Abstract
(1) Background: It is essential to focus attention on sex-specific factors which are clinically relevant in pain management, especially with regards to opioid use disorder (OUD) risk. The aim of this study was to explore potential sex-differences in chronic non-cancer pain (CNCP) outpatients. (2) Methods: An observational cross-sectional study was conducted under CNCP outpatients with long-term prescribed opioids (n = 806), wherein 137 patients had an OUD diagnosis (cases, 64% females) and 669 did not (controls, 66% females). Socio-demographic, clinical, and pharmacological outcomes were analyzed. (3) Results: Female controls presented an older age and less intensive pain therapy but higher psychotropic prescriptions and emergency department visits compared to male controls. Meanwhile, cases demonstrated a younger age, higher work disability, double morphine equivalent daily dose, and benzodiazepine use compared with controls. Here, female cases showed an 8% greater substance use disorder (OR 2.04 [1.11–3.76]) and 24% lower tramadol use, while male cases presented a 22% higher fentanyl use (OR 2.97 [1.52–5.81]) and reported the highest number of adverse drug reactions (24%, OR 2.40 [1.12–5.16]) compared with controls. (4) Conclusions: An OUD individual risk profile was evidenced with sex-differences to take into consideration to design equal prevention programs.
1. Introduction
Chronic pain is one of the leading causes of medical consultation among adults and the main cause of abandonment of their daily activity [1]. It is estimated that 19% of Europeans suffer from chronic pain [2], resulting in important emotional, social, and economic consequences for the patient and his/her environment. Likewise, it is a major health problem associated with great costs and consumption of health resources (around 2.5% of the Spanish GDP) [3]. Normally, it is accompanied by a wide range of comorbidities and risk factors for other adverse health outcomes [4]. It has been demonstrated that women are more vulnerable for developing and maintaining musculoskeletal pain than men [5,6]. Findings from the literature suggest that women are more likely to be prescribed opioids for non-medical use [7], often with higher emotional and affective distress [8] compared with men. As opioid prescription is the usual therapy for CNCP, the question can be raised: Are females at a different risk for developing an opioid use disorder (OUD) than men?
Whilst it is important to clearly distinguish between sex and gender, we also need to understand the mechanisms and pathways underlying the trends we observe, as well as how sex and gender intersect with other factors such as age, income, social status, education, employment, genetics, or personal health practice, and contribute to our health and overall health outcome [9]. There is limited information on sex-differences in OUD risk factors. In general, a young age, past or current substance use, untreated psychiatric disorders, preadolescent sexual abuse, and social or family environments that encourage misuse constitute some of the OUD risk factors previously described [10,11,12]. Nevertheless, the limited presence of women in clinical trials and the lack of stratification by sex -mostly restricted to binary comparisons lacking data on gender dynamics raises questions related to sex-differences [13,14]. In this regard, our aim was to identify potential sex-specific risks and needs in CNCP patients using long-term prescribed opioids. The exploratory nature of this study would help to understand sex-differences in OUD risk for a future gender perspective analysis and allow for more equal clinical assessment and treatments.
2. Materials and Methods
2.1. Study Design
A cross-sectional study was conducted under CNCP outpatients with long-term prescribed opioids (≥6 months) from September 2020 to September 2021 at the Pain Unit (PU) of the Alicante General Hospital. The study is under the umbrella of a master protocol approved by the Ethics Committee of Alicante General Hospital (PI2020-047).
2.2. Participants
A total of 137 patients with OUD (cases) were included from an opioid tapering procedure routinely developed at PU [15] under the following inclusion criteria: adults (>18 years old) with CNCP under long-term prescribed opioids (≥6 months) and a clinical diagnosis of OUD Controls data (n = 669) were obtained from two concomitant observational studies [16,17] with same inclusion criteria except OUD diagnosis. All variables were collected from their original database and, if needed, they were completed using Electronic Health Records (EHRs), which allows for reviewing medical diagnoses, outcomes, and medication use.
2.3. Measures
OUD was diagnosed by a psychiatric expert in pain according to DSM-5 [18] as part of an established opioid tapering procedure [15]. The patient had to meet at least two of the criteria specified in the manual to consider he/she had an OUD.
The independent variable for all of the analysis was the sex of the patient (female/male).
Other socio-demographic characteristics such as age, employment status (active, retired, work disability, unemployed or homemaker) and income (low income as less than €500, middle income as between €500 and 1000, and upper income as more than €1000) were also registered.
A Global Pain State questionnaire [18] measuring, qualitatively, pain, relief, and quality of life was collected at the time of the original interview. Pain intensity and relief were measured using the Visual Analogue Scale (VAS) [19]. Both consist of a horizontal line ranging from 0 (lowest) to 100 mm (highest), where the patient points on the line to the intensity of pain or relief that he/she feels, respectively. Quality of life was evaluated through the EuroQol-5D scale that consists of a VAS (vertical line from 0 (the worst imaginable health status) to 100 mm (the best imaginable) where the patient indicates his/her actual health status. To collect patients’ reports of adverse events (AEs), the most frequent adverse drug reactions (ADRs, selected according to opioids Summary of Product Characteristics frequency as “very common” and “common”) [20], and any other AEs presented, were collected as present/absent. They consisted of the following: sleepiness, dizziness, nausea, vomiting, constipation, itchiness, sexual dysfunction, loss of libido, weight change, headache, skin redness, dry skin, dry mouth, edema, depression, sleep disturbance, nervousness and loss of appetite. In addition, patients were asked about any depression or anxiety symptoms they had. Likewise, all ADRs related to the pain treatment were registered [21]. The presence of history of prior substance use disorder (including tobacco, alcohol and illicit drugs) were registered through the review of medical diagnoses, narratives or any visit to the Addictive Behaviour Unit.
The use (yes/no) of simple analgesics (i.e., paracetamol and metamizole), non-steroidal anti-inflammatory drugs (NSAIDs), and opioids use (i.e., tramadol, codeine, fentanyl, oxycodone, tapentadol, buprenorphine, morphine, hydromorphone and methadone), along with immediate release opioids prescription were registered. In different combinations of opioids, oral morphine equivalent daily dose (MEDD) was estimated using available references [22]. The prescription of antidepressants (i.e., amitriptyline, fluoxetine, escitalopram, and duloxetine), benzodiazepines, and neuromodulators (pregabalin and gabapentin) was also collected.
2.4. Statistical Analysis
Convenience sampling was considered based on the prevalence of OUD diagnosis in our regular clinical routine at PU. Data distribution was analysed with the Kolmogorov-Smirnov test using the Lilliefors correction method. Quantitative parametric data are presented as mean (standard deviation (SD)) whilst the median (interquartile range (IQR)) was used for non-parametric data. Categorical data are expressed as percentages (%). Comparisons of socio-demographic, clinical, pharmacological and safety data were evaluated depending upon their distribution. Bivariate odds ratio (OR) and 95% confidence intervals (CIs) were also calculated. Collinearity between categorical variables was tested depending upon their distribution. Here, results were analyzed by groups (men or women, cases vs. controls) or by sex (i.e., control or cases, men vs. women). A p-value < 0.05 was considered statistically significant. Analyses were carried out using R (Version 3.2.0; the GNU project, Cambridge, MA, USA) and GraphPad Prism (Version 5.0, Dotmatics, Boston, MA, USA).
3. Results
A total of 1452 potential control candidates were explored, whereof 783 were excluded due to patients being duplicated between the studies or not meeting the inclusion criteria. All participants included (Figure 1) were referred to our PU for regular pain management mostly due to somatic pain (85%). Non-specific low back pain was the most common type (associated with radiculopathy, spinal stenosis, or another specific spinal cause), followed by knee pain and other musculoskeletal pain (hip pain or due to other cervical joint dysfunctions).
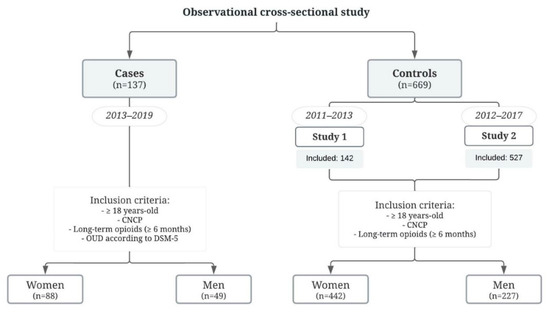
Figure 1.
Flow chart of the patient selection for the study. CNCP, chronic non-cancer pain; OUD, opioid use disorder.
3.1. Socio-Demographic and Clinical Outcomes
A summary of the characteristics of the participants and clinical variables are shown in Table 1 and Table 2. Meanwhile, Figure 2 shows the odds ratios and 95% confidence intervals for risk factors in females (Figure 2a) and males (Figure 2b).

Table 1.
Socio-demographic analysis by sex.

Table 2.
Clinical analysis by sex.
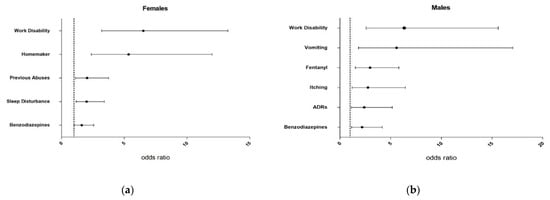
Figure 2.
Odds ratio (OR) with 95% confidence intervals (95% CI) of risk factors for females (a) and males (b). ADRs, adverse drug reactions.
Controls were older than cases, even more in females (66 (56–75) years old) who were the oldest group (vs. female cases: 53 (45–65) years old, p < 0.001; vs. male controls: 53 (45–65), p = 0.001). Thus, controls were more retired than cases (females: 56% vs. 22%, p < 0.001; males: 53% vs. 28%, p = 0.032), whilst the latter presented higher prevalence of work disability (females: 9% vs. 41%, p < 0.001, OR 6.52 [3.21–13.27] and males: 22% vs. 64%, p = 0.009, OR 6.34 [2.57–15.61]) (Figure 2a,b). Females presented the highest household tasks dedication, even more in cases (27% vs. 7% in controls, p < 0.001; OR 5.35 [2.38–12.00]), being 7-times higher than men in both groups. What’s more, a significant 8% greater of SUD was found in female cases relative to controls (19% vs. 11%, p = 0.029; OR 2.04 [1.11–3.76]).
3.2. Pharmacological Outcomes

Table 3.
Analgesic analysis by sex.

Table 4.
Pharmacological and health use analysis by sex.
In control group, sex-differences were observed as females had a greater 8% use of simple analgesics (45% vs. 37% in males, p = 0.039), 12% of tramadol (37% vs. 25%, p = 0.001), along with greater psychotropic drugs use (11%-antidepressants (42% vs. 31%, p = 0.006), 14%-benzodiazepines (41% vs. 27%, p < 0.001)) and emergency room visits (32% vs. 22%, p = 0.017) compared with males. In contrast, males presented a 6% greater use of morphine (10% vs. 4% in females, p = 0.005) and a 13% of neuromodulators prescription (54% vs. 41%, p = 0.002).
In cases, both sexes doubled their MEDD (120–163 mg/day, p < 0.001) compared to controls. As seen in Figure 2, they also presented a 17–15% higher buprenorphine and 12–14% benzodiazepines prescription (females: OR 1.62 [1.02–2.57] and males: OR 2.19 [1.15–4.17]). In contrast, a 24% lower use of tramadol (13% vs. 37% in controls, p < 0.001) was shown in female cases and a 22% greater fentanyl use (40% vs. 18%, p = 0.002; OR 2.97 [1.52–5.81]) was observed in male cases compared to controls.
3.3. Safety Outcomes

Table 5.
Safety variables description by sex.
Women presented a higher number of AEs in both groups, being the highest (median of 6 AEs/patients) in female cases. In fact, ADRs were significantly greater in female controls than men (18% vs. 12%, p = 0.035). On the contrary, although male cases presented the lowest number of AEs (3 (1–6) AEs/patient), they doubled the male controls’ ADRs (24% vs. 12%, p = 0.039; OR 2.40 [1.12–5.16]).
Among reported cases, females presented 23% more dizziness (43% vs. 20% in males, p = 0.038), 17% edema (17% vs. 0%, p = 0.014), and 25% nervousness (52% vs. 27%, p = 0.026) compared to males. They suffered 17% more sleep disturbance compared to controls (52% vs. 35% in female controls, p = 0.012; OR 2.00 [1.18–3.42]). Meanwhile, in male reported cases, 16% higher vomiting (20% vs. 4%, p = 0.005; OR 5.58 [1.83–17.05]) and 18% itching (33% vs. 15%, p = 0.022; OR 2.77 [1.19–6.46]) rates were observed compared to controls. On the other hand, in the control group, females suffered 5% more vomiting (9% vs. 4% in males, p = 0.036), 17% weight change (36% vs. 19% males, p < 0.001), 14% dry skin (38% vs. 24% males, p < 0.001), 12% depression (35% vs. 23% males, p = 0.002), and 12% loss of appetite (28% vs. 16% males, p = 0.001) rates relative to males. Only sexual dysfunction was higher in males (25%) in comparison with females (17%, p < 0.001).
4. Discussion
Younger age, work disability, opioid doses higher than 120 mg/day of morphine equivalent daily dose and benzodiazepine use were found significantly higher in cases compared to controls (similarly for both sexes). Nevertheless, sex-differences in cases were found related to prior SUD, opioid prescription and tolerability. Thus, the use and dose of opioids should be carefully monitored in patients with these underlying factors.
Our data have shown known socio-economic risk factors along with co-medication of high doses of opioids and benzodiazepines [23,24]. However, sex-based differences were observed due to prior SUD [25] and fentanyl prescription [26]. Women have been described to report greater receipt of prescriptions for anxiolytics, sedatives or hypnotics, what could contribute to an OUD [27]. Other clinical evidence suggest that men are more sensitive than women to the abuse-related effects of mu-opioid agonists, as fentanyl, although preclinical studies differ from this evidence [28]. These findings support sex-based tailoring of treatment, but any tailoring should also consider person-level differences [29].
Greater and different pain treatment intensity in females might be a consequence of the normalization of pain symptom between women and family doctors, which may also lead to delays in pain diagnosis or referrals to a PU [30]. In this way, these potential diagnosis and therapeutics delays should be deeply analyzed in terms of biopsychosocial mechanisms, adjusting for confounding by gender, as they may underlie these sex-differences, and considerations for future research should be discussed [31,32]. Besides, among the psychosocial risks that can worsen the state of health of women, we find a significant higher dedication to household tasks. The physical discomfort caused by the overload of domestic work, as well as the physical and mental stress resulting from the double working day as employees and as caregivers for the whole family, calls for further studies to identify appropriate intervention and prevention strategies [33].
The worse analgesic tolerability in females -related to gastrointestinal and nervous systems- and 10% more emergency department visits, falls in line with previously published scientific literature [34,35,36,37]. However, in spite of male cases having the lowest number of AEs, they arose the highest ADRs notification. These sex-differences are not fully elucidated [38]. Possible multifaceted factors seem to be associated. These include neuroanatomical, hormonal, neuroimmunological, but also psychological plus other social and cultural factors which need to be deeper analyzed (along with a gender perspective). In this way, studies should no longer consider men and women as a homogeneous group, given that subjective painkillers’ tolerability substantially differs between sexes [17,39].
The exploratory nature of the study has permitted us to establish differences between women and men in some intersectional factors such as age. However, it did not allow us to collect essential information that would enable us to link, for example, whether this fact was caused by a delayed diagnosis in women [40,41]. For this reason, it is essential to consider potential gender stereotypes threats that could affect our varied experiences and overall health [42].
There are some limitations in this study that need to be acknowledge. First, the sample size was limited by a “convenience sample” due to the low incidence of OUD. Secondly, although the control group came from the same setting, it was composed by subjects from different studies. Moreover, most patients were under other non-opioid centrally acting drugs related to their diverse comorbidities, which might have independently contributed to the observed side-effects. This could introduce a bias mediated by several other variables, such as socio-demographics, that could be more relevant than pain status [43,44]. What’s more, the higher prevalence of buprenorphine among cases could be part of the beginning of medication assisted therapy, prior to the derivation to the PU for the opioid tapering procedure. The data collection of some variables such as prior SUD could have been limited by the poor documentation from healthcare professionals. Nevertheless, this information was gathered through medical diagnoses, narratives, and Addictive Behavior Unit visits. All in all, this study helps to create more information about the needs of these patients to design more equal prevention programs.
5. Conclusions
In light of the above information, sex-differences in pain management and OUD risk have been observed. A deeper analysis of sex-gender interactions may be needed to understand disparities in potential diagnosis delays, analgesic prescription, safety pattern and healthcare resources use. Hence, further research is needed to refine these results and explore potential gender disparities in order to optimize individual pain and OUD management.
Author Contributions
Conceptualization, A.M.P.P., J.M. and C.M.; Methodology, A.M.P.P. and J.M.; Software, D.M.; Validation: A.M.P.P. and D.M.; Formal Analysis, M.E. and L.A.; Investigation, M.E. and L.A.; Resources, A.M.P.P.; Data Curation, M.E.; Writing—Original Draft Preparation, M.E., A.M.P.P. and J.M.; Writing—Review & Editing, M.E., L.A., D.M., C.M., J.M. and A.M.P.P.; Visualization, M.E., L.A., D.M., C.M., J.M. and A.M.P.P.; Supervision, A.M.P.P., D.M. and C.M.; Project Administration, A.M.P.P.; Funding Acquisition, M.E., J.M. and A.M.P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Alicante Institute for Health and Biomedical Research [UGP 21-116] and Spanish Clinical Pharmacology Society.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Dr. Balmis General University Hospital (protocol code PI2020-047 on 05/15/2020).
Informed Consent Statement
Patient consent was waived due to the cross-sectional nature of the study. It was specified that the data was going to be collected from previous approved studies databases whose informed consents were already collected.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to confidentiality concerns of the data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hunt, K.; Adamson, J.; Hewitt, C.; Nazareth, I. Do women consult more than men? A review of gender and consultation for back pain and headache. J. Health Serv. Res. Policy 2011, 16, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef] [PubMed]
- Torralba, A.; Miquel, A.; Darba, J. Situación actual del dolor crónico en España: Iniciativa ‘Pain Proposal’. Rev. la Soc. Esp. del Dolor. 2014, 21, 16–22. [Google Scholar] [CrossRef]
- Husak, A.J.; Bair, M.J. Chronic Pain and Sleep Disturbances: A Pragmatic Review of Their Relationships, Comorbidities, and Treatments. Pain Med. 2020, 21, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Rollman, G.B.; Lautenbacher, S. Sex Differences in Musculoskeletal Pain. Clin. J. Pain 2001, 17, 20–24. [Google Scholar] [CrossRef]
- Larsson, B.; Dragioti, E.; Grimby-Ekman, A.; Gerdle, B.; Björk, J. Predictors of chronic pain intensity, spread, and sensitivity in the general population: A two-year follow-up study from the SWEPAIN cohort. J. Rehabil. Med. 2019, 51, 183–192. [Google Scholar] [CrossRef]
- Osborne, V.; Serdarevic, M.; Crooke, H.; Striley, C.; Cottler, L.B. Non-medical opioid use in youth: Gender differences in risk factors and prevalence. Addict. Behav. 2017, 72, 114–119. [Google Scholar] [CrossRef]
- Jamison, R.N.; Butler, S.F.; Budman, S.H.; Edwards, R.R.; Wasan, A.D. Gender Differences in Risk Factors for Aberrant Prescription Opioid Use. J. Pain 2010, 11, 312–320. [Google Scholar] [CrossRef]
- Canadian Institutes of Health Research, Institute of Gender and Health (Canada). Science Is Better with Sex and Gender: Strategic Plan 2018-2023; Canadian Institutes of Health Research: Ottawa, ON, Canada, 2020. [Google Scholar]
- Webster, L.R. Risk Factors for Opioid-Use Disorder and Overdose. Anesth. Analg. 2017, 125, 1741–1748. [Google Scholar] [CrossRef]
- Votaw, V.R.; McHugh, R.K.; Witkiewitz, K. Alcohol use disorder and motives for prescription opioid misuse: A latent class analysis. Subst. Use Misuse 2019, 54, 1558–1568. [Google Scholar] [CrossRef]
- Nalven, T.; Spillane, N.S.; Schick, M.R. Risk and protective factors for opioid misuse in American Indian adolescents. Drug Alcohol Depend. 2020, 206, 107736. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.R.; Turner, B.E.; Weeks, B.T.; Magnani, C.J.; Wong, B.O.; Rodriguez, F.; Yee, L.M.; Cullen, M.R. Analysis of Female Enrollment and Participant Sex by Burden of Disease in US Clinical Trials Between 2000 and 2020. JAMA Netw. Open 2021, 4, e2113749. [Google Scholar] [CrossRef] [PubMed]
- Zandonai, T.; Escorial, M.; Peiró, A.M. Codeine and Tramadol Use in Athletes: A Potential for Abuse. Front. Pharm. 2021, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Muriel, J.; Margarit, C.; Planelles, B.; Serralta, M.J.; Puga, C.; Inda, M.D.; Cutillas, E.; Morales, D.; Horga, J.F.; Peiró, A.M. OPRM1 influence on and effectiveness of an individualized treatment plan for prescription opioid use disorder patients. Ann. N. Y. Acad. Sci. 2018, 1425, 82–93. [Google Scholar] [CrossRef]
- Planelles, B.; Margarit, C.; Ajo, R.; Sastre, Y.; Muriel, J.; Inda, M.D.; Esteban, M.D.; Peiró, A.M. Health benefits of an adverse events reporting system for chronic pain patients using long-term opioids. Acta Anaesthesiol. Scand. 2018, 63, 248–258. [Google Scholar] [CrossRef]
- Planelles, B.; Margarit, C.; Inda, M.D.; Ballester, P.; Muriel, J.; Barrachina, J.; Ajo, R.; Esteban, M.-D.; Peiró, A.M. Gender based differences, pharmacogenetics and adverse events in chronic pain management. Pharm. J. 2020, 20, 320–328. [Google Scholar] [CrossRef]
- Barrachina, J.; Muriel, J.; Margarit, C.; Planelles, B.; Ballester, P.; Richart-Martínez, M.; Cutillas, E.; Zandonai, T.; Morales, D.; Peiró, A.M. Global Pain State Questionnaire: Reliability, Validity, and Gender Gap. Arch. Intern. Med. Res. 2021, 4, 91–113. [Google Scholar] [CrossRef]
- McCormack, H.M.; Horne, D.J.; Sheather, S. Clinical applications of visual analogue scales: A critical review. Psychol. Med. 1988, 18, 1007–1019. [Google Scholar] [CrossRef]
- AEMPS-CIMA. Online Information Center of Medicines of Spanish Agency of Medicines and Health Products (AEMPS-CIMA) 2021. Available online: https://cima.aemps.es/cima/publico/home.html (accessed on 27 August 2020).
- Wisher, D. Martindale: The Complete Drug Reference. 37th ed. J. Med. Libr. Assoc. 2012, 100, 75–76. [Google Scholar] [CrossRef]
- Pergolizzi, J.; Böger, R.H.; Budd, K.; Dahan, A.; Erdine, S.; Hans, G.; Kress, H.G.; Langford, R.; Likar, R.; Raffa, R.B.; et al. Opioids and the Management of Chronic Severe Pain in the Elderly: Consensus Statement of an International Expert Panel with Focus on the Six Clinically Most Often Used World Health Organization step III Opioids (Buprenorphine, Fentanyl, Hydromorphone, Methadone, Morphine, Oxycodone). Pain Pract. 2008, 8, 287–313. [Google Scholar] [CrossRef]
- Nazarian, A.; Negus, S.S.; Martin, T.J. Factors mediating pain-related risk for opioid use disorder. Neuropharmacology 2021, 186, 108476. [Google Scholar] [CrossRef]
- Sulley, S.; Ndanga, M. Inpatient Opioid Use Disorder and Social Determinants of Health: A Nationwide Analysis of the National Inpatient Sample (2012–2014 and 2016–2017). Cureus 2020, 12, e11311. [Google Scholar] [CrossRef]
- Tong, J.; Chen, Z.; Duan, R.; Lo-Ciganic, W.-H.; Lyu, T.; Tao, C.; Merkel, P.A.; Kranzler, H.R.; Bian, J.; Chen, Y. Identifying Clinical Risk Factors for Opioid Use Disorder using a Distributed Algorithm to Combine Real-World Data from a Large Clinical Data Research Network. AMIA Annu. Symp. Proc. 2020, 2020, 1220–1229. [Google Scholar]
- Han, Y.; Yan, W.; Zheng, Y.; Khan, M.Z.; Yuan, K.; Lu, L. The rising crisis of illicit fentanyl use, overdose, and potential therapeutic strategies. Transl. Psychiatry 2019, 9, 282. [Google Scholar] [CrossRef]
- Peltier, M.R.; Sofuoglu, M.; Petrakis, I.L.; Stefanovics, E.; Rosenheck, R.A. Sex Differences in Opioid Use Disorder Prevalence and Multimorbidity Nationally in the Veterans Health Administration. J. Dual Diagn. 2021, 17, 124–134. [Google Scholar] [CrossRef]
- Townsend, E.A.; Negus, S.S.; Caine, S.B.; Thomsen, M.; Banks, M.L. Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology 2019, 44, 2022–2029. [Google Scholar] [CrossRef]
- Moran, L.M.; Kowalczyk, W.J.; Phillips, K.A.; Vahabzadeh, M.; Lin, J.-L.; Mezghanni, M.; Epstein, D.H.; Preston, K.L. Sex differences in daily life stress and craving in opioid-dependent patients. Am. J. Drug Alcohol Abus 2018, 44, 512–523. [Google Scholar] [CrossRef]
- Ballard, K.; Lowton, K.; Wright, J. What’s the delay? A qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil. Steril. 2006, 86, 1296–1301. [Google Scholar] [CrossRef]
- Rosenblum, A.; Marsch, L.A.; Joseph, H.; Portenoy, R.K. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp. Clin. Psychopharmacol. 2008, 16, 405–416. [Google Scholar] [CrossRef]
- McLean, C.P.; Anderson, E.R. Brave men and timid women? A review of the gender differences in fear and anxiety. Clin. Psychol. Rev. 2009, 29, 496–505. [Google Scholar] [CrossRef]
- Habib, R.R.; Fathallah, F.A.; Messing, K. Full-Time Homemakers: Workers Who Cannot “Go Home and Relax”. Int. J. Occup. Saf. Erg. 2010, 16, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Margarit, C.; Roca, R.; Inda, M.D.M.; Muriel, J.; Ballester, P.; Flor, A.; Morales, D.; Peiro, A.M. Gender Bias and Genotype Influence on Opioid Safety Profile in Chronic Low Back Pain. Clin. J. Pain 2020, 36, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Craft, R.M. Sex differences in opioid analgesia: ‘from mouse to man’. Clin. J. Pain 2003, 19, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Muriel, J.; Margarit, C.; Barrachina, J.; Ballester, P.; Flor, A.; Morales, D.; Horga, J.F.; Fernández, E.; Peiró, A.M. Pharmacogenetics and prediction of adverse events in prescription opioid use disorder patients. Basic Clin. Pharm. Toxicol. 2019, 124, 439–448. [Google Scholar] [CrossRef]
- Lopes, G.S.; Bielinski, S.; Moyer, A.M.; Jacobson, D.J.; Wang, L.; Jiang, R.; Larson, N.B.; Miller, V.M.; Zhu, Y.; Cavanaugh, D.C.; et al. Sex differences in type and occurrence of adverse reactions to opioid analgesics: A retrospective cohort study. BMJ Open 2021, 11, e044157. [Google Scholar] [CrossRef]
- Nasser, S.A.; Afify, E.A. Sex differences in pain and opioid mediated antinociception: Modulatory role of gonadal hormones. Life Sci. 2019, 237, 116926. [Google Scholar] [CrossRef]
- Barbui, C.; Nosè, M.; Bindman, J.; Schene, A.; Becker, T.; Mazzi, M.A.; Kikkert, M.; Camara, J.; Born, A.; Tansella, M. Sex Differences in the Subjective Tolerability of Antipsychotic Drugs. J. Clin. Psychopharmacol. 2005, 25, 521–526. [Google Scholar] [CrossRef]
- Ruiz-Cantero, M.T.; Verdú-Delgado, M. Sesgo de género en el esfuerzo terapéutico. Gac. Sanit. 2004, 18, 118–125. [Google Scholar] [CrossRef]
- Husby, G.K.; Haugen, R.S.; Moen, M.H. Diagnostic delay in women with pain and endometriosis. Acta Obs. Gynecol. Scand. 2003, 82, 649–653. [Google Scholar] [CrossRef]
- Schäfer, G.; Prkachin, K.M.; Kaseweter, K.A.; Williams, A.C. Health care providers’ judgments in chronic pain: The influence of gender and trustworthiness. Pain 2016, 157, 1618–1625. [Google Scholar] [CrossRef]
- Li, Q.; Loke, A.Y. A spectrum of hidden morbidities among spousal caregivers for patients with cancer, and differences between the genders: A review of the literature. Eur. J. Oncol. Nurs. 2013, 17, 578–587. [Google Scholar] [CrossRef]
- Sharma, N.; Chakrabarti, S.; Grover, S. Gender differences in caregiving among family-caregivers of people with mental illnesses. World J. Psychiatry 2016, 6, 7. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).