Calcium Overload or Underload? The Effects of Doxorubicin on the Calcium Dynamics in Guinea Pig Hearts
Abstract
:1. Introduction
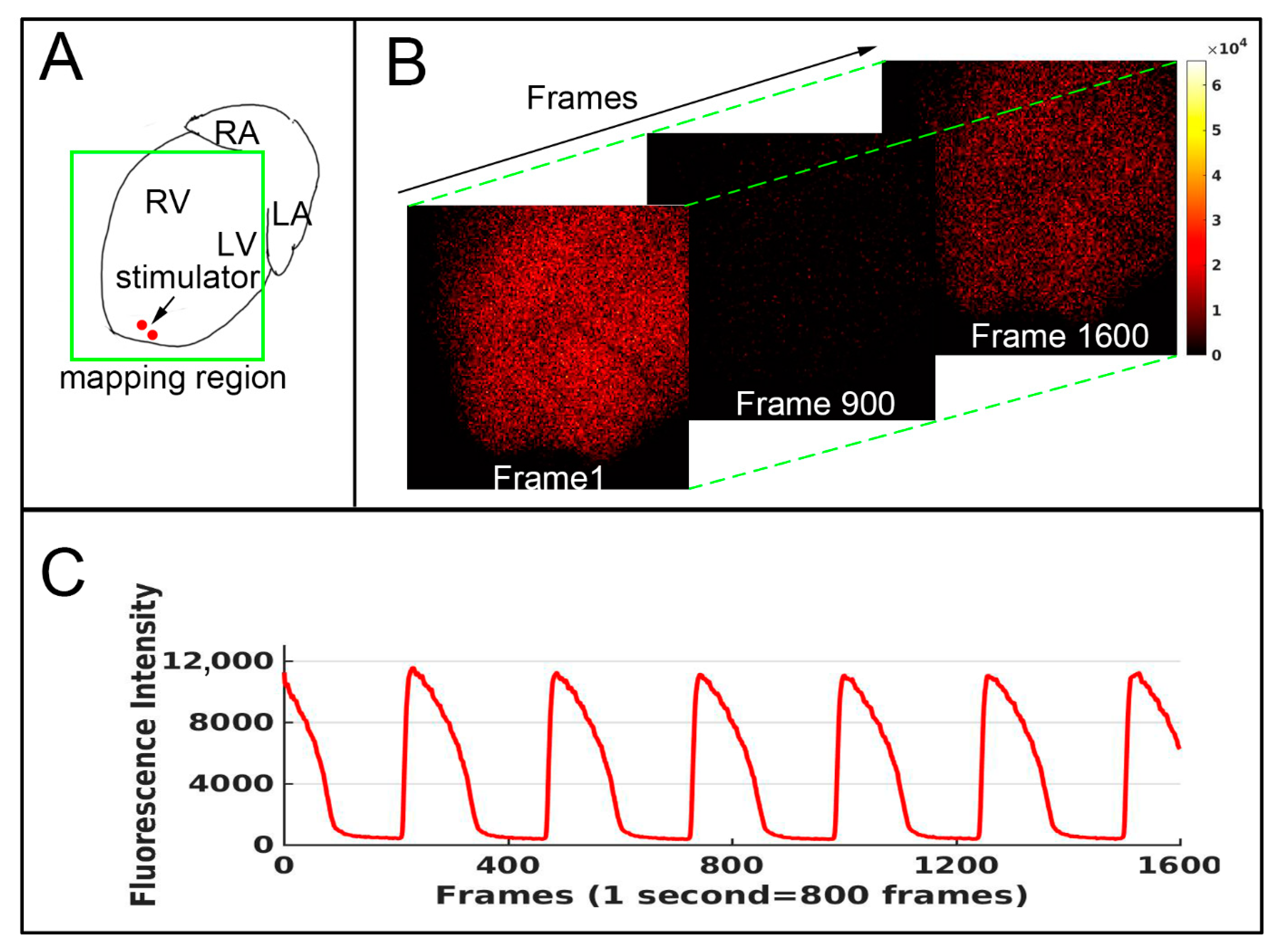
2. Methods
3. Result
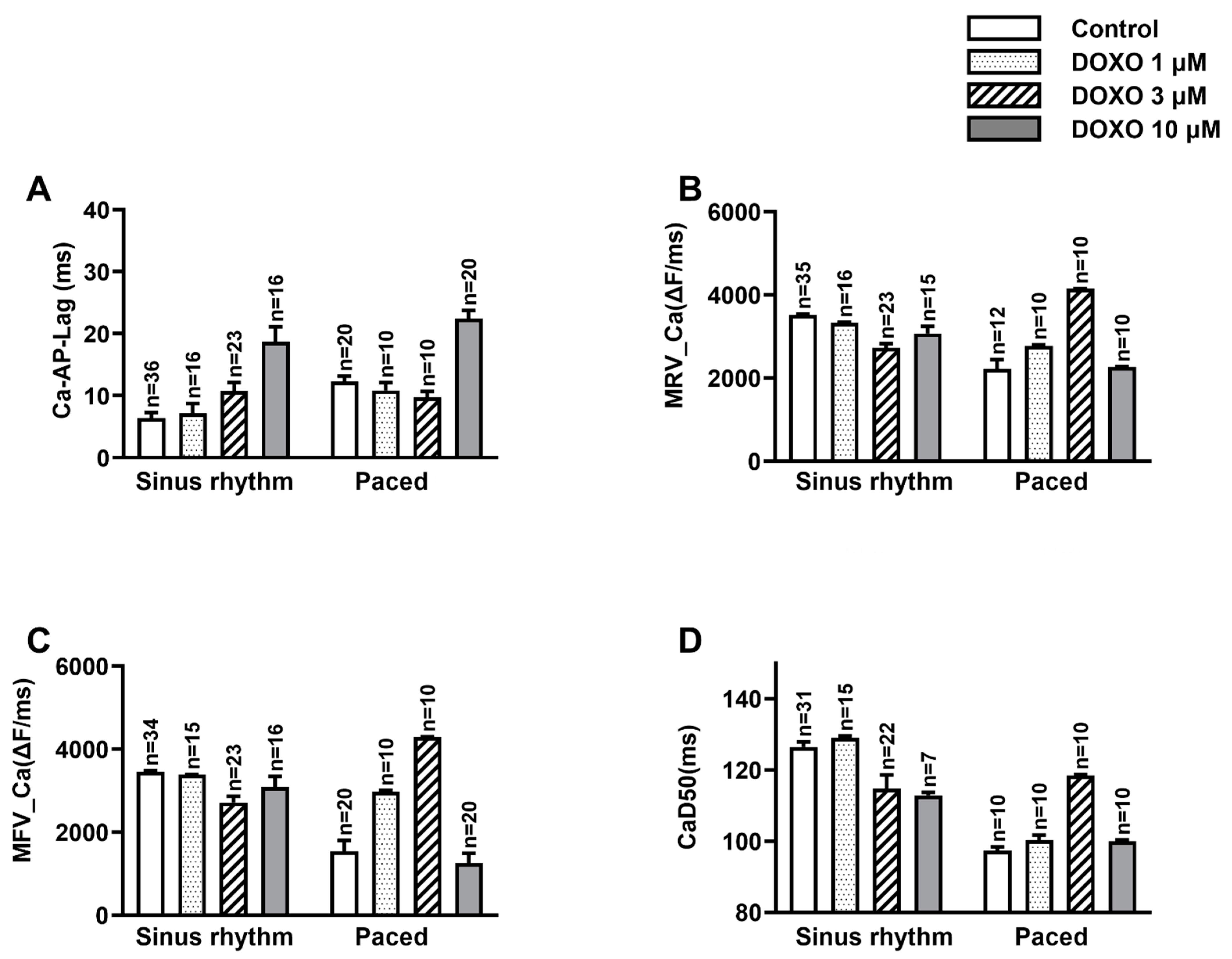
3.1. DOXO Delayed the Ca2+ Activation in Respect of the Occurrence of AP
3.2. DOXO Hampered Both the Releasing and Sequestering Processes of Ca2+
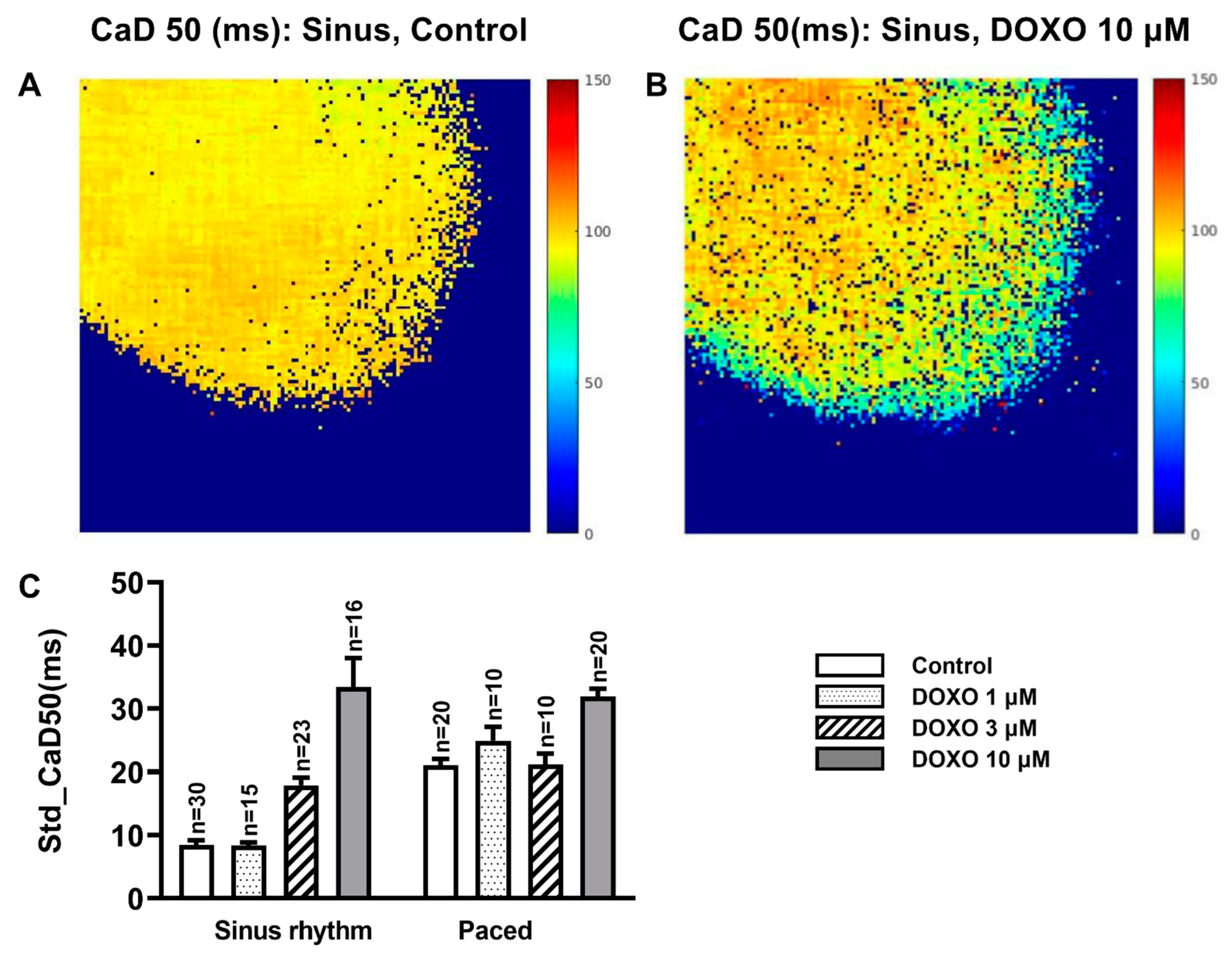
3.3. DOXO Increased the Heterogeneity of the CaD50
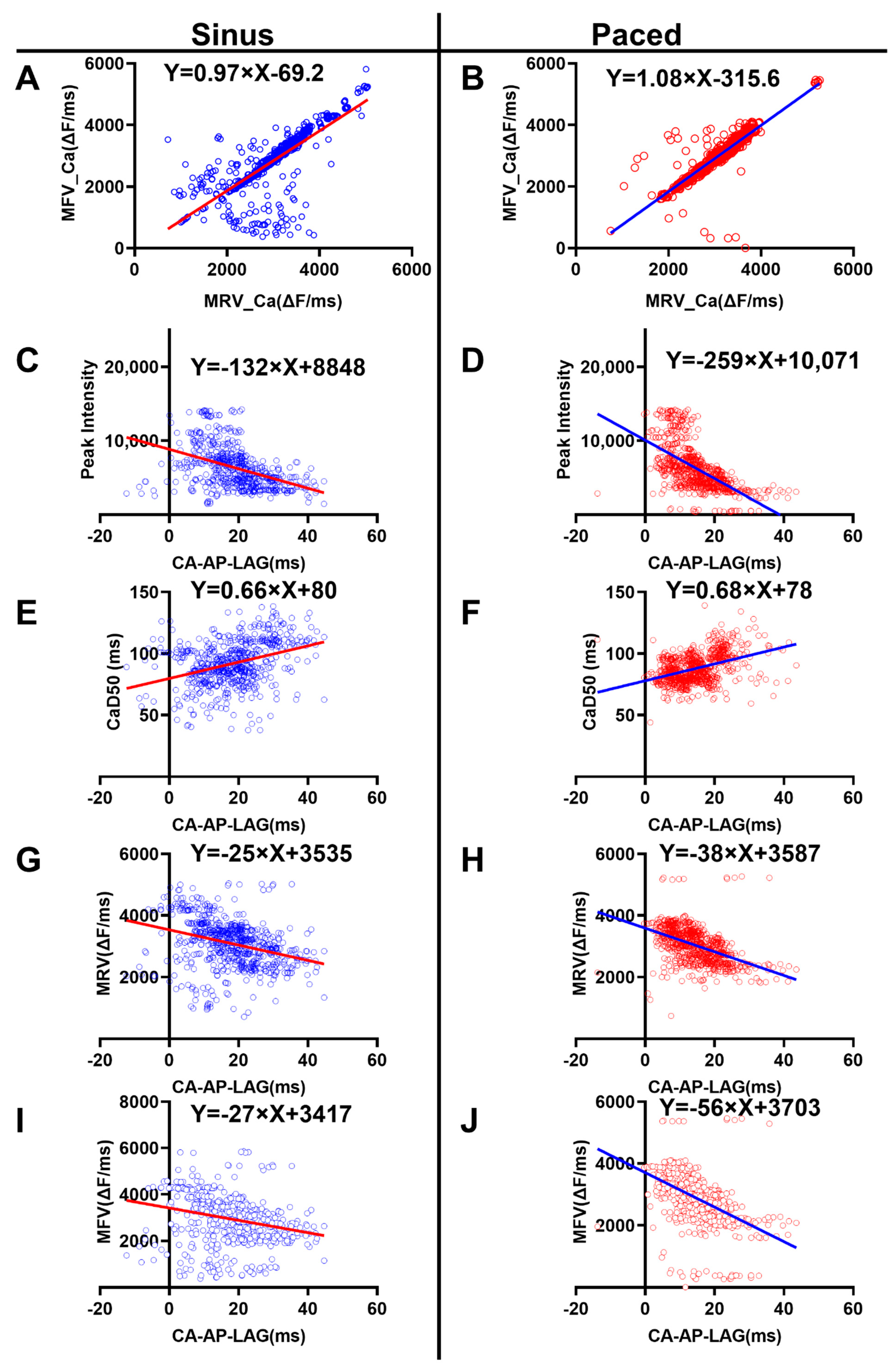
3.4. Correlation Analysis of the Ca2+ Properties
- The MRV_Ca was positively correlated with MFV_Ca
- 2.
- The Ca-AP-Lag was negatively correlated with the peak amplitude of Ca2+ signal
- 3.
- The Ca-AP-Lag was positively correlated with duration of the Ca2+ signal
- 4.
- The Ca-AP-Lag was negatively correlated with the Ca2+ MRV and MFV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, H.; Zhao, X.X.; Lee, S.; Woo, J.S.; Song, M.Y.; Cheng, X.W.; Lee, K.H.; Kim, W. The sGC-cGMP Signaling Pathway as a Potential Therapeutic Target in Doxorubicin-Induced Heart Failure: A Narrative Review. Am. J. Cardiovasc. Drugs 2022, 22, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xian, G.; Zhong, G.; Huang, B.; Liang, S.; Zeng, Q.; Liu, Y. Alleviation of doxorubicin-induced cardiomyocyte death through miR-147-y-mediated mitophagy. Biochem. Biophys. Res. Commun. 2022, 609, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Lee, J.Y.; An, H.S.; Ahn, Y.J.; Jeong, E.A.; Shin, H.J.; Kim, K.E.; Lee, J.; Koh, J.S.; Roh, G.S. LCN2 deficiency ameliorates doxorubicin-induced cardiomyopathy in mice. Biochem. Biophys. Res. Commun. 2022, 588, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef]
- Agustini, F.D.; Arozal, W.; Louisa, M.; Siswanto, S.; Soetikno, V.; Nafrialdi, N.; Suyatna, F. Cardioprotection mechanism of mangiferin on doxorubicin-induced rats: Focus on intracellular calcium regulation. Pharm. Biol. 2016, 54, 1289–1297. [Google Scholar] [CrossRef]
- Shinlapawittayatorn, K.; Chattipakorn, S.C.; Chattipakorn, N. The effects of doxorubicin on cardiac calcium homeostasis and contractile function. J. Cardiol. 2022, 80, 125–132. [Google Scholar] [CrossRef]
- Bupha-Intr, T.; Wattanapermpool, J. Regulatory role of ovarian sex hormones in calcium uptake activity of cardiac sarcoplasmic reticulum. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1101–H1108. [Google Scholar] [CrossRef]
- Pecoraro, M.; Rodríguez-Sinovas, A.; Marzocco, S.; Ciccarelli, M.; Iaccarino, G.; Pinto, A.; Popolo, A. Cardiotoxic Effects of Short-Term Doxorubicin Administration: Involvement of Connexin 43 in Calcium Impairment. Int. J. Mol. Sci. 2017, 18, 2121. [Google Scholar] [CrossRef]
- Olson, R.D.; Gambliel, H.A.; Vestal, R.E.; Shadle, S.E.; Charlier, H.A.; Cusack, B.J. Doxorubicin cardiac dysfunction: Effects on calcium regulatory proteins, sarcoplasmic reticulum, and triiodothyronine. Cardiovasc. Toxicol. 2005, 5, 269–283. [Google Scholar] [CrossRef]
- Olson, H.M.; Young, D.M.; Prieur, D.J.; LeRoy, A.F.; Reagan, R.L. Electrolyte and morphologic alterations of myocardium in adriamycin-treated rabbits. Am. J. Pathol. 1974, 77, 439–454. [Google Scholar] [PubMed]
- Jaenke, R.S. Delayed and progressive myocardial lesions after adriamycin administration in the rabbit. Cancer Res. 1976, 36, 2958–2966. [Google Scholar] [PubMed]
- Buhner, R.; Biedert, S.; Miura, D. [Adriamycin-cardiomyopathy induced by an increment of (Ca2+)] (author’s translation). Klin. Wochenschr. 1980, 58, 747–748. [Google Scholar] [PubMed]
- Chao, Y.K.; Liau, I. One-dimensional scanning multiphoton imaging reveals prolonged calcium transient and sarcomere contraction in a zebrafish model of doxorubicin cardiotoxicity. Biomed. Opt. Express 2021, 12, 7162–7172. [Google Scholar] [CrossRef] [PubMed]
- Sag, C.M.; Köhler, A.C.; Anderson, M.E.; Backs, J.; Maier, L.S. CaMKII-dependent SR Ca leak contributes to doxorubicin-induced impaired Ca handling in isolated cardiac myocytes. J. Mol. Cell. Cardiol. 2011, 51, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Llach, A.; Mazevet, M.; Mateo, P.; Villejouvert, O.; Ridoux, A.; Rucker-Martin, C.; Ribeiro, M.; Fischmeister, R.; Crozatier, B.; Benitah, J.-P.; et al. Progression of excitation-contraction coupling defects in doxorubicin cardiotoxicity. J. Mol. Cell. Cardiol. 2019, 126, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Shinbane, J.S.; A Wood, M.; Jensen, D.; A Ellenbogen, K.; Fitzpatrick, A.P.; Scheinman, M.M. Tachycardia-induced cardiomyopathy: A review of animal models and clinical studies. J. Am. Coll. Cardiol. 1997, 29, 709–715. [Google Scholar] [CrossRef]
- Schüttler, D.; Bapat, A.; Kääb, S.; Lee, K.; Tomsits, P.; Clauss, S.; Hucker, W.J. Animal Models of Atrial Fibrillation. Circ. Res. 2020, 127, 91–110. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Ianuzzo, C.D.; Moe, G.W.; Stopps, T.P.; Armstrong, P. Rapid ventricular pacing of dogs to heart failure: Biochemical and physiological studies. Can. J. Physiol. Pharmacol. 1990, 68, 34–39. [Google Scholar] [CrossRef]
- Perreault, C.L.; Shannon, R.P.; Komamura, K.; Vatner, S.F.; Morgan, J.P. Abnormalities in intracellular calcium regulation and contractile function in myocardium from dogs with pacing-induced heart failure. J. Clin. Investig. 1992, 89, 932–938. [Google Scholar] [CrossRef]
- Wolff, M.R.; Whitesell, L.F.; Moss, R.L. Calcium sensitivity of isometric tension is increased in canine experimental heart failure. Circ. Res. 1995, 76, 781–789. [Google Scholar] [CrossRef]
- Gambliel, H.A.; Burke, B.E.; Cusack, B.J.; Walsh, G.M.; Zhang, Y.L.; Mushlin, P.S.; Olson, R.D. Doxorubicin and C-13 deoxydoxorubicin effects on ryanodine receptor gene expression. Biochem. Biophys. Res. Commun. 2002, 291, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Miwa, N.; Kanaide, H.; Meno, H.; Nakamura, M. Adriamycin and altered membrane functions in rat hearts. Br. J. Exp. Pathol. 1986, 67, 747–755. [Google Scholar] [PubMed]
- Keung, E.C.; Toll, L.; Ellis, M.; A Jensen, R. L-type cardiac calcium channels in doxorubicin cardiomyopathy in rats morphological, biochemical, and functional correlations. J. Clin. Investig. 1991, 87, 2108–2113. [Google Scholar] [CrossRef] [PubMed]
- Ondrias, K.; Borgatta, L.; Kim, D.H.; E Ehrlich, B. Biphasic effects of doxorubicin on the calcium release channel from sarcoplasmic reticulum of cardiac muscle. Circ. Res. 1990, 67, 1167–1174. [Google Scholar] [CrossRef]
- Tocchetti, C.G.; Carpi, A.; Coppola, C.; Quintavalle, C.; Rea, D.; Campesan, M.; Arcari, A.; Piscopo, G.; Cipresso, C.; Monti, M.G.; et al. Ranolazine protects from doxorubicin-induced oxidative stress and cardiac dysfunction. Eur. J. Heart Fail. 2014, 16, 358–366. [Google Scholar] [CrossRef]
- Aslanidi, O.V.; Sleiman, R.N.; Boyett, M.R.; Hancox, J.C.; Zhang, H. Ionic mechanisms for electrical heterogeneity between rabbit Purkinje fiber and ventricular cells. Biophys. J. 2010, 98, 2420–2431. [Google Scholar] [CrossRef]
- Sekar, R.B.; Kizana, E.; Cho, H.C.; Molitoris, J.M.; Hesketh, G.G.; Eaton, B.P.; Marban, E.; Tung, L. IK1 heterogeneity affects genesis and stability of spiral waves in cardiac myocyte monolayers. Circ. Res. 2009, 104, 355–364. [Google Scholar] [CrossRef]
- Yeh, H.-I.; Lai, Y.-J.; Lee, S.-H.; Lee, Y.-N.; Ko, Y.-S.; Chen, S.-A.; Severs, N.J.; Tsai, C.-H. Heterogeneity of myocardial sleeve morphology and gap junctions in canine superior vena cava. Circulation 2001, 104, 3152–3157. [Google Scholar] [CrossRef]
- Antzelevitch, C. Electrical heterogeneity, cardiac arrhythmias, and the sodium channel. Circ. Res. 2000, 87, 964–965. [Google Scholar] [CrossRef]
- Nemec, J. Nonalternans repolarization variability and arrhythmia—The calcium connection. J. Electrocardiol. 2016, 49, 877–882. [Google Scholar] [CrossRef]
- Hazari, M.S.; Haykal-Coates, N.; Winsett, D.W.; Costa, D.L.; Farraj, A.K. Continuous electrocardiogram reveals differences in the short-term cardiotoxic response of Wistar-Kyoto and spontaneously hypertensive rats to doxorubicin. Toxicol. Sci. 2009, 110, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Rudzinski, T.; Ciesielczyk, M.; Religa, W.; Bednarkiewicz, Z.; Krzeminska-Pakula, M. Doxorubicin-induced ventricular arrhythmia treated by implantation of an automatic cardioverter-defibrillator. Europace 2007, 9, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Kharin, S.; Krandycheva, V.; Tsvetkova, A.; Strelkova, M.; Shmakov, D. Remodeling of ventricular repolarization in a chronic doxorubicin cardiotoxicity rat model. Fundam. Clin. Pharmacol. 2013, 27, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Ciccarelli, M.; Fiordelisi, A.; Iaccarino, G.; Pinto, A.; Popolo, A. Diazoxide Improves Mitochondrial Connexin 43 Expression in a Mouse Model of Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2018, 19, 757. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.D.; Lam, A.; Tham, S.; Dulhunty, A.F.; Beard, N.A. Adverse effects of doxorubicin and its metabolic product on cardiac RyR2 and SERCA2A. Mol. Pharmacol. 2014, 86, 438–449. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Gao, L.; Fan, H.; Liu, D.; Lin, M.; Zhu, M.; Deng, T.; Song, Y. Calcium Overload or Underload? The Effects of Doxorubicin on the Calcium Dynamics in Guinea Pig Hearts. Biomedicines 2022, 10, 2197. https://doi.org/10.3390/biomedicines10092197
Wu J, Gao L, Fan H, Liu D, Lin M, Zhu M, Deng T, Song Y. Calcium Overload or Underload? The Effects of Doxorubicin on the Calcium Dynamics in Guinea Pig Hearts. Biomedicines. 2022; 10(9):2197. https://doi.org/10.3390/biomedicines10092197
Chicago/Turabian StyleWu, Jingjing, Linlin Gao, Hong Fan, Deming Liu, Mengxue Lin, Ming Zhu, Tian Deng, and Yuanlong Song. 2022. "Calcium Overload or Underload? The Effects of Doxorubicin on the Calcium Dynamics in Guinea Pig Hearts" Biomedicines 10, no. 9: 2197. https://doi.org/10.3390/biomedicines10092197
APA StyleWu, J., Gao, L., Fan, H., Liu, D., Lin, M., Zhu, M., Deng, T., & Song, Y. (2022). Calcium Overload or Underload? The Effects of Doxorubicin on the Calcium Dynamics in Guinea Pig Hearts. Biomedicines, 10(9), 2197. https://doi.org/10.3390/biomedicines10092197






