The Overlap Syndrome of Obstructive Sleep Apnea and Chronic Obstructive Pulmonary Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Data Extraction
2.3. Risk-of-Bias Assessment
3. Results
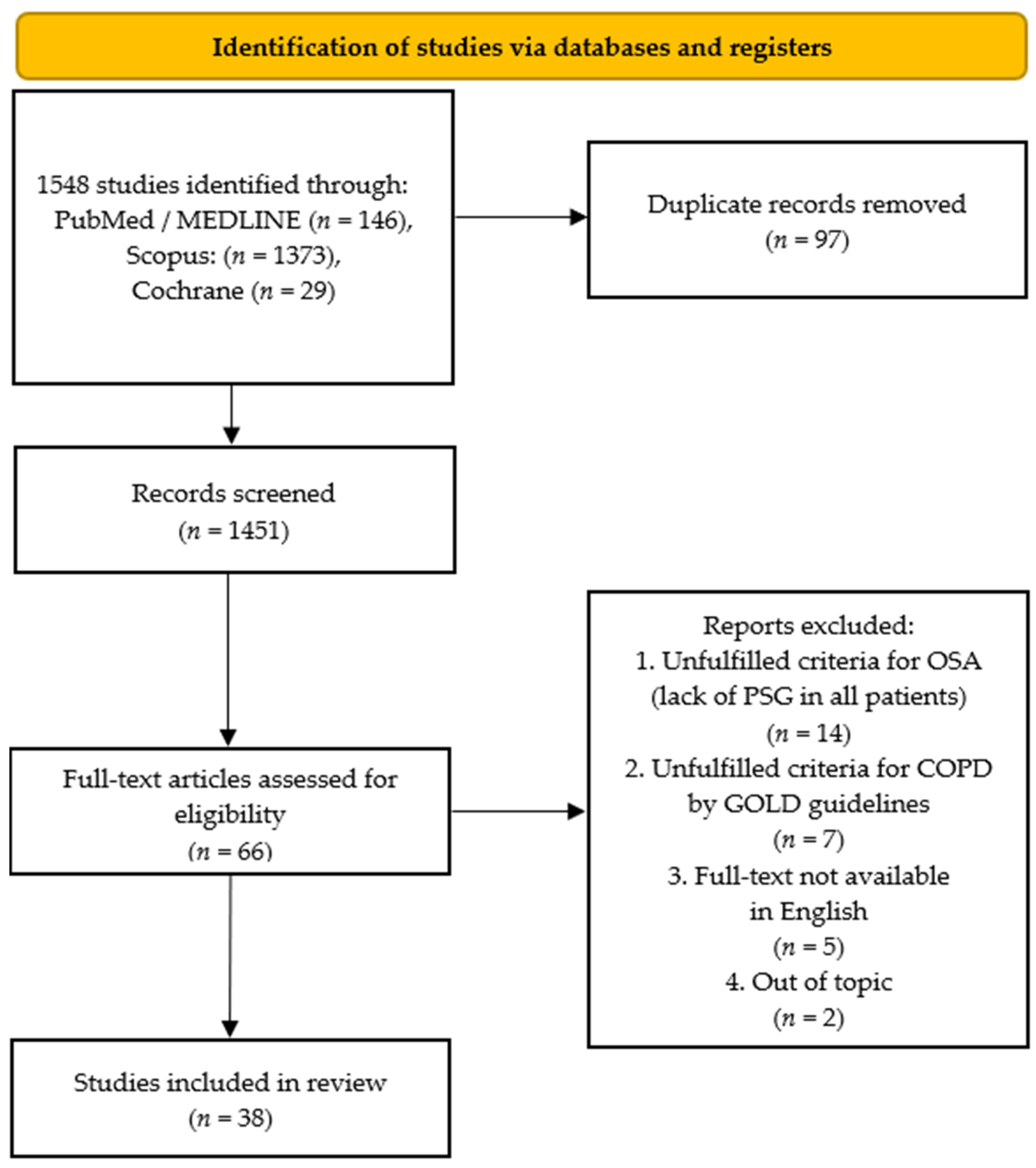
3.1. Search Results
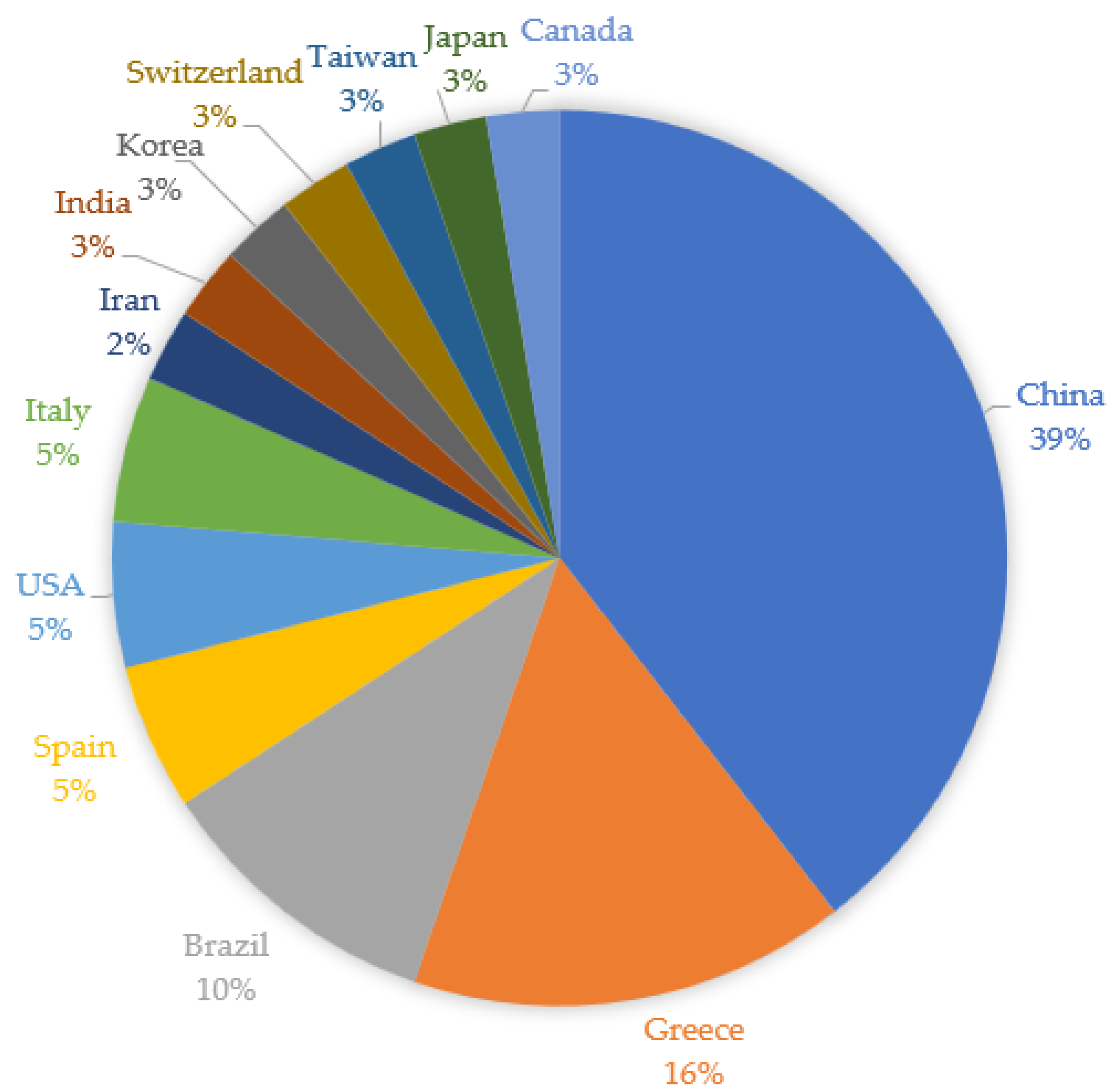
3.2. Study Characteristics and Study Quality
3.3. Systematic Review: Broad Overview and Study Characteristics of All Included Studies
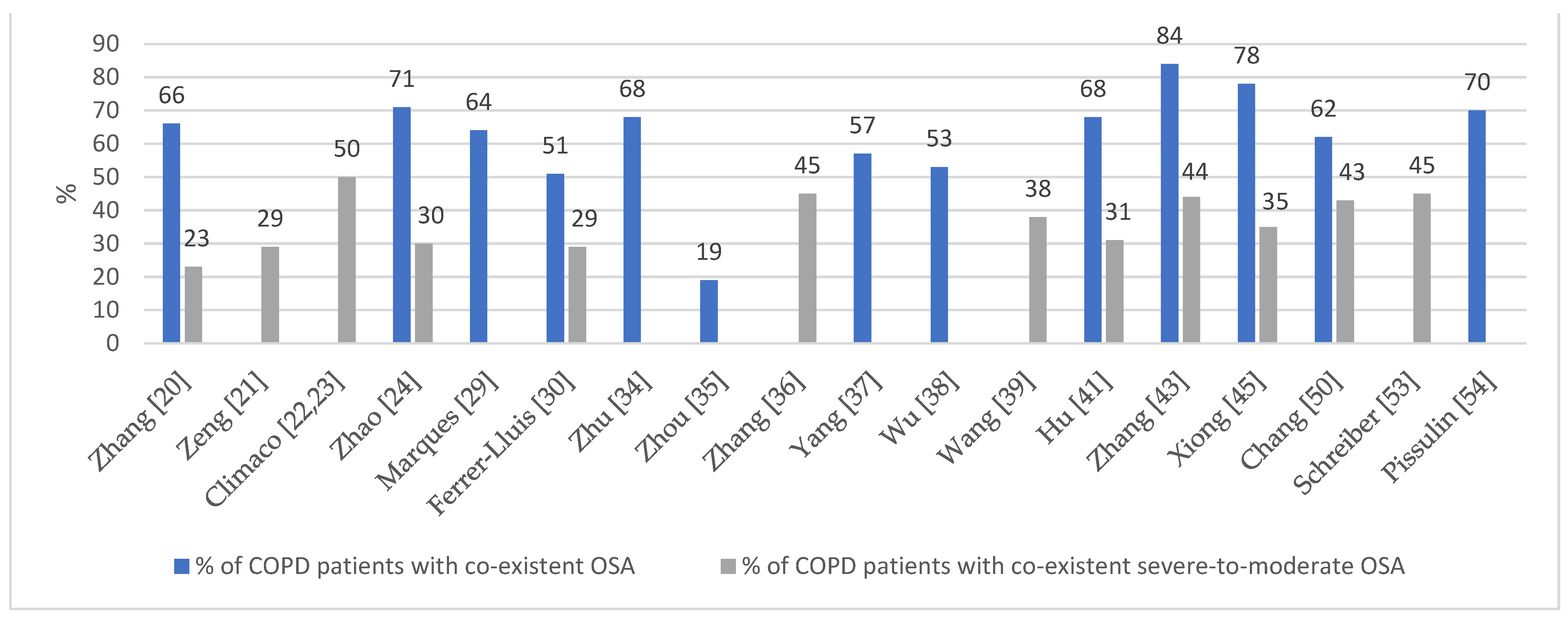
3.3.1. Prevalence of Overlap Syndrome
3.3.2. Meaning of Age and Gender in Overlap Syndrome
3.3.3. Relevance of Body Mass Index, Smoking, and Alcohol Consumption in Overlap Syndrome
3.3.4. Sleep Quality and Other Aspects of Quality of Life in Overlap Syndrome
3.3.5. Polysomnography Findings and Pulmonary Function in Overlap Syndrome
3.3.6. Blood Test Results in Overlap Syndrome
- A.
- Complete blood counts
- B.
- Metabolic and biochemical results
- C.
- Inflammatory indicators
3.3.7. The Role of Comorbidities in Overlap Syndrome
Hypertension, Coronary Heart Disease, and Cardiovascular Diseases in Overlap Syndrome
Role of Diabetes and Metabolic Syndrome in Overlap Syndrome
Depression, Anxiety, and Cognitive Function in Overlap Syndrome
3.3.8. Risk of Mortality in Overlap Syndrome
3.3.9. Treatment in Overlap Syndrome
Tools for Identifying Overlap Syndrome Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AFL | Airflow limitation |
| AHI | Apnea–hypopnea index |
| BMI | Body mass index |
| BQ | Berlin questionnaire |
| CCI | Charlson comorbidity index |
| CHD | Coronary heart disease |
| COPD | Chronic obstructive pulmonary disease |
| CPAP | Continuous positive airway pressure |
| CRP | C-reactive protein |
| CV | Cardiovascular |
| CVD | Cardiovascular disease |
| DM | Diabetes mellitus |
| ESS | Epworth sleepiness scale |
| FEV1 | Forced expiratory volume in 1 s |
| FVC | Forced vital capacity |
| FSS | Fatigue severity scale |
| GOLD | Global Initiative for Obstructive Lung Disease |
| HADS | Hospital anxiety and depression scale |
| HDL-C | High-density lipoprotein cholesterol |
| HbA1c | Glycated hemoglobin |
| MS | Metabolic syndrome |
| NREM | Non-rapid eye movement |
| ODI | Oxygen desaturation index |
| OS | Overlap syndrome of chronic obstructive pulmonary disease and obstructive sleep apnea |
| OSA | Obstructive sleep apnea |
| PaO2 | Partial pressure of oxygen |
| PaCO2 | Partial pressure of carbon dioxide |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PSG | Polysomnography |
| RBC | Red blood cell |
| REM | Rapid eye movement |
| SaO2 | Oxygen saturation |
| SBQ | STOP-BANG questionnaire |
| TG | Triglycerides |
| TS90% | Percentage of total sleep time with SaO2 below 90% |
| TST | Total sleep time |
| VC | Vital capacity |
| QoL | Quality of life |
References
- Malhotra, A.; Schwartz, A.R.; Schneider, H.; Owens, R.L.; DeYoung, P.; Han, M.K.; Wedzicha, J.A.; Hansel, N.N.; Zeidler, M.R.; Wilson, K.C.; et al. Research Priorities in Pathophysiology for Sleep-disordered Breathing in Patients with Chronic Obstructive Pulmonary Disease. An Official American Thoracic Society Research Statement. Am. J. Respir. Crit. Care Med. 2018, 197, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.A.; Eckert, D.J.; Jordan, A.S. Obstructive sleep apnoea pathogenesis from mild to severe: Is it all the same? Respirology 2017, 22, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Mesarwi, O.; Pepin, J.L.; Owens, R.L. Endotypes and phenotypes in obstructive sleep apnea. Curr. Opin. Pulm. Med. 2020, 26, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Vestbo, J.; Hurd, S.S.; Agusti, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Cruz, M.M.; Pereira, M. Epidemiology of Chronic Obstructive Pulmonary Disease in Brazil: A systematic review and meta-analysis. Cienc. Saude Colet. 2020, 25, 4547–4557. [Google Scholar] [CrossRef]
- Biswas, D.; Mukherjee, S.; Chakroborty, R.; Chatterjee, S.; Rath, S.; Das, R.; Begum, S. Occurrence of Anxiety and Depression among Stable COPD Patients and its Impact on Functional Capability. J. Clin. Diagn. Res. 2017, 11, OC24–OC27. [Google Scholar] [CrossRef]
- Stewart, N.H.; Walters, R.; Press, V.; Mokhlesi, B.; Lauderdale, D.; Arora, V. Evaluation of Sleep in Hospitalized Patients with Chronic Obstructive Pulmonary Disease: An Observational Study. Am. J. Respir. Crit. Care Med. 2019, 199, A7111. [Google Scholar] [CrossRef]
- Soriano, J.B.; Abajobir, A.A.; Abate, K.H.; Abera, S.F.; Agrawal, A.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; Aichour, M.T.E.; Alam, K.; et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef]
- Flenley, D.C. Sleep in chronic obstructive lung disease. Clin. Chest Med. 1985, 6, 651–661. [Google Scholar] [CrossRef]
- Weitzenblum, E.; Chaouat, A.; Kessler, R.; Canuet, M. Overlap syndrome: Obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2008, 5, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Khazaie, H.; Abolfathi, M.; Ghasemi, H.; Shabani, S.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. The effect of obstructive sleep apnea on the increased risk of cardiovascular disease: A systematic review and meta-analysis. Neurol. Sci. 2022, 43, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, X.; Guo, M.; Xie, M.; Liu, X. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: Systematic analysis for the Global Burden of Disease Study 2017. BMJ 2020, 368, m234. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Soriano, J.B.; Carrizo, S.J.; Boldova, A.; Celli, B.R. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: The overlap syndrome. Am. J. Respir. Crit. Care Med. 2010, 182, 325–331. [Google Scholar] [CrossRef]
- Epstein, L.J.; Kristo, D.; Strollo, P.J., Jr.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar]
- Chang, J.L.; Goldberg, A.N.; Alt, J.A.; Ashbrook, L.; Auckley, D.; Ayappa, I.; Bakhtiar, H.; Barrera, J.E.; Bartley, B.L.; Billings, M.E.; et al. International consensus statement on obstructive sleep apnea. Int. Forum Allergy Rhinol. 2022. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa quality assessment scale cohort studies. Univ. Ott. 2014. [Google Scholar]
- Herzog, R.; Alvarez-Pasquin, M.; Diaz, C.; Del Barrio, J.; Estrada, J.; Gil, A. Newcastle-Ottawa Scale adapted for cross-sectional studies. BMC Public Health 2013, 13, 154. [Google Scholar]
- Zhang, P.; Chen, B.; Lou, H.; Zhu, Y.; Chen, P.; Dong, Z.; Zhu, X.; Li, T.; Lou, P. Predictors and outcomes of obstructive sleep apnea in patients with chronic obstructive pulmonary disease in China. BMC Pulm. Med. 2022, 22, 16. [Google Scholar] [CrossRef]
- Zeng, Z.; Song, Y.; He, X.; Yang, H.; Yue, F.; Xiong, M.; Hu, K. Obstructive Sleep Apnea is Associated with an Increased Prevalence of Polycythemia in Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Clímaco, D.C.S.; Lustosa, T.C.; Silva, M.; Lins-Filho, O.L.; Rodrigues, V.K.; Oliveira-Neto, L.A.P.; Feitosa, A.D.M.; Queiroga, F.J.P., Jr.; Cabral, M.M.; Pedrosa, R.P. Sleep quality in COPD patients: Correlation with disease severity and health status. J. Bras. Pneumol. 2022, 48, e20210340. [Google Scholar] [CrossRef]
- Clímaco, D.C.S.; Lustosa, T.C.; de F.P., M.V.; Lins-Filho, O.L.; Rodrigues, V.K.; de Oliveira Neto, L.A.P.; Feitosa, A.D.M..; Queiroga Júnior, F.J.P.; Cabral, M.M.; Pedrosa, R.P. Is obstructive sleep apnea associated with increased arterial stiffness in patients with COPD? Sleep Breath. 2022, 1–6. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, D.; Sun, H.; Chang, D.; Lv, X.; Lin, J.; Liu, J.; Wu, X.; Hu, K.; Guo, X.; et al. Anxiety and depression in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: The overlap syndrome. Sleep Breath. 2021, 26, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Voulgaris, A.; Archontogeorgis, K.; Pataka, A.; Flaris, A.N.; Ntolios, P.; Bonsignore, M.R.; Schiza, S.; Steiropoulos, P. Burden of Comorbidities in Patients with OSAS and COPD-OSAS Overlap Syndrome. Medicina 2021, 57, 1201. [Google Scholar] [CrossRef]
- Tang, M.; Wang, Y.; Wang, M.; Tong, R.; Shi, T. Risk for Cardiovascular Disease and One-Year Mortality in Patients With Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea Syndrome Overlap Syndrome. Front. Pharmacol. 2021, 12, 2882. [Google Scholar] [CrossRef] [PubMed]
- Sami, R.; Hashemi, S.; Jalilolghadr, S. Polysomnography findings of patients with overlap syndrome according to severity of lower airway obstruction. J. Res. Med. Sci. 2021, 26, 130. [Google Scholar] [CrossRef] [PubMed]
- Nattusami, L.; Hadda, V.; Khilnani, G.; Madan, K.; Mittal, S.; Tiwari, P.; Mohan, A.; Khan, M.; Guleria, R. Co-existing obstructive sleep apnea among patients with chronic obstructive pulmonary disease. Lung India 2021, 38, 12–17. [Google Scholar] [CrossRef]
- Marques, R.D.; Berton, D.C.; Domnik, N.J.; Driver, H.; Elbehairy, A.F.; Fitzpatrick, M.; O’donnell, D.E.; Fagondes, S.; Neder, J.A. Sleep quality and architecture in copd: The relationship with lung function abnormalities. J. Bras. De Pneumol. 2021, 47, e20200612. [Google Scholar] [CrossRef]
- Ferrer-Lluis, I.; Castillo-Escario, Y.; Glos, M.; Fietze, I.; Penzel, T.; Jane, R. Sleep Apnea & Chronic Obstructive Pulmonary Disease: Overlap Syndrome Dynamics in Patients from an Epidemiological Study. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; Volume 2021, pp. 5574–5577. [Google Scholar] [CrossRef]
- Bae, E.; Kwak, N.; Choi, S.M.; Lee, J.; Park, Y.S.; Lee, C.H.; Lee, S.M.; Yoo, C.G.; Cho, J. Mortality prediction in chronic obstructive pulmonary disease and obstructive sleep apnea. Sleep Med. 2021, 87, 143–150. [Google Scholar] [CrossRef]
- Akinnusi, M.; El-Masri, A.R.; Lawson, Y.; El-Solh, A.A. Association of overlap syndrome with incident atrial fibrillation. Intern Emerg. Med. 2021, 16, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.; Bailly, S.; Soccal, P.M.; Janssens, J.P.; Sapène, M.; Grillet, Y.; Stach, B.; Tamisier, R.; Pépin, J.L. Symptomatic response to CPAP in obstructive sleep apnea versus COPD-obstructive sleep apnea overlap syndrome: Insights from a large national registry. PLoS ONE 2021, 16, e0256230. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhao, Z.; Nie, Q.; Wang, Y.; Fu, Z.; Guo, X.; Hu, K. Effect of lung function on the apnea-hypopnea index in patients with overlap syndrome: A multicenter cross-sectional study. Sleep Breath. 2020, 24, 1059–1066. [Google Scholar] [CrossRef]
- Zhou, W.; Li, C.L.; Cao, J.; Feng, J. Metabolic syndrome prevalence in patients with obstructive sleep apnea syndrome and chronic obstructive pulmonary disease: Relationship with systemic inflammation. Clin. Respir. J. 2020, 14, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Gao, B.; Han, T.; Xiang, B.Y.; Liu, X. Moderate-to-Severe Obstructive Sleep Apnea and Cognitive Function Impairment in Patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1813–1822. [Google Scholar] [CrossRef]
- Yang, X.; Tang, X.; Cao, Y.; Dong, L.; Wang, Y.; Zhang, J.; Cao, J. The Bronchiectasis in COPD-OSA Overlap Syndrome Patients. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 605–611. [Google Scholar] [CrossRef]
- Wu, Q.; Xie, L.; Li, W.; Xiang, G.; Hu, W.; Jiang, H.; Wu, X.; Wu, X.; Li, S. Pulmonary Function Influences the Performance of Berlin Questionnaire, Modified Berlin Questionnaire, and STOP-Bang Score for Screening Obstructive Sleep Apnea in Subjects with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1207–1216. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Li, P.; Gong, T.; Wu, M.; Fu, J.; Nie, M.; Dong, Y.; Hu, K. Severe obstructive sleep apnea in patients with chronic obstructive pulmonary disease is associated with an increased prevalence of mild cognitive impairment. Sleep Med. 2020, 75, 522–530. [Google Scholar] [CrossRef]
- Wang, T.Y.; Tsai, M.H.; Ni, Y.L.; Lin, T.Y.; Huang, S.Y.; Lo, Y.L. A Simplified Screening Questionnaire for Detecting Severe OSA in Chronic Obstructive Airway Disease in Asian Population. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 191–196. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, Z.; Wu, B.; Shi, Z.; Dong, M.; Xiong, M.; Jiang, Y.; Liu, D.; Li, H.; Hu, K. Obstructive Sleep Apnea Increases the Prevalence of Hypertension in Patients with Chronic Obstructive Disease. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 523–532. [Google Scholar] [CrossRef]
- Archontogeorgis, K.; Voulgaris, A.; Papanas, N.; Nena, E.; Xanthoudaki, M.; Pataka, A.; Schiza, S.; Rizzo, M.; Froudarakis, M.E.; Steiropoulos, P. Metabolic Syndrome in Patients with Coexistent Obstructive Sleep Apnea Syndrome and Chronic Obstructive Pulmonary Disease (Overlap Syndrome). Metab. Syndr. Relat. Disord. 2020, 18, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Dai, H.P.; Zhang, H.; Gao, B.; Zhang, L.; Han, T.; Wang, C. Obstructive Sleep Apnea in Patients With Fibrotic Interstitial Lung Disease and COPD. J. Clin. Sleep Med. 2019, 15, 1807–1815. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Shiota, S.; Kusunoki, Y.; Hamaya, H.; Ishii, M.; Kodama, Y.; Akishita, M.; Kida, K.; Takahashi, K.; Nagase, T.; et al. Polysomnographic features of low arousal threshold in overlap syndrome involving obstructive sleep apnea and chronic obstructive pulmonary disease. Sleep Breath. 2019, 23, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Hu, W.; Dong, M.; Wang, M.; Chen, J.; Xiong, H.; Zhong, M.; Jiang, Y.; Liu, D.; Hu, K. The Screening Value Of ESS, SACS, BQ, And SBQ On Obstructive Sleep Apnea In Patients With Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2497–2505. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Hou, W.J.; Dong, L.X.; Cao, J. Endothelial function and T-lymphocyte subsets in patients with overlap syndrome of chronic obstructive pulmonary disease and obstructive sleep apnea. Chin. Med. J. 2019, 132, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Voulgaris, A.; Archontogeorgis, K.; Papanas, N.; Pilitsi, E.; Nena, E.; Xanthoudaki, M.; Mikhailidis, D.P.; Froudarakis, M.E.; Steiropoulos, P. Increased risk for cardiovascular disease in patients with obstructive sleep apnoea syndrome-chronic obstructive pulmonary disease (overlap syndrome). Clin. Respir. J. 2019, 13, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Leung, R.S.; Aaron, S.D.; Ayas, N.; Sandoz, J.S.; Gershon, A.S. Cardiovascular Outcomes and All-Cause Mortality in Patients with Obstructive Sleep Apnea and Chronic Obstructive Pulmonary Disease (Overlap Syndrome). Ann. Am. Thorac. Soc. 2019, 16, 71–81. [Google Scholar] [CrossRef]
- Jaoude, P.; El-Solh, A.A. Predictive factors for COPD exacerbations and mortality in patients with overlap syndrome. Clin. Respir. J. 2019, 13, 643–651. [Google Scholar] [CrossRef]
- Chang, Y.; Xu, L.; Han, F.; Keenan, B.T.; Kneeland-Szanto, E.; Zhang, R.; Zhang, W.; Yu, Y.; Zuo, Y.; Pack, A.I.; et al. Validation of the Nox-T3 Portable Monitor for Diagnosis of Obstructive Sleep Apnea in Patients With Chronic Obstructive Pulmonary Disease. J. Clin. Sleep Med. 2019, 15, 587–596. [Google Scholar] [CrossRef]
- Álvarez, D.; Sánchez-Fernández, A.; Andrés-Blanco, A.M.; Gutiérrez-Tobal, G.C.; Vaquerizo-Villar, F.; Barroso-García, V.; Hornero, R.; del Campo, F. Influence of chronic obstructive pulmonary disease and moderate-to-severe sleep apnoea in overnight cardiac autonomic modulation: Time, frequency and non-linear analyses. Entropy 2019, 21, 381. [Google Scholar] [CrossRef]
- Schreiber, A.; Surbone, S.; Malovini, A.; Mancini, M.; Cemmi, F.; Piaggi, G.; Ceriana, P.; Carlucci, A. The effect of continuous positive airway pressure on pulmonary function may depend on the basal level of forced expiratory volume in 1 second. J. Thorac. Dis. 2018, 10, 6819–6827. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.; Cemmi, F.; Ambrosino, N.; Ceriana, P.; Lastoria, C.; Carlucci, A. Prevalence and Predictors of Obstructive Sleep Apnea in Patients with Chronic Obstructive Pulmonary Disease Undergoing Inpatient Pulmonary Rehabilitation. COPD J. Chronic Obstr. Pulm. Dis. 2018, 15, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Pissulin, F.D.M.; Pacagnelli, F.L.; Aldá, M.A.; Beneti, R.; Barros, J.L.; Minamoto, S.T.; Weber, S.A.T. The triad of obstructive sleep apnea syndrome, COPD, and obesity: Sensitivity of sleep scales and respiratory questionnaires. J. Bras. Pneumol. 2018, 44, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Papachatzakis, I.; Velentza, L.; Zarogoulidis, P.; Kallianos, A.; Trakada, G. Comorbidities in coexisting chronic obstructive pulmonary disease and obstructive sleep apnea-overlap syndrome. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4325–4331. [Google Scholar] [CrossRef]
- Economou, N.T.; Ilias, I.; Velentza, L.; Papachatzakis, Y.; Zarogoulidis, P.; Kallianos, A.; Trakada, G. Sleepiness, fatigue, anxiety and depression in Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea-Overlap-Syndrome, before and after continuous positive airways pressure therapy. PLoS ONE 2018, 13, e0197342. [Google Scholar] [CrossRef]
- Archontogeorgis, K.; Voulgaris, A.; Papanas, N.; Nena, E.; Froudarakis, M.; Mikhailidis, D.P.; Steiropoulos, P. Mean Platelet Volume and Platelet Distribution Width in Patients With Obstructive Sleep Apnea Syndrome and Concurrent Chronic Obstructive Pulmonary Disease. Clin. Appl. Thromb. Hemost. 2018, 24, 1216–1222. [Google Scholar] [CrossRef]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I.; Unit, N.R.G.R.H. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Soler, X.; Gaio, E.; Powell, F.L.; Ramsdell, J.W.; Loredo, J.S.; Malhotra, A.; Ries, A.L. High Prevalence of Obstructive Sleep Apnea in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2015, 12, 1219–1225. [Google Scholar] [CrossRef]
- Liu, W.; Guo, H.; Ding, F.; Cui, Z.; Zhang, J.; Wang, J.; Yuan, Y. Comparison of invasive intubation and noninvasive mechanical ventilation in patients with chronic obstructive pulmonary disease and obstructive sleep apnoea syndrome. J. Int. Med. Res. 2021, 49, 3000605211068312. [Google Scholar] [CrossRef]
- Adle, D.; Bailly, S.; Benmerad, M.; Joyeux-Faure, M.; Jullian-Desayes, I.; Soccal, P.M.; Janssens, J.P.; Sapène, M.; Grillet, Y.; Stach, B.; et al. Clinical presentation and comorbidities of obstructive sleep apnea-COPD overlap syndrome. PLoS ONE 2020, 15, e0235331. [Google Scholar] [CrossRef]
- Easter, M.; Bollenbecker, S.; Barnes, J.W.; Krick, S. Targeting Aging Pathways in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020, 21, 6924. [Google Scholar] [CrossRef]
- Spelta, F.; Fratta Pasini, A.M.; Cazzoletti, L.; Ferrari, M. Body weight and mortality in COPD: Focus on the obesity paradox. Eat Weight Disord 2018, 23, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, D.; Ge, Z.; Yan, M.; Wu, N.; Liu, Y. Body mass index of patients with chronic obstructive pulmonary disease is associated with pulmonary function and exacerbations: A retrospective real world research. J. Thorac. Dis. 2018, 10, 5086–5099. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Milne, S.; Jaw, J.E.; Yang, C.X.; Xu, F.; Li, X.; Obeidat, M.; Sin, D.D. BMI is associated with FEV1 decline in chronic obstructive pulmonary disease: A meta-analysis of clinical trials. Respir. Res. 2019, 20, 236. [Google Scholar] [CrossRef] [PubMed]
- Alter, P.; Kahnert, K.; Trudzinski, F.C.; Bals, R.; Watz, H.; Speicher, T.; Sohler, S.; Andreas, S.; Welte, T.; Rabe, K.F.; et al. Disease Progression and Age as Factors Underlying Multimorbidity in Patients with COPD: Results from COSYCONET. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1703–1713. [Google Scholar] [CrossRef]
- Gunduz, C.; Basoglu, O.K.; Tasbakan, M.S. Prevalence of overlap syndrome in chronic obstructive pulmonary disease patients without sleep apnea symptoms. Clin. Respir. J. 2018, 12, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Qi, H.; Fang, Q.; Tu, J.; Li, Q.; Wang, L. Smoking history and its relationship with comorbidities in patients with obstructive sleep apnea. Tob. Induc. Dis. 2020, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Taveira, K.V.M.; Kuntze, M.M.; Berretta, F.; de Souza, B.D.M.; Godolfim, L.R.; Demathe, T.; De Luca Canto, G.; Porporatti, A.L. Association between obstructive sleep apnea and alcohol, caffeine and tobacco: A meta-analysis. J. Oral Rehabil. 2018, 45, 890–902. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Chiu, N.Y.; Chang, C.C.; Chang, T.G.; Lane, H.Y. The association between cigarette smoking and obstructive sleep apnea. Tob. Induc. Dis. 2019, 17, 27. [Google Scholar] [CrossRef]
- Ben Amar, J.; Ben Mansour, A.; Zaibi, H.; Ben Safta, B.; Dhahri, B.; Aouina, H. Impact of smoking on the severity of Obstructive Sleep Apnea Hypopnea Syndrome. Tunis Med. 2018, 96, 477–482. [Google Scholar] [PubMed]
- Esen, A.D.; Akpinar, M. Relevance of obstructive sleep apnea and smoking: Obstructive sleep apnea and smoking. Fam. Pract. 2021, 38, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Bielicki, P.; Trojnar, A.; Sobieraj, P.; Wasik, M. Smoking status in relation to obstructive sleep apnea severity (OSA) and cardiovascular comorbidity in patients with newly diagnosed OSA. Adv. Respir. Med. 2019, 87, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, M.R.; Martin, J.L.; Kleerup, E.C.; Schneider, H.; Mitchell, M.N.; Hansel, N.N.; Sundar, K.; Schotland, H.; Basner, R.C.; Wells, J.M.; et al. Sleep disruption as a predictor of quality of life among patients in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS). Sleep 2018, 41, zsy044. [Google Scholar] [CrossRef] [PubMed]
- Akinci, B.; Aslan, G.K.; Kiyan, E. Sleep quality and quality of life in patients with moderate to very severe chronic obstructive pulmonary disease. Clin. Respir. J. 2018, 12, 1739–1746. [Google Scholar] [CrossRef]
- Serin, E.K.; Ister, E.D.; Ozdemir, A. The relationship between sleep quality and dyspnoea severity in patients with COPD. Afr. Health Sci. 2020, 20, 1785–1792. [Google Scholar] [CrossRef]
- Vukoja, M.; Kopitovic, I.; Milicic, D.; Maksimovic, O.; Pavlovic-Popovic, Z.; Ilic, M. Sleep quality and daytime sleepiness in patients with COPD and asthma. Clin. Respir. J. 2018, 12, 398–403. [Google Scholar] [CrossRef]
- Shah, A.; Ayas, N.; Tan, W.C.; Malhotra, A.; Kimoff, J.; Kaminska, M.; Aaron, S.D.; Jen, R. Sleep Quality and Nocturnal Symptoms in a Community-Based COPD Cohort. J. Chronic Obstr. Pulm. Dis. 2020, 17, 40–48. [Google Scholar] [CrossRef]
- Ierodiakonou, D.; Bouloukaki, I.; Kampouraki, M.; Papadokostakis, P.; Poulorinakis, I.; Lampraki, I.; Athanasiou, P.; Schiza, S.; Tsiligianni, I.; Greek, U.G. Subjective sleep quality is associated with disease status in COPD patients. The cross-sectional Greek UNLOCK study. Sleep Breath. 2020, 24, 1599–1605. [Google Scholar] [CrossRef]
- Chang, C.H.; Chuang, L.P.; Lin, S.W.; Lee, C.S.; Tsai, Y.H.; Wei, Y.F.; Cheng, S.L.; Hsu, J.Y.; Kuo, P.H.; Yu, C.J.; et al. Factors responsible for poor sleep quality in patients with chronic obstructive pulmonary disease. BMC Pulm. Med. 2016, 16, 118. [Google Scholar] [CrossRef][Green Version]
- Kitajima, T.; Marumo, S.; Shima, H.; Shirata, M.; Kawashima, S.; Inoue, D.; Katayama, Y.; Itotani, R.; Sakuramoto, M.; Fukui, M. Clinical impact of episodic nocturnal hypercapnia and its treatment with noninvasive positive pressure ventilation in patients with stable advanced COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Spicuzza, L.; Campisi, R.; Crimi, C.; Frasca, E.; Crimi, N. Prevalence and determinants of co-morbidities in patients with obstructive apnea and chronic obstructive pulmonary disease. Eur. J. Intern. Med. 2019, 69, e15–e16. [Google Scholar] [CrossRef] [PubMed]
- Stewart, N.H.; Brittan, M.; McElligott, M.; Summers, M.O.; Samson, K.; Press, V.G. Evaluating the Relationship of Airflow Obstruction in COPD with Severity of OSA among Patients with Overlap Syndrome. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1613–1621. [Google Scholar] [CrossRef]
- Naranjo, M.; Willes, L.; Prillaman, B.A.; Quan, S.F.; Sharma, S. Undiagnosed OSA May Significantly Affect Outcomes in Adults Admitted for COPD in an Inner-City Hospital. Chest 2020, 158, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, A.; Goto, T.; Faridi, M.K.; Camargo, C.A.; Hasegawa, K. Association of obstructive sleep apnoea with acute severity of chronic obstructive pulmonary disease exacerbation: A population-based study. Intern. Med. J. 2018, 48, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Ingebrigtsen, T.S.; Marott, J.L.; Lange, P. Witnessed sleep apneas together with elevated plasma glucose are predictors of COPD exacerbations. Eur. Clin. Respir. J. 2020, 7, 1765543. [Google Scholar] [CrossRef] [PubMed]
- El-Gazzar, A.G.; Kamel, M.H.; Elbahnasy, O.K.M.; El-Naggar, M.E. Prognostic value of platelet and neutrophil to lymphocyte ratio in COPD patients. Expert Rev. Respir. Med. 2020, 14, 111–116. [Google Scholar] [CrossRef]
- Brusselle, G.; Pavord, I.D.; Landis, S.; Pascoe, S.; Lettis, S.; Morjaria, N.; Barnes, N.; Hilton, E. Blood eosinophil levels as a biomarker in COPD. Respir. Med. 2018, 138, 21–31. [Google Scholar] [CrossRef]
- Malerba, M.; Olivini, A.; Radaeli, A.; Ricciardolo, F.L.; Clini, E. Platelet activation and cardiovascular comorbidities in patients with chronic obstructive pulmonary disease. Curr. Med. Res. Opin. 2016, 32, 885–891. [Google Scholar] [CrossRef]
- Nena, E.; Papanas, N.; Steiropoulos, P.; Zikidou, P.; Zarogoulidis, P.; Pita, E.; Constantinidis, T.C.; Maltezos, E.; Mikhailidis, D.P.; Bouros, D. Mean Platelet Volume and Platelet Distribution Width in non-diabetic subjects with obstructive sleep apnoea syndrome: New indices of severity? Platelets 2012, 23, 447–454. [Google Scholar] [CrossRef]
- Kalemci, S.; Akin, F.; Sarihan, A.; Sahin, C.; Zeybek, A.; Yilmaz, N. The relationship between hematological parameters and the severity level of chronic obstructive lung disease. Pol. Arch. Intern. Med. 2018, 128, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.; Gaete, D.; Rodriguez, D.; Hoogewijs, D.; Rauner, M.; Sormendi, S.; Wielockx, B. Hypoxia Pathway Proteins are Master Regulators of Erythropoiesis. Int. J. Mol. Sci. 2020, 21, 8131. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Giri, S.; Karmacharya, P.; Aryal, M.R. Obstructive sleep apnea syndrome and secondary polycythemia: Analysis of the nationwide inpatient sample. Sleep Med. 2015, 16, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, H.P.; Wang, P.; Yan, Y.R.; Li, S.Q.; Li, Q.Y. Nocturnal Mean Oxygen Saturation Is Associated with Secondary Polycythemia in Young Adults with Obstructive Sleep Apnea, Especially in Men. Nat. Sci. Sleep 2019, 11, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; DeMeo, D.L.; Silverman, E.K.; Make, B.J.; Wade, R.C.; Wells, J.M.; Cho, M.H.; Hobbs, B.D. Secondary polycythemia in chronic obstructive pulmonary disease: Prevalence and risk factors. BMC Pulm Med. 2021, 21, 235. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbons, C.M.; Goldstein, R.L.; Gottlieb, D.J.; Moy, M.L. Physical Activity in Overlap Syndrome of COPD and Obstructive Sleep Apnea: Relationship with Markers of Systemic Inflammation. J. Clin. Sleep Med. 2019, 15, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Macrea, M.; Campbell, S.; Martin, T.; Oursler, K.A. The peripheral neutrophils in subjects with COPD-OSA overlap syndrome and severe comorbidities: A feasible inflammatory biomarker? Adv. Clin. Exp. Med. 2018, 27, 1677–1682. [Google Scholar] [CrossRef]
- Marin-Oto, M.; Sanz-Rubio, D.; Santamaria-Martos, F.; Benitez, I.; Simon, A.L.; Forner, M.; Cubero, P.; Gil, A.; Sanchez-de-laTorre, M.; Barbe, F.; et al. Soluble RAGE in COPD, with or without coexisting obstructive sleep apnoea. Respir. Res. 2022, 23, 163. [Google Scholar] [CrossRef]
- Salimian, J.; Mirzaei, H.; Moridikia, A.; Harchegani, A.B.; Sahebkar, A.; Salehi, H. Chronic obstructive pulmonary disease: MicroRNAs and exosomes as new diagnostic and therapeutic biomarkers. J. Res. Med. Sci. 2018, 23, 27. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Gozal, D. Circulating exosomes in obstructive sleep apnea as phenotypic biomarkers and mechanistic messengers of end-organ morbidity. Respir. Physiol. Neurobiol. 2018, 256, 143–156. [Google Scholar] [CrossRef]
- Fleming, W.E.; Holty, J.C.; Bogan, R.K.; Hwang, D.; Ferouz-Colborn, A.S.; Budhiraja, R.; Redline, S.; Mensah-Osman, E.; Osman, N.I.; Li, Q.; et al. Use of blood biomarkers to screen for obstructive sleep apnea. Nat. Sci. Sleep 2018, 10, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Lu, X.; Long, H.; Li, T.; Zhang, Y. The association of hemocyte profile and obstructive sleep apnea. J. Clin. Lab. Anal. 2019, 33, e22680. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, F.; Wu, X.; Hou, W. Prevalence of pulmonary embolism in patients with obstructive sleep apnea and chronic obstructive pulmonary disease: The overlap syndrome. Heart. Lung 2019, 48, 261–265. [Google Scholar] [CrossRef]
- Xu, J.; Wei, Z.; Wang, X.; Li, X.; Wang, W. The risk of cardiovascular and cerebrovascular disease in overlap syndrome: A meta-analysis. J. Clin. Sleep Med. 2020, 16, 1199–1207. [Google Scholar] [CrossRef]
- McNicholas, W.T. Comorbid obstructive sleep apnoea and chronic obstructive pulmonary disease and the risk of cardiovascular disease. J. Thorac. Dis. 2018, 10, S4253–S4261. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S. Mechanisms of cardiovascular disease in obstructive sleep apnoea. J. Thorac. Dis. 2018, 10, S4201–S4211. [Google Scholar] [CrossRef]
- Luehrs, R.E.; Moreau, K.L.; Pierce, G.L.; Wamboldt, F.; Aloia, M.; Weinberger, H.D.; Make, B.; Bowler, R.; Crapo, J.D.; Meschede, K.; et al. Cognitive performance is lower among individuals with overlap syndrome than in individuals with COPD or obstructive sleep apnea alone: Association with carotid artery stiffness. J. Appl. Physiol. (1985) 2021, 131, 131–141. [Google Scholar] [CrossRef]
- Gale, N.S.; Albarrati, A.M.; Munnery, M.M.; McDonnell, B.J.; Benson, V.S.; Singer, R.M.T.; Cockcroft, J.R.; Shale, D.J. Aortic Pulse Wave Velocity as a Measure of Cardiovascular Risk in Chronic Obstructive Pulmonary Disease: Two-Year Follow-Up Data from the ARCADE Study. Medicina 2019, 55, 89. [Google Scholar] [CrossRef]
- Joyeux-Faure, M.; Tamisier, R.; Borel, J.C.; Millasseau, S.; Galerneau, L.M.; Destors, M.; Bailly, S.; Pepin, J.L. Contribution of obstructive sleep apnoea to arterial stiffness: A meta-analysis using individual patient data. Thorax 2018, 73, 1146–1151. [Google Scholar] [CrossRef]
- Sun, W.L.; Wang, J.L.; Jia, G.H.; Mi, W.J.; Liao, Y.X.; Huang, Y.W.; Hu, Z.; Zhang, L.Q.; Chen, Y.H. Impact of obstructive sleep apnea on pulmonary hypertension in patients with chronic obstructive pulmonary disease. Chin. Med. J. 2019, 132, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Simons, S.O.; Elliott, A.; Sastry, M.; Hendriks, J.M.; Arzt, M.; Rienstra, M.; Kalman, J.M.; Heidbuchel, H.; Nattel, S.; Wesseling, G.; et al. Chronic obstructive pulmonary disease and atrial fibrillation: An interdisciplinary perspective. Eur. Heart J. 2021, 42, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Moula, A.I.; Parrini, I.; Tetta, C.; Luca, F.; Parise, G.; Rao, C.M.; Mauro, E.; Parise, O.; Matteucci, F.; Gulizia, M.M.; et al. Obstructive Sleep Apnea and Atrial Fibrillation. J. Clin. Med. 2022, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Gershon, A.S.; Atzema, C.; Dorian, P.; Mangat, I.; Hawker, G.; Leung, R.S. Sleep Apnea Increases the Risk of New Hospitalized Atrial Fibrillation: A Historical Cohort Study. Chest 2018, 154, 1330–1339. [Google Scholar] [CrossRef]
- Xiao, X.; Han, H.; Wu, C.; He, Q.; Ruan, Y.; Zhai, Y.; Gao, Y.; Zhao, X.; He, J. Prevalence of Atrial Fibrillation in Hospital Encounters with End-Stage COPD on Home Oxygen: National Trends in the United States. Chest 2019, 155, 918–927. [Google Scholar] [CrossRef]
- Voulgaris, A.; Archontogeorgis, K.; Steiropoulos, P.; Papanas, N. Cardiovascular Disease in Patients with Chronic Obstructive Pulmonary Disease, Obstructive Sleep Apnoea Syndrome and Overlap Syndrome. Curr. Vasc. Pharmacol. 2021, 19, 285–300. [Google Scholar] [CrossRef]
- Sharma, B.; Neilan, T.G.; Kwong, R.Y.; Mandry, D.; Owens, R.L.; McSharry, D.; Bakker, J.P.; Malhotra, A. Evaluation of right ventricular remodeling using cardiac magnetic resonance imaging in co-existent chronic obstructive pulmonary disease and obstructive sleep apnea. J. Chronic Obstr. Pulm. Dis. 2013, 10, 4–10. [Google Scholar] [CrossRef]
- Chalegre, S.T.; Lins-Filho, O.L.; Lustosa, T.C.; Franca, M.V.; Couto, T.L.G.; Drager, L.F.; Lorenzi-Filho, G.; Bittencourt, M.S.; Pedrosa, R.P. Impact of CPAP on arterial stiffness in patients with obstructive sleep apnea: A meta-analysis of randomized trials. Sleep Breath. 2021, 25, 1195–1202. [Google Scholar] [CrossRef]
- Cowie, M.R.; Linz, D.; Redline, S.; Somers, V.K.; Simonds, A.K. Sleep Disordered Breathing and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 608–624. [Google Scholar] [CrossRef]
- Castan-Abad, M.T.; Montserrat-Capdevila, J.; Godoy, P.; Marsal, J.R.; Ortega, M.; Alseda, M.; Barbe, F. Diabetes as a risk factor for severe exacerbation and death in patients with COPD: A prospective cohort study. Eur. J. Public Health 2020, 30, 822–827. [Google Scholar] [CrossRef]
- Katsiki, N.; Steiropoulos, P.; Papanas, N.; Mikhailidis, D.P. Diabetes Mellitus and Chronic Obstructive Pulmonary Disease: An Overview. Exp. Clin. Endocrinol. Diabetes 2021, 129, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Lacedonia, D.; Carpagnano, G.E.; Patricelli, G.; Carone, M.; Gallo, C.; Caccavo, I.; Sabato, R.; Depalo, A.; Aliani, M.; Capozzolo, A.; et al. Prevalence of comorbidities in patients with obstructive sleep apnea syndrome, overlap syndrome and obesity hypoventilation syndrome. Clin. Respir. J. 2018, 12, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Müllerova, H.; Locantore, N.W.; Vestbo, J.; Watkins, M.L.; Wouters, E.F.M.; Rennard, S.I.; Sharafkhaneh, A. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am. J. Respir. Crit. Care Med. 2011, 183, 604–611. [Google Scholar] [CrossRef]
- Underner, M.; Cuvelier, A.; Peiffer, G.; Perriot, J.; Jaafari, N. The influence of anxiety and depression on COPD exacerbations. Rev. Mal. Respir. 2018, 35, 604–625. [Google Scholar] [CrossRef]
- Yohannes, A.M.; Mulerova, H.; Lavoie, K.; Vestbo, J.; Rennard, S.I.; Wouters, E.; Hanania, N.A. The Association of Depressive Symptoms with Rates of Acute Exacerbations in Patients with COPD: Results From a 3-year Longitudinal Follow-up of the ECLIPSE Cohort. J. Am. Med. Dir. Assoc. 2017, 18, 955–959.e956. [Google Scholar] [CrossRef]
- Huang, J.; Bian, Y.; Zhao, Y.; Jin, Z.; Liu, L.; Li, G. The Impact of Depression and Anxiety on Chronic Obstructive Pulmonary Disease Acute Exacerbations: A prospective cohort study. J. Affect. Disord. 2021, 281, 147–152. [Google Scholar] [CrossRef]
- Chen, Y.H.; Keller, J.K.; Kang, J.H.; Hsieh, H.J.; Lin, H.C. Obstructive sleep apnea and the subsequent risk of depressive disorder: A population-based follow-up study. J. Clin. Sleep Med. 2013, 9, 417–423. [Google Scholar] [CrossRef]
- Ben Thabet, J.; Gassara, I.; Smaoui, N.; Msaad, S.; Maalej Bouali, M.; Yaich, S.; Omri, S.; Feki, R.; Zouari, L.; Charfi, N.; et al. Effects of continuous positive airway pressure on depression, anxiety and quality of life in obstructive sleep apnea hypopnea syndrome patients. Encephale 2022, 48, 397–403. [Google Scholar] [CrossRef]
- Olaithe, M.; Bucks, R.S.; Hillman, D.R.; Eastwood, P.R. Cognitive deficits in obstructive sleep apnea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med. Rev. 2018, 38, 39–49. [Google Scholar] [CrossRef]
- Lin, Y.N.; Zhou, L.N.; Zhang, X.J.; Li, Q.Y.; Wang, Q.; Xu, H.J. Combined effect of obstructive sleep apnea and chronic smoking on cognitive impairment. Sleep Breath. 2016, 20, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qin, Z.; Li, W.; Li, X.; Shen, H.; Wang, W. Effects of somatotropic axis on cognitive dysfunction of obstructive sleep apnea. Sleep Breath. 2020, 24, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tulek, B.; Atalay, N.B.; Yildirim, G.; Kanat, F.; Suerdem, M. Cognitive function in chronic obstructive pulmonary disease: Relationship to global initiative for chronic obstructive lung disease 2011 categories. Respirology 2014, 19, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Crum, R.M.; Anthony, J.C.; Bassett, S.S.; Folstein, M.F. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993, 269, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.P.; Emch, J.T.; Rueschman, M.; Sands, S.A.; Shea, S.A.; Wellman, A.; Redline, S. Apnea-Hypopnea Event Duration Predicts Mortality in Men and Women in the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2019, 199, 903–912. [Google Scholar] [CrossRef]
- Du, W.; Liu, J.; Zhou, J.; Ye, D.; OuYang, Y.; Deng, Q. Obstructive sleep apnea, COPD, the overlap syndrome, and mortality: Results from the 2005–2008 National Health and Nutrition Examination Survey. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 665–674. [Google Scholar] [CrossRef]
- Kendzerska, T.; Povitz, M.; Bai, X.; Pakhale, S.; Wen, S.W.; Gershon, A.S. Coexistence of clinically significant obstructive sleep apnea with physician-diagnosed asthma or chronic obstructive pulmonary disease: A population study of prevalence and mortality. Can. J. Respir. Crit. Care Sleep Med. 2022, 6, 24–34. [Google Scholar] [CrossRef]
- Ioachimescu, O.C.; Janocko, N.J.; Ciavatta, M.M.; Howard, M.; Warnock, M.V. Obstructive Lung Disease and Obstructive Sleep Apnea (OLDOSA) cohort study: 10-year assessment. J. Clin. Sleep Med. 2020, 16, 267–277. [Google Scholar] [CrossRef]
- Singh, G.; Agarwal, A.; Zhang, W.; Kuo, Y.F.; Sultana, R.; Sharma, G. Impact of PAP therapy on hospitalization rates in Medicare beneficiaries with COPD and coexisting OSA. Sleep Breath. 2019, 23, 193–200. [Google Scholar] [CrossRef]
- Sterling, K.L.; Pepin, J.L.; Linde-Zwirble, W.; Chen, J.; Benjafield, A.V.; Cistulli, P.A.; Cole, K.V.; Emami, H.; Woodford, C.; Armitstead, J.P.; et al. Impact of Positive Airway Pressure Therapy Adherence on Outcomes in Patients with Obstructive Sleep Apnea and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2022, 206, 197–205. [Google Scholar] [CrossRef]
- Oktay Arslan, B.; Ucar Hosgor, Z.Z.; Orman, M.N. Which Screening Questionnaire is Best for Predicting Obstructive Sleep Apnea in the Sleep Clinic Population Considering Age, Gender, and Comorbidities? Turk. Thorac. J. 2020, 21, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Amra, B.; Rahmati, B.; Soltaninejad, F.; Feizi, A. Screening Questionnaires for Obstructive Sleep Apnea: An Updated Systematic Review. Oman. Med. J. 2018, 33, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.M.; Feemster, L.C.; Udris, E.M.; Griffith, M.F.; Spece, L.J.; Palen, B.N.; He, K.; Parthasarathy, S.; Strohl, K.P.; Kapur, V.K.; et al. Poor Outcomes among Patients with Chronic Obstructive Pulmonary Disease with Higher Risk for Undiagnosed Obstructive Sleep Apnea in the LOTT Cohort. J. Clin. Sleep Med. 2019, 15, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Soler, X.; Liao, S.Y.; Marin, J.M.; Lorenzi-Filho, G.; Jen, R.; De Young, P.; Owens, R.L.; Ries, A.L.; Malhotra, A. Age, gender, neck circumference, and Epworth sleepiness scale do not predict obstructive sleep apnea (OSA) in moderate to severe chronic obstructive pulmonary disease (COPD): The challenge to predict OSA in advanced COPD. PLoS ONE 2017, 12, e0177289. [Google Scholar] [CrossRef] [PubMed]
- Jen, R.; Orr, J.E.; Li, Y.; DeYoung, P.; Smales, E.; Malhotra, A.; Owens, R.L. Accuracy of WatchPAT for the Diagnosis of Obstructive Sleep Apnea in Patients with Chronic Obstructive Pulmonary Disease. J. Chronic Obstr. Pulm. Dis. 2020, 17, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kaur, H.; Singh, S.; Khawaja, I. The Overlap Syndrome. Cureus 2018, 10, e3453. [Google Scholar] [CrossRef]
- Olszewska, E.; Vasilenok, N.; Polecka, A.; Strozynski, A.; Olszewska, N.; Rogowski, M.; Fiedorczuk, P. Long-term outcomes of pharyngoplasty for Obstructive Sleep Apnea Syndrome. Otolaryngol. Pol. 2022, 76, 18–25. [Google Scholar] [CrossRef]
- Lajoie, A.C.; Sériès, F.; Bernard, S.; Bernard, E.; Santaolalla, C.J.E.; Abad Fernández, A.; Maltais, F.; Lacasse, Y. Reliability of Home Nocturnal Oximetry in the Diagnosis of Overlap Syndrome in COPD. Respiration 2020, 99, 132–139. [Google Scholar] [CrossRef]
- Sforza, E.; Roche, F.; Chapelle, C.; Pichot, V. Internight Variability of Apnea-Hypopnea Index in Obstructive Sleep Apnea Using Ambulatory Polysomnography. Front. Physiol. 2019, 10, 849. [Google Scholar] [CrossRef]
- Roeder, M.; Bradicich, M.; Schwarz, E.I.; Thiel, S.; Gaisl, T.; Held, U.; Kohler, M. Night-to-night variability of respiratory events in obstructive sleep apnoea: A systematic review and meta-analysis. Thorax 2020, 75, 1095–1102. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Completed, published | Unfinished, unpublished |
| Original articles | Reviews, letters to the editor, conference papers, case reports, book chapters, expert opinions |
| Observational studies | Experimental studies |
| Full text available in English | Language other than English or only abstract available in English |
| Human studies | Animal studies |
| Studies concerning the OS | Studies not related to the OS |
| Diagnosis of COPD by GOLD guidelines [4] | Diagnosis of COPD not matching the GOLD criteria [4] |
| Diagnosis of OSA based on PSG | Diagnosis of OSA based on other sleep studies than PSG, such as polygraphy or a questionnaire survey |
| Good-quality studies | Poor-quality studies |
| Studies published from January 2018 to 26 October 2022 | Studies published before January 2018 |
| At least 50 participants | Fewer than 50 participants |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwaty, K.; Dżaman, K.; Sobczyk, K.M.; Sikorska, K.I. The Overlap Syndrome of Obstructive Sleep Apnea and Chronic Obstructive Pulmonary Disease: A Systematic Review. Biomedicines 2023, 11, 16. https://doi.org/10.3390/biomedicines11010016
Czerwaty K, Dżaman K, Sobczyk KM, Sikorska KI. The Overlap Syndrome of Obstructive Sleep Apnea and Chronic Obstructive Pulmonary Disease: A Systematic Review. Biomedicines. 2023; 11(1):16. https://doi.org/10.3390/biomedicines11010016
Chicago/Turabian StyleCzerwaty, Katarzyna, Karolina Dżaman, Krystyna Maria Sobczyk, and Katarzyna Irmina Sikorska. 2023. "The Overlap Syndrome of Obstructive Sleep Apnea and Chronic Obstructive Pulmonary Disease: A Systematic Review" Biomedicines 11, no. 1: 16. https://doi.org/10.3390/biomedicines11010016
APA StyleCzerwaty, K., Dżaman, K., Sobczyk, K. M., & Sikorska, K. I. (2023). The Overlap Syndrome of Obstructive Sleep Apnea and Chronic Obstructive Pulmonary Disease: A Systematic Review. Biomedicines, 11(1), 16. https://doi.org/10.3390/biomedicines11010016







