Abstract
Sorafenib has been used to treat advanced hepatocellular carcinoma (aHCC). However, there is no evidence for a response of different target lesions to sorafenib administration. Therefore, we aimed to evaluate the effect of sorafenib on various aHCC target lesions. The outcomes of sorafenib treatment on aHCC, i.e., treatment response for all Child A status patients receiving the drug, were analyzed. Of 377 aHCC patients, 73 (19.3%) had complete/partial response to sorafenib, while 134 (35.4%) and 171 (45.2) had a stable or progressive disease, respectively, in the first six months. Of the evaluated metastatic lesions, 149 (39.4%), 48 (12.7%), 123 (32.5%), 98 (25.9%), 83 (22.0%), and 45 (11.9%) were present in liver, bone, lung, portal/hepatic vein thrombus, lymph nodes, and peritoneum, respectively. The overall survival and duration of treatment were 16.9 ± 18.3 and 8.1 ± 10.5 months (with median times of 11.4 and 4.6, respectively). Our analysis showed poor outcomes in macroscopic venous thrombus and bone, higher AFP, and multiple target lesions. ALBI grade A had a better outcome. Sorafenib administration showed good treatment outcomes in selected situations. PD patients with thrombus or multiple metastases should be considered for sorafenib second-line treatment. The ALBI liver function test should be selected as a treatment criterion.
1. Introduction
Curative treatment for resectable hepatocellular carcinoma (HCC) leads to the best outcome, while recurrence, including intrahepatic, disseminated spread, or distant metastases, require systemic treatment, i.e., sorafenib and regorafenib target therapy [1,2,3,4,5]. The existence of several options for first- and second-line systemic treatments of advanced HCC improves the overall survival [6,7]. Regorafenib second-line treatment has been used for advanced HCC. This approach was shown to prolong overall survival to 32 months in a resource study [8,9]. However, there was no concensus about the survival benefit in different target lesions, such as bone, lung, lymph node and peritoneal metastasis [10,11,12]. Therefore, although HCC with different organ metastasis was assumed as stage IV, varying clinical responses should be studied.
Albumin-bilirubin (ALBI) grade has been shown to be an indicator of liver dysfunction [13,14]. A combination of ALBI and APRI showed superior predicting power for postoperative hepatic failure [15]. ALBI grade 2 was shown to be a significant negative predictor when patients were treated with eluting embolic chemoembolization [16]. Ramucirumab has been shown to be of survival benefit in ALBI grade 1, and patients with grade 2 or 3 expressed liver-specific adverse events in a REACH study [17]. ALBI grade was found to be a predictor for HCC outcome in regional ablation therapy and systemic administration [18]. Alpha-fetoprotein (AFP) is a standard diagnostic marker; serum level over 400 ng/mL is a selection criterion for sequential therapy with ramucirumab [19]. The role of AFP in tyrosine kinase inhibition is debated [20]. Although several biomarkers have been developed for clinical application, AFP remains the most important one [21,22].
This study is the first to explore the organs which are susceptible to target lesions. It examined the differences of responses and long-term outcomes of sorafenib systemic treatment in advanced HCC. Liver fucntion, ALBI grading and AFP response and clinical relevance were also studied.
2. Materials and Methods
2.1. Patients and Samples
This study was approved by the Institute Review Board (IRB) of Chang Gung Memorial Hospital (CGMH), Linkou (IRB 201600513B0). Since 2012, when the National Health Insurance system approved the administration of sorafenib as a first-line treatment for advanced HCC, 377 HCC patients have been treated. Treatment indications included extrahepatic spreading (EHS), macroscopic venous invasion (MVI), or refractory response to transarterial chemoembolization (TACE). All enrolled patients showed good performance status, with ECOG scores between 0 to 1 and Child-Pugh A status, and received sorafenib-based drugs as target therapy with or without combination treatment. The clinical and pathological variables were collected for analysis. Progression-free and overall survival were compared using the log-rank test and Kaplan-Meier survival analysis. The clinical response to sorafenib was measured according to the RECIST criteria and classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) [23].
2.2. Statistical Analysis
Categorical data were analyzed using the chi-square test or Fisher’s exact test. Continuous variables were analyzed using a t-test. Survival rates in each group were determined by the Kaplan-Meier method. Differences between groups were analyzed using the log-rank test. All calculated p-values were two-tailed, with the significance defined at the 95% level (p < 0.05). Statistical analyses were performed using SPSS statistical software version 19.0 (SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. Analysis of Clinical Response to Sorafenib Administration
In the CR, PR, SD, and PD clinical responses of the 377 patients to sorafenib, the outcomes were 18 (4.8%), 55 (14.6%), 133 (35.3%), and 171 (45.4%), and the overall clinical responses were 15 (4.0%), 12 (3.2%), 62 (16.4%) and 288 (76.4%), respectively. The objective response rate in the first 6 months was 54.6%. Positivity of hepatitis B and C infection was 61.3% and 29.7%, respectively, all in Child-Pugh A status. Within the sample, 133 (35.5%) showed AFP levels more than 400 ng/mL, and 215 (57.4%), 150 (40.1%), and 9 (2.4%) showed ALBI grades 1, 2, and 3, respectively. The most common extrahepatic spread was to bone, lung, peritoneum, and lymph nodes, in the form of target lesions; 157 (41.6%) patients had multiple target lesions (Table 1). The mean duration of treatment and overall survival were 8.1 ± 10.5 and 16.9 ± 18.3 months, respectively. The overall survival rate was 84.8%, 68.5%, 51.6%, 39.5%, 38.5%, and 33.5% in 6 months and 1, 2, 3, 4 and 5 years, respectively. The progression-free survival rate was 48.4%, 27.9%, 13.3%, 11.1%, 7.1%, and 5.4% in 6 months and 1, 2, 3, 4, and 5 years, respectively (Figure 1). There were significant differences in aspartate transaminase (AST), AFP and ALBI grade among the best responders (p = 0.002, 0.021, and 0.011, respectively).

Table 1.
Demographic data of advanced HCC patients receiving sorafenib, and correlation with clinical responses.
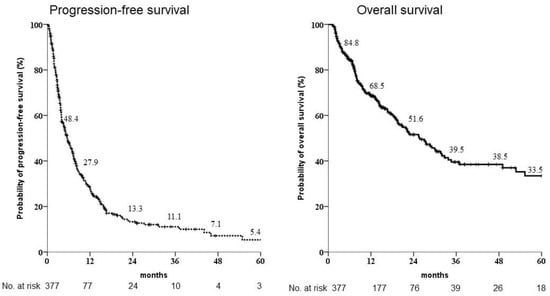
Figure 1.
Kaplan-Meier estimates of survival rates of the 377 patients enrolled for sorafenib administration as first-line treatment. The progression-free survival rates at 1, 2 and 3 years were 27.9%, 13.3% and 11.1%, respectively, whereas the overall survival rates after 1, 2 and 3 years were 68.5%, 51.6% and 39.5%, respectively.
Based on the guidelines of the Taiwan Liver Cancer Association and National Health Insurance of Taiwan, the indications for sorafenib as a first line prescription are extrahepatic spreading (EHS) and MVI involved the first generation and TACE refractoriness (64.7%, 21.0%, and 14.3%, respectively). There were no significant differences in indication, dosage or sequential treatment among the best clinical responders. Fourteen cases underwent surgery or radiofrequency ablation (RFA), while 81 received TACE, 72 received radiotherapy (RT) and 11 received chemotherapy as part of a combination treatment. The combination of sorafenib with surgical resection, TACE, RFA, or radiotherapy could significantly increase the probability of favorable oncologic outcome. There were significantly poorer responses in cases with bone metastasis, lung metastasis and/or multiple target lesions.
3.2. Differential Oncological Outcomes Based on the Presence of Organ-Specific Target Lesions and ALBI Grade
Our analysis of progression-free survival (PFS) showed that significantly poorer prognostic factors included multiple targets, bone, lung, MVI, ALBI grade 2 or 3, AFP > 400 ng/mL, AST two times elevation and combined treatment (Figure 2; p = 0.009, 0.021, 0.036, 0.038, <0.001, 0.002, 0.004, and 0.019, respectively). The Cox regression multivariate analysis showed that bone, lung, MVI, ALBI grade 2 or 3, AFP > 400 ng/mL and combined treatment were independent prognostic factors (Table 2, p = 0.001, 0.008, 0.008, <0.001, 0.008 and 0.026, respectively).
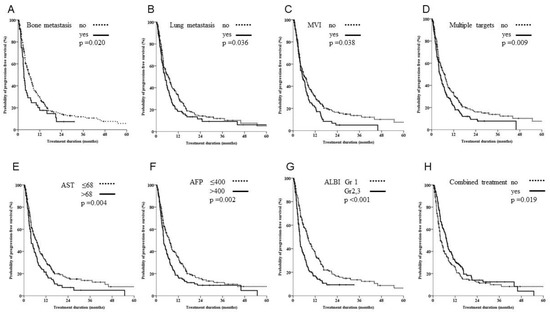
Figure 2.
Kaplan-Meier estimates of survival rates stratified by different organ metastasis, vascular involvement, hepatitis or combined treatment. (A–D) Patients with bone metastasis, lung metastasis, macroscopic venous invasion (MVI) or multiple lesions had significantly poorer outcomes in terms of progression-free survival. (E,F) Patients with aspartate transaminase (AST) levels twice those of normal values and alpha fetoprotein (AFP) levels of >400 ng/mL had significantly poorer outcomes in terms of progression-free survival. (G) Patients with grade III albumin-bilirubin had poor outcomes compared with patients with grade I/II. (H) A combination of sorafenib and ablation treatment yielded significantly better outcomes.

Table 2.
Results of univariate and multivariate Cox regression analysis for progression-free survival following sorafenib treatment for 377 advanced HCC patients.
In a subgroup analysis, when patients with multiple targets were excluded from the single-target subgroup analysis, there were significantly better outcomes for patients with LN metastasis. Patients with MVI still demonstrated the worst outcome in both PFS and OS. Furthermore, those with bone or lung metastases showed significant survival benefits in OS but not PFS; this was attributed to sequential therapy (see Figure 3).
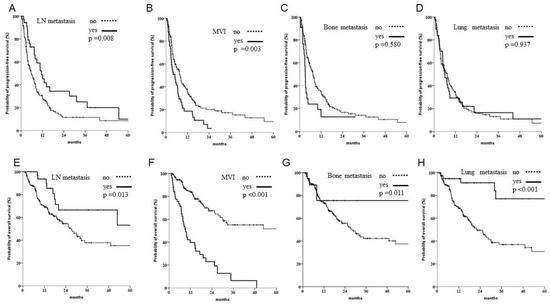
Figure 3.
Subgroup analysis of progression and overall survival when patients with multiple targets were excluded. (A–D) Of 220 patients with single organ involvement, those with lymph node (LN) metastasis had better outcomes, whereas those with bone metastasis or macroscopic venous invasion (MVI) had worse outcomes. (E–H) Significant better OS was noted in patients with single organ involvement, including LNs, bones and lungs, but worse outcomes were observed in patients with MVI.
3.3. Sequential Target Therapy Resulted in Better Survival Outcome
Of the 345 patients with survival follow-up when PD was recorded, 132 had the option of to taking the second line treatment, including target therapy, chemotherapy, ablation, surgery and checkpoint inhibitors. The survival benefit was identified in OS for sequential therapy. Different survival responses were observed with different modality treatment bases. A better survival outcome was noted in sequential target therapy and ablation/surgical treatments (Figure 4).
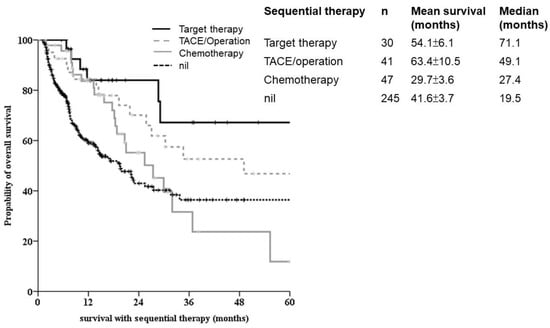
Figure 4.
Sequential therapy for advanced HCC. Kaplan-Meier survival analysis showed significantly better outcomes in the tyrosine kinase inhibitor group (solid line). Patients with chemotherapy-based second line-treatment for aHCC showed significantly poorer outcomes.
4. Discussion
Over the past decade, new systemic treatments for advanced HCC have led to improved patient outcomes. Clinical practice has several treatment regimens for first- and second-line therapies [24]. A combination of immune checkpoint inhibitors and tyrosine kinase inhibitors could be a breakthrough treatment modality, but defects in interferon-γ or insufficient tumor antigen immunosuppressive cells in the tumor microenvironment develop resistance to immune checkpoint inhibitors [25,26]. Sorafenib and other TKIs have been used globally, and molecular biomarkers and genome changes in pathobiological issues have been emphasized [27]. However, varying outcomes in different metastatic locations have been studied. The current evidence indicates the worst outcomes with bone and lung metastases. Patients with aHCC usually had multiple target lesions for treatment (41.6%), and it is hard to determine the cause of cancer-related mortality and organ involvement. However, the LN and peritoneal appear to benefit or show non-inferiority with sorafenib administration, compared with cases in which other organs are involved.
AFP is not only a diagnostic serologic surveillance lab test, but also a tool for the prognostic analysis of surgical outcomes [23]. AFP > 400 ng/mL and AFP response in the REACH study showed better outcomes in OS and PFS, demonstrating the importance of biomarker studies for HCC [28]. Elevated AFP represents a subgroup in HCC tumor heterogeneity [29]. A combination of ALBI grade and AFP level was used as a tool for patient outcome prediction in 88 real-world retrospective cohort study cases [21]. All treated patients showed Child-Pugh grade A status, and ALBI grading was the best evaluation factor for liver function tests. Patients with two-fold elevations of AST were not enrolled in the early clinical phase III studies, and few reports have focused on the impact of chronic hepatitis. However, an elevation of AST representing hepatitis B reactivation and antiviral treatment could prevent prevent reactivation and prolong overall survival for hepatic artery infusion chemotherapy in HCC [30,31,32]. Therefore, AST elevation was also a negative predicting factor, suggesting that patients with liver damage should be carefully monitored in real-world practice.
Combination and multimodality treatments offer a better treatment option for advanced HCC. Radiation or ablation therapies are commonly used for local tumor control. Treating patients at the optimum physiologic status with minimal damage could offer better oncologic outcomes. Combination immunotherapy with immune checkpoint inhibitors (ICI) yielded limited data, and no definite conclusion could be reached. However, development in ICI and tyrosine kinase inhibitors is expected to provide new primary treatment choices in the future.
Though all metastatic HCC could be treated with TKI or dual therapy, no evidence of treatment efficiency at different metastatic sites was found. TMmain venous tumor thrombosis showed poor outcome. Hence, a combined treatment with ICI could be considered. Surgical resection could be considered a combined or sequential strategy for single metastatic lung lesions to improve outcomes. Though HCC with LN metastasis showed no significant benefit in total, better outcomes were observed in single target lesion analysis in our study. A longer DOR was reported in [33,34].
Our study has some limitations. Firstly, it is not a prospective study. Further, there was some bias in the study period. Treatment options have increased in recent years, with other TKIs or dual therapies becoming available as first-line treatments. However, the study revealed that patients with different metastatic target lesions could present different outcomes, providing justification for alternative treatment protocols.
5. Conclusions
Advanced HCC with sorafenib as a first-line regimen had poor outcomes in bone or lung metastasis, MVI, ALBI grade 2 or 3, and AFP > 400 ng/mL. Combined treatment of a local tumor control showed better outcomes. Add-on sorafenib treatment in lung or bone metastasis should be considered.
Although the number of cases enrolled in this study was limited, the data offer some insights. Firstly, independent poor prognostic factors included bone, lung, MVI, ALBI grade 2 or 3, and AFP > 400 ng/mL; combined treatment offered better outcomes. Secondly, bone and lung involvement indicated a higher probability of progression, but sequential therapy including ablation/surgery and second-line targeting resulted in relatively higher long-term survival outcomes.
Author Contributions
Conceptualization, M.-C.Y.; methodology, S.-F.H.; validation, S.-W.C., H.-Y.H. and C.-W.H.; formal analysis, M.-C.Y.; investigation, M.-C.Y.; resources, C.-F.H.; data curation, K.-T.P., C.-T.W., P.-M.C.; writing—original draft preparation, H.-Y.H.; writing—review and editing, C.-W.L. and T.-H.W.; visualization, C.-H.H.; supervision, M.-C.Y.; project administration, M.-C.Y.; funding acquisition, M.-C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by Chang Gung Medical Foundation CMRPVVJ0022 and CMRPG1J0121 for Yu MC.
Institutional Review Board Statement
The retrospective cohort study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Chang Gung Memorial Hospital, Linkou (IRB 201600513B0) and approved on 20 May 2016.
Informed Consent Statement
The study is approved with IRB and waiver of inform consent.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful for the technological support of the Department of Cancer Center, Chang Gung Memorial Hospital, Linkou branch.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cha, C.; Fong, Y.; Jarnagin, W.R.; Blumgart, L.H.; DeMatteo, R.P. Predictors and Patterns of Recurrence After Resection of Hepatocellular Carcinoma. J. Am. Coll. Surg. 2003, 197, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Cleary, S.P.; Wei, A.C.; Yang, I.; Taylor, B.R.; Hemming, A.W.; Langer, B.; Grant, D.R.; Greig, P.D.; Gallinger, S. Recurrence After Liver Resection for Hepatocellular Carcinoma: Risk Factors, Treatment, and Outcomes. Surgery 2007, 141, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Peng, T.; Guan, R.; Zhou, Y.; Zeng, C.; Lin, Y.; Wu, Z.; Tan, H. Development of a Novel Prognostic Nomogram for the Early Recurrence of Liver Cancer After Curative Hepatectomy. Ann. Transl. Med. 2021, 9, 1541. [Google Scholar] [CrossRef]
- Wu, J.C.; Huang, Y.H.; Chau, G.Y.; Su, C.W.; Lai, C.R.; Lee, P.C.; Huo, T.I.; Sheen, I.J.; Lee, S.D.; Lui, W.Y. Risk Factors for Early and Late Recurrence in Hepatitis B-Related Hepatocellular Carcinoma. J. Hepatol. 2009, 51, 890–897. [Google Scholar] [CrossRef]
- Hayashi, M.; Shimizu, T.; Hirokawa, F.; Inoue, Y.; Komeda, K.; Asakuma, M.; Miyamoto, Y.; Takeshita, A.; Shibayama, Y.; Tanigawa, N. Clinicopathological Risk Factors for Recurrence Within One Year After Initial Hepatectomy for Hepatocellular Carcinoma. Am. Surg. 2011, 77, 572–578. [Google Scholar] [CrossRef]
- Shen, A.; Tang, C.; Wang, Y.; Chen, Y.; Yan, X.; Zhang, C.; Liu, R.; Wei, X.; Zhu, Y.; Zhang, H.; et al. A Systematic Review of Sorafenib in Child-Pugh A Patients with Unresectable Hepatocellular Carcinoma. J. Clin. Gastroenterol. 2013, 47, 871–880. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Sherrow, C.; Attwood, K.; Zhou, K.; Mukherjee, S.; Iyer, R.; Fountzilas, C. Sequencing Systemic Therapy Pathways for Advanced Hepatocellular Carcinoma: A Cost Effectiveness Analysis. Liver Cancer 2020, 9, 549–562. [Google Scholar] [CrossRef]
- Takada, H.; Kurosaki, M.; Tsuchiya, K.; Komiyama, Y.; Itakura, J.; Takahashi, Y.; Nakanishi, H.; Yasui, Y.; Tamaki, N.; Maeyashiki, C.; et al. Baseline and Early Predictors of Good Patient Candidates for Second-Line After Sorafenib Treatment in Unresectable Hepatocellular Carcinoma. Cancers 2019, 11, 1256. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, S.S.; Wang, H.J.; Kim, B.W.; Cho, H.; Jung, J.; Cho, S.S.; Kim, J.K.; Lee, J.H.; Kim, Y.B.; et al. Detection of Novel Genomic Markers for Predicting Prognosis in Hepatocellular Carcinoma Patients by Integrative Analysis of Copy Number Aberrations and Gene Expression Profiles: Results from a Long-Term Follow-Up. DNA Cell Biol. 2016, 35, 71–80. [Google Scholar] [CrossRef]
- Zhu, Z.Z.; Bao, L.L.; Zhao, K.; Xu, Q.; Zhu, J.Y.; Zhu, K.X.; Wen, B.J.; Ye, Y.Q.; Wan, X.X.; Wang, L.L.; et al. Copy Number Aberrations of Multiple Genes Identified as Prognostic Markers for Extrahepatic Metastasis-Free Survival of Patients with Hepatocellular Carcinoma. Curr. Med. Sci. 2019, 39, 759–765. [Google Scholar] [CrossRef]
- Yu, M.C.; Lee, C.W.; Lee, Y.S.; Lian, J.H.; Tsai, C.L.; Liu, Y.P.; Wu, C.H.; Tsai, C.N. Prediction of Early-Stage Hepatocellular Carcinoma Using OncoScan Chromosomal Copy Number Aberration Data. World J. Gastroenterol. 2017, 23, 7818–7829. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of Liver Function in Patients with Hepatocellular Carcinoma: A New Evidence-Based Approach-the ALBI Grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Shimose, S.; Hiraoka, A.; Nakano, M.; Iwamoto, H.; Tanaka, M.; Tanaka, T.; Noguchi, K.; Aino, H.; Ogata, K.; Kajiwara, M.; et al. First-Line Sorafenib Sequential Therapy and Liver Disease Etiology for Unresectable Hepatocellular Carcinoma Using Inverse Probability Weighting: A Multicenter Retrospective Study. Cancer Med. 2021, 10, 8530–8541. [Google Scholar] [CrossRef] [PubMed]
- Mai, R.Y.; Wang, Y.Y.; Bai, T.; Chen, J.; Xiang, B.D.; Wu, G.B.; Wu, F.X.; Li, L.Q.; Ye, J.Z. Combination of ALBI and Apri to Predict Post-hepatectomy Liver Failure After Liver Resection for HBV-Related HCC Patients. Cancer Manag. Res. 2019, 11, 8799–8806. [Google Scholar] [CrossRef]
- Carling, U.; Røsok, B.; Line, P.D.; Dorenberg, E.J. ALBI and P-ALBI grade in Child-Pugh A patients treated with drug eluting embolic chemoembolization for hepatocellular carcinoma. Acta Radiol. 2019, 60, 702–709. [Google Scholar] [CrossRef]
- Kudo, M.; Galle, P.R.; Brandi, G.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Llovet, J.M.; Assenat, E.; Merle, P.; Chan, S.L.; et al. Effect of ramucirumab on ALBI grade in patients with advanced HCC: Results from REACH and REACH-2. JHEP Rep. 2021, 3, 100215. [Google Scholar] [CrossRef]
- Zhang, W. Sorafenib in adjuvant setting: Call for precise and personalized therapy. Transl. Gastroenterol. Hepatol. 2016, 1, 13. [Google Scholar] [CrossRef]
- Kudo, M.; Galle, P.R.; Llovet, J.M.; Finn, R.S.; Vogel, A.; Motomura, K.; Assenat, E.; Merle, P.; Brandi, G.; Daniele, B.; et al. Ramucirumab in elderly patients with hepatocellular carcinoma and elevated alpha-fetoprotein after sorafenib in REACH and REACH-2. Liver Int. 2020, 40, 2008–2020. [Google Scholar] [CrossRef]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J. Clin. Oncol. 2020, 20, 4317–4345. [Google Scholar] [CrossRef]
- Wang, H.W.; Chuang, P.H.; Su, W.P.; Kao, J.T.; Hsu, W.F.; Lin, C.C.; Huang, G.T.; Lin, J.T.; Lai, H.C.; Peng, C.Y. On-Treatment Albumin-Bilirubin Grade: Predictor of Response and Outcome of Sorafenib-Regorafenib Sequential Therapy in Patients with Unresectable Hepatocellular Carcinoma. Cancers 2021, 13, 3758. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; Chao, Y.; Lee, P.C.; Chen, S.C.; Chi, C.T.; Wu, C.J.; Wu, K.C.; Hou, M.C.; Huang, Y.H. Determinants of Survival and Post-Progression Outcomes by Sorafenib-Regorafenib Sequencing for Unresectable Hepatocellular Carcinoma. Cancers 2022, 14, 2014. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.C.; Chan, K.M.; Lee, C.F.; Lee, Y.S.; Eldeen, F.Z.; Chou, H.S.; Lee, W.C.; Chen, M.F. Alkaline phosphatase: Does it have a role in predicting hepatocellular carcinoma recurrence? J. Gastrointest. Surg. 2011, 15, 1440–1449. [Google Scholar] [CrossRef]
- Llovet, J.M.; Villanueva, A.; Marrero, J.A.; Schwartz, M.; Meyer, T.; Galle, P.R.; Lencioni, R.; Greten, T.F.; Kudo, M.; Mandrekar, S.J.; et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology 2021, 73, 158–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Jiang, M.J.; Deng, Z.J.; Li, L.; Huang, J.L.; Liu, Z.X.; Li, L.Q.; Zhong, J.H. Immune checkpoint inhibitors in hepatocellular carcinoma: Current progresses and challenges. Front. Oncol. 2021, 11, 4456. [Google Scholar] [CrossRef]
- Peterson-Dana, C. Shared decisions and agency: Better engagement tools. Psychiatr. Serv. 2019, 1, 857. [Google Scholar] [CrossRef]
- Mou, L.; Tian, X.; Zhou, B.; Zhan, Y.; Chen, J.; Lu, Y.; Deng, J.; Deng, Y.; Wu, Z.; Li, Q.; et al. Improving outcomes of tyrosine kinase inhibitors in hepatocellular carcinoma: New data and ongoing trials. Front. Oncol. 2021, 11, 4183. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. REACH-2 study investigators Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Lee, C.W.; Yu, M.C.; Lin, G.; Chiu, J.C.; Chiang, M.H.; Sung, C.M.; Hsieh, Y.C.; Kuo, T.; Lin, C.Y.; Tsai, H.I. Serum metabolites may be useful markers to assess vascular invasion and identify normal alpha-fetoprotein in hepatocellular carcinoma undergoing liver resection: A pilot study. World J. Surg. Oncol. 2020, 18, 121. [Google Scholar] [CrossRef]
- Kudo, M.; Kawamura, Y.; Hasegawa, K.; Tateishi, R.; Kariyama, K.; Shiina, S.; Toyoda, H.; Imai, Y.; Hiraoka, A.; Ikeda, M.; et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021, 10, 181–223. [Google Scholar] [CrossRef]
- Piñero, F.; Dirchwolf, M.; Pessôa, M.G. Biomarkers in Hepatocellular Carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. Cells 2020, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lai, J.; Lyu, N.; Xie, Q.; Cao, H.; Chen, D.; He, M.; Zhang, B.; Zhao, M. Effects of Antiviral Therapy on HBV Reactivation and Survival in Hepatocellular Carcinoma Patients Undergoing Hepatic Artery Infusion Chemotherapy. Front. Oncol. 2020, 10, 582504. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.I.; Kim, B.H.; Kim, Y.J.; Yoon, J.H.; Jung, Y.J.; Chie, E.K. Role of radiotherapy in Barcelona Clinic Liver Cancer stage C hepatocellular carcinoma treated with sorafenib. J. Gastroenterol. Hepatol. 2022, 37, 387–394. [Google Scholar] [CrossRef]
- Lee, C.W.; Chan, K.M.; Lee, C.F.; Yu, M.C.; Lee, W.C.; Wu, T.J.; Chen, M.F. Hepatic resection for hepatocellular carcinoma with lymph node metastasis: Clinicopathological analysis and survival outcome. Asian J. Surg. 2011, 34, 53–62. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).