Impact of Hypermannosylation on the Structure and Functionality of the ER and the Golgi Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. Histological Analysis of Murine Tissue
2.2. Mouse Embryonic Fibroblast (MEF) Experiments
2.3. Western Blot
2.4. Sugar Measurements
2.5. Furin Activity Assay
2.6. GDP-Mannose Measurements
2.7. Glycoprotein Enrichment and Mass Spectrometry
2.8. Cell Fractionation of Transfected Cells
2.9. Electron Microscopy (EM) Analysis
2.10. Statistical Analysis
3. Results
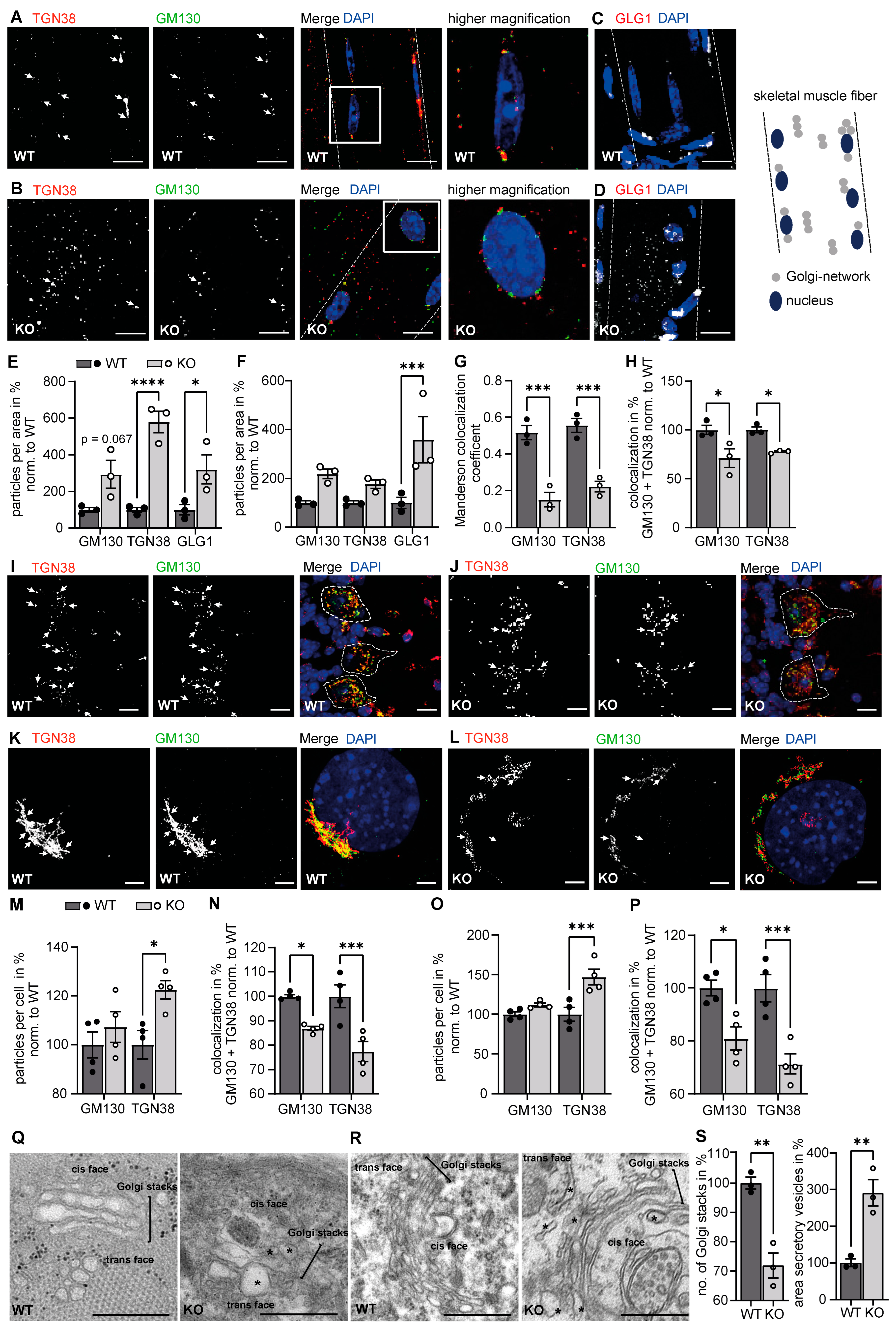
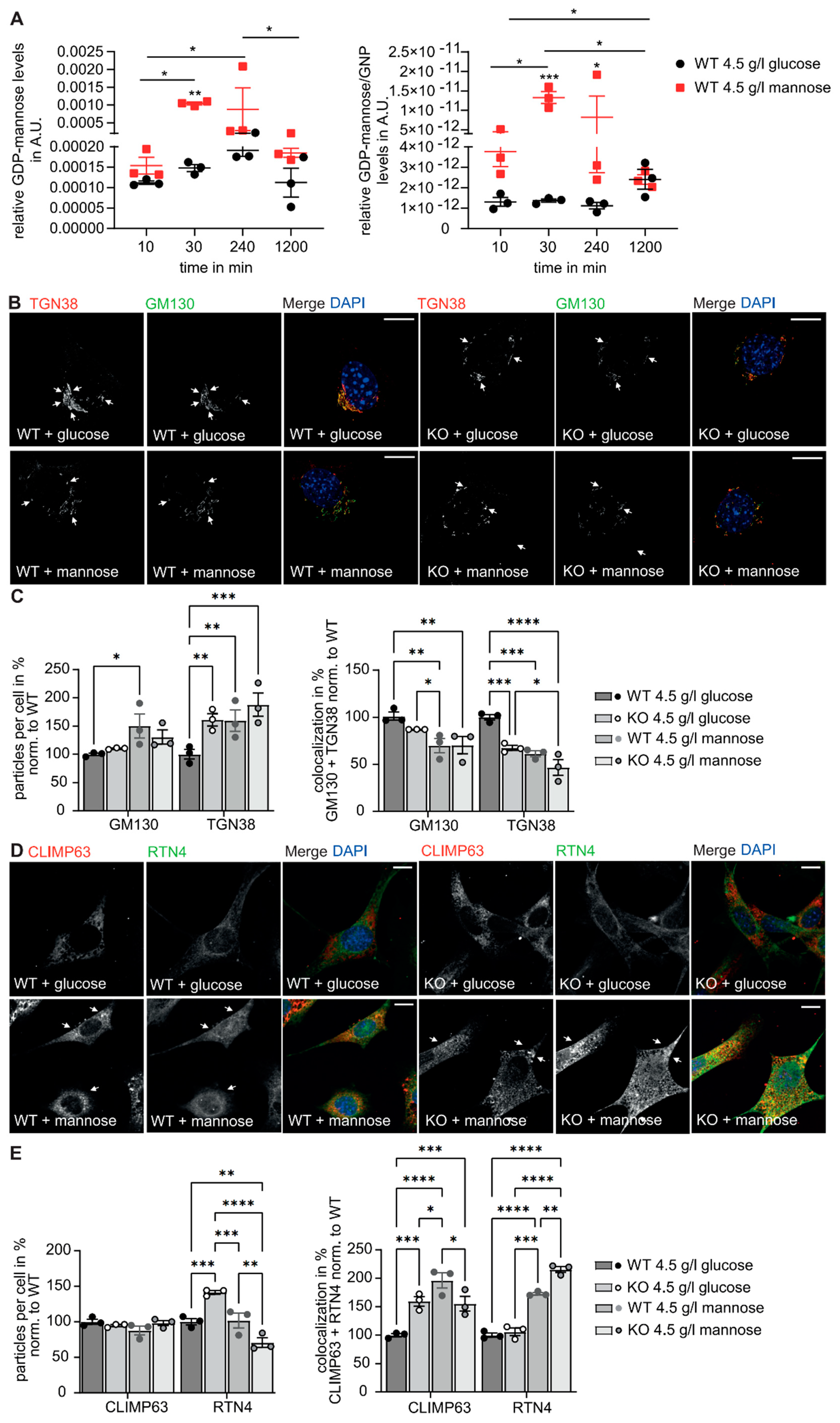
3.1. The Structure of the Golgi Apparatus Is Altered upon Disruption of GMPPA
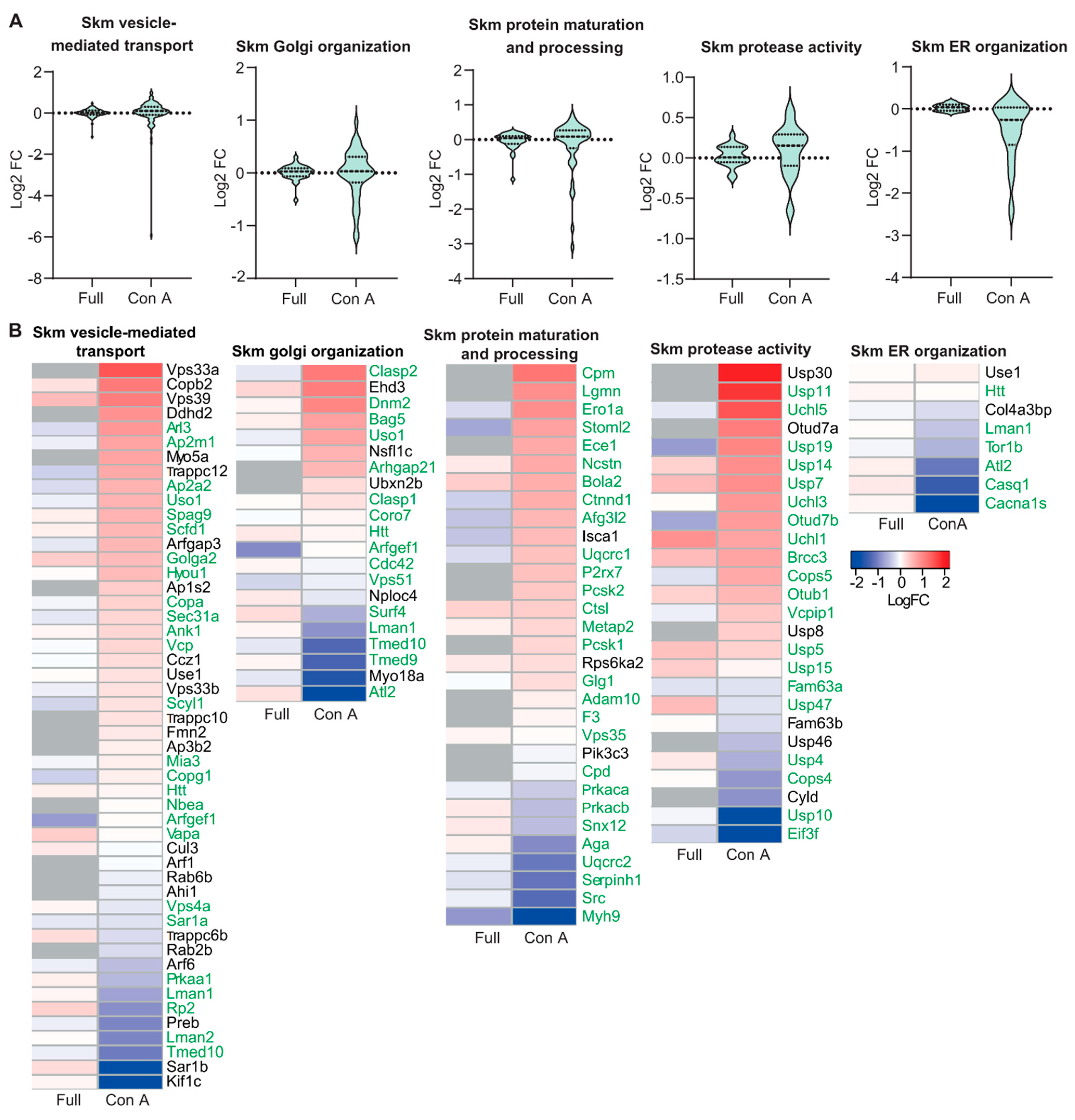
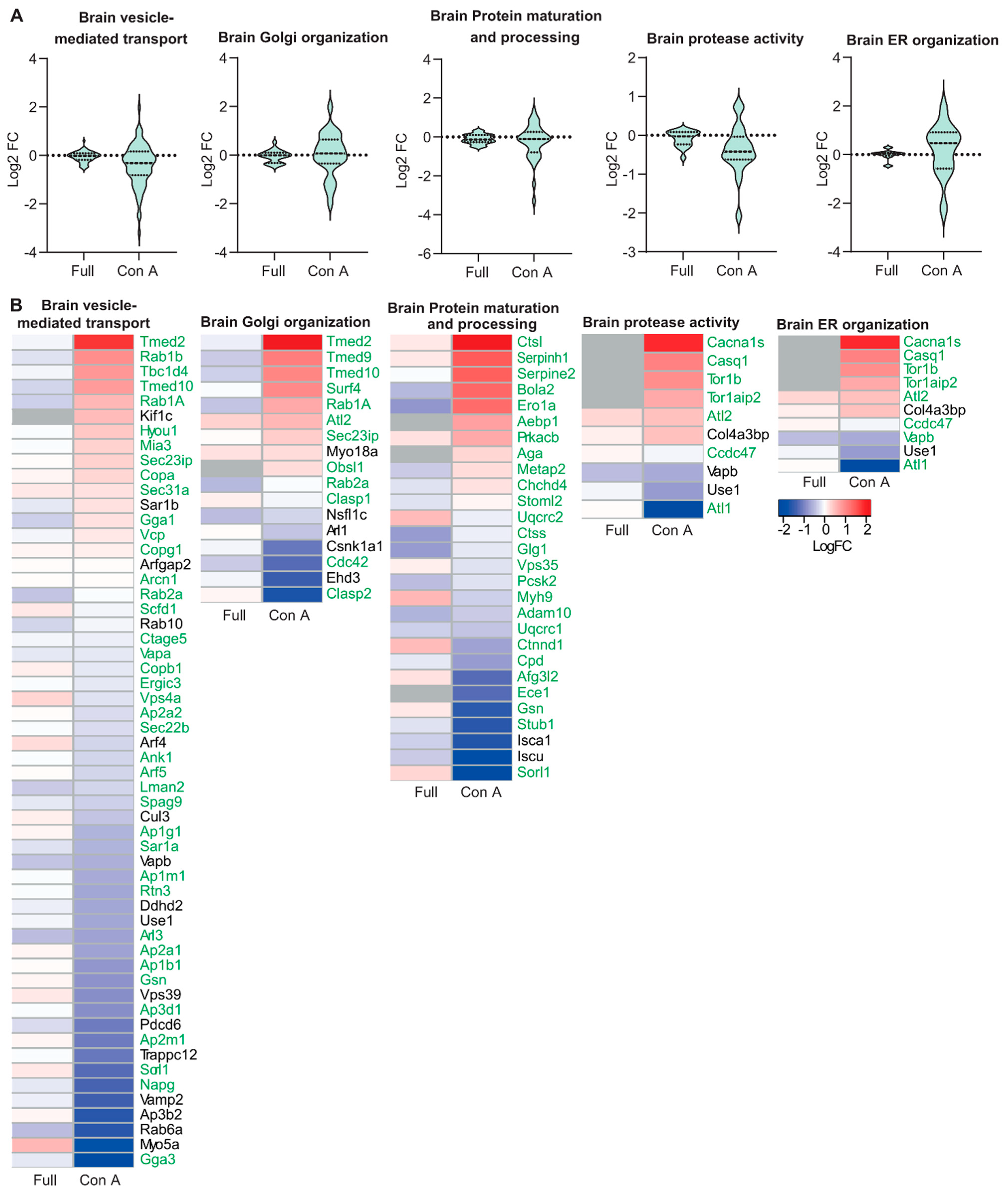
3.2. Proteomic Analysis of ER and Golgi Related Glycoproteins in Brain and Muscle Tissues of GMPPA KO Mice
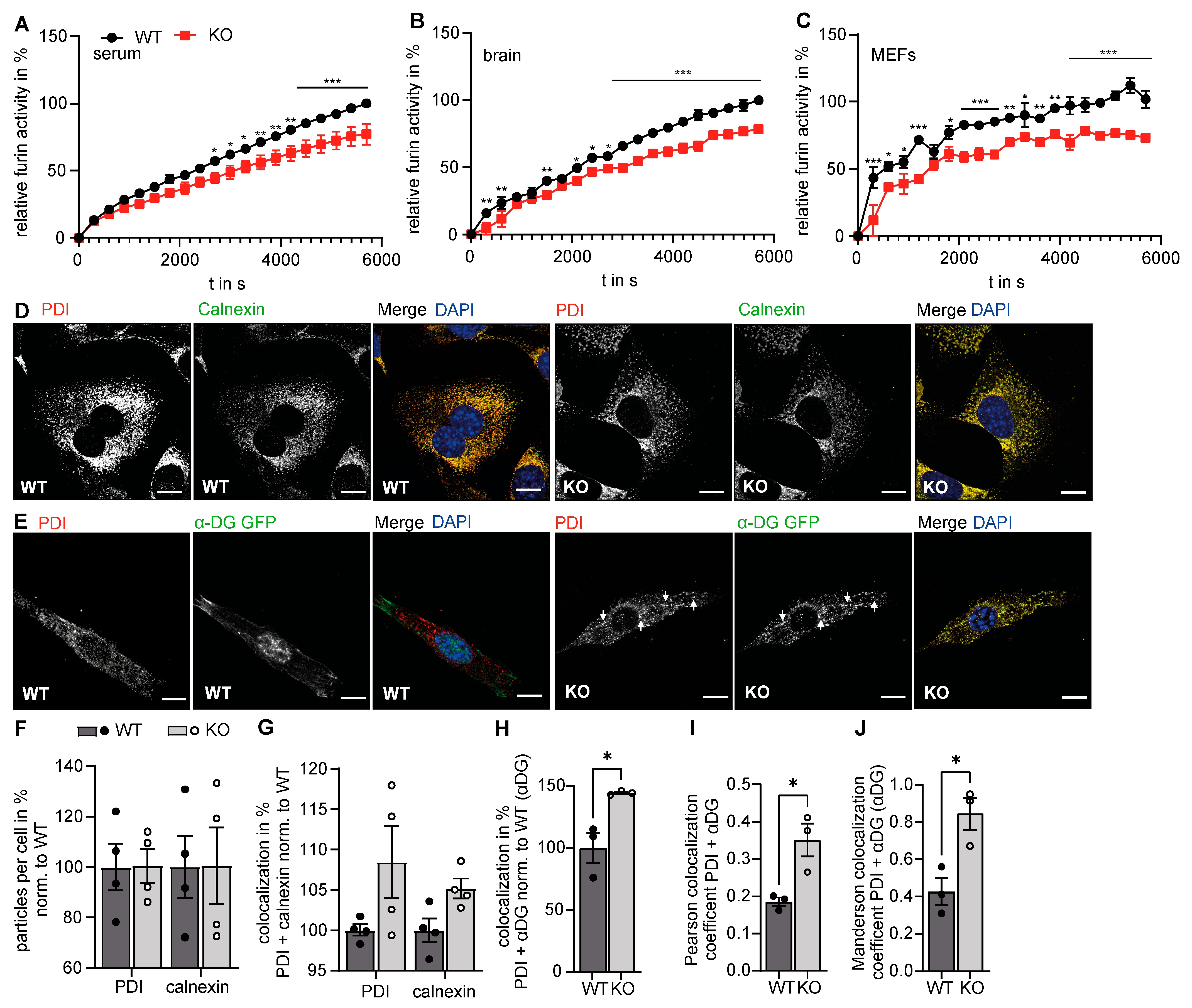
3.3. Golgi Function Is Altered in GMPPA KO Mice
3.4. Increased Retention of α-DG in the Absence of GMPPA
3.5. GMPPA Associated Alterations of the ER and Golgi Apparatus Can Be Mimicked by Increasing the Extracellular Mannose Concentration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breloy, I.; Hanisch, F.-G. Functional Roles of O-Glycosylation. Molecules 2018, 23, 3063. [Google Scholar] [CrossRef]
- Shental-Bechor, D.; Levy, Y. Effect of glycosylation on protein folding: A close look at thermodynamic stabilization. Proc. Natl. Acad. Sci. USA 2008, 105, 8256–8261. [Google Scholar] [CrossRef] [PubMed]
- Traini, M.; Kumaran, R.; Thaysen-Andersen, M.; Kockx, M.; Jessup, W.; Kritharides, L. N-glycosylation of human sphingomyelin phosphodiesterase acid-like 3A (SMPDL3A) is essential for stability, secretion and activity. Biochem. J. 2017, 474, 1071–1092. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.K.; Zhang, Z.; Baksi, K.; Serrano-Negrón, J.E. Dolichol phosphate mannose synthase: A Glycosyltransferase with Unity in molecular diversities. Glycoconj. J. 2017, 34, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Freeze, H.H.; Boyce, M.; Zachara, N.E.; Hart, G.W.; Schnaar, R.L. Glycosylation Precursors. In Essentials of Glycobiology [Internet], 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; Chapter 5. [Google Scholar]
- Leszek, A.; Kleczkowski, D.D. Sugar Activation for Production of Nucleotide Sugars as Substrates for Glycosyltransferases in Plants. J. Appl. Glycosci. 2015, 62, 25–36. [Google Scholar]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. (Eds.) Essentials of Glycobiology [Internet], 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015–2017. [Google Scholar]
- Stanley, P.; Schachter, H.; Taniguchi, N. N-Glycans. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009; Chapter 8. [Google Scholar]
- Stanley, P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 2011, 3, a005199. [Google Scholar] [CrossRef]
- Péanne, R.; de Lonlay, P.; Foulquier, F.; Kornak, U.; Lefeber, D.J.; Morava, E.; Pérez, B.; Seta, N.; Thiel, C.; Van Schaftingen, E.; et al. Congenital disorders of glycosylation (CDG): Quo vadis? Eur. J. Med. Genet. 2018, 61, 643–663. [Google Scholar] [CrossRef]
- Belaya, K.; Cruz, P.M.R.; Liu, W.W.; Maxwell, S.; McGowan, S.; Farrugia, M.E.; Petty, R.; Walls, T.J.; Sedghi, M.; Basiri, K.; et al. Mutations in GMPPB cause congenital myasthenic syndrome and bridge myasthenic disorders with dystroglycanopathies. Brain 2015, 138 Pt 9, 2493–2504. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.; Malik, M.; Mahmood, S.; Gießelmann, S.; Beetz, C.; Hennings, J.C.; Huebner, A.K.; Grahn, A.; Reunert, J.; Nürnberg, G.; et al. Mutations in GMPPA cause a glycosylation disorder characterized by intellectual disability and autonomic dysfunction. Am. J. Hum. Genet. 2013, 93, 727–734. [Google Scholar] [CrossRef]
- Franzka, P.; Henze, H.; Jung, M.J.; Schüler, S.C.; Mittag, S.; Biskup, K.; Liebmann, L.; Kentache, T.; Morales, J.; Martínez, B.; et al. GMPPA defects cause a neuromuscular disorder with α-dystroglycan hyperglycosylation. J. Clin. Investig. 2021, 131, e139076. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, Z.; Wang, Y.; Yang, F.; Wang, J.; Huang, W.; Qin, J.; Tian, M.; Cai, X.; Liu, X.; et al. Cryo-EM structures of human GMPPA-GMPPB complex reveal how cells maintain GDP-mannose homeostasis. Nat. Struct. Mol. Biol. 2021, 28, 1–12. [Google Scholar] [CrossRef]
- Reynders, E.; Foulquier, F.; Annaert, W.; Matthijs, G. How Golgi glycosylation meets and needs trafficking: The case of the COG complex. Glycobiology 2010, 21, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.; Lupashin, V.V. Role of the conserved oligomeric Golgi (COG) complex in protein glycosylation. Carbohydr. Res. 2008, 343, 2024–2031. [Google Scholar] [CrossRef]
- Linders, P.T.A.; Gerretsen, E.C.F.; Ashikov, A.; Vals, M.-A.; de Boer, R.; Revelo, N.H.; Arts, R.; Baerenfaenger, M.; Zijlstra, F.; Huijben, K.; et al. Congenital disorder of glycosylation caused by starting site-specific variant in syntaxin-5. Nat. Commun. 2021, 12, 6227. [Google Scholar] [CrossRef] [PubMed]
- Franzka, P.; Krüger, L.; Schurig, M.K.; Olecka, M.; Hoffmann, S.; Blanchard, V.; Hübner, C.A. Altered Glycosylation in the Aging Heart. Front. Mol. Biosci. 2021, 8, 673044. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Rabouille, C.; Watson, R.; Nilsson, T.; Hui, N.; Slusarewicz, P.; Kreis, T.E.; Warren, G. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 1995, 131 Pt 2, 1715–1726. [Google Scholar] [CrossRef]
- Luzio, J.P.; Brake, B.; Banting, G.; Howell, K.E.; Braghetta, P.; Stanley, K.K. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38). Biochem. J. 1990, 270, 97–102. [Google Scholar] [CrossRef]
- Kawano, J.-I.; Nakayama, T.; Kotani, T.; Matsubayashi, H.; Yamamoto, M.-T.; Suganuma, T. Identification and characterization of an insect homologue of the vertebrate Golgi apparatus protein 1 (MG-160/cysteine-rich fibroblast growth factor receptor/E-selectin ligand-1/latent transforming growth factor-beta complex protein-1) with a Golgi-specific monoclonal antibody. Histochem. Cell Biol. 2002, 117, 381–389. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Riley, N.M.; Hebert, A.S.; Westphall, M.S.; Coon, J.J. Capturing site-specific heterogeneity with large-scale N-glycoproteome analysis. Nat. Commun. 2019, 10, 1311. [Google Scholar] [CrossRef]
- Sanchez, E.J.; Lewis, K.M.; Munske, G.R.; Nissen, M.S.; Kang, C. Glycosylation of skeletal calsequestrin: Implications for its function. J. Biol. Chem. 2012, 287, 3042–3050. [Google Scholar] [CrossRef]
- Takeuchi, H.; Haltiwanger, R.S. Significance of glycosylation in Notch signaling. Biochem. Biophys. Res. Commun. 2014, 453, 235–242. [Google Scholar] [CrossRef]
- Wan, L.; Molloy, S.S.; Thomas, L.; Liu, G.; Xiang, Y.; Rybak, S.L.; Thomas, G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 1998, 94, 205–216. [Google Scholar] [CrossRef] [PubMed]
- van Nispen Tot Pannerden, H.E.; van Dijk, S.M.; Du, V.; Heijnen, H.F. Platelet protein disulfide isomerase is localized in the dense tubular system and does not become surface expressed after activation. Blood 2009, 114, 4738–4740. [Google Scholar] [CrossRef]
- Shibata, Y.; Shemesh, T.; Prinz, W.A.; Palazzo, A.F.; Kozlov, M.M.; Rapoport, T.A. Mechanisms determining the morphology of the peripheral ER. Cell 2010, 143, 774–788. [Google Scholar] [CrossRef] [PubMed]
- Jozsef, L.; Tashiro, K.; Kuo, A.; Park, E.J.; Skoura, A.; Albinsson, S.; Rivera-Molina, F.; Harrison, K.D.; Iwakiri, Y.; Toomre, D.; et al. Reticulon 4 is necessary for endoplasmic reticulum tubulation, STIM1-Orai1 coupling, and store-operated calcium entry. J. Biol. Chem. 2014, 289, 9380–9395. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zheng, P.; Qian, N.; Chen, Q.; Zhou, X.; Hu, J.; Chen, J.; Teng, J. Calumenin-1 Interacts with Climp63 to Cooperatively Determine the Luminal Width and Distribution of Endoplasmic Reticulum Sheets. Iscience 2019, 22, 70–80. [Google Scholar] [CrossRef]
- Kerselidou, D.; Dohai, B.S.; Nelson, D.R.; Daakour, S.; De Cock, N.; Hassoun, Z.A.O.; Kim, D.-K.; Olivet, J.; El Assal, D.C.; Jaiswal, A.; et al. Alternative glycosylation controls endoplasmic reticulum dynamics and tubular extension in mammalian cells. Sci. Adv. 2021, 7, eabe8349. [Google Scholar] [CrossRef]
- Robbins, E.; Gonatas, N.K. The ultrastructure of a mammalian cell during the mitotic cycle. J. Cell Biol. 1964, 21, 429–463. [Google Scholar] [CrossRef]
- Warren, G. Membrane partitioning during cell division. Annu. Rev. Biochem. 1993, 62, 323–348. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczyk, K.; Kaczmarek, R.; Czerwinski, M. How glycosylation affects glycosylation: The role of N-glycans in glycosyltransferase activity. Glycobiology 2020, 30, 941–969. [Google Scholar] [CrossRef]
- Bravo, R.; Parra, V.; Gatica, D.; Rodriguez, A.E.; Torrealba, N.; Paredes, F.; Wang, Z.V.; Zorzano, A.; Hill, J.A.; Jaimovich, E.; et al. Endoplasmic reticulum and the unfolded protein response: Dynamics and metabolic integration. Int. Rev. Cell Mol. Biol. 2013, 301, 215–290. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, Y. Golgi structure formation, function, and post-translational modifications in mammalian cells. F1000Research 2017, 6, 2050. [Google Scholar] [CrossRef]
- Witkos, T.M.; Lowe, M. The Golgin Family of Coiled-Coil Tethering Proteins. Front. Cell Dev. Biol. 2016, 3, 86. [Google Scholar] [CrossRef]
- De Matteis, M.A.; Morrow, J.S. The role of ankyrin and spectrin in membrane transport and domain formation. Curr. Opin. Cell Biol. 1998, 10, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Machamer, C.E. Golgi disassembly in apoptosis: Cause or effect? Trends Cell Biol. 2003, 13, 279–281. [Google Scholar] [CrossRef]
- Anwar, M.U.; Sergeeva, O.A.; Abrami, L.; Mesquita, F.S.; Lukonin, I.; Amen, T.; Chuat, A.; Capolupo, L.; Liberali, P.; D’Angelo, G.; et al. ER-Golgi-localized proteins TMED2 and TMED10 control the formation of plasma membrane lipid nanodomains. Dev. Cell 2022, 57, 2334–2346.e8. [Google Scholar] [CrossRef]
- Mitrovic, S.; Ben-Tekaya, H.; Koegler, E.; Gruenberg, J.; Hauri, H.-P. The cargo receptors Surf4, endoplasmic reticulum-Golgi intermediate compartment (ERGIC)-53, and p25 are required to maintain the architecture of ERGIC and Golgi. Mol. Biol. Cell 2008, 19, 1976–1990. [Google Scholar] [CrossRef]
- Fujita, Y.; Ohama, E.; Takatama, M.; Al-Sarraj, S.; Okamoto, K. Fragmentation of Golgi apparatus of nigral neurons with α-synuclein-positive inclusions in patients with Parkinson’s disease. Acta Neuropathol. 2006, 112, 261–265. [Google Scholar] [CrossRef]
- Gonatas, N.K.; Stieber, A.; Gonatas, J.O. Fragmentation of the Golgi apparatus in neurodegenerative diseases and cell death. J. Neurol. Sci. 2006, 246, 21–30. [Google Scholar] [CrossRef]
- Hallenberger, S.; Bosch, V.; Angliker, H.; Shaw, E.; Klenk, H.-D.; Garten, W. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature 1992, 360, 358–361. [Google Scholar] [CrossRef]
- Thomas, G. Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002, 3, 753–766. [Google Scholar] [CrossRef]
- Vidricaire, G.; Denault, J.; LeDuc, R. Characterization of a secreted form of human furin endoprotease. Biochem. Biophys. Res. Commun. 1993, 195, 1011–1018. [Google Scholar] [CrossRef]
- Vey, M.; Schäfer, W.; Berghöfer, S.; Klenk, H.-D.; Garten, W. Maturation of the trans-Golgi network protease furin: Compartmentalization of propeptide removal, substrate cleavage, and COOH-terminal truncation. J. Cell Biol. 1994, 127 (6 Pt 2), 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, A.; Crivelli, S.N.; Singh, M.; Lingappa, V.R.; Muschler, J.L. SEA domain proteolysis determines the functional composition of dystroglycan. FASEB J. 2008, 22, 612–621. [Google Scholar] [CrossRef]
- Singh, J.; Itahana, Y.; Knight-Krajewski, S.; Kanagawa, M.; Campbell, K.P.; Bissell, M.J.; Muschler, J. Proteolytic enzymes and altered glycosylation modulate dystroglycan function in carcinoma cells. Cancer Res. 2004, 64, 6152–6159. [Google Scholar] [CrossRef] [PubMed]
- Ervasti, J.M.; Campbell, K.P. Membrane organization of the dystrophin-glycoprotein complex. Cell 1991, 66, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Ibraghimov-Beskrovnaya, O.; Ervasti, J.M.; Leveille, C.J.; Slaughter, C.A.; Sernett, S.W.; Campbell, K.P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 1992, 355, 696–702. [Google Scholar] [CrossRef]
- Hounsell, E. Glycoproteins. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Sharma, V.; Freeze, H.H. Mannose efflux from the cells: A potential source of mannose in blood. J. Biol. Chem. 2011, 286, 10193–10200. [Google Scholar] [CrossRef]
- Sharma, V.; Ichikawa, M.; Freeze, H.H. Mannose metabolism: More than meets the eye. Biochem. Biophys. Res. Commun. 2014, 453, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Alton, G.; Hasilik, M.; Niehues, R.; Panneerselvam, K.; Etchison, J.R.; Fana, F.; Freeze, H.H. Direct utilization of mannose for mammalian glycoprotein biosynthesis. Glycobiology 1998, 8, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kinoshita, M.; Yamada, A.; Kawano, S.; Liu, H.; Kamimura, S.; Nakagawa, M.; Nagasawa, S.; Taguchi, T.; Yamada, S.; et al. Mannose and phosphomannose isomerase regulate energy metabolism under glucose starvation in leukemia. Cancer Sci. 2021, 112, 4944–4956. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzka, P.; Schüler, S.C.; Kentache, T.; Storm, R.; Bock, A.; Katona, I.; Weis, J.; Buder, K.; Kaether, C.; Hübner, C.A. Impact of Hypermannosylation on the Structure and Functionality of the ER and the Golgi Complex. Biomedicines 2023, 11, 146. https://doi.org/10.3390/biomedicines11010146
Franzka P, Schüler SC, Kentache T, Storm R, Bock A, Katona I, Weis J, Buder K, Kaether C, Hübner CA. Impact of Hypermannosylation on the Structure and Functionality of the ER and the Golgi Complex. Biomedicines. 2023; 11(1):146. https://doi.org/10.3390/biomedicines11010146
Chicago/Turabian StyleFranzka, Patricia, Svenja Caren Schüler, Takfarinas Kentache, Robert Storm, Andrea Bock, Istvan Katona, Joachim Weis, Katrin Buder, Christoph Kaether, and Christian A. Hübner. 2023. "Impact of Hypermannosylation on the Structure and Functionality of the ER and the Golgi Complex" Biomedicines 11, no. 1: 146. https://doi.org/10.3390/biomedicines11010146
APA StyleFranzka, P., Schüler, S. C., Kentache, T., Storm, R., Bock, A., Katona, I., Weis, J., Buder, K., Kaether, C., & Hübner, C. A. (2023). Impact of Hypermannosylation on the Structure and Functionality of the ER and the Golgi Complex. Biomedicines, 11(1), 146. https://doi.org/10.3390/biomedicines11010146







