Connexins and Pannexins: Important Players in Neurodevelopment, Neurological Diseases, and Potential Therapeutics
Abstract
1. Introduction
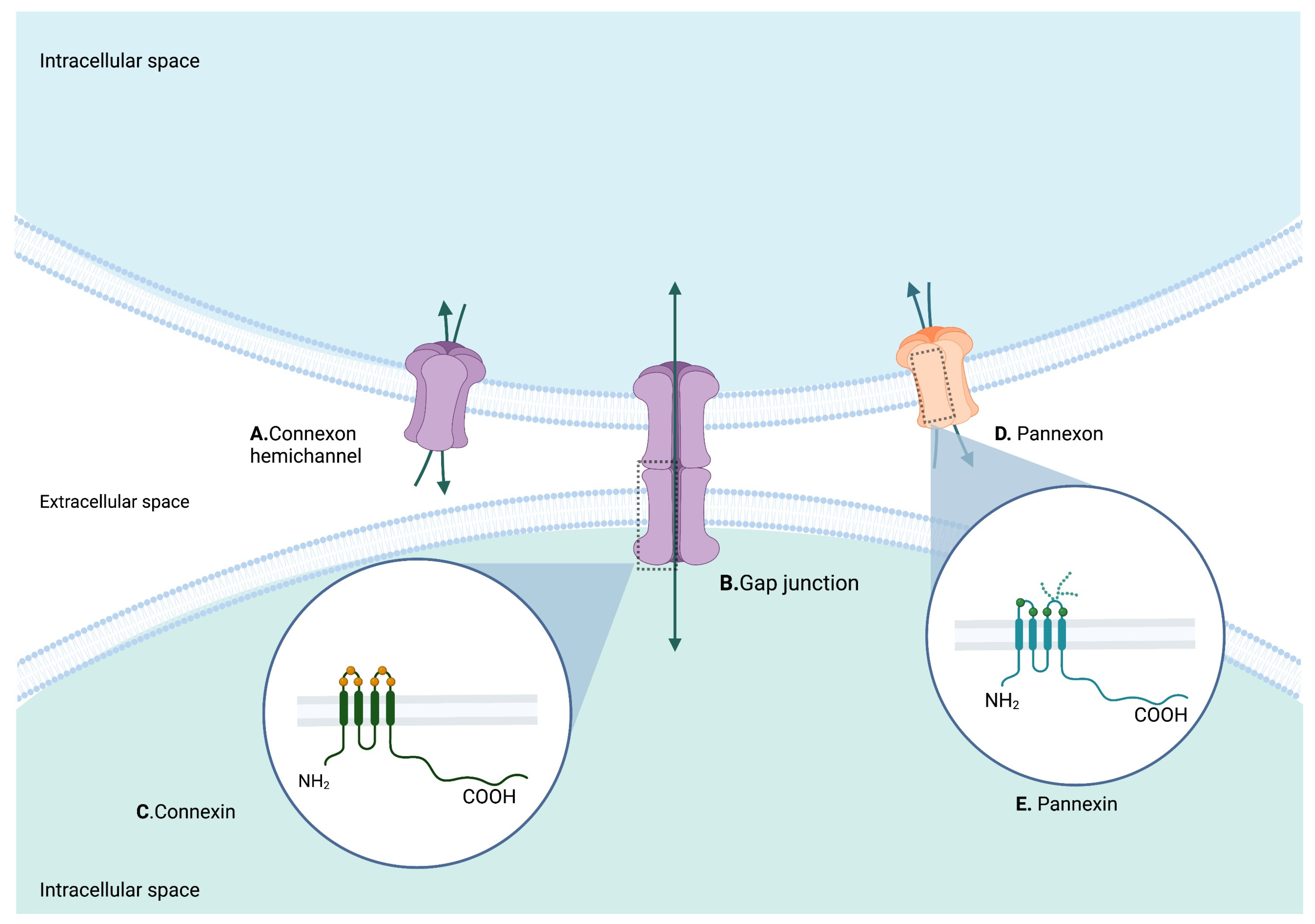
2. Structure of Cxs and Panxs
2.1. Cx43
2.2. Panx1
3. General Role of Cxs and Panxs in the Nervous System
| Cx | Gene | Relevant Function during Development | Relevant Function in Adult CNS or PNS | Related Diseases and Functional Alteration | Reference |
|---|---|---|---|---|---|
| Cx43 | Gap junction protein alpha 1 |
|
|
| [5,53,56,57] |
| CX37 | Gap junction protein alpha 4 | - |
| - | [52] |
| CX40 | Gap junction protein alpha 5 |
|
| - | [52,58] |
| CX32 | Gap junction protein beta 1 |
|
|
| [50,59] |
| CX26 | Gap junction protein beta 2 |
|
|
| [60,61] |
| CX30 | Gap junction protein beta 6 |
|
| Upregulation in neurodegenerative diseases | [62,63] |
| CX45 | Gap junction protein gamma 1 |
|
| - | [58] |
| CX47 CX46.6 | Gap junction protein gamma 2 | - |
| Pelizaeus-Merzbacher-like disease 1 | [64,65] |
| CX36 | Gap junction protein delta 2 |
|
| - | [66,67] |
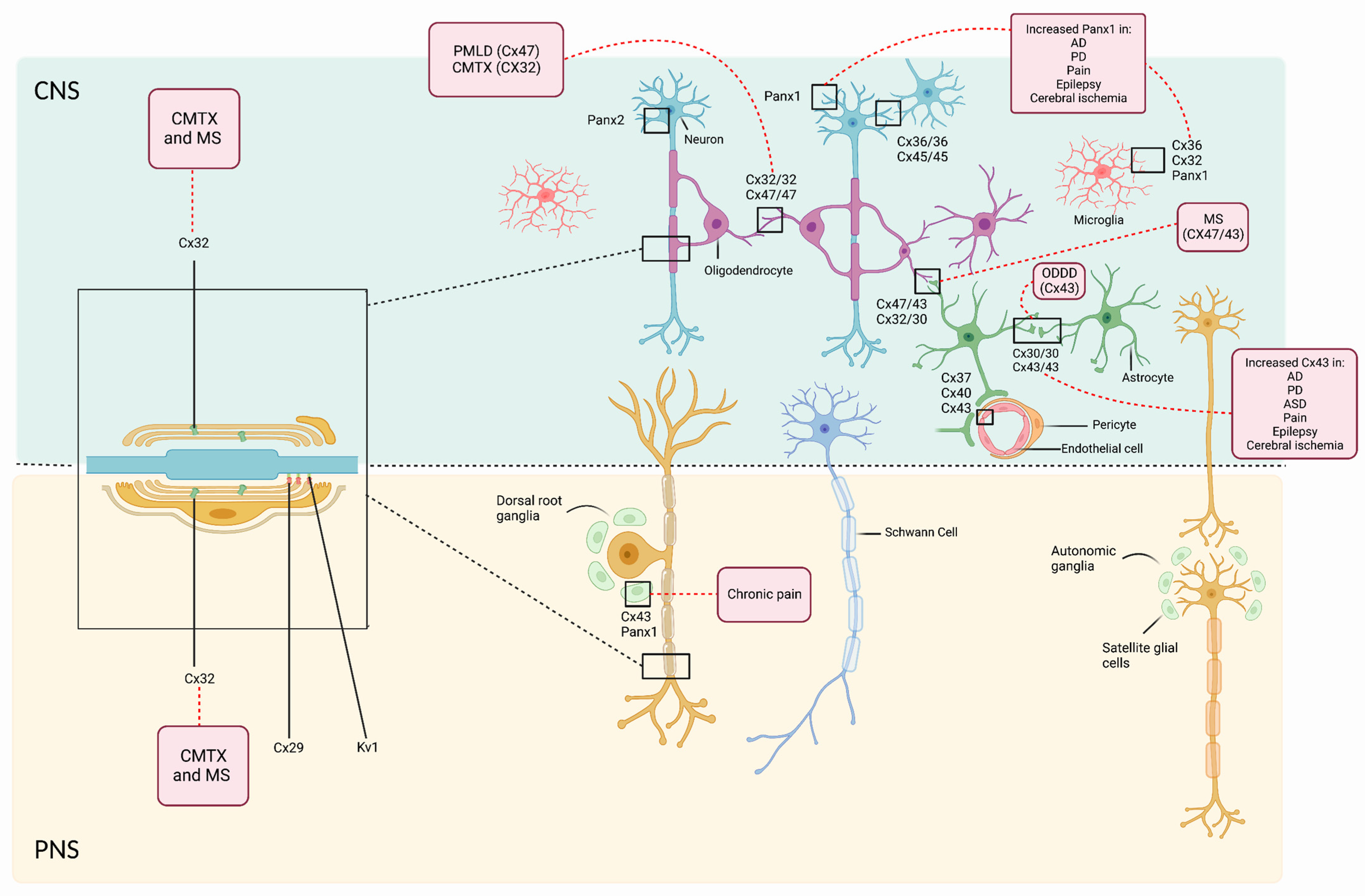
3.1. Role of Cxs and Panxs in Cells of the CNS
3.1.1. Neurons
3.1.2. Astrocytes
3.1.3. Oligodendrocytes
3.1.4. Microglia
3.2. Role of Cxs and Panxs in Cells of the PNS
3.2.1. Schwann Cells
3.2.2. Satellite Glial Cells (SGC)
4. Clinical Correlation
4.1. Congenital Neurological Diseases Associated with Cx and Panx Alterations
4.1.1. Charcot-Marie-Tooth Disease
4.1.2. Oculodentodigital Dysplasia
4.1.3. Pelizaeus-Merzbacher-like Disease Type 1 (PMLD1)
4.2. Acquired Neurological Diseases
4.2.1. Multiple Sclerosis (MS)
4.2.2. Neurodegenerative Diseases: Alzheimer’s Disease (AD), Parkinson’s Disease (PD)
4.2.3. Pain
4.2.4. Epilepsy
4.2.5. Autism Spectrum Disorder (ASD)
4.2.6. Cerebral Ischemia
4.2.7. Neuroinflammation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, S. Induction and Competence. In Developmental Biology, 6th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2000. [Google Scholar]
- Sonnen, K.F.; Janda, C.Y. Signalling Dynamics in Embryonic Development. Biochem. J. 2021, 478, 4045–4070. [Google Scholar] [CrossRef]
- Basson, M.A. Signaling in Cell Differentiation and Morphogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008151. [Google Scholar] [CrossRef]
- Sanz-Ezquerro, J.J.; Münsterberg, A.E.; Stricker, S. Signaling Pathways in Embryonic Development. Front. Cell Dev. Biol. 2017, 5, 76. [Google Scholar] [CrossRef]
- Jourdeuil, K.; Taneyhill, L.A. The Gap Junction Protein Connexin 43 Controls Multiple Aspects of Cranial Neural Crest Cell Development. J. Cell Sci. 2020, 133, jcs235440. [Google Scholar] [CrossRef]
- Weber, P.A.; Chang, H.C.; Spaeth, K.E.; Nitsche, J.M.; Nicholson, B.J. The Permeability of Gap Junction Channels to Probes of Different Size Is Dependent on Connexin Composition and Permeant-Pore Affinities. Biophys. J. 2004, 87, 958. [Google Scholar] [CrossRef]
- Lukowicz-Bedford, R.M.; Farnsworth, D.R.; Miller, A.C. Connexinplexity: The Spatial and Temporal Expression of Connexin Genes during Vertebrate Organogenesis. bioRxiv 2021, 12, jkac062. [Google Scholar] [CrossRef]
- Koval, M.; Molina, S.A.; Burt, J.M. Mix and Match: Investigating Heteromeric and Heterotypic Gap Junction Channels in Model Systems and Native Tissues. FEBS Lett. 2014, 588, 1193. [Google Scholar] [CrossRef]
- Mondal, A.; Sachse, F.B.; Moreno, A.P. Modulation of Asymmetric Flux in Heterotypic Gap Junctions by Pore Shape, Particle Size and Charge. Front. Physiol. 2017, 8, 206. [Google Scholar] [CrossRef]
- Sorgen, P.L.; Trease, A.J.; Spagnol, G.; Delmar, M.; Nielsen, M.S. Protein–Protein Interactions with Connexin 43: Regulation and Function. Int. J. Mol. Sci. 2018, 19, 1428. [Google Scholar] [CrossRef]
- Xie, H.Y.; Cui, Y.; Deng, F.; Feng, J.C. Connexin: A Potential Novel Target for Protecting the Central Nervous System? Neural Regen. Res. 2015, 10, 659–666. [Google Scholar] [CrossRef]
- Decrock, E.; de Bock, M.; Wang, N.; Bultynck, G.; Giaume, C.; Naus, C.C.; Green, C.R.; Leybaert, L. Connexin and Pannexin Signaling Pathways, an Architectural Blueprint for CNS Physiology and Pathology? Cell. Mol. Life Sci. 2015, 72, 2823–2851. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Orozco, I.J.; Du, J.; Lü, W. Structures of Human Pannexin 1 Reveal Ion Pathways and Mechanism of Gating. Nature 2020, 584, 646. [Google Scholar] [CrossRef]
- Michalski, K.; Henze, E.; Nguyen, P.; Lynch, P.; Kawate, T. The Weak Voltage Dependence of Pannexin 1 Channels Can Be Tuned by N-Terminal Modifications. J. Gen. Physiol. 2018, 150, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Sosinsky, G.E.; Boassa, D.; Dermietzel, R.; Duffy, H.S.; Laird, D.W.; MacVicar, B.A.; Naus, C.C.; Penuela, S.; Scemes, E.; Spray, D.C.; et al. Pannexin Channels Are Not Gap Junction Hemichannels. Channels 2011, 5, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Sahu, G.; Sukumaran, S.; Bera, A.K. Pannexins Form Gap Junctions with Electrophysiological and Pharmacological Properties Distinct from Connexins. Sci. Rep. 2014, 4, 4955. [Google Scholar] [CrossRef]
- Dahl, G.; Locovei, S. Pannexin: To Gap or Not to Gap, Is That a Question? IUBMB Life 2006, 58, 409–419. [Google Scholar] [CrossRef]
- Abudara, V.; Retamal, M.A.; del Rio, R.; Orellana, J.A. Synaptic Functions of Hemichannels and Pannexons: A Double-Edged Sword. Front. Mol. Neurosci. 2018, 11, 435. [Google Scholar] [CrossRef]
- Suadicani, S.O.; Iglesias, R.; Wang, J.; Dahl, G.; Spray, D.C.; Scemes, E. ATP Signaling Is Deficient in Cultured Pannexin1-Null Mouse Astrocytes. Glia 2012, 60, 1106–1116. [Google Scholar] [CrossRef]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin Membrane Channels Are Mechanosensitive Conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef]
- Fujii, Y.; Maekawa, S.; Morita, M. Astrocyte Calcium Waves Propagate Proximally by Gap Junction and Distally by Extracellular Diffusion of ATP Released from Volume-Regulated Anion Channels. Sci. Rep. 2017, 7, 131115. [Google Scholar] [CrossRef]
- Dahl, G. ATP Release through Pannexon Channels. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140191. [Google Scholar] [CrossRef] [PubMed]
- Wörsdörfer, P.; Maxeiner, S.; Markopoulos, C.; Kirfel, G.; Wulf, V.; Auth, T.; Urschel, S.; von Maltzahn, J.; Willecke, K. Connexin Expression and Functional Analysis of Gap Junctional Communication in Mouse Embryonic Stem Cells. Stem Cells 2008, 26, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Ohtaka-Maruyama, C. Subplate Neurons as an Organizer of Mammalian Neocortical Development. Front. Neuroanat. 2020, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Jurić, M.; Zeitler, J.; Vukojević, K.; Bočina, I.; Grobe, M.; Kretzschmar, G.; Saraga-Babić, M.; Filipović, N. Expression of Connexins 37, 43 and 45 in Developing Human Spinal Cord and Ganglia. Int. J. Mol. Sci. 2020, 21, 9356. [Google Scholar] [CrossRef]
- Park, Y.J.; Yoo, S.A.; Kim, M.; Kim, W.U. The Role of Calcium–Calcineurin–NFAT Signaling Pathway in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 195. [Google Scholar] [CrossRef]
- Davidson, J.O.; Green, C.R.; Bennet, L.; Gunn, A.J. Battle of the Hemichannels—Connexins and Pannexins in Ischemic Brain Injury. Int. J. Dev. Neurosci. 2015, 45, 66–74. [Google Scholar] [CrossRef]
- Sánchez, O.F.; Rodríguez, A.v.; Velasco-España, J.M.; Murillo, L.C.; Sutachan, J.J.; Albarracin, S.L. Role of Connexins 30, 36, and 43 in Brain Tumors, Neurodegenerative Diseases, and Neuroprotection. Cells 2020, 9, 846. [Google Scholar] [CrossRef]
- Charvériat, M.; Mouthon, F.; Rein, W.; Verkhratsky, A. Connexins as Therapeutic Targets in Neurological and Neuropsychiatric Disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166098. [Google Scholar] [CrossRef]
- Wicki-Stordeur, X.E.; Sanchez-Arias, X.C.; Dhaliwal, J.; Carmona-Wagner, E.O.; Shestopalov, X.I.; Lagace, D.C.; Swayne, L.A. Pannexin 1 Differentially Affects Neural Precursor Cell Maintenance in the Ventricular Zone and Peri-Infarct Cortex. J. Neurosci. 2016, 36, 1203–1210. [Google Scholar] [CrossRef]
- Li, N.; Leung, G.K.K. Oligodendrocyte Precursor Cells in Spinal Cord Injury: A Review and Update. BioMed Res. Int. 2015, 2015, 235195. [Google Scholar] [CrossRef]
- Kirichenko, E.Y.; Skatchkov, S.N.; Ermakov, A.M. Structure and Functions of Gap Junctions and Their Constituent Connexins in the Mammalian CNS. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2021, 15, 107–119. [Google Scholar] [CrossRef]
- Maeda, S.; Nakagawa, S.; Suga, M.; Yamashita, E.; Oshima, A.; Fujiyoshi, Y.; Tsukihara, T. Structure of the Connexin 26 Gap Junction Channel at 3.5 Å Resolution. Nature 2009, 458, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.B.; Haddad, B.G.; O’Neill, S.E.; Chorev, D.S.; Yoshioka, C.C.; Robinson, C.v.; Zuckerman, D.M.; Reichow, S.L. Structure of Native Lens Connexin 46/50 Intercellular Channels by Cryo-EM. Nature 2018, 564, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jeong, H.; Hyun, J.; Ryu, B.; Park, K.; Lim, H.H.; Yoo, J.; Woo, J.S. Cryo-EM Structure of Human Cx31.3/GJC3 Connexin Hemichannel. Sci. Adv. 2020, 6, eaba4996. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Acosta-Gutierrez, S.; Lavriha, P.; Othman, A.; Lopez-Pigozzi, D.; Bayraktar, E.; Schuster, D.; Picotti, P.; Zamboni, N.; Bortolozzi, M.; et al. Structure of the Connexin-43 Gap Junction Channel Reveals a Closed Sieve-like Molecular Gate. bioRxiv 2022. [Google Scholar] [CrossRef]
- Johnstone, S.R.; Billaud, M.; Lohman, A.W.; Taddeo, E.P.; Isakson, B.E. Posttranslational Modifications in Connexins and Pannexins. J. Membr. Biol. 2012, 245, 319–332. [Google Scholar] [CrossRef]
- Woo, J.-S.; Lee, H.-J.; Cha, H.J.; Jeong, H.; Lee, C.-W.; Kim, M.; Yoo, J. Structural Insights into the Gating Mechanism of Human Cx43/GJA1 Gap Junction Channel. 2021; preprints. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Zhang, L.; Chen, B.; Yang, L.; Li, X.; Li, Y.; Yu, H. Inhibition of Connexin 43 Hemichannels Alleviates Cerebral Ischemia/Reperfusion Injury via the TLR4 Signaling Pathway. Front. Cell. Neurosci. 2018, 12, 372. [Google Scholar] [CrossRef]
- Walrave, L.; Pierre, A.; Albertini, G.; Aourz, N.; de Bundel, D.; van Eeckhaut, A.; Vinken, M.; Giaume, C.; Leybaert, L.; Smolders, I. Inhibition of Astroglial Connexin43 Hemichannels with TAT-Gap19 Exerts Anticonvulsant Effects in Rodents. Glia 2018, 66, 1788–1804. [Google Scholar] [CrossRef]
- Mou, L.; Ke, M.; Song, M.; Shan, Y.; Xiao, Q.; Liu, Q.; Li, J.; Sun, K.; Pu, L.; Guo, L.; et al. Structural Basis for Gating Mechanism of Pannexin 1 Channel. Cell Res. 2020, 30, 452–454. [Google Scholar] [CrossRef]
- Deng, Z.; He, Z.; Maksaev, G.; Bitter, R.M.; Rau, M.; Fitzpatrick, J.A.J.; Yuan, P. Cryo-EM Structures of the ATP Release Channel Pannexin 1. Nat. Struct. Mol. Biol. 2020, 27, 373–381. [Google Scholar] [CrossRef]
- Qu, R.; Dong, L.; Zhang, J.; Yu, X.; Wang, L.; Zhu, S. Cryo-EM Structure of Human Heptameric Pannexin 1 Channel. Cell Res. 2020, 30, 446–448. [Google Scholar] [CrossRef] [PubMed]
- DeLalio, L.J.; Billaud, M.; Ruddiman, C.A.; Johnstone, S.R.; Butcher, J.T.; Wolpe, A.G.; Jin, X.; Keller, T.C.S.; Keller, A.S.; Rivière, T.; et al. Constitutive SRC-Mediated Phosphorylation of Pannexin 1 at Tyrosine 198 Occurs at the Plasma Membrane. J. Biol. Chem. 2019, 294, 6940–6956. [Google Scholar] [CrossRef] [PubMed]
- López, X.; Escamilla, R.; Fernández, P.; Duarte, Y.; González-Nilo, F.; Palacios-Prado, N.; Martinez, A.D.; Sáez, J.C. Stretch-Induced Activation of Pannexin 1 Channels Can Be Prevented by PKA-Dependent Phosphorylation. Int. J. Mol. Sci. 2020, 21, 9180. [Google Scholar] [CrossRef]
- Sandilos, J.K.; Chiu, Y.H.; Chekeni, F.B.; Armstrong, A.J.; Walk, S.F.; Ravichandran, K.S.; Bayliss, D.A. Pannexin 1, an ATP Release Channel, Is Activated by Caspase Cleavage of Its Pore-Associated C-Terminal Autoinhibitory Region. J. Biol. Chem. 2012, 287, 11303–11311. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.; Barr, K.; Jones, H.; Zhu, D.; Kidder, G. Multiple Members of the Connexin Gene Family Participate in Preimplantation Development of the Mouse. Genesis 1996, 18, 234–243. [Google Scholar] [CrossRef]
- Huettner, J.E.; Lu, A.; Qu, Y.; Wu, Y.; Kim, M.; McDonald, J.W. Gap Junctions and Connexon Hemichannels in Human Embryonic Stem Cells. Stem Cells 2006, 24, 1654–1667. [Google Scholar] [CrossRef]
- Wong, R.C.B.; Pébay, A.; Nguyen, L.T.V.; Koh, K.L.L.; Pera, M.F. Presence of Functional Gap Junctions in Human Embryonic Stem Cells. Stem Cells 2004, 22, 883–889. [Google Scholar] [CrossRef]
- Moore, A.R.; Zhou, W.L.; Sirois, C.L.; Belinsky, G.S.; Zecevic, N.; Antic, S.D. Connexin Hemichannels Contribute to Spontaneous Electrical Activity in the Human Fetal Cortex. Proc. Natl. Acad. Sci. USA 2014, 111, E3919–E3928. [Google Scholar] [CrossRef]
- Crabtree, G.R.; Olson, E.N. NFAT Signaling: Choreographing the Social Lives of Cells. Cell 2002, 109, S67–S79. [Google Scholar] [CrossRef]
- Zhao, Y.; Xin, Y.; He, Z.; Hu, W. Function of Connexins in the Interaction between Glial and Vascular Cells in the Central Nervous System and Related Neurological Diseases. Neural Plast. 2018, 2018, 6323901. [Google Scholar] [CrossRef]
- Hösli, L.; Binini, N.; Ferrari, K.D.; Thieren, L.; Looser, Z.J.; Zuend, M.; Zanker, H.S.; Berry, S.; Holub, M.; Möbius, W.; et al. Decoupling Astrocytes in Adult Mice Impairs Synaptic Plasticity and Spatial Learning. Cell Rep. 2022, 38, 110484. [Google Scholar] [CrossRef] [PubMed]
- Clasadonte, J.; Scemes, E.; Wang, Z.; Boison, D.; Haydon, P.G. Connexin 43-Mediated Astroglial Metabolic Networks Contribute to the Regulation of the Sleep-Wake Cycle. Neuron 2017, 95, 1365–1380.e5. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Conzelmann, C.; Riaz, M.A.; Noll, T.; Gündüz, D. Inhibition of Cx43 Attenuates ERK1/2 Activation, Enhances the Expression of Cav-1 and Suppresses Cell Proliferation. Int. J. Mol. Med. 2018, 42, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Kotini, M.; Barriga, E.H.; Leslie, J.; Gentzel, M.; Rauschenberger, V.; Schambon, A.; Mayor, R. Gap Junction Protein Connexin-43 Is a Direct Transcriptional Regulator of N-Cadherin in Vivo. Nat. Commun. 2018, 9, 3846. [Google Scholar] [CrossRef]
- Cooper, M.L.; Pasini, S.; Lambert, W.S.; D’Alessandro, K.B.; Yao, V.; Risner, M.L.; Calkins, D.J. Redistribution of Metabolic Resources through Astrocyte Networks Mitigates Neurodegenerative Stress. Proc. Natl. Acad. Sci. USA 2020, 117, 18810–18821. [Google Scholar] [CrossRef]
- Dilger, N.; Neehus, A.L.; Grieger, K.; Hoffmann, A.; Menssen, M.; Ngezahayo, A. Gap Junction Dependent Cell Communication Is Modulated During Transdifferentiation of Mesenchymal Stem/Stromal Cells Towards Neuron-Like Cells. Front. Cell Dev. Biol. 2020, 8, 869. [Google Scholar] [CrossRef]
- Tomaselli, P.J.; Rossor, A.M.; Horga, A.; Jaunmuktane, Z.; Carr, A.; Saveri, P.; Piscosquito, G.; Pareyson, D.; Laura, M.; Blake, J.C.; et al. Mutations in Noncoding Regions of GJB1 Are a Major Cause of X-Linked CMT. Neurology 2017, 88, 1445–1453. [Google Scholar] [CrossRef]
- Cohn, E.; Kelley, P. Clinical Phenotype and Mutations in Connexin 26 (DFNB1/GJB2), the Most Common Cause of Childhood Hearing Loss. Am. J. Med. Genet. 1999, 89, 130–136. [Google Scholar] [CrossRef]
- Su, X.; Chen, J.J.; Liu, L.Y.; Huang, Q.; Zhang, L.Z.; Li, X.Y.; He, X.N.; Lu, W.; Sun, S.; Li, H.; et al. Neonatal CX26 Removal Impairs Neocortical Development and Leads to Elevated Anxiety. Proc. Natl. Acad. Sci. USA 2017, 114, 3228–3233. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin Signaling in Plant Development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Wallraff, A.; Köhling, R.; Heinemann, U.; Theis, M.; Willecke, K.; Steinhäuser, C. The Impact of Astrocytic Gap Junctional Coupling on Potassium Buffering in the Hippocampus. J. Neurosci. 2006, 26, 5438–5447. [Google Scholar] [CrossRef] [PubMed]
- Owczarek-Lipska, M.; Mulahasanovic, L.; Obermaier, C.D.; Hörtnagel, K.; Neubauer, B.A.; Korenke, G.C.; Biskup, S.; Neidhardt, J. Novel Mutations in the GJC2 Gene Associated with Pelizaeus–Merzbacher-like Disease. Mol. Biol. Rep. 2019, 46, 4507–4516. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; das Sarma, J. Connexin 43/47 Channels Are Important for Astrocyte/Oligodendrocyte Cross-Talk in Myelination and Demyelination. J. Biosci. 2018, 43, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Frisch, C.; Souza-Silva, M.A.D.; Söhl, G.; Güldenagel, M.; Willecke, K.; Huston, J.P.; Dere, E. Stimulus Complexity Dependent Memory Impairment and Changes in Motor Performance after Deletion of the Neuronal Gap Junction Protein Connexin36 in Mice. Behav. Brain Res. 2005, 157, 177–185. [Google Scholar] [CrossRef]
- Hartfield, E.M.; Rinaldi, F.; Glover, C.P.; Wong, L.F.; Caldwell, M.A.; Uney, J.B. Connexin 36 Expression Regulates Neuronal Differentiation from Neural Progenitor Cells. PLoS ONE 2011, 6, e14746. [Google Scholar] [CrossRef]
- Cavaliere, F.; Donno, C.; D’Ambrosi, N. Purinergic Signaling: A Common Pathway for Neural and Mesenchymal Stem Cell Maintenance and Differentiation. Front. Cell. Neurosci. 2015, 9, 211. [Google Scholar] [CrossRef]
- Wilkaniec, A.; Gąssowska, M.; Czapski, G.A.; Cieślik, M.; Sulkowski, G.; Adamczyk, A. P2X7 Receptor-Pannexin 1 Interaction Mediates Extracellular Alpha-Synuclein-Induced ATP Release in Neuroblastoma SH-SY5Y Cells. Purinergic Signal. 2017, 13, 347. [Google Scholar] [CrossRef]
- Boyce, A.K.J.; Swayne, L.A. P2X7 Receptor Cross-Talk Regulates ATP-Induced Pannexin 1 Internalization. Biochem. J. 2017, 474, 2133–2144. [Google Scholar] [CrossRef]
- Wicki-Stordeur, L.E.; Dzugalo, A.D.; Swansburg, R.M.; Suits, J.M.; Swayne, L.A. Pannexin 1 Regulates Postnatal Neural Stem and Progenitor Cell Proliferation. Neural Dev. 2012, 7, 11. [Google Scholar] [CrossRef]
- Hainz, N.; Beckmann, A.; Schubert, M.; Haase, A.; Martin, U.; Tschernig, T.; Meier, C. Human Stem Cells Express Pannexins. BMC Res. Notes 2018, 11, 54. [Google Scholar] [CrossRef]
- Freitas-Andrade, M.; Bechberger, J.F.; MacVicar, B.A.; Viau, V.; Naus, C.C.; Freitas-Andrade, M.; Bechberger, J.F.; MacVicar, B.A.; Viau, V.; Naus, C.C. Pannexin1 Knockout and Blockade Reduces Ischemic Stroke Injury in Female, but Not in Male Mice. Oncotarget 2017, 8, 36973–36983. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.J. Pannexin Channels and Ischaemia. J. Physiol. 2015, 593, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Flores-Muñoz, C.; Gómez, B.; Mery, E.; Mujica, P.; Gajardo, I.; Córdova, C.; Lopez-Espíndola, D.; Durán-Aniotz, C.; Hetz, C.; Muñoz, P.; et al. Acute Pannexin 1 Blockade Mitigates Early Synaptic Plasticity Defects in a Mouse Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2020, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zang, Z.; He, J.; Chen, X.; Yu, S.; Pei, Y.; Hou, Z.; An, N.; Yang, H.; Zhang, C.; et al. Expression of Pannexin 1 and 2 in Cortical Lesions from Intractable Epilepsy Patients with Focal Cortical Dysplasia. Oncotarget 2016, 8, 6883–6895. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cerdeño, V.; Noctor, S.C. Neural Progenitor Cell Terminology. Front. Neuroanat. 2018, 12, 104. [Google Scholar] [CrossRef]
- Qi, G.J.; Chen, Q.; Chen, L.J.; Shu, Y.; Bu, L.L.; Shao, X.Y.; Zhang, P.; Jiao, F.J.; Shi, J.; Tian, B. Phosphorylation of Connexin 43 by Cdk5 Modulates Neuronal Migration During Embryonic Brain Development. Mol. Neurobiol. 2015, 53, 2969–2982. [Google Scholar] [CrossRef]
- Swayne, L.A.; Sorbara, C.D.; Bennett, S.A.L. Pannexin 2 Is Expressed by Postnatal Hippocampal Neural Progenitors and Modulates Neuronal Commitment. J. Biol. Chem. 2010, 285, 24977–24986. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.J.; Huang, Q.; Su, X.; Yu, Y.C.; Liu, L.Y. Connexin43 in Neonatal Excitatory Neurons Is Important for Short-Term Motor Learning. Brain Res. 2019, 1720, 146287. [Google Scholar] [CrossRef]
- Talaverón, R.; Fernández, P.; Escamilla, R.; Pastor, A.M.; Matarredona, E.R.; Sáez, J.C. Neural Progenitor Cells Isolated from the Subventricular Zone Present Hemichannel Activity and Form Functional Gap Junctions with Glial Cells. Front. Cell. Neurosci. 2015, 9, 411. [Google Scholar] [CrossRef]
- Genet, N.; Bhatt, N.; Bourdieu, A.; Hirschi, K.K. Multifaceted Roles of Connexin 43 in Stem Cell Niches. Curr. Stem Cell Rep. 2018, 4, 1–12. [Google Scholar] [CrossRef]
- Zhang, J.; Griemsmann, S.; Wu, Z.; Dobrowolski, R.; Willecke, K.; Theis, M.; Steinhäuser, C.; Bedner, P. Connexin43, but Not Connexin30, Contributes to Adult Neurogenesis in the Dentate Gyrus. Brain Res. Bull. 2018, 136, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Olk, S.; Turchinovich, A.; Grzendowski, M.; Stühler, K.; Meyer, H.E.; Zoidl, G.; Dermietzel, R. Proteomic Analysis of Astroglial Connexin43 Silencing Uncovers a Cytoskeletal Platform Involved in Process Formation and Migration. Glia 2010, 58, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Ghézali, G.; Calvo, C.-F.; Pillet, L.-E.; Llense, F.; Ezan, P.; Pannash, U.; Bemelman, A.-P.; Menneville, S.-E.; Rouach, N. Connexin 30 Controls Astroglial Polarization during Postnatal Brain Development. Development 2018, 4, dev155275. [Google Scholar] [CrossRef]
- Rouach, N.; Koulakoff, A.; Abudara, V.; Willecke, K.; Giaume, C. Astroglial Metabolic Networks Sustain Hippocampal Synaptic Transmission. Science 2008, 322, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Egawa, K.; Yamada, J.; Furukawa, T.; Yanagawa, Y.; Fukuda, A. Cl− Homeodynamics in Gap Junction-Coupled Astrocytic Networks on Activation of GABAergic Synapses. J. Physiol. 2013, 591, 3901–3917. [Google Scholar] [CrossRef]
- Bazzigaluppi, P.; Weisspapir, I.; Stefanovic, B.; Leybaert, L.; Carlen, P.L. Astrocytic Gap Junction Blockade Markedly Increases Extracellular Potassium without Causing Seizures in the Mouse Neocortex. Neurobiol. Dis. 2017, 101, 1–7. [Google Scholar] [CrossRef]
- Meunier, C.; Wang, N.; Yi, C.; Dallerac, G.; Ezan, P.; Koulakoff, A.; Leybaert, L.; Giaume, C. Contribution of Astroglial Cx43 Hemichannels to the Modulation of Glutamatergic Currents by D-Serine in the Mouse Prefrontal Cortex. J. Neurosci. 2017, 37, 9064–9075. [Google Scholar] [CrossRef]
- Ezan, P.; André, P.; Cisternino, S.; Saubaméa, B.; Boulay, A.C.; Doutremer, S.; Thomas, M.A.; Quenech’Du, N.; Giaume, C.; Cohen-Salmon, M. Deletion of Astroglial Connexins Weakens the Blood-Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1457–1467. [Google Scholar] [CrossRef]
- Philips, T.; Rothstein, J.D. Oligodendroglia: Metabolic Supporters of Neurons. J. Clin. Investig. 2017, 127, 3271–3280. [Google Scholar] [CrossRef]
- Niu, J.; Li, T.; Yi, C.; Huang, N.; Koulakoff, A.; Weng, C.; Li, C.; Zhao, C.J.; Giaume, C.; Xiao, L. Connexin-Based Channels Contribute to Metabolic Pathways in the Oligodendroglial Lineage. J. Cell Sci. 2016, 129, 1902–1914. [Google Scholar] [CrossRef]
- Claus Stolt, C.; Rehberg, S.; Ader, M.; Lommes, P.; Riethmacher, D.; Schachner, M.; Bartsch, U.; Wegner, M. Terminal Differentiation of Myelin-Forming Oligodendrocytes Depends on the Transcription Factor Sox10. Genes Dev. 2002, 16, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Kleopa, K.A.; Orthmann, J.L.; Enriquez, A.; Paul, D.L.; Scherer, S.S. Unique Distributions of the Gap Junction Proteins Connexin29, Connexin32, and Connexin47 in Oligodendrocytes. Glia 2004, 47, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Kostuk, E.W.; Cai, J.; Iacovitti, L. Subregional Differences in Astrocytes Underlie Selective Neurodegeneration or Protection in Parkinson’s Disease Models in Culture. Glia 2019, 67, 1542–1557. [Google Scholar] [CrossRef] [PubMed]
- Fasciani, I.; Pluta, P.; González-Nieto, D.; Martínez-Montero, P.; Molano, J.; Paíno, C.L.; Millet, O.; Barrio, L.C. Directional Coupling of Oligodendrocyte Connexin-47 and Astrocyte Connexin-43 Gap Junctions. Glia 2018, 66, 2340–2352. [Google Scholar] [CrossRef]
- Magnotti, L.M.; Goodenough, D.A.; Paul, D.L. Deletion of Oligodendrocyte Cx32 and Astrocyte Cx43 Causes White Matter Vacuolation, Astrocyte Loss and Early Mortality. Glia 2011, 59, 1064–1074. [Google Scholar] [CrossRef]
- Ahn, M.; Lee, J.; Gustafsson, A.; Enriquez, A.; Lancaster, E.; Sul, J.Y.; Haydon, P.G.; Paul, D.L.; Huang, Y.; Abrams, C.K.; et al. Cx29 and Cx32, Two Connexins Expressed by Myelinating Glia, Do Not Interact and Are Functionally Distinct. J. Neurosci. Res. 2008, 86, 992–1006. [Google Scholar] [CrossRef]
- Altevogt, B.M.; Kleopa, K.A.; Postma, F.R.; Scherer, S.S.; Paul, D.L. Connexin29 Is Uniquely Distributed within Myelinating Glial Cells of the Central and Peripheral Nervous Systems. J. Neurosci. 2002, 22, 6458–6470. [Google Scholar] [CrossRef]
- Mendes, M.S.; Majewska, A.K. An Overview of Microglia Ontogeny and Maturation in the Homeostatic and Pathological Brain. Eur. J. Neurosci. 2021, 53, 3525–3547. [Google Scholar] [CrossRef]
- Bennett, F.C.; Bennett, M.L.; Yaqoob, F.; Mulinyawe, S.B.; Grant, G.A.; Hayden Gephart, M.; Plowey, E.D.; Barres, B.A. A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron 2018, 98, 1170–1183.e8. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nelson, L.H. Microglia and beyond: Innate Immune Cells as Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef]
- Bennett, M.L.; Bennett, F.C.; Liddelow, S.A.; Ajami, B.; Zamanian, J.L.; Fernhoff, N.B.; Mulinyawe, S.B.; Bohlen, C.J.; Adil, A.; Tucker, A.; et al. New Tools for Studying Microglia in the Mouse and Human CNS. Proc. Natl. Acad. Sci. USA 2016, 113, E1738–E1746. [Google Scholar] [CrossRef] [PubMed]
- Fontainhas, A.M.; Wang, M.; Liang, K.J.; Chen, S.; Mettu, P.; Damani, M.; Fariss, R.N.; Li, W.; Wong, W.T. Microglial Morphology and Dynamic Behavior Is Regulated by Ionotropic Glutamatergic and GABAergic Neurotransmission. PLoS ONE 2011, 6, e15973. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.B.; Uy, B.; Perera, A.; Nicholson, L.F.B. AGEs–RAGE Mediated up-Regulation of Connexin43 in Activated Human Microglial CHME-5 Cells. Neurochem. Int. 2012, 60, 640–651. [Google Scholar] [CrossRef]
- Ioghen, O.; Manole, E.; Gherghiceanu, M.; Popescu, B.O.; Ceafalan, L.C. Non-Myelinating Schwann Cells in Health and Disease; InTechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Theveneau, E.; Mayor, R. Neural Crest Cell Migration: Guidance, Pathways, and Cell–Cell Interactions. In Neural Crest Cells: Evolution, Development and Disease; Academic Press: Cambridge, MA, USA, 2014; pp. 73–88. [Google Scholar] [CrossRef]
- Mehrotra, P.; Tseropoulos, G.; Bronner, M.E.; Andreadis, S.T. Adult Tissue–Derived Neural Crest-like Stem Cells: Sources, Regulatory Networks, and Translational Potential. Stem Cells Transl. Med. 2020, 9, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. Schwann Cell Precursors; Multipotent Glial Cells in Embryonic Nerves. Front. Mol. Neurosci. 2019, 12, 69. [Google Scholar] [CrossRef]
- Bortolozzi, M. What’s the Function of Connexin 32 in the Peripheral Nervous System? Front. Mol. Neurosci. 2018, 11, 227. [Google Scholar] [CrossRef]
- Ino, D.; Sagara, H.; Suzuki, J.; Kanemaru, K.; Okubo, Y.; Iino, M. Neuronal Regulation of Schwann Cell Mitochondrial Ca2+ Signaling during Myelination. Cell Rep. 2015, 12, 1951–1959. [Google Scholar] [CrossRef]
- Menichella, D.M.; Majdan, M.; Awatramani, R.; Goodenough, D.A.; Sirkowski, E.; Scherer, S.S.; Paul, D.L. Genetic and Physiological Evidence That Oligodendrocyte Gap Junctions Contribute to Spatial Buffering of Potassium Released during Neuronal Activity. J. Neurosci. 2006, 26, 10984–10991. [Google Scholar] [CrossRef]
- Menichella, D.M.; Goodenough, D.A.; Sirkowski, E.; Scherer, S.S.; Paul, D.L. Connexins Are Critical for Normal Myelination in the CNS. J. Neurosci. 2003, 23, 5963–5973. [Google Scholar] [CrossRef]
- Milosavljević, A.; Jančić, J.; Mirčić, A.; Dožić, A.; Boljanović, J.; Milisavljević, M.; Ćetković, M. Morphological and Functional Characteristics of Satellite Glial Cells in the Peripheral Nervous System. Folia Morphol. 2021, 80, 745–755. [Google Scholar] [CrossRef]
- Amoretti, M.; Amsler, C.; Bonomi, G.; Bouchta, A.; Bowe, P.; Carraro, C.; Cesar, C.L.; Chaliton, M.; Collier, M.J.T.; Doser, M.; et al. Production and Detection of Cold Antihydrogen Atoms. Nature 2002, 419, 456–459. [Google Scholar] [CrossRef] [PubMed]
- George, D.; Ahrens, P.; Lambert, S. Satellite Glial Cells Represent a Population of Developmentally Arrested Schwann Cells. Glia 2018, 66, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A.; Riquelme, M.A.; Stehberg, J.; Alcayaga, J. Connexin43 Hemichannels in Satellite Glial Cells, Can They Influence Sensory Neuron Activity? Front. Mol. Neurosci. 2017, 10, 374. [Google Scholar] [CrossRef]
- Ohara, P.T.; Vit, J.P.; Bhargava, A.; Romero, M.; Sundberg, C.; Charles, A.C.; Jasmin, L. Gliopathic Pain: When Satellite Glial Cells Go Bad. Neuroscientist 2009, 15, 450–463. [Google Scholar] [CrossRef]
- Zhang, Y.; Laumet, G.; Chen, S.R.; Hittelman, W.N.; Pan, H.L. Pannexin-1 Up-Regulation in the Dorsal Root Ganglion Contributes to Neuropathic Pain Development. J. Biol. Chem. 2015, 290, 14647–14655. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M.; Spray, D.C. Emerging Importance of Satellite Glia in Nervous System Function and Dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, R.; Saigusa, T.; Aono, Y.; Hayashi, Y.; Hitomi, S.; Shimada, M.; Iwata, K.; Shinoda, M. Pannexin 1 Role in the Trigeminal Ganglion in Infraorbital Nerve Injury-Induced Mechanical Allodynia. Oral Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Morena, J.; Gupta, A.; Hoyle, J.C. Charcot-Marie-Tooth: From Molecules to Therapy. Int. J. Mol. Sci. 2019, 20, 3419. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Wang, E.; Wang, M.; Sin, W.C.; Brennand, K.J.; Schadt, E.; Naus, C.C.; Buxbaum, J.; Zhang, B. GJA1 (Connexin43) Is a Key Regulator of Alzheimer’s Disease Pathogenesis. Acta Neuropathol. Commun. 2018, 6, 144. [Google Scholar] [CrossRef]
- Sáez, P.J.; Shoji, K.F.; Retamal, M.A.; Harcha, P.A.; Ramírez, G.; Jiang, J.X.; von Bernhardi, R.; Sáez, J.C. ATP Is Required and Advances Cytokine-Induced Gap Junction Formation in Microglia in Vitro. Mediat. Inflamm. 2013, 2013, 216402. [Google Scholar] [CrossRef]
- Doshi, D.C.; Limdi, P.K.; Parekh, N.v.; Gohil, N.R. Oculodentodigital Dysplasia. Indian J. Ophthalmol. 2016, 64, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Han, S.A.; Kim, S.J. X-Linked Charcot-Marie-Tooth Disease with GJB1 Mutation Presenting as Acute Disseminated Encephalomyelitis-like Illness: A Case Report. Medicine 2017, 96, e9176. [Google Scholar] [CrossRef]
- Sargiannidou, I.; Kagiava, A.; Bashiardes, S.; Richter, J.; Christodoulou, C.; Scherer, S.S.; Kleopa, K.A. Intraneural GJB1 Gene Delivery Improves Nerve Pathology in a Model of X-Linked Charcot–Marie–Tooth Disease. Ann. Neurol. 2015, 78, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Kagiava, A.; Sargiannidou, I.; Theophilidis, G.; Karaiskos, C.; Richter, J.; Bashiardes, S.; Schiza, N.; Nearchou, M.; Christodoulou, C.; Scherer, S.S.; et al. Intrathecal Gene Therapy Rescues a Model of Demyelinating Peripheral Neuropathy. Proc. Natl. Acad. Sci. USA 2016, 113, E2421–E2429. [Google Scholar] [CrossRef] [PubMed]
- Taşdelen, E.; Durmaz, C.D.; Karabulut, H.G. Autosomal Recessive Oculodentodigital Dysplasia: A Case Report and Review of the Literature. Cytogenet. Genome Res. 2018, 154, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Couser, N.L.; Pandya, A. Oculodentodigital Dysplasia: A Case Report and Major Review of the Eye and Ocular Adnexa Features of 295 Reported Cases. Case Rep. Ophthalmol. Med. 2020, 2020, 6535974. [Google Scholar] [CrossRef]
- Furuta, N.; Ikeda, M.; Hirayanagi, K.; Fujita, Y.; Amanuma, M.; Okamoto, K. A Novel GJA1 Mutation in Oculodentodigital Dysplasia with Progressive Spastic Paraplegia and Sensory Deficits. Intern. Med. 2012, 51, 93–98. [Google Scholar] [CrossRef]
- Harting, I.; Karch, S.; Moog, U.; Seitz, A.; Pouwels, P.J.W.; Wolf, N.I. Oculodentodigital Dysplasia: A Hypomyelinating Leukodystrophy with a Characteristic MRI Pattern of Brain Stem Involvement. Am. J. Neuroradiol. 2019, 40, 903–907. [Google Scholar] [CrossRef]
- De Bock, M.; Kerrebrouck, M.; Wang, N.; Leybaert, L. Neurological Manifestations of Oculodentodigital Dysplasia: A Cx43 Channelopathy of the Central Nervous System? Front. Pharmacol. 2013, 4, 120. [Google Scholar] [CrossRef]
- Ashrafi, M.R.; Amanat, M.; Garshasbi, M.; Kameli, R.; Nilipour, Y.; Heidari, M.; Rezaei, Z.; Tavasoli, A.R. An Update on Clinical, Pathological, Diagnostic, and Therapeutic Perspectives of Childhood Leukodystrophies. Expert Rev. Neurother. 2020, 20, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wang, J.; Jiang, Y.; Wu, Y.; Hao, H.; Ji, T. Different Mutations of Gap Junction Connexin 47 Lead to Discrepant Activation of Unfolded Protein Response Pathway in Pelizaeus–Merzbacher-Like Disease. Neuropediatrics 2017, 48, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Bose, A.; Thomas, D.; Sarma, J. das Microtubule-Assisted Altered Trafficking of Astrocytic Gap Junction Protein Connexin 43 Is Associated with Depletion of Connexin 47 during Mouse Hepatitis Virus Infection. J. Biol. Chem. 2017, 292, 14747–14763. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Li, R. Cellular Immunology of Relapsing Multiple Sclerosis: Interactions, Checks, and Balances. Lancet Neurol. 2021, 20, 470–483. [Google Scholar] [CrossRef]
- Comi, G.; Bar-Or, A.; Lassmann, H.; Uccelli, A.; Hartung, H.P.; Montalban, X.; Sørensen, P.S.; Hohlfeld, R.; Hauser, S.L. Role of B Cells in Multiple Sclerosis and Related Disorders. Ann. Neurol. 2021, 89, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.v.; Brewer, R.C.; Ho, P.P.; Moon, J.S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.S.; Bartley, C.M.; et al. Clonally Expanded B Cells in Multiple Sclerosis Bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Wekerle, H. Epstein–Barr Virus Sparks Brain Autoimmunity in Multiple Sclerosis. Nature 2022, 603, 230–232. [Google Scholar] [CrossRef]
- Bove, R.M.; Green, A.J. Remyelinating Pharmacotherapies in Multiple Sclerosis. Neurotherapeutics 2017, 14, 894–904. [Google Scholar] [CrossRef]
- Tress, O.; Maglione, M.; May, D.; Pivneva, T.; Richter, N.; Seyfarth, J.; Binder, S.; Zlomuzica, A.; Seifert, G.; Theis, M.; et al. Panglial Gap Junctional Communication Is Essential for Maintenance of Myelin in the CNS. J. Neurosci. 2012, 32, 7499–7518. [Google Scholar] [CrossRef]
- Papaneophytou, C.P.; Georgiou, E.; Karaiskos, C.; Sargiannidou, I.; Markoullis, K.; Freidin, M.M.; Abrams, C.K.; Kleopa, K.A. Regulatory Role of Oligodendrocyte Gap Junctions in Inflammatory Demyelination. Glia 2018, 66, 2589–2603. [Google Scholar] [CrossRef]
- Markoullis, K.; Sargiannidou, I.; Schiza, N.; Roncaroli, F.; Reynolds, R.; Kleopa, K.A. Oligodendrocyte Gap Junction Loss and Disconnection from Reactive Astrocytes in Multiple Sclerosis Gray Matter. J. Neuropathol. Exp. Neurol. 2014, 73, 865–879. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, D.; Zhang, W.J.; Tang, Y.; Peng, Y. Astrocytes Induce Proliferation of Oligodendrocyte Progenitor Cells via Connexin 47-Mediated Activation of Chi3l1 Expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3012–3020. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Yamasaki, R.; Li, G.; Masaki, K.; Yamaguchi, H.; Fujita, A.; Isobe, N.; Kira, J.I. Connexin 30 Deficiency Attenuates Chronic but Not Acute Phases of Experimental Autoimmune Encephalomyelitis through Induction of Neuroprotective Microglia. Front. Immunol. 2018, 9, 2588. [Google Scholar] [CrossRef]
- Nataf, S.; Barritault, M.; Pays, L. A Unique TGFB1-Driven Genomic Program Links Astrocytosis, Low-Grade Inflammation and Partial Demyelination in Spinal Cord Periplaques from Progressive Multiple Sclerosis Patients. Int. J. Mol. Sci. 2017, 18, 2097. [Google Scholar] [CrossRef] [PubMed]
- Une, H.; Yamasaki, R.; Nagata, S.; Yamaguchi, H.; Nakamuta, Y.; Indiasari, U.C.; Cui, Y.; Shinoda, K.; Masaki, K.; Götz, M.; et al. Brain Gray Matter Astroglia-Specific Connexin 43 Ablation Attenuates Spinal Cord Inflammatory Demyelination. J. Neuroinflammation 2021, 18, 126. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Niu, J.; Yu, G.; Ezan, P.; Yi, C.; Wang, X.; Koulakoff, A.; Gao, X.; Chen, X.; Sáez, J.C.; et al. Connexin 43 Deletion in Astrocytes Promotes CNS Remyelination by Modulating Local Inflammation. Glia 2020, 68, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, Z.; Li, R.; Qiu, W.; Hu, X.; Zhou, Z. The Effects of Carbenoxolone against Experimental Autoimmune Encephalomyelitis in a Mouse Model. Neuroimmunomodulation 2020, 27, 19–27. [Google Scholar] [CrossRef]
- Angeli, S.; Kousiappa, I.; Stavrou, M.; Sargiannidou, I.; Georgiou, E.; Papacostas, S.S.; Kleopa, K.A. Altered Expression of Glial Gap Junction Proteins Cx43, Cx30, and Cx47 in the 5XFAD Model of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 1060. [Google Scholar] [CrossRef]
- Maulik, M.; Vasan, L.; Bose, A.; Chowdhury, S.D.; Sengupta, N.; Sarma, J. das Amyloid-b Regulates Gap Junction Protein Connexin 43 Trafficking in Cultured Primary Astrocytes. J. Biol. Chem. 2020, 295, 15097–15111. [Google Scholar] [CrossRef]
- Yi, C.; Ezan, P.; Fernández, P.; Schmitt, J.; Sáez, J.C.; Giaume, C.; Koulakoff, A. Inhibition of Glial Hemichannels by Boldine Treatment Reduces Neuronal Suffering in a Murine Model of Alzheimer’s Disease. Glia 2017, 65, 1607–1625. [Google Scholar] [CrossRef]
- Díaz, E.F.; Labra, V.C.; Alvear, T.F.; Mellado, L.A.; Inostroza, C.A.; Oyarzún, J.E.; Salgado, N.; Quintanilla, R.A.; Orellana, J.A. Connexin 43 Hemichannels and Pannexin-1 Channels Contribute to the α-Synuclein-Induced Dysfunction and Death of Astrocytes. Glia 2019, 67, 1598–1619. [Google Scholar] [CrossRef]
- Maatouk, L.; Yi, C.; Carrillo-de Sauvage, M.A.; Compagnion, A.C.; Hunot, S.; Ezan, P.; Hirsch, E.C.; Koulakoff, A.; Pfrieger, F.W.; Tronche, F.; et al. Glucocorticoid Receptor in Astrocytes Regulates Midbrain Dopamine Neurodegeneration through Connexin Hemichannel Activity. Cell Death Differ. 2018, 26, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Z.; Hu, Y.; Lu, Y.; Yang, Q.; Fu, D.; Chen, F.; Li, Y. man CaMKII and CaV3.2 T-Type Calcium Channel Mediate Connexin-43-Dependent Inflammation by Activating Astrocytes in Vincristine-Induced Neuropathic Pain. Cell Biol. Toxicol. 2021, 1–24. [Google Scholar] [CrossRef]
- Aquilino, M.S.; Whyte-Fagundes, P.; Lukewich, M.K.; Zhang, L.; Bardakjian, B.L.; Zoidl, G.R.; Carlen, P.L. Pannexin-1 Deficiency Decreases Epileptic Activity in Mice. Int. J. Mol. Sci. 2020, 21, 7510. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D.; Reutiman, T.J.; Lee, S. Expression of Astrocytic Markers Aquaporin 4 and Connexin 43 Is Altered in Brains of Subjects with Autism. Synapse 2008, 62, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Deture, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Madeira, D.; Dias, L.; Santos, P.; Cunha, R.A.; Canas, P.M.; Agostinho, P. Association Between Adenosine A 2A Receptors and Connexin 43 Regulates Hemichannels Activity and ATP Release in Astrocytes Exposed to Amyloid-β Peptides. Mol. Neurobiol. 2021, 58, 6232–6248. [Google Scholar] [CrossRef]
- Mei, X.; Ezan, P.; Giaume, C.; Koulakoff, A. Astroglial Connexin Immunoreactivity Is Specifically Altered at β-Amyloid Plaques in β-Amyloid Precursor Protein/Presenilin1 Mice. Neuroscience 2010, 171, 92–105. [Google Scholar] [CrossRef]
- Yi, C.; Mei, X.; Ezan, P.; Mato, S.; Matias, I.; Giaume, C.; Koulakoff, A. Astroglial Connexin43 Contributes to Neuronal Suffering in a Mouse Model of Alzheimer’s Disease. Cell Death Differ. 2016, 23, 1691–1701. [Google Scholar] [CrossRef]
- Orellana, J.A.; Shoji, K.F.; Abudara, V.; Ezan, P.; Amigou, E.; Sáez, P.J.; Jiang, J.X.; Naus, C.C.; Sáez, J.C.; Giaume, C. Amyloid β-Induced Death in Neurons Involves Glial and Neuronal Hemichannels. J. Neurosci. 2011, 31, 4962–4977. [Google Scholar] [CrossRef]
- Santello, M.; Toni, N.; Volterra, A. Astrocyte Function from Information Processing to Cognition and Cognitive Impairment. Nat. Neurosci. 2019, 22, 154–166. [Google Scholar] [CrossRef]
- Fujita, A.; Yamaguchi, H.; Yamasaki, R.; Cui, Y.; Matsuoka, Y.; Yamada, K.-i.; Kira, J.-i. Connexin 30 Deficiency Attenuates A2 Astrocyte Responses and Induces Severe Neurodegeneration in a 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Hydrochloride Parkinson’s Disease Animal Model. J. Neuroinflamm. 2018, 15, 227. [Google Scholar] [CrossRef]
- Muñoz, M.F.; Griffith, T.N.; Contreras, J.E. Mechanisms of ATP Release in Pain: Role of Pannexin and Connexin Channels. Purinergic Signal. 2021, 17, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Cheong, Y.K.; He, S.Q.; Tiwari, V.; Liu, J.; Wang, Y.; Raja, S.N.; Li, J.; Guan, Y.; Li, W. Suppression of Spinal Connexin 43 Expression Attenuates Mechanical Hypersensitivity in Rats after an L5 Spinal Nerve Injury. Neurosci. Lett. 2014, 566, 194–199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, M.J.; Kress, B.; Han, X.; Moll, K.; Peng, W.; Ji, R.R.; Nedergaard, M. Astrocytic CX43 Hemichannels and Gap Junctions Play a Crucial Role in Development of Chronic Neuropathic Pain Following Spinal Cord Injury. Glia 2012, 60, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Roh, D.H.; Yoon, S.Y.; Kwon, S.G.; Choi, H.S.; Han, H.J.; Beitz, A.J.; Lee, J.H. Astrocyte Sigma-1 Receptors Modulate Connexin 43 Expression Leading to the Induction of below-Level Mechanical Allodynia in Spinal Cord Injured Mice. Neuropharmacology 2016, 111, 34–46. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Zhou, Y.; Yu, H.; Zhang, M.; Du, X.; Wang, D.; Zhang, F.; Xu, Y.; Zhang, J.; et al. Inhibition of Phosphodiesterase-4 in the Spinal Dorsal Horn Ameliorates Neuropathic Pain via CAMP-Cytokine-Cx43 Signaling in Mice. CNS Neurosci. Ther. 2022, 28, 749–760. [Google Scholar] [CrossRef]
- Koyama, R.; Iwata, K.; Hayashi, Y.; Hitomi, S.; Shibuta, I.; Furukawa, A.; Asano, S.; Kaneko, T.; Yonehara, Y.; Shinoda, M. Pannexin 1-Mediated ATP Signaling in the Trigeminal Spinal Subnucleus Caudalis Is Involved in Tongue Cancer Pain. Int. J. Mol. Sci. 2021, 22, 11404. [Google Scholar] [CrossRef]
- Gusso, D.; Cruz, F.F.; Fritsch, P.M.; Gobbo, M.O.; Morrone, F.B.; Bonan, C.D. Pannexin Channel 1, P2×7 Receptors, and Dimethyl Sulfoxide Mediate Pain Responses in Zebrafish. Behav. Brain Res. 2022, 423, 113786. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, M.; Burma, N.E.; Lee, K.Y.; Leduc-Pessah, H.; Kwok, C.H.T.; Reid, A.R.; O’Brien, M.; Sagalajev, B.; Stratton, J.A.; Patrick, N.; et al. Microglial Pannexin-1 Channel Activation Is a Spinal Determinant of Joint Pain. Sci. Adv. 2018, 4, eaas9846. [Google Scholar] [CrossRef]
- Onodera, M.; Meyer, J.; Furukawa, K.; Hiraoka, Y.; Aida, T.; Tanaka, K.; Tanaka, K.F.; Rose, C.R.; Matsui, K. Exacerbation of Epilepsy by Astrocyte Alkalization and Gap Junction Uncoupling. J. Neurosci. 2021, 41, 2106–2118. [Google Scholar] [CrossRef]
- Deshpande, T.; Li, T.; Henning, L.; Wu, Z.; Müller, J.; Seifert, G.; Steinhäuser, C.; Bedner, P. Constitutive Deletion of Astrocytic Connexins Aggravates Kainate-Induced Epilepsy. Glia 2020, 68, 2136–2147. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gonzalo, M.; Losi, G.; Chiavegato, A.; Zonta, M.; Cammarota, M.; Brondi, M.; Vetri, F.; Uva, L.; Pozzan, T.; de Curtis, M.; et al. An Excitatory Loop with Astrocytes Contributes to Drive Neurons to Seizure Threshold. PLoS Biol. 2010, 8, e1000352. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, L.; Feng, J.; Qiu, J.; Lin, W. Expression of Connexin 32 and Connexin 43 in the Cerebral Cortex of Patients with Refractory Epilepsy. LaboratoriumsMedizin 2017, 41, 33–40. [Google Scholar] [CrossRef]
- Vincze, R.; Péter, M.; Szabó, Z.; Kardos, J.; Héja, L.; Kovács, Z. Connexin 43 Differentially Regulates Epileptiform Activity in Models of Convulsive and Non-Convulsive Epilepsies. Front. Cell. Neurosci. 2019, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.M.; Voss, L.J.; Melin, S.M.; Mason, J.P.; Cursons, R.T.; Steyn-Ross, D.A.; Steyn-Ross, M.L.; Sleigh, J.W. Connexin36 Knockout Mice Display Increased Sensitivity to Pentylenetetrazol-Induced Seizure-like Behaviors. Brain Res. 2010, 1360, 198–204. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.Q.; Jia, J.N.; Liu, Z.Q.; Zhou, H.H.; Mao, X.Y. Targeting Gap Junction in Epilepsy: Perspectives and Challenges. Biomed. Pharmacother. 2019, 109, 57–65. [Google Scholar] [CrossRef]
- Brunal, A.A.; Clark, K.C.; Ma, M.; Woods, I.G.; Pan, Y.A. Effects of Constitutive and Acute Connexin 36 Deficiency on Brain-Wide Susceptibility to PTZ-Induced Neuronal Hyperactivity. Front. Mol. Neurosci. 2021, 13, 239. [Google Scholar] [CrossRef]
- Jiang, T.; Long, H.; Ma, Y.; Long, L.; Li, Y.; Li, F.; Zhou, P.; Yuan, C.; Xiao, B. Altered Expression of Pannexin Proteins in Patients with Temporal Lobe Epilepsy. Mol. Med. Rep. 2013, 8, 1801–1806. [Google Scholar] [CrossRef]
- Song, P.; Hu, J.; Liu, X.; Deng, X. Increased Expression of the P2X7 Receptor in Temporal Lobe Epilepsy: Animal Models and Clinical Evidence. Mol. Med. Rep. 2019, 19, 5433–5439. [Google Scholar] [CrossRef]
- Cepeda, C.; Chang, J.W.; Owens, G.C.; Huynh, M.N.; Chen, J.Y.; Tran, C.; Vinters, H.v.; Levine, M.S.; Mathern, G.W. In Rasmussen Encephalitis, Hemichannels Associated with Microglial Activation Are Linked to Cortical Pyramidal Neuron Coupling: A Possible Mechanism for Cellular Hyperexcitability. CNS Neurosci. Ther. 2015, 21, 152–163. [Google Scholar] [CrossRef]
- Sauer, A.K.; Stanton, J.; Hans, S.; x Grabrucker, A. Autism Spectrum Disorders: Etiology and Pathology; Exon Publications: Brisbane, Australia, 2021; pp. 1–15. [Google Scholar] [CrossRef]
- Wang, Q.; Kong, Y.; Wu, D.Y.; Liu, J.H.; Jie, W.; You, Q.L.; Huang, L.; Hu, J.; de Chu, H.; Gao, F.; et al. Impaired Calcium Signaling in Astrocytes Modulates Autism Spectrum Disorder-like Behaviors in Mice. Nat. Commun. 2021, 12, 3321. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, J.; He, F.; Cai, C.; Wang, H.; Wang, Y.; Lin, Y.; Rong, H.; Cheng, G.; Xu, R.; et al. Alteration of Gut Microbiota-Associated Epitopes in Children with Autism Spectrum Disorders. Brain Behav. Immun. 2019, 75, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, X.; Hao, Y.; Qiu, L.; Lou, Y.; Zhang, Y.; Ma, D.; Feng, J. The Multifaceted Role of Astrocyte Connexin 43 in Ischemic Stroke Through Forming Hemichannels and Gap Junctions. Front. Neurol. 2020, 11, 703. [Google Scholar] [CrossRef] [PubMed]
- Sáez, J.C.; Leybaert, L. Hunting for Connexin Hemichannels. FEBS Lett. 2014, 588, 1205–1211. [Google Scholar] [CrossRef]
- Wei, R.; Bao, W.; He, F.; Meng, F.; Liang, H.; Luo, B. Pannexin1 Channel Inhibitor (10 Panx) Protects Against Transient Focal Cerebral Ischemic Injury by Inhibiting RIP3 Expression and Inflammatory Response in Rats. Neuroscience 2020, 437, 23–33. [Google Scholar] [CrossRef]
- Gómez, G.I.; Falcon, R.v.; Maturana, C.J.; Labra, V.C.; Salgado, N.; Rojas, C.A.; Oyarzun, J.E.; Cerpa, W.; Quintanilla, R.A.; Orellana, J.A. Heavy Alcohol Exposure Activates Astroglial Hemichannels and Pannexons in the Hippocampus of Adolescent Rats: Effects on Neuroinflammation and Astrocyte Arborization. Front. Cell. Neurosci. 2018, 12, 472. [Google Scholar] [CrossRef]
- Yin, X.; Feng, L.; Ma, D.; Yin, P.; Wang, X.; Hou, S.; Hao, Y.; Zhang, J.; Xin, M.; Feng, J. Roles of Astrocytic Connexin-43, Hemichannels, and Gap Junctions in Oxygen-Glucose Deprivation/Reperfusion Injury Induced Neuroinflammation and the Possible Regulatory Mechanisms of Salvianolic Acid B and Carbenoxolone. J. Neuroinflamm. 2018, 15, 97. [Google Scholar] [CrossRef]
- Wang, X.; Feng, L.; Xin, M.; Hao, Y.; Wang, X.; Shang, P.; Zhao, M.; Hou, S.; Zhang, Y.; Xiao, Y.; et al. Mechanisms Underlying Astrocytic Connexin-43 Autophagy Degradation during Cerebral Ischemia Injury and the Effect on Neuroinflammation and Cell Apoptosis. Biomed. Pharmacother. 2020, 127, 110125. [Google Scholar] [CrossRef]
- Zeng, W.; Fu, L.; Xu, H. MicroRNA-206 Relieves Irradiation-induced Neuroinflammation by Regulating Connexin 43. Exp. Ther. Med. 2021, 22, 1186. [Google Scholar] [CrossRef]


| Panx | Gene | Relevant Function during Development | Relevant Function in the Adult Brain | Related Diseases and Functional Alteration | Reference |
|---|---|---|---|---|---|
| Panx1 | PANX1 |
|
|
| [19,30,75,76] |
| Panx2 | PANX2 | - | - |
| [76] |
| Disease | Cx or Panx Involved | Mechanism for Disease | Channel Modulation Studies | Reference |
|---|---|---|---|---|
| MS | Cx47, Cx43, Cx30, Cx32 |
|
| [144,150] |
| AD | Cx43, Panx1 |
|
| [75,151,152,153] |
| PD | Cx43, Panx1 |
|
| [154,155] |
| Pain | Cx43, Panx1 |
|
| [156] |
| Epilepsy | Cx43, Cx30, Panx1, Panx2 |
|
| [157] |
| ASD | Cx43 |
| - | [158] |
| Cerebral ischemia | Cx43, Panx1 |
|
| [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baracaldo-Santamaría, D.; Corrales-Hernández, M.G.; Ortiz-Vergara, M.C.; Cormane-Alfaro, V.; Luque-Bernal, R.-M.; Calderon-Ospina, C.-A.; Cediel-Becerra, J.-F. Connexins and Pannexins: Important Players in Neurodevelopment, Neurological Diseases, and Potential Therapeutics. Biomedicines 2022, 10, 2237. https://doi.org/10.3390/biomedicines10092237
Baracaldo-Santamaría D, Corrales-Hernández MG, Ortiz-Vergara MC, Cormane-Alfaro V, Luque-Bernal R-M, Calderon-Ospina C-A, Cediel-Becerra J-F. Connexins and Pannexins: Important Players in Neurodevelopment, Neurological Diseases, and Potential Therapeutics. Biomedicines. 2022; 10(9):2237. https://doi.org/10.3390/biomedicines10092237
Chicago/Turabian StyleBaracaldo-Santamaría, Daniela, María Gabriela Corrales-Hernández, Maria Camila Ortiz-Vergara, Valeria Cormane-Alfaro, Ricardo-Miguel Luque-Bernal, Carlos-Alberto Calderon-Ospina, and Juan-Fernando Cediel-Becerra. 2022. "Connexins and Pannexins: Important Players in Neurodevelopment, Neurological Diseases, and Potential Therapeutics" Biomedicines 10, no. 9: 2237. https://doi.org/10.3390/biomedicines10092237
APA StyleBaracaldo-Santamaría, D., Corrales-Hernández, M. G., Ortiz-Vergara, M. C., Cormane-Alfaro, V., Luque-Bernal, R.-M., Calderon-Ospina, C.-A., & Cediel-Becerra, J.-F. (2022). Connexins and Pannexins: Important Players in Neurodevelopment, Neurological Diseases, and Potential Therapeutics. Biomedicines, 10(9), 2237. https://doi.org/10.3390/biomedicines10092237






