRef]
- Cuminetti, G.; Gelsomino, S.; Curello, S.; Lorusso, R.; Maessen, J.G.; Hoorntje, J.C. Contemporary use of arterial and venous conduits in coronary artery bypass grafting: Anatomical, functional and clinical aspects. Neth. Heart J. 2017, 25, 4–13. [Google Scholar] [CrossRef]
- Lavee, J.; Schneiderman, J.; Yorav, S.; Shewach-Millet, M.; Adar, R. Complications of saphenous vein harvesting following coronary artery bypass surgery. J. Cardiovasc. Surg. 1989, 30, 989–991. [Google Scholar]
- Sabik, J.F.; Lytle, B.W.; Blackstone, E.H.; Houghtaling, P.L.; Cosgrove, D.M. Comparison of saphenous vein and internal thoracic artery graft patency by coronary system. Ann. Thorac. Surg. 2005, 79, 544–551. [Google Scholar] [CrossRef]
- Harskamp, R.E.; Lopes, R.D.; Baisden, C.E.; de Winter, R.J.; Alexander, J.H. Saphenous vein graft failure after coronary artery bypass surgery: Pathophysiology, management, and future directions. Ann. Surg. 2013, 257, 824–833. [Google Scholar] [CrossRef]
- Paz, M.A.; Lupon, J.; Bosch, X.; Pomar, J.L.; Sanz, G. Predictors of early saphenous vein aortocoronary bypass graft occlusion. Ann. Thorac. Surg. 1993, 56, 1101–1106. [Google Scholar] [CrossRef]
- Goldman, S.; Zadina, K.; Moritz, T.; Ovitt, T.; Sethi, G.; Copeland, J.G.; Thottapurathu, L.; Krasnicka, B.; Ellis, N.; Anderson, R.J.; et al. VA Cooperative Study Group Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a Department of Veterans Affairs Cooperative Study. J. Am. Coll. Cardiol. 2004, 44, 2149–2156. [Google Scholar] [CrossRef]
- Shah, P.J.; Gordon, I.; Fuller, J.; Seevanayagam, S.; Rosalion, A.; Tatoulis, J.; Raman, J.S.; Buxton, B.F. Factors affecting saphenous vein graft patency: Clinical and angiographic study in 1402 symptomatic patients operated on between 1977 and 1999. J. Thorac. Cardiovasc. Surg. 2003, 126, 1972–1977. [Google Scholar] [CrossRef]
- Gitto, L.; Bonaccorso, L.; Maiese, A.; dell’Aquila, M.; Arena, V.; Bolino, G. A scream from the past: A multidisciplinary approach in a concealment of a corpse found mummified. J. Forensic Leg. Med. 2015, 32, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Gitto, L.; Serinelli, S.; Busardò, F.P.; Panebianco, V.; Bolino, G.; Maiese, A. Can post-mortem computed tomography be considered an alternative for autopsy in deaths due to hemopericardium? J. Geriatr. Cardiol. 2014, 11(4), 363–367. [Google Scholar]
- Weman, S.M.; Salminen, U.S.; Penttilä, A.; Männikkö, A.; Karhunen, P.J. Post-mortem cast angiography in the diagnostics of graft complications in patients with fatal outcome following coronary artery bypass grafting (CABG). Int. J. Legal Med. 1999, 112(2), 107–114. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Jagen, M.A.; Tunick, P.A.; Kronzon, I. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J. Am. Soc. Echocardiogr. 2003, 16, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.Q.; Huang, R.I.; Marcus, F.; Xavier, L.; Alpert, J. Saphenous vein graft aneurysm: Case report and review. Cardiol. Rev. 2003, 11, 26–34. [Google Scholar] [CrossRef]
- al-Harthi, S.S.; Nouh, M.S.; Arafa, M.; al-Nozha, M. Aneurysmal dilatation of the coronary arteries: Diagnostic patterns and clinical significance. Int. J. Cardiol. 1991, 30, 191–194. [Google Scholar] [CrossRef]
- Hassantash, S.A.; Bikdeli, B.; Kalantarian, S.; Sadeghian, M.; Afshar, H. Pathophysiology of aortocoronary saphenous vein bypass graft disease. Asian Cardiovasc. Thorac. Ann. 2008, 16, 331–336. [Google Scholar] [CrossRef]
- Cummings, P.M.; Trelka, D.P.; Springer, K.M. Atlas of Forensic Histopathology, 1st ed.; Cambridge University Press: Cambridge, UK, 2011; pp. 2–5. [Google Scholar]
- Raffetto, J.D.; Khalil, R.A. Mechanisms of varicose vein formation: Valve dysfunction and wall dilation. Phlebology 2008, 23, 85–98. [Google Scholar] [CrossRef]
- Agenzia Sanitaria Regionale. Descrizione degli interventi e delle caratteristiche cliniche dei pazienti cardiochirurgici in Regione Emilia-Romagna. In Area di Programma Governo Clinico; Agenzia Sanitaria Regionale: Pescara, Italy, 2007. [Google Scholar]
- Schumer, E.M.; Chaney, J.H.; Trivedi, J.R.; Linsky, P.L.; Williams, M.L.; Slaughter, M.S. Emergency Coronary Artery Bypass Grafting: Indications and Outcomes from 2003 through 2013. Tex. Heart Inst. J. 2016, 43, 214–219. [Google Scholar] [CrossRef]
- Muñoz, M.; Acheson, A.G.; Bisbe, E.; Butcher, A.; Gómez-Ramírez, S.; Khalafallah, A.A.; Kehlet, H.; Kietaibl, S.; Liumbruno, G.M.; Meybohm, P.; et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018, 73, 1418–1431. [Google Scholar] [CrossRef]
| Reference | n° cases | Age (y) and Gender | Time | Rupture Modality | Outcome | Diagnostic Investigations | Autopsy | Symptoms | Description | Morbidities |
|---|---|---|---|---|---|---|---|---|---|---|
| Aneurysms (14) | ||||||||||
| Riahi M et al. 1975 [6] | 1 | 40 M | 3 years | Aneurysmal dilatation and rupture of SVG | Alive | RX, Aortography | No | Asymptomatic | Vein graft aneurysm which appeared 6 months after surgery. The surgical operation consisted of an aortic valve replacement and a right coronary artery SVG. | Severe aortic insufficiency |
| Benchimol et al. 1975 [7] | 1 | 62 M | 4 months | Aneurysmal dilations and rupture of SVG | Alive | Angiography | No | Asymptomatic | After aorto-coronary SVG implantation, the patient underwent aortocoronary graft angiography demonstrating multiple venous aneurysmal dilations. | Diabetes Mellitus |
| Douglas et al. 1979 [8] | 1 | 33 M | 7 days | Rupture of mycotic aneurysm in the midportion of the SVG | Dead | NA | Yes | Fever, leukocytosis, hemorrhage | After SVG surgery, the patients developed an infective SVG aneurysm. Initially, no infection was documented. After 5 days, later tamponade secondary to the recurrence of hemorrhage was fatal. | Smoker, Coronary artery disease |
| Bramlet DA et al. 1982 [10] | 1 | 46 M | 8 years | Aneurysmal dilatation and rupture of SVG | Dead | Angiography | Yes | Nocturnal recurrent chest pain | Patients resulted affected by multiple coronary artery aneurysms in which a dissecting SVG aneurysm developed. | Hypertension, Smoker, Myocardial infarction, Aneurysms of coronaries |
| Shapeero LG et al. 1983 [11] | 1 | 56 M | 9 years | Aneurysmal dilatation and rupture of SVG | Alive | RX, CT, Cardiac catheterization, Angiography | No | Unstable Angina | The SVG mass was developed 3 months after the last radiological control. The mass compressed the main and left pulmonary arteries. Inside, a thrombotic formation was present. During this period patient suffered a heart attack. | NA |
| Murphy JP et al. 1986 [12] | 1 | 65 M | 14 years | Aneurysmal dilations and rupture of SVG | Alive | RX | No | Intermittent anterior pleural chest pain, Hemothorax | Reversed SV grafts had been placed to the right and the left anterior descending artery. After the SVG rupture, 1600 mL of fresh blood was evacuated from the right chest. The patient was operated on with systemic cooling. | NA |
| Yousem D et al. 1986 [13] | 1 | 23 F | 5 years | Rupture | Dead | RX, CT, Transthoracic echocardiogram | Yes | NA | CT revealed two large fusiform structures of low attenuation. Echocardiography showed vascular origin. Several months later, the patient died suddenly at home. | Nonspecific Vasculitis; Chronic renal failure |
| Forster DA et al. 1991 [9] | 1 | 62 M | 17 years | Intraoperative rupture of aneurismatic SVG | Alive | RX, CT | No | Difficulty breathing and chest pain | The patient was operated on for a thoracic mass suspicious of a cyst or teratoma. During right thoracotomy, after the removal of a clot and bleeding, it was identified as an SVG aneurysm. | NA |
| Steg PG et al. 1997 [14] | 1 | 40 M | 8 years | SVG rupture and false aneurysm formation | Alive | Contrast enhanced CT; Coronal spin-echo ECG-gated MRI; Aortography | No | Atypical chest pain | A sudden rupture of a thrombotic SVG developed a false aneurism. Recurrent bleedings were detected. | NA |
| Távora FR et al. 2007 [15] | 1 | 39 M | 10 years | Aneurysmal dilations and rupture of SVG | Dead | CT | Yes | Chest pain, hematemesis, severe respiratory distress, pleural effusion. | Admitted to the hospital with hematemesis ten years after aortocoronary bypass surgery. CT images revealed three aortocoronary SVG aneurysms but failed to detect any rupture. His subsequent death due to a rupture of the SVG aneurysm was documented at autopsy. | NA |
| Taguchi E et al. 2010 [16] | 1 | 82 F | 18 years | Aneurysmal dilations and rupture of SVG | Dead | CT, Coronary angiography | Yes | Shock state | The patient showed a sudden state of shock. Serological examinations were normal except for a low creatinine increase. CT revealed a mediastinal mass. The patient fell into cardiac arrest during an arteriography procedure. | Coronary artery disease |
| Salcedo JD et al. 2013 [17] | 1 | 83 M | 12 years | SVG aneurismatic rupture during angiography examination | Alive | Coronary angiography | No | Asymptomatic | The patient was operated on for cardiac ischemia. During the contrast injection for diagnostic coronary angiography, the graft erupted with contrast extravasation into the surrounding tissue. | Peripheric and Coronary arterial disease |
| Koshy GB et al. 2020 [18] | 1 | 65 M | 16 years | Aneurysmal dilations and rupture of SVG | Alive | CT, Transthoracic echocardiography, Left heart catheterization, Coronary angiography | No | Chest pain and shortness of breath | Chest pain occurred for 3 days then it was accompanied by chest pressure. Serological examinations were normal. During the angiography, extravasation of contrast was demonstrated. The patient was treated by catheterization. | Hypertension, Hyperlipidemia, Chronic obstructive pulmonary disease |
| Montgomery R et al. 2021 [19] | 1 | 60 M | 12 years | Aneurysmal Dilations, infection and rupture of SVG | Alive | Coronary angiography, Echocardiogram | No | Chest pain and shortness of breath, hypotension | Two months after the occurrence of Staphylococcus aureus Bacteremia, the patient developed a rupture of an infected SVG aneurysm resulting in pericardial tamponade. | Surgical site infection |
| Pseudoaneurysms (9) | ||||||||||
| Rosin MD et al. 1989 [20] | 1 | 46 M | 8 years | SVG spontaneous rupture and pseudoaneurysm formation | Alive | Aortography | No | Chest pain with collapse | The patient manifested sudden hypotension. On CT examination, a mediastinal mass was evident, which was identified as a pericardial cyst. On radiological testing, bleeding near the right atrium was evident. Emergency surgery showed the presence of a pseudoaneurysm with fresh blood and thrombi in the mediastinum. | NA |
| Karwande SV et al. 1990 [21] | 1 | 45 M | 13 years | SVG spontaneous rupture and pseudoaneurysm formation | Alive | RX, CT | No | Chest and back pain | A mediastinal mass was discovered during radiological examinations. After the patient suffered an episode of heart attack a source of brisk bleeding was found during the operation, and it was identified as an SVG pseudoaneurism. | Hyperlipidemia; Hypertension |
| Werthman PE et al. 1991 [22] | 1 | 63 M | 3 years | SVG spontaneous rupture and pseudoaneurysm formation | Alive | CT, Aortography | No | Chest pain | After a heart attack episode, a mediastinal mass was discovered. After four months, the mass was discovered to be a pseudoaneurysm secondary a rupture of an SVG. | Coronary artery disease, Peripheral vascular disease |
| Dimitri WR et al. 1992 [23] | 1 | 63 M | NA | Pseudoaneurysm SVG formation and rupture | Alive | CT, Aortography, Vein graft angiography | No | Massive hemorrhage and hypokinesia of the cardiac inferior segment | A SVG pseudo aneurysm caused numerous episodes of profuse intermittent bleeding through the sternum with a dehiscence of sternal wound healing. | Extensive triple vessels disease; Peripheral vascular disease |
| Kallis P et al. 1993 [24] | 1 | 45 M | 13 years | SVG pseudo aneurysmatic spontaneous rupture | Dead | RX, CT, Cardiac catheterization, Angiography | Yes | Dyspnea | Radiological examination showed critical stenosis of vein graft and coronaries but also a mass on the left side of the pulmonary artery. The aneurismal part was resected. Only histological examination revealed a pseudo-aneurismatic SVG rupture. | Triple vessel cardiac disease, Severe aortic valve dilatation. |
| Mohara J et al. 1998 [25] | 1 | 73 F | 1 month | SVG rupture and pseudoaneurysm formation | Alive | NA | No | Cardiogenic tamponade | The patient was urgently operated on for triple vessel cardiac disease. One month later angiography resulted in normal but after a few days, a posterior pseudoaneurysmal rupture was verified. The patient was urgently operated | Triple vessel cardiac disease |
| Puri R et al. 2009 [26] | 1 | 61 M | 13 years | SVG pseudo aneurysmatic spontaneous rupture | Alive | RX, CT, Transthoracic echocardiography, Angiography | No | Cardiogenic shock | The patient developed dyspnea but all radiological examinations resulted negative for SVG alterations. After five days echocardiography revealed cardiac tamponade. The haematoma was evacuated. | NA |
| Smer et al. 2015 [27] | 2 | 91 F | NA | Pseudoaneurysm SVG formation and rupture | Alive | RX, CT, Transthoracic echocardiography | No | Hemotorax | An incidental mediastinal mass on chest X-ray with a continuous flow at the eco doppler was found. It was complicated three months later with a rupture. Conservative treatment was elected. | Advanced dementia, Hypertension, Three-vessel CABG |
| 80 M | 2 weeks | Pseudoaneurysm SVG formation and rupture | Alive | CT, Transthoracic echocardiography | No | Chest pain, respiratory distress, peripheral edema, jugular venous distension, bibasilar crackles | The patient was operated on for a valvular replacement. After the operation, an SVG rupture with a pericardial hematoma formation developed. The aneurism was resected and the dehiscence sutured. | Severe aortic insufficiency, Two-vessel CABG | ||
| Type of Lesion | Dead | Alive | Age (y) | Rupture Time (y) | ||||
|---|---|---|---|---|---|---|---|---|
| Aneurysm | Pseudoaneurysm | ≤45 | >45 | ≤5 | >5 | |||
| Male | 12 (52.2) | 7 (30.4) | 4 (17.4) | 15 (65.2) | 6 (26.1) | 13 (56.5) | 5 (21.7) | 13 (56.5) |
| Female | 2 (8.7) | 2 (8.7) | 2 (8.7) | 2 (8.7) | 1 (4.3) | 3 (13) | 2 (8.7) | 1 (4.3) |
| NA | - | - | - | - | - | - | 2 (8.7) | |
| Total | 14 (60.9) | 9 (39.1) | 6 | 17 (73.9) | 7 (30.4) | 16 (69.6) | 7 (30.4) | 14 (60.9) |
| Diabetes Mellitus | Hypertension | Hyperlipidemia | COPD | Other Vascular Disease | |
|---|---|---|---|---|---|
| Male | 1 | 3 | 2 | 1 | 7 |
| Female | - | 1 | - | - | 5 |
| Rupture Time (y) | |||
|---|---|---|---|
| Age (y) | ≤5 | >5 | NA |
| ≤45 | 3 (13.4) | 4 (17.4) | - |
| >45 | 4 (17.4) | 10 (43.5) | 2 (8.7) |
| Aneurysm | Pseudoaneurysm | |||
|---|---|---|---|---|
| Age (y) | Alive | Dead | Alive | Dead |
| ≤45 | 2 (8.7) | 4 (17.4) | 1 (4.3) | 1 (4.3) |
| >45 | 7 (30.4) | 1 (4.3) | 7 (30.4) | - |
| Total (19) | 9 (39.1) | 5 (21.7) | 8 (34.9) | 1 (4.3) |
| Autopsy | ||
|---|---|---|
| Performed | Not Performed | |
| Male | 4 (17.4) | 15 (65.2) |
| Female | 2 (8.7) | 2 (8.7) |
| Total | 6 (26.1) | 17 (73.9) |
| Autoptic Data | Aneurysm (n = 5) | Pseudoaneurysm (n = 1) |
|---|---|---|
| Internal Examination | ||
| Pericarditis | 1 | 0 |
| Hemorrhage | 1 | 0 |
| Graft rupture | 2 | 1 |
| Graft occlusion | 1 | 1 |
| Graft aneurysm | 5 | 0 |
| Graft pseudoaneurysm | 0 | 1 |
| Dissecting aneurysm (graft) | 1 | 0 |
| Coronary atherosclerosis | 2 | 0 |
| Hemopericardium | 1 | 0 |
| Hemomediastinum | 2 | 0 |
| Heart weight grown | 2 | 0 |
| Left ventricle dilatation | 2 | 0 |
| Lung congestion | 1 | 0 |
| Histology | ||
| Heart: Polymorphonuclear infiltrate | 1 | 0 |
| Acute phlebitis (graft) | 1 | 0 |
| Coronary atherosclerosis | 2 | 0 |
| Graft atherosclerosis | 2 | 1 |
| Graft dissection | 1 | 0 |
| Intramural hemorrhagic dissection (graft) | 1 | 0 |
| Myocardial infarct | 2 | 0 |
| Thrombi | 1 | 1 |
| Hemorrhage | 2 | 0 |
| Fibrin | 2 | 0 |
| Calcification | 2 | 0 |
| Myocardial interstitial fibrosis | 1 | 0 |
| Myocyte hypertrophy | 1 | 0 |
| Graft occlusion | 1 | 1 |
| Graft aneurysm | 5 | 0 |
| Graft pseudoaneurysm | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezzetti, E.; Maiese, A.; Spina, F.; Del Duca, F.; De Matteis, A.; Di Paolo, M.; La Russa, R.; Turillazzi, E.; Fineschi, V. Early Saphenous Vein Graft Aneurysm Rupture: A Not So-Late Complication. Case Report and Comprehensive Literature Review. Biomedicines 2023, 11, 220. https://doi.org/10.3390/biomedicines11010220
Mezzetti E, Maiese A, Spina F, Del Duca F, De Matteis A, Di Paolo M, La Russa R, Turillazzi E, Fineschi V. Early Saphenous Vein Graft Aneurysm Rupture: A Not So-Late Complication. Case Report and Comprehensive Literature Review. Biomedicines. 2023; 11(1):220. https://doi.org/10.3390/biomedicines11010220
Chicago/Turabian StyleMezzetti, Eleonora, Aniello Maiese, Federica Spina, Fabio Del Duca, Alessandra De Matteis, Marco Di Paolo, Raffaele La Russa, Emanuela Turillazzi, and Vittorio Fineschi. 2023. "Early Saphenous Vein Graft Aneurysm Rupture: A Not So-Late Complication. Case Report and Comprehensive Literature Review" Biomedicines 11, no. 1: 220. https://doi.org/10.3390/biomedicines11010220
APA StyleMezzetti, E., Maiese, A., Spina, F., Del Duca, F., De Matteis, A., Di Paolo, M., La Russa, R., Turillazzi, E., & Fineschi, V. (2023). Early Saphenous Vein Graft Aneurysm Rupture: A Not So-Late Complication. Case Report and Comprehensive Literature Review. Biomedicines, 11(1), 220. https://doi.org/10.3390/biomedicines11010220








