Autoantibodies as Biomarker and Therapeutic Target in Systemic Sclerosis
Abstract
1. Introduction
- Which mechanisms are involved in maturation of autoreactive B cells and secretion of autoantibodies in SSc?
- Which autoantibodies might be useful as predictors of disease course and which role do autoantibodies play in the pathophysiology of SSc?
- Which therapeutic approaches have been evaluated to target autoantibody-mediated pathologies in SSc?
2. Which Mechanisms Are Involved in Maturation of Autoreactive B Cells and Autoantibody Secretion in SSc?
2.1. Tolerance Mechanisms in B-Cell Maturation
2.2. Natural and Pathogenic Autoantibodies
2.3. Autoreactive B Cells and Autoantibodies
3. Which Autoantibodies Might Be Useful as Predictors of Disease Course and Which Role Do Autoantibodies Play in the Pathophysiology of SSc?
3.1. Autoantibodies against Nuclear Antigens (ANAs)
3.2. Anti-Neutrophil Cytoplasmic Antibodies (ANCAs)
3.3. Anti-Phospholipid Antibodies (aPL)
3.4. Autoantibodies Recognizing G-Protein-Coupled Receptors, Growth Factors and Their Respective Receptors
3.4.1. Functional Autoantibodies against GPCR
Anti-AT1R and Anti-ETAR Autoantibodies
Anti-Muscarinic-3 Acetylcholine Receptor (M3R) Autoantibodies
Anti-CXCR3 and Anti-CXCR4 Autoantibodies
Anti-PAR-1 Autoantibodies
4. Which Therapeutic Approaches Have Been Evaluated to Target Autoantibody-Mediated Pathologies in SSc?
4.1. B-Cell- and Plasma Cell-Mediated Strategies
4.1.1. Anti-CD19 Antibody
4.1.2. Anti-CD20 Antibody
4.1.3. Anti-BAFF Antibody
4.1.4. Proteasome Inhibitor
4.1.5. Anti-CD38 Antibody
4.1.6. Inhibitor of Bruton Tyrosine Kinase
4.1.7. Therapeutic Approaches Targeting PAMP- and DAMP-Mediated Activation of the B-Cell Compartment
4.2. Autologous Hematopoietic Stem-Cell Transplantation (aHSCT)
4.3. Unspecific Approaches for the Removal of Antibodies
4.3.1. Therapeutic Plasma Exchange, Plasmapheresis and Rheopheresis
4.3.2. Immunoadsorption
4.3.3. Intravenous Gammaglobulin (IVIg)
4.4. Specific Approaches for the Removal of Antibodies
4.4.1. Selective Removal of Autoantibodies by Lysosomal Degradation
4.4.2. Selective Removal of Autoantibodies Using Aptamer BC007
4.5. Therapeutics Targeting B-Cell-Secreted Cytokines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zmorzyński, S.; Wojcierowska-Litwin, M.; Kowal, M.; Michalska-Jakubus, M.; Styk, W.; Filip, A.A.; Walecka, I.; Krasowska, D. NOTCH3 T6746C and TP53 P72R Polymorphisms Are Associated with the Susceptibility to Diffuse Cutaneous Systemic Sclerosis. BioMed Res. Int. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Hanson, A.L.; Sahhar, J.; Ngian, G.-S.; Roddy, J.; Walker, J.; Stevens, W.; Nikpour, M.; Assassi, S.; Proudman, S.; Mayes, M.D.; et al. Contribution of HLA and KIR Alleles to Systemic Sclerosis Susceptibility and Immunological and Clinical Disease Subtypes. Front. Genet. 2022, 13. [Google Scholar] [CrossRef]
- Watad, A.; Rosenberg, V.; Tiosano, S.; Tervaert, J.W.C.; Yavne, Y.; Shoenfeld, Y.; Shalev, V.; Chodick, G.; Amital, H. Silicone breast implants and the risk of autoimmune/rheumatic disorders: A real-world analysis. Int. J. Epidemiol. 2018, 47, 1846–1854. [Google Scholar] [CrossRef]
- Kassamali, B.; Kassamali, A.A.; Muntyanu, A.; Netchiporouk, E.; Vleugels, R.A.; LaChance, A. Geographic distribution and environmental triggers of systemic sclerosis cases from 2 large academic tertiary centers in Massachusetts. J. Am. Acad. Dermatol. 2021, 86, 925–927. [Google Scholar] [CrossRef]
- Aguila, L.A.; da Silva, H.C.; Medeiros-Ribeiro, A.C.; Bunjes, B.G.; Luppino-Assad, A.P.; Sampaio-Barros, P.D. Is exposure to environmental factors associated with a characteristic clinical and laboratory profile in systemic sclerosis? A retrospective analysis. Rheumatol. Int. 2020, 41, 1143–1150. [Google Scholar] [CrossRef]
- Lazzaroni, M.G.; Campochiaro, C.; Bertoldo, E.; De Luca, G.; Caimmi, C.; Tincani, A.; Franceschini, F.; Airò, P. Association of anti-RNA polymerase III antibody with silicone breast implants rupture in a multicentre series of Italian patients with systemic sclerosis. Clin. Exp. Rheumatol. 2021, 39, 25–28. [Google Scholar] [CrossRef]
- Skiba, M.A.; Kruse, A.C. Autoantibodies as Endogenous Modulators of GPCR Signaling. Trends Pharmacol. Sci. 2020, 42, 135–150. [Google Scholar] [CrossRef]
- Whitfield, M.L.; Finlay, D.R.; Murray, J.I.; Troyanskaya, O.G.; Chi, J.-T.; Pergamenschikov, A.; McCalmont, T.H.; Brown, P.O.; Botstein, D.; Connolly, M.K. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc. Natl. Acad. Sci. USA 2003, 100, 12319–12324. [Google Scholar] [CrossRef]
- Roberson, E.D.; Carns, M.; Cao, L.; Aren, K.; Goldberg, I.A.; Morales-Heil, D.J.; Korman, B.D.; Atkinson, J.P.; Varga, J. RNA-Seq analysis identifies alterations of the primary cilia gene SPAG17 and SOX9 locus non-coding RNAs in systemic sclerosis. Arthritis Rheumatol. 2022. [Google Scholar] [CrossRef]
- Lafyatis, R.; O’Hara, C.; Feghali-Bostwick, C.A.; Matteson, E. B cell infiltration in systemic sclerosis–associated interstitial lung disease. Arthritis Care Res. 2007, 56, 3167–3168. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Gordon, S.M.; Waldman, M.; Edison, J.D.; Little, D.J.; Stitt, R.S.; Bailey, W.T.; Hughes, J.B.; Olson, S.W. Autoantibodies are present before the clinical diagnosis of systemic sclerosis. PLoS ONE 2019, 14, e0214202. [Google Scholar] [CrossRef]
- Koenig, M.; Joyal, F.; Fritzler, M.J.; Roussin, A.; Abrahamowicz, M.; Boire, G.; Goulet, J.-R.; Rich, É.; Grodzicky, T.; Raymond, Y.; et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: A twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Care Res. 2008, 58, 3902–3912. [Google Scholar] [CrossRef]
- Bellando-Randone, S.; Del Galdo, F.; Lepri, G.; Minier, T.; Huscher, D.; Furst, D.E.; Allanore, Y.; Distler, O.; Czirják, L.; Bruni, C.; et al. Progression of patients with Raynaud’s phenomenon to systemic sclerosis: A five-year analysis of the European Scleroderma Trial and Research group multicentre, longitudinal registry study for Very Early Diagnosis of Systemic Sclerosis (VEDOSS). Lancet Rheumatol. 2021, 3, e834–e843. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Wardemann, H.; Yurasov, S.; Schaefer, A.; Young, J.W.; Meffre, E.; Nussenzweig, M.C. Predominant Autoantibody Production by Early Human B Cell Precursors. Science 2003, 301, 1374–1377. [Google Scholar] [CrossRef]
- Nemazee, D. Mechanisms of central tolerance for B cells. Nat. Rev. Immunol. 2017, 17, 281–294. [Google Scholar] [CrossRef]
- Getahun, A.; Smith, M.J.; Cambier, J.C. Mechanisms of Peripheral B Cell Tolerance. In Encyclopedia of Immunobiology; Academic Press: Oxford, UK, 2016; pp. 83–91. [Google Scholar] [CrossRef]
- Hornung, V.; Rothenfusser, S.; Britsch, S.; Krug, A.; Jahrsdörfer, B.; Giese, T.; Endres, S.; Hartmann, G. Quantitative Expression of Toll-Like Receptor 1–10 mRNA in Cellular Subsets of Human Peripheral Blood Mononuclear Cells and Sensitivity to CpG Oligodeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar] [CrossRef]
- Bourke, E.; Bosisio, D.; Golay, J.; Polentarutti, N.; Mantovani, A. The toll-like receptor repertoire of human B lymphocytes: Inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood 2003, 102, 956–963. [Google Scholar] [CrossRef]
- Dorner, M.; Brandt, S.; Tinguely, M.; Zucol, F.; Bourquin, J.-P.; Zauner, L.; Berger, C.; Bernasconi, M.; Speck, R.; Nadal, D. Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production. Immunology 2009, 128, 573–579. [Google Scholar] [CrossRef]
- Browne, E.P. Regulation of B-cell responses by Toll-like receptors. Immunology 2012, 136, 370–379. [Google Scholar] [CrossRef]
- O’Reilly, S. Toll-like receptor triggering in systemic sclerosis: Time to target. Rheumatology 2022. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S. Toll Like Receptors in systemic sclerosis: An emerging target. Immunol. Lett. 2018, 195, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Frasca, L.; Lande, R. Toll-like receptors in mediating pathogenesis in systemic sclerosis. Clin. Exp. Immunol. 2020, 201, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Koelsch, K.; Zheng, N.-Y.; Zhang, Q.; Duty, A.; Helms, C.; Mathias, M.D.; Jared, M.; Smith, K.; Capra, J.D.; Wilson, P.C. Mature B cells class switched to IgD are autoreactive in healthy individuals. J. Clin. Investig. 2007, 117, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Marques, O.C.; Marques, A.; Giil, L.M.; De Vito, R.; Rademacher, J.; Günther, J.; Lange, T.; Humrich, J.Y.; Klapa, S.; Schinke, S.; et al. GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Siloşi, I.; Siloşi, C.A.; Boldeanu, M.V.; Cojocaru, M.; Biciuşcă, V.; Avrămescu, C.S.; Cojocaru, I.M.; Bogdan, M.; Folcuţi, R.M. The role of autoantibodies in health and disease. Romanian J. Morphol. Embryol. 2016, 57, 633–638. [Google Scholar]
- Riemekasten, G.; Petersen, F.; Heidecke, H. What Makes Antibodies Against G Protein-Coupled Receptors so Special? A Novel Concept to Understand Chronic Diseases. Front. Immunol. 2020, 11, 564526. [Google Scholar] [CrossRef]
- Devitt, A.; Marshall, L.J. The innate immune system and the clearance of apoptotic cells. J. Leukoc. Biol. 2011, 90, 447–457. [Google Scholar] [CrossRef]
- Silverman, G.J.; Vas, J.; Grönwall, C. Protective autoantibodies in the rheumatic diseases: Lessons for therapy. Nat. Rev. Rheumatol. 2013, 9, 291–300. [Google Scholar] [CrossRef]
- Elkon, K.; Casali, P. Nature and functions of autoantibodies. Nat. Clin. Pract. Rheumatol. 2008, 4, 491–498. [Google Scholar] [CrossRef]
- Mehrani, T.; Petri, M. IgM Anti-ß2 Glycoprotein I Is Protective Against Lupus Nephritis and Renal Damage in Systemic Lupus Erythematosus. J. Rheumatol. 2010, 38, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, K.; Xu, Y.; Chen, C. Natural autoantibodies and associated B cells in immunity and autoimmunity. Autoimmunity 2013, 46, 138–147. [Google Scholar] [CrossRef]
- Casali, P.; Schettino, E.W. Structure and Function of Natural Antibodies. Immunol. Silicones 1996, 210, 167–179. [Google Scholar] [CrossRef]
- Duddy, M.E.; Alter, A.; Bar-Or, A. Distinct Profiles of Human B Cell Effector Cytokines: A Role in Immune Regulation? J. Immunol. 2004, 172, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Astorri, E.; Bombardieri, M.; Gabba, S.; Peakman, M.; Pozzilli, P.; Pitzalis, C. Evolution of Ectopic Lymphoid Neogenesis and In Situ Autoantibody Production in Autoimmune Nonobese Diabetic Mice: Cellular and Molecular Characterization of Tertiary Lymphoid Structures in Pancreatic Islets. J. Immunol. 2010, 185, 3359–3368. [Google Scholar] [CrossRef] [PubMed]
- Nacionales, D.C.; Weinstein, J.S.; Yan, X.-J.; Albesiano, E.; Lee, P.Y.; Kelly-Scumpia, K.M.; Lyons, R.; Satoh, M.; Chiorazzi, N.; Reeves, W.H. B Cell Proliferation, Somatic Hypermutation, Class Switch Recombination, and Autoantibody Production in Ectopic Lymphoid Tissue in Murine Lupus. J. Immunol. 2009, 182, 4226–4236. [Google Scholar] [CrossRef]
- Hampe, C.S. B Cells in Autoimmune Diseases. Scientifica 2012, 2012, 1–18. [Google Scholar] [CrossRef]
- Naparstek, Y.; Plotz, P.H. The Role of Autoantibodies in Autoimmune Disease. Annu. Rev. Immunol. 1993, 11, 79–104. [Google Scholar] [CrossRef]
- Senécal, J.-L.; Hoa, S.; Yang, R.; Koenig, M. Pathogenic roles of autoantibodies in systemic sclerosis: Current understandings in pathogenesis. J. Scleroderma Relat. Disord. 2019, 5, 103–129. [Google Scholar] [CrossRef]
- Freitag, H.; Szklarski, M.; Lorenz, S.; Sotzny, F.; Bauer, S.; Philippe, A.; Kedor, C.; Grabowski, P.; Lange, T.; Riemekasten, G.; et al. Autoantibodies to Vasoregulative G-Protein-Coupled Receptors Correlate with Symptom Severity, Autonomic Dysfunction and Disability in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Clin. Med. 2021, 10, 3675. [Google Scholar] [CrossRef]
- Notkins, A.L. Polyreactivity of antibody molecules. Trends Immunol. 2004, 25, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-H.; Zhang, Y.; Hu, Y.-F.; Wahl, L.M.; Cisar, J.O.; Notkins, A.L. The Broad Antibacterial Activity of the Natural Antibody Repertoire Is Due to Polyreactive Antibodies. Cell Host Microbe 2007, 1, 51–61. [Google Scholar] [CrossRef]
- Köhler, F.; Hug, E.; Eschbach, C.; Meixlsperger, S.; Hobeika, E.; Kofer, J.; Wardemann, H.; Jumaa, H. Autoreactive B Cell Receptors Mimic Autonomous Pre-B Cell Receptor Signaling and Induce Proliferation of Early B Cells. Immunity 2008, 29, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, M.; Kuchen, S.; Distler, O.; Michel, B.A.; Gay, R.E.; Gay, S. Increased serum levels of antibodies against human cytomegalovirus and prevalence of autoantibodies in systemic sclerosis. Arthritis Care Res. 1999, 42, 389–392. [Google Scholar] [CrossRef]
- Gkoutzourelas, A.; Liaskos, C.; Simopoulou, T.; Katsiari, C.; Efthymiou, G.; Scheper, T.; Meyer, W.; Tsirogianni, A.; Tsigalou, C.; Dardiotis, E.; et al. A study of antigen-specific anti-cytomegalovirus antibody reactivity in patients with systemic sclerosis and concomitant anti-Ro52 antibodies. Rheumatol. Int. 2020, 40, 1–11. [Google Scholar] [CrossRef]
- Efthymiou, G.; Dardiotis, E.; Liaskos, C.; Marou, E.; Scheper, T.; Meyer, W.; Daponte, A.; Daoussis, D.; Hadjigeorgiou, G.; Bogdanos, D.P.; et al. A comprehensive analysis of antigen-specific antibody responses against human cytomegalovirus in patients with systemic sclerosis. Clin. Immunol. 2019, 207, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Gourh, P.; Safran, S.A.; Alexander, T.; Boyden, S.E.; Morgan, N.D.; Shah, A.A.; Mayes, M.D.; Doumatey, A.; Bentley, A.R.; Shriner, D.; et al. HLA and autoantibodies define scleroderma subtypes and risk in African and European Americans and suggest a role for molecular mimicry. Proc. Natl. Acad. Sci. USA 2019, 117, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Glauzy, S.; Olson, B.; May, C.K.; Parisi, D.; Massad, C.; Hansen, J.E.; Ryu, C.; Herzog, E.L.; Meffre, E. Defective Early B Cell Tolerance Checkpoints in Patients With Systemic Sclerosis Allow the Production of Self Antigen –Specific Clones. Arthritis Rheumatol. 2021, 74, 307–317. [Google Scholar] [CrossRef]
- Matsushita, T.; Hamaguchi, Y.; Hasegawa, M.; Takehara, K.; Fujimoto, M. Decreased levels of regulatory B cells in patients with systemic sclerosis: Association with autoantibody production and disease activity. Rheumatology 2015, 55, 263–267. [Google Scholar] [CrossRef]
- Steri, M.; Orrù, V.; Idda, M.L.; Pitzalis, M.; Pala, M.; Zara, I.; Sidore, C.; Faà, V.; Floris, M.; Deiana, M.; et al. Overexpression of the Cytokine BAFF and Autoimmunity Risk. N. Engl. J. Med. 2017, 376, 1615–1626. [Google Scholar] [CrossRef]
- Yoshizaki, A. Pathogenic roles of B lymphocytes in systemic sclerosis. Immunol. Lett. 2018, 195, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Mavropoulos, A.; Simopoulou, T.; Varna, A.; Sakkas, L.I.; Liaskos, C.; Katsiari, C.G.; Bogdanos, D.P. Breg Cells Are Numerically Decreased and Functionally Impaired in Patients With Systemic Sclerosis. Arthritis Rheumatol. 2016, 68, 494–504. [Google Scholar] [CrossRef]
- Ricard, L.; Malard, F.; Riviere, S.; Laurent, C.; Fain, O.; Mohty, M.; Gaugler, B.; Mekinian, A. Regulatory B cell imbalance correlates with Tfh expansion in systemic sclerosis. Clin. Exp. Rheumatol. 2021, 39, 20–24. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Daoussis, D.; Mavropoulos, A.; Liossis, S.-N.; Bogdanos, D.P. Regulatory B cells: New players in inflammatory and autoimmune rheumatic diseases. Semin. Arthritis Rheum. 2018, 48, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Fujimoto, M.; Hasegawa, M.; Takehara, K. Altered blood B lymphocyte homeostasis in systemic sclerosis: Expanded naive B cells and diminished but activated memory B cells. Arthritis Care Res. 2004, 50, 1918–1927. [Google Scholar] [CrossRef]
- Forestier, A.; Guerrier, T.; Jouvray, M.; Giovannelli, J.; Lefèvre, G.; Sobanski, V.; Hauspie, C.; Hachulla, E.; Hatron, P.-Y.; Zéphir, H.; et al. Altered B lymphocyte homeostasis and functions in systemic sclerosis. Autoimmun. Rev. 2018, 17, 244–255. [Google Scholar] [CrossRef]
- Marrapodi, R.; Pellicano, C.; Radicchio, G.; Leodori, G.; Colantuono, S.; Iacolare, A.; Gigante, A.; Visentini, M.; Rosato, E. CD21low B cells in systemic sclerosis: A possible marker of vascular complications. Clin. Immunol. 2020, 213, 108364. [Google Scholar] [CrossRef]
- Thorarinsdottir, K.; Camponeschi, A.; Gjertsson, I.; Mårtensson, I.-L. CD21−/lowB cells: A Snapshot of a Unique B Cell Subset in Health and Disease. Scand. J. Immunol. 2015, 82, 254–261. [Google Scholar] [CrossRef]
- Thorarinsdottir, K.; Camponeschi, A.; Jonsson, C.; Granhagen Önnheim, K.; Nilsson, J.; Forslind, K.; Visentini, M.; Jacobsson, L.; Mårtensson, I.L.; Gjertsson, I. CD21−/low B cells associate with joint damage in rheumatoid arthritis patients. Scand. J. Immunol. 2019, 90, e12792. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, D.; Terrier, B.; Bannock, J.; Vazquez, T.; Massad, C.; Kang, I.; Joly, F.; Rosenzwajg, M.; Sene, D.; Benech, P.; et al. Expansion of Autoreactive Unresponsive CD21−/lowB Cells in Sjögren’s Syndrome-Associated Lymphoproliferation. Arthritis Care Res. 2012, 65, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Wehr, C.; Eibel, H.; Masilamani, M.; Illges, H.; Schlesier, M.; Peter, H.-H.; Warnatz, K. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin. Immunol. 2004, 113, 161–171. [Google Scholar] [CrossRef]
- Wang, J.; Watanabe, T. Expression and function of Fas during differentiation and activation of B cells. Int. Rev. Immunol. 1999, 18, 367–379. [Google Scholar] [CrossRef]
- Soto, L.; Ferrier, A.; Aravena, O.A.; Fonseca, E.; Berendsen, J.; Biere, A.; Bueno, D.; Ramos, V.; Aguillón, J.C.; Catalán, D.F. Systemic Sclerosis Patients Present Alterations in the Expression of Molecules Involved in B-Cell Regulation. Front. Immunol. 2015, 6, 496. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Hasegawa, M.; Fujimoto, M.; Tedder, T.F.; Takehara, K. Quantitative Genetic Variation in CD19 Expression Correlates with Autoimmunity. J. Immunol. 2000, 165, 6635–6643. [Google Scholar] [CrossRef]
- Zhou, L.J.; Smith, H.M.; Waldschmidt, T.J.; Schwarting, R.; Daley, J.; Tedder, T.F. Tissue-specific expression of the human CD19 gene in transgenic mice inhibits antigen-independent B-lymphocyte development. Mol. Cell. Biol. 1994, 14. [Google Scholar] [CrossRef]
- Asano, N.; Fujimoto, M.; Yazawa, N.; Shirasawa, S.; Hasegawa, M.; Okochi, H.; Tamaki, K.; Tedder, T.F.; Sato, S. B Lymphocyte Signaling Established by the CD19/CD22 Loop Regulates Autoimmunity in the Tight-Skin Mouse. Am. J. Pathol. 2004, 165, 641–650. [Google Scholar] [CrossRef]
- Inaoki, M.; Sato, S.; Weintraub, B.C.; Goodnow, C.; Tedder, T.F. CD19-Regulated Signaling Thresholds Control Peripheral Tolerance and Autoantibody Production in B Lymphocytes. J. Exp. Med. 1997, 186, 1923–1931. [Google Scholar] [CrossRef]
- Sato, S.; Ono, N.; Steeber, D.A.; Pisetsky, D.S.; Tedder, T.F. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J. Immunol. 1996, 157, 4371–4378. [Google Scholar]
- Komura, K.; Yanaba, K.; Ogawa, F.; Shimizu, K.; Takehara, K.; Sato, S. Elevation of IgG levels is a serological indicator for pulmonary fibrosis in systemic sclerosis with anti-topoisomerase I antibodies and those with anticentromere antibody. Clin. Exp. Dermatol. 2008, 33, 329–332. [Google Scholar] [CrossRef]
- Cavazzana, I.; Vojinovic, T.; Airo’, P.; Fredi, M.; Ceribelli, A.; Pedretti, E.; Lazzaroni, M.G.; Garrafa, E.; Franceschini, F. Systemic Sclerosis-Specific Antibodies: Novel and Classical Biomarkers. Clin. Rev. Allergy Immunol. 2022, 1–19. [Google Scholar] [CrossRef]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef]
- Mierau, R.; Moinzadeh, P.; Riemekasten, G.; Melchers, I.; Meurer, M.; Reichenberger, F.; Buslau, M.; Worm, M.; Blank, N.; Hein, R.; et al. Frequency of disease-associated and other nuclear autoantibodies in patients of the German network for systemic scleroderma: Correlation with characteristic clinical features. Arthritis Res. Ther. 2011, 13, R172. [Google Scholar] [CrossRef]
- Tan, E.M. Antinuclear Antibodies: Diagnostic Markers for Autoimmune Diseases and Probes for Cell Biology. Adv. Immunol. 1989, 44, 93–151. [Google Scholar] [CrossRef]
- Salazar, G.A.; Assassi, S.; Wigley, F.; Hummers, L.; Varga, J.; Hinchcliff, M.; Khanna, D.; Schiopu, E.; Phillips, K.; Furst, D.E.; et al. Antinuclear antibody-negative systemic sclerosis. Semin. Arthritis Rheum. 2014, 44, 680–686. [Google Scholar] [CrossRef]
- Serling-Boyd, N.; Chung, M.P.-S.; Li, S.; Becker, L.; Fernandez-Becker, N.; Clarke, J.; Fiorentino, D.; Chung, L. Gastric antral vascular ectasia in systemic sclerosis: Association with anti-RNA polymerase III and negative anti-nuclear antibodies. Semin. Arthritis Rheum. 2020, 50, 938–942. [Google Scholar] [CrossRef]
- Patterson, K.A.; Roberts-Thomson, P.J.; Lester, S.; Tan, J.A.; Hakendorf, P.; Rischmueller, M.; Zochling, J.; Sahhar, J.; Nash, P.; Roddy, J.; et al. Interpretation of an Extended Autoantibody Profile in a Well-Characterized Australian Systemic Sclerosis (Scleroderma) Cohort Using Principal Components Analysis. Arthritis Rheumatol. 2015, 67, 3234–3244. [Google Scholar] [CrossRef]
- Clark, K.E.N.; Campochiaro, C.; Csomor, E.; Taylor, A.; Nevin, K.; Galwey, N.; Morse, M.A.; Singh, J.; Teo, Y.V.; Ong, V.H.; et al. Molecular basis for clinical diversity between autoantibody subsets in diffuse cutaneous systemic sclerosis. Ann. Rheum. Dis. 2021, 80, 1584–1593. [Google Scholar] [CrossRef]
- Stochmal, A.; Czuwara, J.; Trojanowska, M.; Rudnicka, L. Antinuclear Antibodies in Systemic Sclerosis: An Update. Clin. Rev. Allergy Immunol. 2019, 58, 40–51. [Google Scholar] [CrossRef]
- Adler, B.L.; Boin, F.; Wolters, P.J.; Bingham, C.O.; Shah, A.A.; Greider, C.; Casciola-Rosen, L.; Rosen, A. Autoantibodies targeting telomere-associated proteins in systemic sclerosis. Ann. Rheum. Dis. 2021, 80, 912–919. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, P.; Pappas, G.; Cassani, F.; Worthington, J.; Ferri, S.; Quarneti, C.; Cipriano, V.; De Molo, C.; Lenzi, M.; et al. Antibodies to SS-A/Ro-52kD and centromere in autoimmune liver disease: A clue to diagnosis and prognosis of primary biliary cirrhosis. Aliment. Pharmacol. Ther. 2007, 26, 831–838. [Google Scholar] [CrossRef]
- Zheng, B.; Vincent, C.; Fritzler, M.J.; Senécal, J.-L.; Koenig, M.; Joyal, F. Prevalence of Systemic Sclerosis in Primary Biliary Cholangitis Using the New ACR/EULAR Classification Criteria. J. Rheumatol. 2016, 44, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Henes, J.; Glaeser, L.; Kötter, I.; Vogel, W.; Kanz, L.; Klein, R. Analysis of anti–topoisomerase I antibodies in patients with systemic sclerosis before and after autologous stem cell transplantation. Rheumatology 2016, 56, 451–456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jarzabek-Chorzelska, M.; Błaszczyk, M.; Kołacińska-Strasz, Z.; Jabłońska, S.; Chorzelski, T.; Maul, G. Are ACA and Scl 70 antibodies mutually exclusive? Br. J. Dermatol. 1990, 122, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, I.A.F.M.; Foocharoen, C.; Bannert, B.; Carreira, P.E.; Caporali, R.F.; Smith, V.; Kumánovics, G.; Becker, M.O.; Vanthuyne, M.; Simsek, I.; et al. Clinical significance of coexisting antitopoisomerase I and anticentromere antibodies in patients with systemic sclerosis: A EUSTAR group-based study. Clin. Exp. Rheumatol. 2012, 31, S96–S102. [Google Scholar]
- Harvey, G.R.; Butts, S.; Rands, A.L.; Patel, Y.; McHugh, N.J. Clinical and serological associations with anti-RNA polymerase antibodies in systemic sclerosis. Clin. Exp. Immunol. 1999, 117, 395–402. [Google Scholar] [CrossRef]
- Harris, M.L.; Rosen, A. Autoimmunity in scleroderma: The origin, pathogenetic role, and clinical significance of autoantibodies. Curr. Opin. Rheumatol. 2003, 15, 778–784. [Google Scholar] [CrossRef]
- Chen, M.; Dittmann, A.; Kuhn, A.; Ruzicka, T.; von Mikecz, A. Recruitment of topoisomerase I (Scl-70) to nucleoplasmic proteasomes in response to xenobiotics suggests a role for altered antigen processing in scleroderma. Arthritis Care Res. 2005, 52, 877–884. [Google Scholar] [CrossRef]
- Perera, A.; Fertig, N.; Lucas, M.; Rodriguez-Reyna, T.S.; Hu, P.; Steen, V.D.; Medsger, T.A., Jr. Clinical subsets, skin thickness progression rate, and serum antibody levels in systemic sclerosis patients with anti–topoisomerase I antibody. Arthritis Care Res. 2007, 56, 2740–2746. [Google Scholar] [CrossRef]
- Kuwana, M.; Kaburaki, J.; Mimori, T.; Kawakami, Y.; Tojo, T. Longitudinal analysis of autoantibody response to topoisomerase I in systemic sclerosis. Arthritis Care Res. 2000, 43, 1074–1084. [Google Scholar] [CrossRef]
- Rudnicka, L.; Czuwara, J.; Barusińska, A.; Nowicka, U.; Makiela, B.; Jablonska, S. Implications for the Use of Topoisomerase I Inhibitors in Treatment of Patients with Systemic Sclerosis. Ann. N. Y. Acad. Sci. 1996, 803, 318–320. [Google Scholar] [CrossRef]
- Hénault, J.; Tremblay, M.; Clément, I.; Raymond, Y.; Senécal, J.-L. Direct binding of anti-DNA topoisomerase I autoantibodies to the cell surface of fibroblasts in patients with systemic sclerosis. Arthritis Care Res. 2004, 50, 3265–3274. [Google Scholar] [CrossRef] [PubMed]
- Hénault, J.; Robitaille, G.; Senécal, J.-L.; Raymond, Y. DNA topoisomerase I binding to fibroblasts induces monocyte adhesion and activation in the presence of anti–topoisomerase I autoantibodies from systemic sclerosis patients. Arthritis Care Res. 2006, 54, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Corallo, C.; Cheleschi, S.; Cutolo, M.; Soldano, S.; Fioravanti, A.; Volpi, N.; Franci, D.; Nuti, R.; Giordano, N. Antibodies against specific extractable nuclear antigens (ENAs) as diagnostic and prognostic tools and inducers of a profibrotic phenotype in cultured human skin fibroblasts: Are they functional? Arthritis Res. Ther. 2019, 21, 1–11. [Google Scholar] [CrossRef]
- Suwanchote, S.; Rachayon, M.; Rodsaward, P.; Wongpiyabovorn, J.; Deekajorndech, T.; Wright, H.L.; Edwards, S.W.; Beresford, M.W.; Rerknimitr, P.; Chiewchengchol, D. Anti-neutrophil cytoplasmic antibodies and their clinical significance. Clin. Rheumatol. 2018, 37, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Walls, C.A.; Basu, N.; Hutcheon, G.; Erwig, L.P.; Little, M.A.; Kidder, D. A novel 4-dimensional live-cell imaging system to study leukocyte-endothelial dynamics in ANCA-associated vasculitis. Autoimmunity 2019, 53, 148–155. [Google Scholar] [CrossRef]
- Kettritz, R. How anti-neutrophil cytoplasmic autoantibodies activate neutrophils. Clin. Exp. Immunol. 2012, 169, 220–228. [Google Scholar] [CrossRef]
- Jerke, U.; Hernandez, D.P.; Beaudette, P.; Korkmaz, B.; Dittmar, G.; Kettritz, R. Neutrophil serine proteases exert proteolytic activity on endothelial cells. Kidney Int. 2015, 88, 764–775. [Google Scholar] [CrossRef]
- Ralston, D.R.; Marsh, C.B.; Lowe, M.P.; Wewers, M.D. Antineutrophil cytoplasmic antibodies induce monocyte IL-8 release. Role of surface proteinase-3, alpha1-antitrypsin, and Fcgamma receptors. J. Clin. Investig. 1997, 100, 1416–1424. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J. ANCAs Are Also Antimonocyte Cytoplasmic Autoantibodies. Clin. J. Am. Soc. Nephrol. 2014, 10, 4–6. [Google Scholar] [CrossRef]
- Sibelius, U.; Hattar, K.; Schenkel, A.; Noll, T.; Csernok, E.; Gross, W.L.; Mayet, W.-J.; Piper, H.-M.; Seeger, W.; Grimminger, F. Wegener’s Granulomatosis: Anti–proteinase 3 Antibodies Are Potent Inductors of Human Endothelial Cell Signaling and Leakage Response. J. Exp. Med. 1998, 187, 497–503. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, P.; Tovoli, F.; Muratori, L. Anti-neutrophil cytoplasm antibodies (ANCA) in autoimmune diseases: A matter of laboratory technique and clinical setting. Autoimmun. Rev. 2021, 20, 102787. [Google Scholar] [CrossRef]
- Khanna, D.; Aggarwal, A.; Bhakuni, D.S.; Dayal, R.; Misra, R. Bactericidal/permeability-increasing protein and cathepsin G are the major antigenic targets of antineutrophil cytoplasmic autoantibodies in systemic sclerosis. J. Rheumatol. 2003, 30, 1248–1252. [Google Scholar] [PubMed]
- Ruffatti, A.; Sinico, R.A.; Radice, A.; Ossi, E.; Cozzi, F.; Tonello, M.; Grypiotis, P.; Todesco, S. Autoantibodies to proteinase 3 and myeloperoxidase in systemic sclerosis. J. Rheumatol. 2002, 29, 918–923. [Google Scholar] [PubMed]
- Caramaschi, P.; Biasi, D.; Tonolli, E.; Carletto, A.; Bambara, L.M. Antineutrophil cytoplasmic antibodies in scleroderma patients: First report of a case with anti-proteinase 3 antibodies and review of the literature. Jt. Bone Spine 2002, 69, 177–180. [Google Scholar] [CrossRef]
- Quéméneur, T.; Mouthon, L.; Cacoub, P.; Meyer, O.; Michon-Pasturel, U.; Vanhille, P.; Hatron, P.-Y.; Guillevin, L.; Hachulla, E. Systemic Vasculitis During the Course of Systemic Sclerosis. Medicine 2013, 92, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Derrett-Smith, E.C.; Nihtyanova, S.I.; Harvey, J.; Salama, A.D.; Denton, C.P. Revisiting ANCA-associated vasculitis in systemic sclerosis: Clinical, serological and immunogenetic factors. Rheumatology 2013, 52, 1824–1831. [Google Scholar] [CrossRef]
- Trang, G.; Steele, R.; Baron, M.; Hudson, M. Corticosteroids and the risk of scleroderma renal crisis: A systematic review. Rheumatol. Int. 2010, 32, 645–653. [Google Scholar] [CrossRef]
- Vega-Ostertag, M.; Casper, K.; Swerlick, R.; Ferrara, D.; Harris, E.N.; Pierangeli, S.S. Involvement of p38 MAPK in the up-regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Care Res. 2005, 52, 1545–1554. [Google Scholar] [CrossRef]
- Liestøl, S.; Sandset, P.M.; Jacobsen, E.M.; Mowinckel, M.-C.; Wisløff, F. Decreased anticoagulant response to tissue factor pathway inhibitor type 1 in plasmas from patients with lupus anticoagulants. Br. J. Haematol. 2007, 136, 131–137. [Google Scholar] [CrossRef]
- Seed, P.; Parmar, K.; Moore, G.W.; Stuart-Smith, S.E.; Hunt, B.J.; Breen, K.A. Complement activation in patients with isolated antiphospholipid antibodies or primary antiphospholipid syndrome. Thromb. Haemost. 2012, 107, 423–429. [Google Scholar] [CrossRef]
- Espinola, R.G.; Ghara, A.E.; Harris, E.N.; Pierangeli, S.S. Hydroxychloroquine Reverses Platelet Activation Induced by Human IgG Antiphospholipid Antibodies. Thromb. Haemost. 2002, 87, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Yalavarthi, S.; Gould, T.J.; Rao, A.N.; Mazza, L.F.; Morris, A.E.; Nunezalvarez, C.A.; Hernández-Ramírez, D.; Bockenstedt, P.L.; Liaw, P.C.; Cabral, A.R.; et al. Release of Neutrophil Extracellular Traps by Neutrophils Stimulated With Antiphospholipid Antibodies: A Newly Identified Mechanism of Thrombosis in the Antiphospholipid Syndrome. Arthritis Rheumatol. 2015, 67, 2990–3003. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Yalavarthi, S.; Kanthi, Y.; Mazza, L.F.; Elfline, M.A.; Luke, C.E.; Pinsky, D.J.; Henke, P.K.; Knight, J.S. In Vivo Role of Neutrophil Extracellular Traps in Antiphospholipid Antibody-Mediated Venous Thrombosis. Arthritis Rheumatol. 2016, 69, 655–667. [Google Scholar] [CrossRef] [PubMed]
- López-Pedrera, C.; Buendía, P.; Cuadrado, M.J.; Siendones, E.; Aguirre, M.A.; Barbarroja, N.; Duarte, C.M.; Torres, A.; Khamashta, M.; Velasco, F. Antiphospholipid antibodies from patients with the antiphospholipid syndrome induce monocyte tissue factor expression through the simultaneous activation of NF-κB/Rel proteins via the p38 mitogen-activated protein kinase pathway, and of the MEK-1/ERK pathway. Arthritis Care Res. 2005, 54, 301–311. [Google Scholar] [CrossRef]
- Sobanski, V.; Lemaire-Olivier, A.; Giovannelli, J.; Dauchet, L.; Simon, M.; Lopez, B.; Yelnik, C.; Lambert, M.; Hatron, P.-Y.; Hachulla, E.; et al. Prevalence and Clinical Associations of Antiphospholipid Antibodies in Systemic Sclerosis: New Data From a French Cross-Sectional Study, Systematic Review, and Meta-Analysis. Front. Immunol. 2018, 9, 2457. [Google Scholar] [CrossRef]
- Martin, M.; Martinez, C.; Arnaud, L.; Weber, J.-C.; Poindron, V.; Blaison, G.; Kieffer, P.; Bonnotte, B.; Berthier, S.; Wahl, D.; et al. Association of antiphospholipid antibodies with active digital ulceration in systemic sclerosis. RMD Open 2019, 5, e001012. [Google Scholar] [CrossRef]
- Merashli, M.; Alves, J.; Ames, P.R. Clinical relevance of antiphospholipid antibodies in systemic sclerosis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2017, 46, 615–624. [Google Scholar] [CrossRef]
- Murthy, S.; Wannick, M.; Eleftheriadis, G.; Müller, A.; Luo, J.; Busch, H.; Dalmann, A.; Riemekasten, G.; Sadik, C.D. Immunoglobulin G of systemic sclerosis patients programs a pro-inflammatory and profibrotic phenotype in monocyte-like THP-1 cells. Rheumatology 2020, 60, 3012–3022. [Google Scholar] [CrossRef]
- Rosetti, F.; Tsuboi, N.; Chen, K.; Nishi, H.; Ernandez, T.; Sethi, S.; Croce, K.; Stavrakis, G.; Alcocer-Varela, J.; Gómez-Martin, D.; et al. Human Lupus Serum Induces Neutrophil-Mediated Organ Damage in Mice That Is Enabled by Mac-1 Deficiency. J. Immunol. 2012, 189, 3714–3723. [Google Scholar] [CrossRef]
- Stern, M.E.; Schaumburg, C.S.; Siemasko, K.F.; Gao, J.; Wheeler, L.A.; Grupe, D.A.; de Paiva, C.; Calder, V.L.; Calonge, M.; Niederkorn, J.Y.; et al. Autoantibodies Contribute to the Immunopathogenesis of Experimental Dry Eye Disease. Investig. Opthalmol. Vis. Sci. 2012, 53, 2062–2075. [Google Scholar] [CrossRef]
- Scofield, R.H.; Asfa, S.; Obeso, D.; Jonsson, R.; Kurien, B.T. Immunization with Short Peptides from the 60-kDa Ro Antigen Recapitulates the Serological and Pathological Findings as well as the Salivary Gland Dysfunction of Sjögren’s Syndrome. J. Immunol. 2005, 175, 8409–8414. [Google Scholar] [CrossRef]
- Rosenberg, N.L.; Ringel, S.P.; Kotzin, B.L. Experimental autoimmune myositis in SJL/J mice. Clin. Exp. Immunol. 1987, 68, 117–129. [Google Scholar] [PubMed]
- Becker, M.O.; Kill, A.; Kutsche, M.; Guenther, J.; Rose, A.; Tabeling, C.; Witzenrath, M.; Kühl, A.A.; Heidecke, H.; Ghofrani, H.A.; et al. Vascular Receptor Autoantibodies in Pulmonary Arterial Hypertension Associated with Systemic Sclerosis. Am. J. Respir. Crit. Care Med. 2014, 190, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Kill, A.; Tabeling, C.; Undeutsch, R.; Kühl, A.A.; Günther, J.; Radic, M.; Becker, M.O.; Heidecke, H.; Worm, M.; Witzenrath, M.; et al. Autoantibodies to angiotensin and endothelin receptors in systemic sclerosis induce cellular and systemic events associated with disease pathogenesis. Arthritis Res. Ther. 2014, 16, R29. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Yin, J.; Wang, X.; Heidecke, H.; Hackel, A.M.; Dong, X.; Kasper, B.; Wen, L.; Zhang, L.; Schulze-Forster, K.; et al. Induced antibodies directed to the angiotensin receptor type 1 provoke skin and lung inflammation, dermal fibrosis and act species overarching. Ann. Rheum. Dis. 2022, 81, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Petersen, F.; Shu, Y.; Kasper, B.; Magatsin, J.D.T.; Ahmadi, M.; Yin, J.; Wax, J.; Wang, X.; Heidecke, H.; et al. Transfer of PBMC From SSc Patients Induces Autoantibodies and Systemic Inflammation in Rag2-/-/IL2rg-/- Mice. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Zhang, Z.; Zhang, Y.; Lei, Z.; Jin, W.; Cao, J.; Jiao, X. Autoantibody against angiotensin II type I receptor induces pancreatic β-cell apoptosis via enhancing autophagy. Acta Biochim. Biophys. Sin. 2021, 53, 784–795. [Google Scholar] [CrossRef]
- Mejia-Vilet, J.M.; López-Hernández, Y.J.; Santander-Vélez, J.I.; Trujeque-Matos, M.; Cruz, C.; Torre, C.A.C.D.L.; Espinosa-Cruz, V.; Espinosa-González, R.; Uribe-Uribe, N.O.; Morales-Buenrostro, L.E. Angiotensin II receptor agonist antibodies are associated with microvascular damage in lupus nephritis. Lupus 2020, 29, 371–378. [Google Scholar] [CrossRef]
- Jiang, Y.; Duffy, F.; Hadlock, J.; Raappana, A.; Styrchak, S.; Beck, I.; Mast, F.D.; Miller, L.R.; Chour, W.; Houck, J.; et al. Angiotensin II receptor I auto-antibodies following SARS-CoV-2 infection. PLoS ONE 2021, 16, e0259902. [Google Scholar] [CrossRef]
- Wallukat, G.; Homuth, V.; Fischer, T.; Lindschau, C.; Horstkamp, B.; Jüpner, A.; Baur, E.; Nissen, E.; Vetter, K.; Neichel, D.; et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J. Clin. Investig. 1999, 103, 945–952. [Google Scholar] [CrossRef]
- Dragun, D.; Müller, D.N.; Bräsen, J.H.; Fritsche, L.; Nieminen-Kelhä, M.; Dechend, R.; Kintscher, U.; Rudolph, B.; Hoebeke, J.; Eckert, D.; et al. Angiotensin II Type 1–Receptor Activating Antibodies in Renal-Allograft Rejection. N. Engl. J. Med. 2005, 352, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Dragun, D.; Catar, R.; Philippe, A. Non-HLA antibodies against endothelial targets bridging allo- and autoimmunity. Kidney Int. 2016, 90, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Lukitsch, I.; Kehr, J.; Chaykovska, L.; Wallukat, G.; Nieminen-Kelhä, M.; Batuman, V.; Dragun, D.; Gollasch, M. Renal Ischemia and Transplantation Predispose to Vascular Constriction Mediated by Angiotensin II Type 1 Receptor-Activating Antibodies. Transplantation 2012, 94, 8–13. [Google Scholar] [CrossRef]
- Rasini, E.; Cosentino, M.; Marino, F.; Legnaro, M.; Ferrari, M.; Guasti, L.; Venco, A.; Lecchini, S. Angiotensin II type 1 receptor expression on human leukocyte subsets: A flow cytometric and RT-PCR study. Regul. Pept. 2006, 134, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, S.; Stumpf, M.; Strehlow, K.; Schmid, A.; Schieffer, B.; Böhm, M.; Nickenig, G. Interleukin-6 Induces Oxidative Stress and Endothelial Dysfunction by Overexpression of the Angiotensin II Type 1 Receptor. Circ. Res. 2004, 94, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Pearl, M.H.; Grotts, J.; Rossetti, M.; Zhang, Q.; Gjertson, D.W.; Weng, P.; Elashoff, D.; Reed, E.F.; Chambers, E.T. Cytokine Profiles Associated With Angiotensin II Type 1 Receptor Antibodies. Kidney Int. Rep. 2018, 4, 541–550. [Google Scholar] [CrossRef]
- Ceolotto, G.; Papparella, I.; Bortoluzzi, A.; Strapazzon, G.; Ragazzo, F.; Bratti, P.; Fabricio, A.; Squarcina, E.; Gion, M.; Palatini, P.; et al. Interplay Between miR-155, AT1R A1166C Polymorphism, and AT1R Expression in Young Untreated Hypertensives. Am. J. Hypertens. 2011, 24, 241–246. [Google Scholar] [CrossRef]
- Hrenak, J.; Simko, F. Renin–Angiotensin System: An Important Player in the Pathogenesis of Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2020, 21, 8038. [Google Scholar] [CrossRef]
- Sorohan, B.M.; Ismail, G.; Leca, N.; Tacu, D.; Obrișcă, B.; Constantinescu, I.; Baston, C.; Sinescu, I. Angiotensin II type 1 receptor antibodies in kidney transplantation: An evidence-based comprehensive review. Transplant. Rev. 2020, 34, 100573. [Google Scholar] [CrossRef]
- Lei, J.; Zhang, S.; Wang, P.; Liao, Y.; Bian, J.; Yin, X.; Wu, Y.; Bai, L.; Wang, F.; Yang, X.; et al. Long-term presence of angiotensin II type 1 receptor autoantibody reduces aldosterone production by triggering Ca2+ overload in H295R cells. Immunol. Res. 2017, 66, 44–51. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Takagi, K.; Hara, M.; Fukasawa, C.; Sugiura, T.; Nishimagi, E.; Harigai, M.; Kamatani, N. Angiotensin II in the lesional skin of systemic sclerosis patients contributes to tissue fibrosis via angiotensin II type 1 receptors. Arthritis Care Res. 2004, 50, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Miyauchi, T.; Suzuki, N.; Yuhara, T.; Akama, T.; Suzuki, H.; Kashiwagi, H. Significance of plasma endothelin-1 levels in patients with systemic sclerosis. J. Rheumatol. 1992, 19, 1566–1571. [Google Scholar] [PubMed]
- Denton, C.P.; Pope, J.E.; Peter, H.-H.; Gabrielli, A.; Boonstra, A.; Hoogen, F.H.J.V.D.; Riemekasten, G.; De Vita, S.; Morganti, A.; Dolberg, M.; et al. Long-term effects of bosentan on quality of life, survival, safety and tolerability in pulmonary arterial hypertension related to connective tissue diseases. Ann. Rheum. Dis. 2007, 67, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Tillon, J.; Hervé, F.; Chevallier, D.; Muir, J.-F.; Levesque, H.; Marie, I. Successful treatment of systemic sclerosis-related digital ulcers and sarcoidosis with endothelin receptor antagonist (bosentan) therapy. Br. J. Dermatol. 2006, 154, 1000–1002. [Google Scholar] [CrossRef] [PubMed]
- Arefiev, K.; Fiorentino, D.; Chung, L. Endothelin Receptor Antagonists for the Treatment of Raynaud’s Phenomenon and Digital Ulcers in Systemic Sclerosis. Int. J. Rheumatol. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Dhillon, S. Bosentan. Drugs 2009, 69, 2005–2024. [Google Scholar] [CrossRef]
- Korn, J.H.; Mayes, M.; Cerinic, M.M.; Rainisio, M.; Pope, J.; Hachulla, E.; Rich, E.; Carpentier, P.; Molitor, J.; Seibold, J.R.; et al. Digital ulcers in systemic sclerosis: Prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Care Res. 2004, 50, 3985–3993. [Google Scholar] [CrossRef]
- Riemekasten, G.; Philippe, A.; Näther, M.; Slowinski, T.; Muller, D.N.; Heidecke, H.; Matucci-Cerinic, M.; Czirják, L.; Lukitsch, I.; Becker, M.; et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann. Rheum. Dis. 2010, 70, 530–536. [Google Scholar] [CrossRef]
- Günther, J.; Kill, A.; Becker, M.O.; Heidecke, H.; Rademacher, J.; Siegert, E.; Radić, M.; Burmester, G.-R.; Dragun, D.; Riemekasten, G. Angiotensin receptor type 1 and endothelin receptor type A on immune cells mediate migration and the expression of IL-8 and CCL18 when stimulated by autoantibodies from systemic sclerosis patients. Arthritis Res. Ther. 2014, 16, R65. [Google Scholar] [CrossRef]
- Avouac, J.; Riemekasten, G.; Meune, C.; Ruiz, B.; Kahan, A.; Allanore, Y. Autoantibodies against Endothelin 1 Type A Receptor Are Strong Predictors of Digital Ulcers in Systemic Sclerosis. J. Rheumatol. 2015, 42, 1801–1807. [Google Scholar] [CrossRef]
- Cabral-Marques, O.; Riemekasten, G. Vascular hypothesis revisited: Role of stimulating antibodies against angiotensin and endothelin receptors in the pathogenesis of systemic sclerosis. Autoimmun. Rev. 2016, 15, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Nakamura, Y.; Matsumoto, I.; Nishimagi, E.; Satoh, T.; Kuwana, M.; Sumida, T.; Hara, M. Muscarinic-3 acetylcholine receptor autoantibody in patients with systemic sclerosis: Contribution to severe gastrointestinal tract dysmotility. Ann. Rheum. Dis. 2008, 68, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, J.; Kedika, R.; Mendoza, F.; Jimenez, S.; Blomain, E.S.; Dimarino, A.J.; Cohen, S.; Rattan, S. Role of muscarinic-3 receptor antibody in systemic sclerosis: Correlation with disease duration and effects of IVIG. Am. J. Physiol. Liver Physiol. 2016, 310, G1052–G1060. [Google Scholar] [CrossRef] [PubMed]
- Raja, J.; Nihtyanova, S.I.; Murray, C.; Denton, C.P.; Ong, V.H. Sustained benefit from intravenous immunoglobulin therapy for gastrointestinal involvement in systemic sclerosis. Rheumatology 2015, 55, 115–119. [Google Scholar] [CrossRef]
- Weigold, F.; Günther, J.; Pfeiffenberger, M.; Marques, O.C.; Siegert, E.; Dragun, D.; Philippe, A.; Regensburger, A.-K.; Recke, A.; Yu, X.; et al. Antibodies against chemokine receptors CXCR3 and CXCR4 predict progressive deterioration of lung function in patients with systemic sclerosis. Arthritis Res. Ther. 2018, 20, 52. [Google Scholar] [CrossRef]
- Recke, A.; Regensburger, A.-K.; Weigold, F.; Müller, A.; Heidecke, H.; Marschner, G.; Hammers, C.; Ludwig, R.; Riemekasten, G. Autoantibodies in Serum of Systemic Scleroderma Patients: Peptide-Based Epitope Mapping Indicates Increased Binding to Cytoplasmic Domains of CXCR3. Front. Immunol. 2018, 9, 428. [Google Scholar] [CrossRef]
- Simon, M.; Lücht, C.; Hosp, I.; Zhao, H.; Wu, D.; Heidecke, H.; Witowski, J.; Budde, K.; Riemekasten, G.; Catar, R. Autoantibodies from Patients with Scleroderma Renal Crisis Promote PAR-1 Receptor Activation and IL-6 Production in Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 11793. [Google Scholar] [CrossRef]
- Shima, Y.; Kuwahara, Y.; Murota, H.; Kitaba, S.; Kawai, M.; Hirano, T.; Arimitsu, J.; Narazaki, M.; Hagihara, K.; Ogata, A.; et al. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology 2010, 49, 2408–2412. [Google Scholar] [CrossRef]
- Schiopu, E.; Chatterjee, S.; Hsu, V.; Flor, A.; Cimbora, D.; Patra, K.; Yao, W.; Li, J.; Streicher, K.; McKeever, K.; et al. Safety and tolerability of an anti-CD19 monoclonal antibody, MEDI-551, in subjects with systemic sclerosis: A phase I, randomized, placebo-controlled, escalating single-dose study. Arthritis Res. Ther. 2016, 18, 131. [Google Scholar] [CrossRef]
- Mei, H.E.; Frölich, D.; Giesecke, C.; Loddenkemper, C.; Reiter, K.; Schmidt, S.; Feist, E.; Daridon, C.; Tony, H.-P.; Radbruch, A.; et al. Steady-state generation of mucosal IgA+ plasmablasts is not abrogated by B-cell depletion therapy with rituximab. Blood 2010, 116, 5181–5190. [Google Scholar] [CrossRef]
- Zamanian, R.T.; Badesch, D.; Chung, L.; Domsic, R.T.; Medsger, T.; Pinckney, A.; Keyes-Elstein, L.; D’Aveta, C.; Spychala, M.; White, R.J.; et al. Safety and Efficacy of B-Cell Depletion with Rituximab for the Treatment of Systemic Sclerosis–associated Pulmonary Arterial Hypertension: A Multicenter, Double-Blind, Randomized, Placebo-controlled Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, M.; Meijs, J.; Dorjée, A.L.; Marsan, N.A.; Schouffoer, A.; Ninaber, M.K.; Quint, K.D.; Bonte-Mineur, F.; Huizinga, T.W.J.; Scherer, H.U.; et al. Rituximab in early systemic sclerosis. RMD Open 2017, 3, e000384. [Google Scholar] [CrossRef] [PubMed]
- Sircar, G.; Goswami, R.P.; Sircar, D.; Ghosh, A.; Ghosh, P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: Open label, randomized, controlled trial. Rheumatology 2018, 57, 2106–2113. [Google Scholar] [CrossRef]
- Goswami, R.P.; Ray, A.; Chatterjee, M.; Mukherjee, A.; Sircar, G.; Ghosh, P. Rituximab in the treatment of systemic sclerosis–related interstitial lung disease: A systematic review and meta-analysis. Rheumatology 2020, 60, 557–567. [Google Scholar] [CrossRef]
- Kaegi, C.; Wuest, B.; Crowley, C.; Boyman, O. Systematic Review of Safety and Efficacy of Second- and Third-Generation CD20-Targeting Biologics in Treating Immune-Mediated Disorders. Front. Immunol. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Mahévas, M.; Michel, M.; Weill, J.-C.; Reynaud, C.-A. Long-Lived Plasma Cells in Autoimmunity: Lessons from B-Cell Depleting Therapy. Front. Immunol. 2013, 4, 494. [Google Scholar] [CrossRef]
- Ehrenstein, M.; Wing, M.R.E.C. The BAFFling effects of rituximab in lupus: Danger ahead? Nat. Rev. Rheumatol. 2016, 12, 367–372. [Google Scholar] [CrossRef]
- Matsushita, T.; Kobayashi, T.; Mizumaki, K.; Kano, M.; Sawada, T.; Tennichi, M.; Okamura, A.; Hamaguchi, Y.; Iwakura, Y.; Hasegawa, M.; et al. BAFF inhibition attenuates fibrosis in scleroderma by modulating the regulatory and effector B cell balance. Sci. Adv. 2018, 4, eaas9944. [Google Scholar] [CrossRef]
- Gordon, J.K.; Martyanov, V.; Franks, J.M.; Bernstein, E.J.; Szymonifka, J.; Magro, C.; Wildman, H.F.; Wood, T.A.; Whitfield, M.L.; Spiera, R.F. Belimumab for the Treatment of Early Diffuse Systemic Sclerosis. Arthritis Rheumatol. 2017, 70, 308–316. [Google Scholar] [CrossRef]
- Fineschi, S.; Reith, W.; Guerne, P.A.; Dayer, J.; Chizzolini, C. Proteasome blockade exerts an antifibrotic activity by coordinately down-regulating type I collagen and tissue inhibitor of metalloproteinase-1 and up-regulating metalloproteinase-1 production in human dermal fibroblasts. FASEB J. 2006, 20, 562–564. [Google Scholar] [CrossRef]
- Fineschi, S.; Bongiovanni, M.; Donati, Y.; Djaafar, S.; Naso, F.; Goffin, L.; Argiroffo, C.B.; Pache, J.-C.; Dayer, J.-M.; Ferrari-Lacraz, S.; et al. In Vivo Investigations on Anti-Fibrotic Potential of Proteasome Inhibition in Lung and Skin Fibrosis. Am. J. Respir. Cell Mol. Biol. 2008, 39, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Benfaremo, D.; Gabrielli, A. Is There a Future for Anti-CD38 Antibody Therapy in Systemic Autoimmune Diseases? Cells 2019, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Pavlasova, G.; Borsky, M.; Seda, V.; Cerna, K.; Osickova, J.; Doubek, M.; Mayer, J.; Calogero, R.; Trbusek, M.; Pospisilova, S.; et al. Ibrutinib inhibits CD20 upregulation on CLL B cells mediated by the CXCR4/SDF-1 axis. Blood 2016, 128, 1609–1613. [Google Scholar] [CrossRef]
- Einhaus, J.; Pecher, A.-C.; Asteriti, E.; Schmid, H.; Secker, K.-A.; Duerr-Stoerzer, S.; Keppeler, H.; Klein, R.; Schneidawind, C.; Henes, J.; et al. Inhibition of effector B cells by ibrutinib in systemic sclerosis. Arthritis Res. Ther. 2020, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Servaas, N.H.; Spierings, J.; Pandit, A.; van Laar, J.M. The role of innate immune cells in systemic sclerosis in the context of autologous hematopoietic stem cell transplantation. Clin. Exp. Immunol. 2020, 201, 34–39. [Google Scholar] [CrossRef]
- Gernert, M.; Tony, H.-P.; Schwaneck, E.C.; Gadeholt, O.; Schmalzing, M. Autologous hematopoietic stem cell transplantation in systemic sclerosis induces long-lasting changes in B cell homeostasis toward an anti-inflammatory B cell cytokine pattern. Arthritis Res. Ther. 2019, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Arruda, L.C.M.; Lima-Júnior, J.R.; Clave, E.; Moraes, D.A.; Douay, C.; Fournier, I.; Moins-Teisserenc, H.; Covas, D.T.; Malmegrim, K.; Farge, D.; et al. Newly-Generated Regulatory B- and T-Cells Are Associated with Clinical Improvement and Reversal of Dermal Fibrosis in Systemic Sclerosis Patients after Autologous Hematopoietic Stem Cell Transplantation. Blood 2016, 128, 4625. [Google Scholar] [CrossRef]
- Tyndall, A.; Black, C.; Finke, J.; Winkler, J.; Mertlesmann, R.; Peter, H.; Gratwohl, A. Treatment of systemic sclerosis with autologous haemopoietic stem cell transplantation. Lancet 1997, 349, 254. [Google Scholar] [CrossRef]
- Shah, A.; Spierings, J.; van Laar, J.; Sullivan, K.M. Re-evaluating inclusion criteria for autologous hematopoietic stem cell transplantation in advanced systemic sclerosis: Three successful cases and review of the literature. J. Scleroderma Relat. Disord. 2021, 6, 199–205. [Google Scholar] [CrossRef]
- Moraes, D.A.; Oliveira, M.C. Life after Autologous Hematopoietic Stem Cell Transplantation for Systemic Sclerosis. J. Blood Med. 2021, 12, 951–964. [Google Scholar] [CrossRef]
- Burt, R.K.; Shah, S.J.; Dill, K.; Grant, T.; Gheorghiade, M.; Schroeder, J.; Craig, R.; Hirano, I.; Marshall, K.; Ruderman, E.; et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): An open-label, randomised phase 2 trial. Lancet 2011, 378, 498–506. [Google Scholar] [CrossRef]
- Van Laar, J.M.; Farge, D.; Sont, J.; Naraghi, K.; Marjanovic, Z.; Larghero, J.; Schuerwegh, A.J.; Marijt, E.W.A.; Vonk, M.C.; Schattenberg, A.V.; et al. Autologous Hematopoietic Stem Cell Transplantation vs Intravenous Pulse Cyclophosphamide in Diffuse Cutaneous Systemic Sclerosis. JAMA 2014, 311, 2490–2498. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Goldmuntz, E.A.; Keyes-Elstein, L.; McSweeney, P.A.; Pinckney, A.; Welch, B.; Mayes, M.D.; Nash, R.A.; Crofford, L.J.; Eggleston, B.; et al. Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma. N. Engl. J. Med. 2018, 378, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Henes, J.; Oliveira, M.C.; Labopin, M.; Badoglio, M.; Scherer, H.U.; Del Papa, N.; Daikeler, T.; Schmalzing, M.; Schroers, R.; Martin, T.; et al. Autologous stem cell transplantation for progressive systemic sclerosis: A prospective non-interventional study from the European Society for Blood and Marrow Transplantation Autoimmune Disease Working Party. Haematologica 2020, 106, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Gernert, M.; Tony, H.-P.; Schwaneck, E.C.; Fröhlich, M.; Schmalzing, M. Low B cell counts as risk factor for infectious complications in systemic sclerosis after autologous hematopoietic stem cell transplantation. Arthritis Res. Ther. 2020, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Strunz, P.-P.; Froehlich, M.; Gernert, M.; Schwaneck, E.C.; Fleischer, A.; Pecher, A.-C.; Tony, H.-P.; Henes, J.C.; Schmalzing, M. Immunological Adverse Events After Autologous Hematopoietic Stem Cell Transplantation in Systemic Sclerosis Patients. Front. Immunol. 2021, 12, 3526. [Google Scholar] [CrossRef]
- Alexander, T.; Henes, J.; Distler, J.H.W.; Schmalzing, M.; Blank, N.; Kötter, I.; Hiepe, F. Autologe hämatopoetische Stammzelltransplantation bei systemischer Sklerose. Z. Rheumatol. 2020, 79, 429–436. [Google Scholar] [CrossRef]
- Harris, E.S.; Meiselman, H.J.; Moriarty, P.M.; Metzger, A.; Malkovsky, M. Therapeutic plasma exchange for the treatment of systemic sclerosis: A comprehensive review and analysis. J. Scleroderma Relat. Disord. 2018, 3, 132–152. [Google Scholar] [CrossRef]
- Patten, E.; Berkman, E.M. Therapeutic Plasmapheresis and Plasma Exchange. CRC Crit. Rev. Clin. Lab. Sci. 1986, 23, 147–175. [Google Scholar] [CrossRef]
- Åkesson, A.; Wollheim, F.A.; Thysell, H.; Gustafson, T.; Forsberg, L.; Pahlm, O.; Wollmer, P.; Åkesson, B.; And, P.W. Visceral Improvement Following Combined Plasmapheresis and Immunosuppressive Drug Therapy in Progressive Systemic Sclerosis. Scand. J. Rheumatol. 1988, 17, 313–323. [Google Scholar] [CrossRef]
- Suga, K.; Yamashita, H.; Takahashi, Y.; Katagiri, D.; Hinoshita, F.; Kaneko, H. Therapeutic efficacy of combined glucocorticoid, intravenous cyclophosphamide, and double-filtration plasmapheresis for skin sclerosis in diffuse systemic sclerosis. Medicine 2020, 99, e19301. [Google Scholar] [CrossRef] [PubMed]
- Korsten, P.; Müller, G.A.; Rademacher, J.-G.; Zeisberg, M.; Tampe, B. Rheopheresis for Digital Ulcers and Raynaud’s Phenomenon in Systemic Sclerosis Refractory to Conventional Treatments. Front. Med. 2019, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Lutze, S.; Daeschlein, G.; Konschake, W.; Jünger, M. Rheopheresis as a causal therapy option for systemic scleroderma (SSc). Clin. Hemorheol. Microcirc. 2017, 67, 229–240. [Google Scholar] [CrossRef]
- Cid, J.; Carbassé, G.; Andreu, B.; Baltanás, A.; Garcia-Carulla, A.; Lozano, M. Efficacy and safety of plasma exchange: An 11-year single-center experience of 2730 procedures in 317 patients. Transfus. Apher. Sci. 2014, 51, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Mokrzycki, M.H.; Balogun, R.A. Therapeutic apheresis: A review of complications and recommendations for Prevention and management. J. Clin. Apher. 2011, 26, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Abos, J.; Roquer, J.; Mazzara, R.; Pereira, A. Effect of plasmapheresis on serum and CSF autoantibody levels in CNS paraneoplastic syndromes. Neurology 1990, 40, 1621. [Google Scholar] [CrossRef]
- Hohenstein, B.; Bornstein, S.; Aringer, M. Immunoadsorption for connective tissue disease. Atheroscler. Suppl. 2013, 14, 185–189. [Google Scholar] [CrossRef]
- Mulhearn, B.; Bruce, I.N. Indications for IVIG in rheumatic diseases. Rheumatology 2014, 54, 383–391. [Google Scholar] [CrossRef]
- Jolles, S. A review of high-dose intravenous immunoglobulin (hdIVIg) in the treatment of the autoimmune blistering disorders. Clin. Exp. Dermatol. 2001, 26, 127–131. [Google Scholar] [CrossRef]
- Sewell, W.A.C.; Jolles, S. Immunomodulatory action of intravenous immunoglobulin. Immunology 2002, 107, 387–393. [Google Scholar] [CrossRef]
- Agostini, E.; De Luca, G.; Bruni, C.; Bartoli, F.; Tofani, L.; Campochiaro, C.; Pacini, G.; Moggi-Pignone, A.; Guiducci, S.; Bellando-Randone, S.; et al. Intravenous immunoglobulins reduce skin thickness in systemic sclerosis: Evidence from Systematic Literature Review and from real life experience. Autoimmun. Rev. 2021, 20, 102981. [Google Scholar] [CrossRef]
- Sanges, S.; Rivière, S.; Mekinian, A.; Martin, T.; Le Quellec, A.; Chatelus, E.; Lescoat, A.; Jego, P.; Cazalets, C.; Quéméneur, T.; et al. Intravenous immunoglobulins in systemic sclerosis: Data from a French nationwide cohort of 46 patients and review of the literature. Autoimmun. Rev. 2017, 16, 377–384. [Google Scholar] [CrossRef]
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J.; et al. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of Covid-19. N. Engl. J. Med. 2022, 386, 2188–2200. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, M.M. Etanercept, a novel drug for the treatment of patients with severe, active rheumatoid arthritis. Clin. Ther. 1999, 21, 75–87. [Google Scholar] [CrossRef]
- Vaccaro, C.; Zhou, J.; Ober, R.J.; Ward, E.S. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat. Biotechnol. 2005, 23, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Goebeler, M.; Bata-Csörgő, Z.; De Simone, C.; Didona, B.; Remenyik, E.; Reznichenko, N.; Stoevesandt, J.; Ward, E.; Parys, W.; de Haard, H.; et al. Treatment of pemphigus vulgaris and foliaceus with efgartigimod, a neonatal Fc receptor inhibitor: A phase II multicentre, open-label feasibility trial*. Br. J. Dermatol. 2021, 186, 429–439. [Google Scholar] [CrossRef]
- Maho-Vaillant, M.; Sips, M.; Golinski, M.-L.; Vidarsson, G.; Goebeler, M.; Stoevesandt, J.; Bata-Csörgő, Z.; Balbino, B.; Verheesen, P.; Joly, P.; et al. FcRn Antagonism Leads to a Decrease of Desmoglein-Specific B Cells: Secondary Analysis of a Phase 2 Study of Efgartigimod in Pemphigus Vulgaris and Pemphigus Foliaceus. Front. Immunol. 2022, 13, 863095. [Google Scholar] [CrossRef]
- Werth, V.P.; Culton, D.A.; Concha, J.S.; Graydon, J.S.; Blumberg, L.J.; Okawa, J.; Pyzik, M.; Blumberg, R.S.; Hall, R.P. Safety, Tolerability, and Activity of ALXN1830 Targeting the Neonatal Fc Receptor in Chronic Pemphigus. J. Investig. Dermatol. 2021, 141, 2858–2865.e4. [Google Scholar] [CrossRef]
- Robak, T.; Kaźmierczak, M.; Jarque, I.; Musteata, V.; Treliński, J.; Cooper, N.; Kiessling, P.; Massow, U.; Woltering, F.; Snipes, R.; et al. Phase 2 multiple-dose study of an FcRn inhibitor, rozanolixizumab, in patients with primary immune thrombocytopenia. Blood Adv. 2020, 4, 4136–4146. [Google Scholar] [CrossRef]
- Newland, A.C.; Sánchez-González, B.; Rejtő, L.; Egyed, M.; Romanyuk, N.; Godar, M.; Verschueren, K.; Gandini, D.; Ulrichts, P.; Beauchamp, J.; et al. Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia. Am. J. Hematol. 2019, 95, 178–187. [Google Scholar] [CrossRef]
- Sun, W.; Khare, P.; Wang, X.; Challa, D.K.; Greenberg, B.M.; Ober, R.J.; Ward, E.S. Selective Depletion of Antigen-Specific Antibodies for the Treatment of Demyelinating Disease. Mol. Ther. 2021, 29, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, Y.; Hong, H.; Zhang, Y.; Cai, W.; Fang, D. Aptamers as therapeutics in cardiovascular diseases. Curr. Med. Chem. 2011, 18, 4169–4174. [Google Scholar] [CrossRef] [PubMed]
- Famulok, M.; Mayer, G. Aptamers and SELEX in Chemistry & Biology. Chem. Biol. 2014, 21, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Haberland, A.; Wallukat, G.; Schimke, I. Aptamer Binding and Neutralization of β1-Adrenoceptor Autoantibodies: Basics and a Vision of Its Future in Cardiomyopathy Treatment☆ ☆This work was supported by the European Regional Development Fund (10141685; Berlin, Germany) and Stiftung Pathobiochemie, Deutsche Gesellschaft für Klinische Chemie und Laboratoriumsmedizin (66/2007) and 48/2011, Germany). Trends Cardiovasc. Med. 2011, 21, 177–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef]
- Ng, E.W.M.; Shima, D.T.; Calias, P.; Cunningham, E.T.C., Jr.; Guyer, D.R.; Adamis, A.P. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov. 2006, 5, 123–132. [Google Scholar] [CrossRef]
- Paborsky, L.; McCurdy, S.; Griffin, L.; Toole, J.; Leung, L. The single-stranded DNA aptamer-binding site of human thrombin. J. Biol. Chem. 1993, 268, 20808–20811. [Google Scholar] [CrossRef]
- Haberland, A.; Holtzhauer, M.; Schlichtiger, A.; Bartel, S.; Schimke, I.; Müller, J.; Dandel, M.; Luppa, P.B.; Wallukat, G. Aptamer BC 007—A broad spectrum neutralizer of pathogenic autoantibodies against G-protein-coupled receptors. Eur. J. Pharmacol. 2016, 789, 37–45. [Google Scholar] [CrossRef]
- Wallukat, G.; Müller, J.; Haberland, A.; Berg, S.; Schulz, A.; Freyse, E.-J.; Vetter, R.; Salzsieder, E.; Kreutz, R.; Schimke, I. Aptamer BC007 for neutralization of pathogenic autoantibodies directed against G-protein coupled receptors: A vision of future treatment of patients with cardiomyopathies and positivity for those autoantibodies. Atherosclerosis 2015, 244, 44–47. [Google Scholar] [CrossRef]
- Cabral-Marques, O.; Halpert, G.; Schimke, L.F.; Ostrinski, Y.; Vojdani, A.; Baiocchi, G.C.; Freire, P.P.; Filgueiras, I.S.; Zyskind, I.; Lattin, M.T.; et al. Autoantibodies targeting GPCRs and RAS-related molecules associate with COVID-19 severity. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Hohberger, B.; Harrer, T.; Mardin, C.; Kruse, F.; Hoffmanns, J.; Rogge, L.; Heltmann, F.; Moritz, M.; Szewczykowski, C.; Schottenhamml, J.; et al. Case Report: Neutralization of Autoantibodies Targeting G-Protein-Coupled Receptors Improves Capillary Impairment and Fatigue Symptoms After COVID-19 Infection. Front. Med. 2021, 8, 2008. [Google Scholar] [CrossRef]
- Zhu, X.; Li, L.; Zou, L.; Zhu, X.; Xian, G.; Li, H.; Tan, Y.; Xie, L. A Novel Aptamer Targeting TGF-β Receptor II Inhibits Transdifferentiation of Human Tenon’s Fibroblasts into Myofibroblast. Investig. Opthalmol. Vis. Sci. 2012, 53, 6897–6903. [Google Scholar] [CrossRef]
- Khanna, D.; Denton, C.P.; Jahreis, A.; van Laar, J.M.; Frech, T.M.; Anderson, M.E.; Baron, M.; Chung, L.; Fierlbeck, G.; Lakshminarayanan, S.; et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): A phase 2, randomised, controlled trial. Lancet 2016, 387, 2630–2640. [Google Scholar] [CrossRef] [PubMed]
- Roofeh, D.; Lin, C.J.F.; Goldin, J.; Kim, G.H.; Furst, D.E.; Denton, C.P.; Huang, S.; Khanna, D.; the focuSSced Investigators. Tocilizumab Prevents Progression of Early Systemic Sclerosis–Associated Interstitial Lung Disease. Arthritis Rheumatol. 2021, 73, 1301–1310. [Google Scholar] [CrossRef]
- Denton, C.P.; Ong, V.H.; Xu, S.; Chen-Harris, H.; Modrusan, Z.; Lafyatis, R.; Khanna, D.; Jahreis, A.; Siegel, J.; Sornasse, T. Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: Insights from the faSScinate clinical trial in systemic sclerosis. Ann. Rheum. Dis. 2018, 77, 1362–1371. [Google Scholar] [CrossRef]
- Cardoneanu, A.; Burlui, A.M.; Macovei, L.A.; Bratoiu, I.; Richter, P.; Rezus, E. Targeting Systemic Sclerosis from Pathogenic Mechanisms to Clinical Manifestations: Why IL-6? Biomedicines 2022, 10, 318. [Google Scholar] [CrossRef]
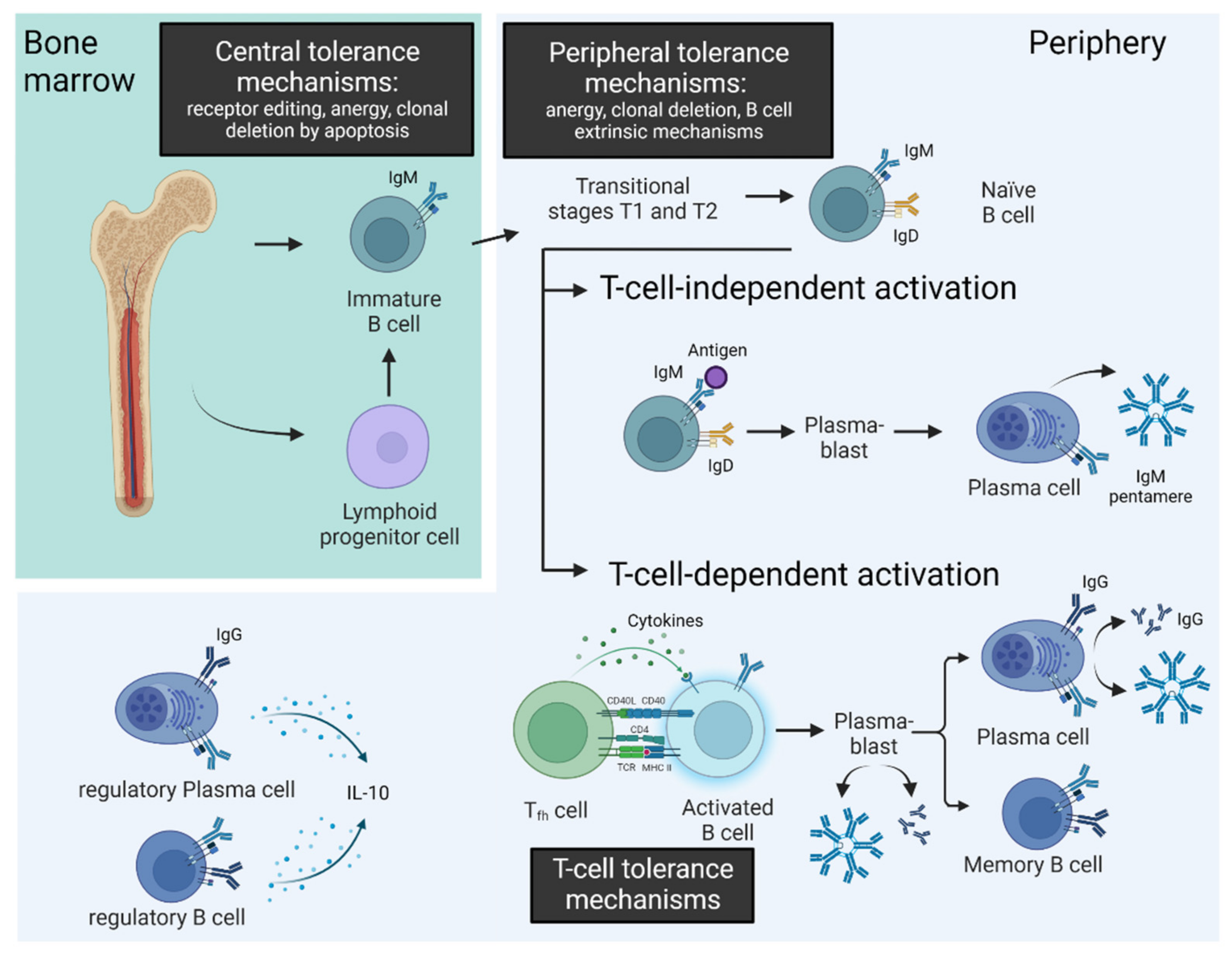
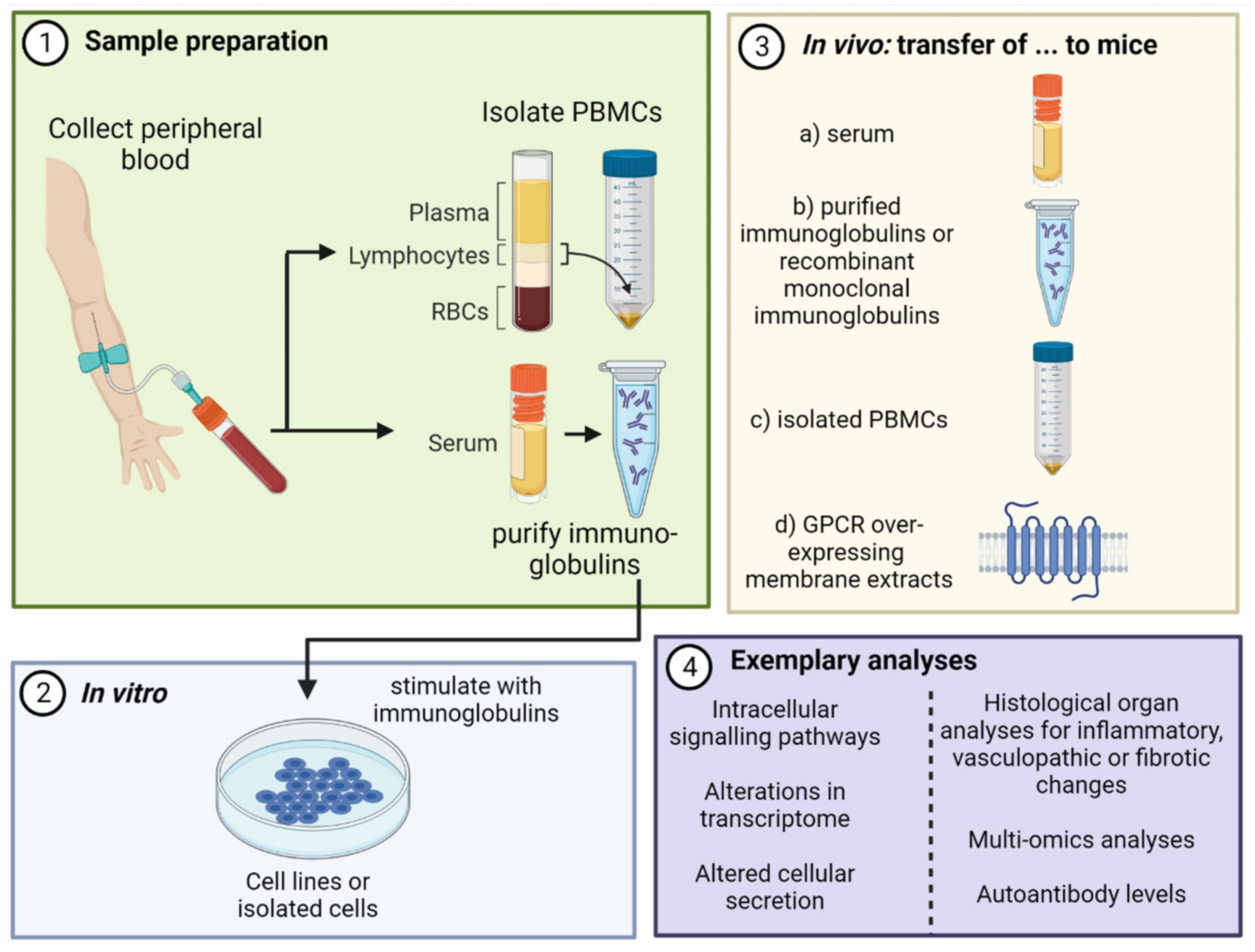
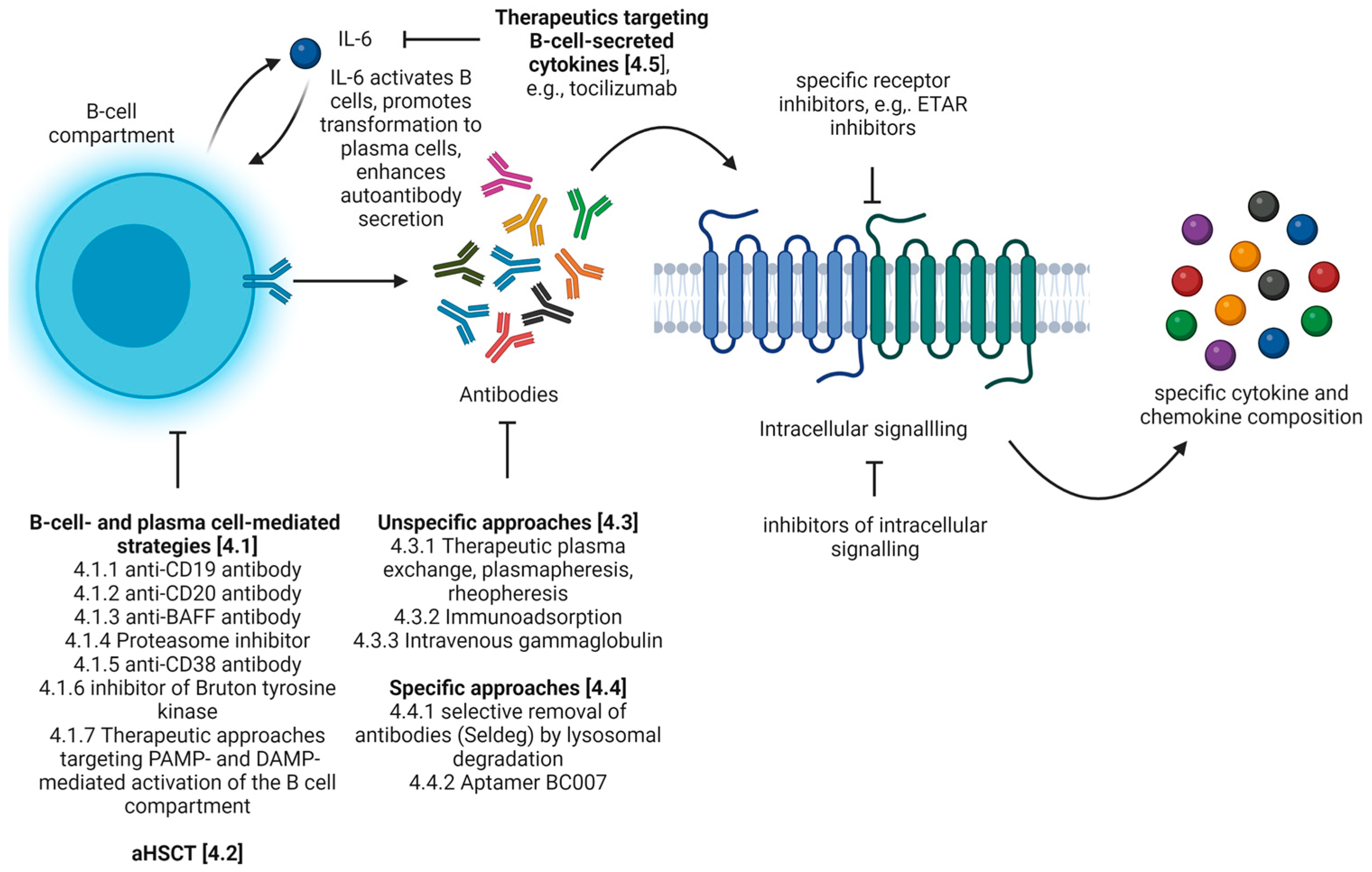
| Natural Autoantibodies | Pathogenic Autoantibodies | |
|---|---|---|
| Isotype | IgM, less frequently IgA or IgG | IgG, less frequently IgA or IgM |
| Generation of antibody diversity | Unmutated V(D)J recombination | V(D)J recombination, somatic hypermutation |
| Affinity | low | high |
| Mechanism of action | maintenance of immune homeostasis, amelioration of risk and severity of autoimmune diseases | contribution to autoimmune diseases via autoantibody-dependent and autoantibody-independent pathways |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graßhoff, H.; Fourlakis, K.; Comdühr, S.; Riemekasten, G. Autoantibodies as Biomarker and Therapeutic Target in Systemic Sclerosis. Biomedicines 2022, 10, 2150. https://doi.org/10.3390/biomedicines10092150
Graßhoff H, Fourlakis K, Comdühr S, Riemekasten G. Autoantibodies as Biomarker and Therapeutic Target in Systemic Sclerosis. Biomedicines. 2022; 10(9):2150. https://doi.org/10.3390/biomedicines10092150
Chicago/Turabian StyleGraßhoff, Hanna, Konstantinos Fourlakis, Sara Comdühr, and Gabriela Riemekasten. 2022. "Autoantibodies as Biomarker and Therapeutic Target in Systemic Sclerosis" Biomedicines 10, no. 9: 2150. https://doi.org/10.3390/biomedicines10092150
APA StyleGraßhoff, H., Fourlakis, K., Comdühr, S., & Riemekasten, G. (2022). Autoantibodies as Biomarker and Therapeutic Target in Systemic Sclerosis. Biomedicines, 10(9), 2150. https://doi.org/10.3390/biomedicines10092150





