Epithelial-to-Mesenchymal Transition in Metastasis: Focus on Laryngeal Carcinoma
Abstract
1. Introduction
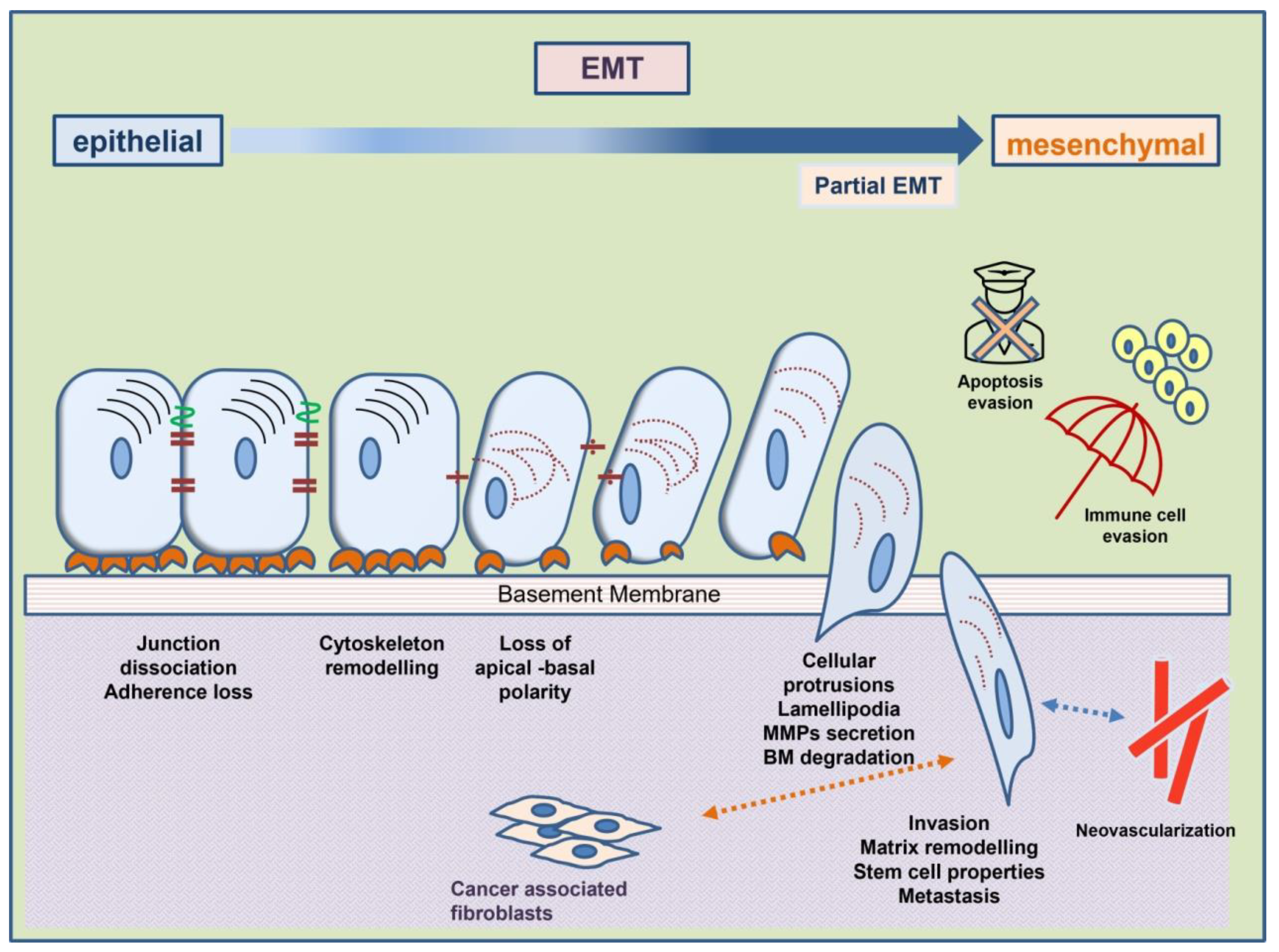
2. Epithelial-to-Mesenchymal Transition (EMT)
2.1. The Role of EMT Effectors in EMT Characteristics
2.1.1. Loss of Adhesion
2.1.2. Remodeling of the Cytoskeleton
2.1.3. Evasion of Apoptosis
2.1.4. Evasion of Immune Surveillance
2.1.5. Upregulation of Metalloproteinases (MMPs)
2.1.6. Neovascularization
2.1.7. Acquisition of Stem-Cell Traits
2.1.8. Altered Interaction of EMT Cells with Tumor Stroma
2.2. Inducers and Pathways of EMT
2.2.1. The Pathway of TGF-β
2.2.2. Molecular Pathway of Wnt
2.2.3. Molecular Pathways of Growth Factors
2.2.4. Signaling of EMT via Integrins
2.2.5. Epigenetic Regulation of EMT by microRNAs
2.2.6. Regulation of EMT by Inflammation and Hypoxia
3. Epithelial-to-Mesenchymal Transition—The Contemporary Approach
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nocini, R.; Molteni, G.; Mattiuzzi, C.; Lippi, G. Updates on larynx cancer epidemiology. Chin. J. Cancer Res. 2020, 32, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Suhail, Y.; Cain, M.P.; Vanaja, K.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R.; Kshitiz. Systems Biology of Cancer Metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Chopin, D. Epithelial cell plasticity in development and tumor progression. Cancer Metastasis Rev. 1999, 18, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Lambert, A.W.; Weinberg, R.A. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat. Rev. Cancer 2021, 21, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar] [CrossRef]
- Fan, X.; Jin, S.; Li, Y.; Khadaroo, P.A.; Dai, Y.; He, L.; Zhou, D.; Lin, H. Genetic and Epigenetic Regulation of E-Cadherin Signaling in Human Hepatocellular Carcinoma. Cancer Manag. Res. 2019, 11, 8947–8963. [Google Scholar] [CrossRef]
- Masterson, J.; O’Dea, S. Posttranslational truncation of E-cadherin and significance for tumour progression. Cells Tissues Organs 2007, 185, 175–179. [Google Scholar] [CrossRef]
- Cai, J.; Culley, M.K.; Zhao, Y.; Zhao, J. The role of ubiquitination and deubiquitination in the regulation of cell junctions. Protein Cell 2018, 9, 754–769. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Cappellesso, R.; Marioni, G.; Crescenzi, M.; Giacomelli, L.; Guzzardo, V.; Mussato, A.; Staffieri, A.; Martini, A.; Blandamura, S.; Fassina, A. The prognostic role of the epithelial-mesenchymal transition markers E-cadherin and Slug in laryngeal squamous cell carcinoma. Histopathology 2015, 67, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.J.; Song, P.P.; Zhou, H.; Shen, X.H.; Wang, J.G.; Ma, X.F.; Gu, Y.J.; Liu, D.D.; Feng, A.N.; Qian, X.Y.; et al. Role of epithelial-mesenchymal transition markers E-cadherin, N-cadherin, β-catenin and ZEB2 in laryngeal squamous cell carcinoma. Oncol. Lett. 2018, 15, 3472–3481. [Google Scholar] [CrossRef] [PubMed]
- Nardi, C.E.; Dedivitis, R.A.; Camillo de Almeida, R.; de Matos, L.L.; Cernea, C.R. The role of E-cadherin and β-catenin in laryngeal cancer. Oncotarget 2018, 9, 30199–30209. [Google Scholar] [CrossRef][Green Version]
- Yu, L.; Li, H.Z.; Lu, S.M.; Tian, J.J.; Ma, J.K.; Wang, H.B.; Xu, W. Downregulation of TWIST decreases migration and invasion of laryngeal carcinoma Hep-2 cells by regulating the E-cadherin, N-cadherin expression. J. Cancer Res. Clin. Oncol. 2011, 137, 1487–1493. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, D.; Yang, J.; Xue, K.; Liu, Y.; Jin, C. Knockdown of Snail inhibits epithelial-mesenchymal transition of human laryngeal squamous cell carcinoma Hep-2 cells through the vitamin D receptor signaling pathway. Biochem. Cell Biol. 2017, 95, 672–678. [Google Scholar] [CrossRef]
- Leggett, S.E.; Hruska, A.M.; Guo, M.; Wong, L.Y. The epithelial-mesenchymal transition and the cytoskeleton in bioengineered systems. Cell Commun. Signal. 2021, 19, 32. [Google Scholar] [CrossRef]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Research 2018, 7, F1000 Faculty Rev-1796. [Google Scholar] [CrossRef]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; The, M.T.; Waseem, A. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef]
- Strouhalova, K.; Přechová, M.; Gandalovičová, A.; Brábek, J.; Gregor, M.; Rosel, D. Vimentin Intermediate Filaments as Potential Target for Cancer Treatment. Cancers 2020, 12, 184. [Google Scholar] [CrossRef]
- Karamanou, K.; Franchi, M.; Vynios, D.; Brézillon, S. Epithelial-to-mesenchymal transition and invadopodia markers in breast cancer: Lumican a key regulator. Semin. Cancer Biol. 2020, 62, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Manuelli, V.; Cahill, F.; Wylie, H.; Gillett, C.; Correa, I.; Heck, S.; Rimmer, A.; Haire, A.; Van Hemelrijck, M.; Rudman, S.; et al. Invadopodia play a role in prostate cancer progression. BMC Cancer 2022, 22, 386. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, L.A.; Schaafsma, H.E.; Manni, J.J.; Ruiter, D.J.; Ramaekers, F.C.; Kuijpers, W. Cytokeratin and vimentin expression in normal epithelium and squamous cell carcinomas of the larynx. Eur. Arch. Otorhinolaryngol. 1997, 254, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Odero-Marah, V.; Hawsawi, O.; Henderson, V.; Sweeney, J. Epithelial-Mesenchymal Transition (EMT) and Prostate Cancer. Adv. Exp. Med. Biol. 2018, 1095, 101–110. [Google Scholar]
- Liu, S.; Zhou, F.; Shen, Y.; Zhang, Y.; Yin, H.; Zeng, Y.; Liu, J.; Yan, Z.; Liu, X. Fluid shear stress induces epithelial-mesenchymal transition (EMT) in Hep-2 cells. Oncotarget 2016, 7, 32876–32892. [Google Scholar] [CrossRef]
- Ali, A.; Soares, A.B.; Eymael, D.; Magalhaes, M. Expression of invadopodia markers can identify oral lesions with a high risk of malignant transformation. J. Pathol. Clin. Res. 2021, 7, 61–74. [Google Scholar] [CrossRef]
- Castillo Ferrer, C.; Berthenet, K.; Ichim, G.; Paoli, P.; Giannoni, E.; Chiarugi, P. Apoptosis-Fueling the oncogenic fire. FEBS J. 2021, 288, 4445–4463. [Google Scholar] [CrossRef]
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta. 2013, 1833, 3481–3498. [Google Scholar] [CrossRef]
- Cao, Z.; Livas, T.; Kyprianou, N. Anoikis and EMT: Lethal “Liaisons” during Cancer Progression. Crit. Rev. Oncog. 2016, 21, 155–168. [Google Scholar] [CrossRef]
- Alanko, J.; Mai, A.; Jacquemet, G.; Schauer, K.; Kaukonen, R.; Saari, M.; Goud, B.; Ivaska, J. Integrin endosomal signalling suppresses anoikis. Nat. Cell Biol. 2015, 17, 1412–1421. [Google Scholar] [CrossRef]
- Kilinc, A.N.; Han, S.; Barrett, L.A.; Anandasivam, N.; Nelson, C.M. Integrin-linked kinase tunes cell-cell and cell-matrix adhesions to regulate the switch between apoptosis and EMT downstream of TGFβ1. Mol. Biol. Cell 2021, 32, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Tsirtsaki, K.; Gkretsi, V. The focal adhesion protein Integrin-Linked Kinase (ILK) as an important player in breast cancer pathogenesis. Cell Adh. Migr. 2020, 14, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Song, T.; Li, C.; Mao, W. GSK-3β in DNA repair, apoptosis, and resistance of chemotherapy, radiotherapy of cancer. Biochim. Acta Mol. Cell Res. 2020, 1867, 118659. [Google Scholar] [CrossRef] [PubMed]
- Gundamaraju, R.; Lu, W.; Paul, M.K.; Jha, N.K.; Gupta, P.K.; Ojha, S.; Chattopadhyay, I.; Rao, P.V.; Ghavami, S. Autophagy and EMT in cancer and metastasis: Who controls whom? Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166431. [Google Scholar] [CrossRef] [PubMed]
- Babaei, G.; Aziz, S.G.; Jaghi, N.Z.Z. EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed.Pharm. 2021, 133, 110909. [Google Scholar] [CrossRef]
- Gugnoni, M.; Sancisi, V.; Manzotti, G.; Gandolfi, G.; Ciarrocchi, A. Autophagy and epithelial-mesenchymal transition: An intricate interplay in cancer. Cell Death Dis. 2016, 7, e2520. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X. Role of Focal Adhesion Kinase in Head and Neck Squamous Cell Carcinoma and Its Therapeutic Prospect. OncoTargets Ther. 2020, 13, 10207–10220. [Google Scholar] [CrossRef]
- Chrysovergis, A.; Papanikolaou, V.; Tsiambas, E.; Kikidis, D.; Maragoudakis, P.; Ragos, V.; Kyrodimos, E. Caspase complex in laryngeal squamous cell carcinoma. J. BUON 2019, 24, 1–4. [Google Scholar]
- Han, B.B.; Li, S.; Tong, M.; Holpuch, A.S.; Spinney, R.; Wang, D.; Border, M.B.; Liu, Z.; Sarode, S.; Pei, P.; et al. Fenretinide Perturbs Focal Adhesion Kinase in Premalignant and Malignant Human Oral Keratinocytes. Fenretinide’sChemopreventive Mechanisms Include ECM Interactions. Cancer Prev. Res. 2015, 8, 419–430. [Google Scholar] [CrossRef]
- Xu, Y.T.; Chen, R.Q.; Lin, G.B.; Fang, X.L.; Yu, S.J.; Liang, X.H.; Zhang, R. Defining the regulatory role of programmed cell death 4 in laryngeal squamous cell carcinoma. Biochem. Cell Biol. 2018, 96, 522–538. [Google Scholar] [CrossRef]
- Larson, C.; Oronsky, B.; Carter, C.A.; Oronsky, A.; Knox, S.J.; Sher, D.; Reid, T.R. TGF-beta: A master immune regulator. Expert Opin. Ther. Target 2020, 24, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Liu, M.; Kuo, F.; Capistrano, K.J.; Kang, D.; Nixon, B.G.; Shi, W.; Chou, C.; Do, M.H.; Stamatiades, E.G.; Gao, S. TGF-β suppresses type 2 immunity to cancer. Nature 2020, 587, 115–120. [Google Scholar] [CrossRef]
- Plaschka, M.; Benboubker, V.; Grimont, M.; Berthet, J.; Tonon, L.; Lopez, J.; Le-Bouar, M.; Balme, B.; Tondeur, G.; de la Fouchardiere, A. ZEB1 transcription factor promotes immune escape in melanoma. J. Immunother. Cancer 2022, 10, e003484. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Tang, Y.L.; Liang, X.H. Transforming growth factor-β signaling in head and neck squamous cell carcinoma: Insights into cellular responses. Oncol. Lett. 2018, 16, 4799–4806. [Google Scholar] [CrossRef] [PubMed]
- Di Gioacchino, M.; Della Valle, L.; Allegra, A.; Pioggia, G.; Gangemi, S. AllergoOncology: Role of immune cells and immune proteins. Clin. Transl. Allergy 2022, 12, e12133. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.B.; Patel, S.; Matossian, M.D.; Ucar, D.A.; Miele, L.; Burow, M.E.; Flaherty, P.T.; Cavanaugh, J.E. Molecular Mechanisms of Epithelial to Mesenchymal Transition Regulated by ERK5 Signaling. Biomolecules 2021, 11, 183. [Google Scholar] [CrossRef]
- Liu, J.F.; Crépin, M.; Liu, J.M.; Barritault, D.; Ledoux, D. FGF-2 and TPA induce matrix metalloproteinase-9 secretion in MCF-7 cells through PKC activation of the Ras/ERK pathway. Biochem. Biophys. Res. Commun. 2002, 293, 1174–1182. [Google Scholar] [CrossRef]
- Zuo, J.H.; Zhu, W.; Li, M.Y.; Li, X.H.; Yi, H.; Zeng, G.Q.; Wan, X.X.; He, Q.Y.; Li, J.H.; Qu, J.Q.; et al. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin. J. Cell. Biochem. 2011, 112, 2508–2517. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; García-Hernández, A.A.; Ramos, C. Matrix Metalloproteinases’ Role in Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1245, 97–131. [Google Scholar]
- Turunen, S.P.; Tatti-Bugaeva, O.; Lehti, K. Membrane-type matrix metalloproteases as diverse effectors of cancer progression. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1974–1988. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Liu, J.; Han, X.; Zhang, X.; Lin, T.; Wang, Y.; Bai, J.; Han, J. FBXO22 Suppresses Metastasis in Human Renal Cell Carcinoma via Inhibiting MMP-9-Mediated Migration and Invasion and VEGF-Mediated Angiogenesis. Int. J. Biol. Sci. 2019, 15, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Heinz, A. Elastases and elastokines: Elastin degradation and its significance in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273. [Google Scholar] [CrossRef]
- Christopoulos, T.A.; Papageorgakopoulou, N.; Ravazoula, P.; Mastronikolis, N.S.; Papadas, T.A.; Theocharis, D.A.; Vynios, D.H. Expression of metalloproteinases and their tissue inhibitors in squamous cell laryngeal carcinoma. Oncol. Rep. 2007, 18, 855–860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Danilewicz, M.; Sikorska, B.; Wagrowska-Danilewicz, M. Prognostic significance of the immunoexpression of matrix metalloproteinase MMP2 and its inhibitor TIMP2 in laryngeal cancer. Med. Sci. Monit. 2003, 9, 42–47. [Google Scholar]
- Wittekindt, C.; Jovanovic, N.; Guntinas-Lichius, O. Expression of matrix metalloproteinase-9 (MMP-9) and blood vessel density in laryngeal squamous cell carcinomas. Acta Otolaryngol. 2011, 131, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.Q.; Du, W.L.; Cai, M.H.; Yao, J.Y.; Zhao, Y.Y.; Mou, X.Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef]
- Gyftopoulos, K.; Vourda, K.; Sakellaropoulos, G.; Perimenis, P.; Athanasopoulos, A.; Papadaki, E. The angiogenic switch for vascular endothelial growth factor-A and cyclooxygenase-2 in prostate carcinoma: Correlation with microvessel density, androgen receptor content and Gleason grade. Urol. Int. 2011, 87, 464–469. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, L.; Lai, W.; Zeng, Y.; Xu, H.; Lan, Q.; Su, P.; Chu, Z. Interaction with tumor associated macrophages promotes PRL 3 induced invasion of colorectal cancer cells via MAPK pathway induced EMT and NF κB signaling induced angiogenesis. Oncol. Rep. 2019, 41, 2790–2802. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; He, Y.; Yao, B.; Zhang, Y. Regulation of matrix metalloproteinases 2 and 9 in corneal neovascularization. Chem. Biol. Drug Des. 2020, 95, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Stacker, S.A.; Achen, M.G. Emerging Roles for VEGF-D in Human Disease. Biomolecules 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Xu, C.; Li, X.; Yang, X. Twist1 promotes astrocytoma development by stimulating vasculogenic mimicry. Oncol. Lett. 2019, 18, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, J.; Zhao, W.; Peng, Z.; Liu, X.; Li, B.; Zhang, H.; Shan, B.; Zhang, C.; Duan, C. Vasculogenic mimicry in carcinogenesis and clinical applications. J.Hematol. Oncol. 2020, 13, 19. [Google Scholar] [CrossRef]
- Franz, L.; Nicolè, L.; Frigo, A.C.; Ottaviano, G.; Gaudioso, P.; Saccardo, T.; Visconti, F.; Cappellesso, R.; Blandamura, S.; Fassina, A.; et al. Epithelial-to-Mesenchymal Transition and Neoangiogenesis in Laryngeal Squamous Cell Carcinoma. Cancers 2021, 13, 3339. [Google Scholar] [CrossRef]
- Lin, P.; Wang, W.; Sun, B.C.; Cai, W.J.; Li, L.; Lu, H.H.; Han, C.R.; Zhang, J.M. Vasculogenic mimicry is a key prognostic factor for laryngeal squamous cell carcinoma: A new pattern of blood supply. Chin. Med. J. 2012, 125, 3445–3449. [Google Scholar]
- BolzoniVillaret, A.; Barbieri, D.; Peretti, G.; Schreiber, A.; Fisogni, S.; Lonardi, S.; Facchetti, F.; Nicolai, P. Angiogenesis and lymphangiogenesis in early-stage laryngeal carcinoma: Prognostic implications. Head Neck 2013, 35, 1132–1137. [Google Scholar] [CrossRef]
- Oshimori, N. Cancer stem cells and their niche in the progression of squamous cell carcinoma. Cancer Sci. 2020, 111, 3985–3992. [Google Scholar] [CrossRef]
- Karatas, O.F.; Suer, I.; Yuceturk, B.; Yilmaz, M.; Hajiyev, Y.; Creighton, C.J.; Ittmann, M.; Ozen, M. The role of miR-145 in stem cell characteristics of human laryngeal squamous cell carcinoma Hep-2 cells. Tumour Biol. 2016, 37, 4183–4192. [Google Scholar] [CrossRef]
- Szafarowski, T.; Sierdziński, J.; Ludwig, N.; Głuszko, A.; Filipowska, A.; Szczepański, M.J. Assessment of cancer stem cell marker expression in primary head and neck squamous cell carcinoma shows prognostic value for aldehyde dehydrogenase (ALDH1A1). Eur. J. Pharm. 2020, 867, 172837. [Google Scholar] [CrossRef]
- Elkashty, O.A.; Abu Elghanam, G.; Su, X.; Liu, Y.; Chauvin, P.J.; Tran, S.D. Cancer stem cells enrichment with surface markers CD271 and CD44 in human head and neck squamous cell carcinomas. Carcinogenesis 2020, 41, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Joshua, B.; Kaplan, M.J.; Doweck, I.; Pai, R.; Weissman, I.L.; Prince, M.E.; Ailles, L.E. Frequency of cells expressing CD44, a head and neck cancer stem cell marker: Correlation with tumor aggressiveness. Head Neck 2012, 34, 42–49. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Catalano, V.; Turdo, A.; Di Franco, S.; Dieli, F.; Todaro, M.; Stassi, G. Tumor and its microenvironment: A synergistic interplay. Semin. Cancer Biol. 2013, 23, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Caja, L.; Dituri, F.; Mancarella, S.; Caballero-Diaz, D.; Moustakas, A.; Giannelli, G.; Fabregat, I. TGF-β and the Tissue Microenvironment: Relevance in Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 1294. [Google Scholar] [CrossRef]
- Asif, P.J.; Longobardi, C.; Hahne, M.; Medema, J.P. The Role of Cancer-Associated Fibroblasts in Cancer Invasion and Metastasis. Cancers 2021, 13, 4720. [Google Scholar] [CrossRef]
- Akhtar, M.; Haider, A.; Rashid, S.; Al-Nabet, A.D.M.H. Paget’s “Seed and Soil” Theory of Cancer Metastasis: An Idea Whose Time has Come. Adv. Anat. Pathol. 2019, 26, 69–74. [Google Scholar] [CrossRef]
- Custódio, M.; Biddle, A.; Tavassoli, M. Portrait of a CAF: The story of cancer-associated fibroblasts in head and neck cancer. Oral Oncol. 2020, 110, 104972. [Google Scholar] [CrossRef]
- OrgenCalli, A.; Dere, Y.; Sari, A.; Dirilenoglu, F.; Onur, I.; İmre, K. Evaluation of Stromal Myofibroblasts in Laryngeal Dysplasia and Invasive Squamous Cell Carcinoma. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 233–238. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, J.; Zheng, J.; Hsueh, C.; Guo, Y.; Zhou, L. Characterization of selective exosomal microRNA expression profile derived from laryngeal squamous cell carcinoma detected by next generation sequencing. Oncol. Rep. 2018, 40, 2584–2594. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, M.; Huang, Q.; Guo, Y.; Gong, H.; Hu, C.; Zhou, L. Aberrant expression profiles and bioinformatic analysis of CAF-derived exosomal miRNAs from three moderately differentiated supraglottic LSCC patients. J. Clin. Lab. Anal. 2022, 36, e24108. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, X.; Guo, H.; Xue, X.; Zhang, Y.; Niu, M.; Cui, J.; Liu, H.; Luo, H.; Yang, D.; et al. Serum Exosomal miR-941 as a promising Oncogenic Biomarker for Laryngeal Squamous Cell Carcinoma. J. Cancer 2020, 11, 5329–5344. [Google Scholar] [CrossRef]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Sader, F.; Denis, J.F.; Laref, H.; Roy, S. Epithelial to mesenchymal transition is mediated by both TGF-β canonical and non-canonical signaling during axolotl limb regeneration. Sci. Rep. 2019, 9, 1144. [Google Scholar] [CrossRef]
- Hagedorn, H.; Sauer, U.; Schleicher, E.; Nerlich, A. Expression of TGF-beta 1 protein and mRNA and the effect on the tissue remodeling in laryngeal carcinomas. Anticancer Res. 1999, 19, 4265–4272. [Google Scholar]
- Zheng, L.; Guan, Z.; Xue, M. TGF-β Signaling Pathway-Based Model to Predict the Subtype and Prognosis of Head and Neck Squamous Cell Carcinoma. Front. Genet. 2022, 13, 862860. [Google Scholar] [CrossRef]
- Franchi, A.; Gallo, O.; Sardi, I.; Santucci, M. Downregulation of transforming growth factor beta type II receptor in laryngeal carcinogenesis. J. Clin. Pathol. 2001, 54, 201–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Castellone, M.D.; Laukkanen, M.O. TGF-beta1, WNT, and SHH signaling in tumor progression and in fibrotic diseases. Front. Biosci. 2017, 9, 31–45. [Google Scholar]
- Patel, S.; Alam, A.; Pant, R.; Chattopadhyay, S. Wnt Signaling and Its Significance Within the Tumor Microenvironment: Novel Therapeutic Insights. Front. Immunol. 2019, 10, 2872. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Gong, C.; Yuan, K. LncRNA UCA1 promotes cell proliferation, invasion and migration of laryngeal squamous cell carcinoma cells by activating Wnt/β-catenin signaling pathway. Exp. Ther. Med. 2019, 17, 1182–1189. [Google Scholar] [CrossRef]
- Zhang, F.; Mao, D.; He, Z.; Li, W.; Zhang, X.; Li, L. SLCO4A1-AS1 regulates laryngeal squamous cell carcinoma cell phenotypes via the Wnt pathway. Oral Dis. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Cui, X.; Fang, N.; Cui, Y.; Xiao, D.; Wang, X. Long non-coding RNA NEF inhibits proliferation and promotes apoptosis of laryngeal squamous cell carcinoma cells by inhibiting Wnt/β-catenin signaling. Oncol. Lett. 2019, 17, 4928–4934. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Cheng, H.; Liu, S.; Hao, X.; Lan, L.; Wu, G.; Liu, M.; Zhao, Y. FOXP4 promotes laryngeal squamous cell carcinoma progression through directly targeting LEF 1. Mol. Med. Rep. 2021, 24, 831. [Google Scholar] [CrossRef]
- Tang, X.; Sun, Y.; Wan, G.; Sun, J.; Sun, J.; Pan, C. Knockdown of YAP inhibits growth in Hep-2 laryngeal cancer cells via epithelial-mesenchymal transition and the Wnt/β-catenin pathway. BMC Cancer 2019, 19, 654. [Google Scholar] [CrossRef]
- Psyrri, A.; Kotoula, V.; Fountzilas, E.; Alexopoulou, Z.; Bobos, M.; Televantou, D.; Karayannopoulou, G.; Krikelis, D.; Markou, K.; Karasmanis, I.; et al. Prognostic significance of the Wnt pathway in squamous cell laryngeal cancer. Oral Oncol. 2014, 50, 298–305. [Google Scholar] [CrossRef]
- Ghosh, S.; Marrocco, I.; Yarden, Y. Roles for receptor tyrosine kinases in tumor progression and implications for cancer treatment. Adv. Cancer Res. 2020, 147, 1–57. [Google Scholar]
- Tripathi, K.; Garg, M. Mechanistic regulation of epithelial-to-mesenchymal transition through RAS signaling pathway and therapeutic implications in human cancer. J. Cell Commun. Signal. 2018, 12, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Gosens, R.; Wieland, T.; Schmidt, M. Paving the Rho in cancer metastasis: Rho GTPases and beyond. Pharmacol. Ther. 2018, 183, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M. Src signaling in cancer invasion. J. Cell. Physiol. 2010, 223, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yun, F.; Shi, L.; Li, Z.H.; Luo, N.R.; Jia, Y.F. Roles of Signaling Pathways in the Epithelial-Mesenchymal Transition in Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 6201–6206. [Google Scholar] [CrossRef]
- Song, Y.; Dong, Y.D.; Bai, W.L.; Ma, X.L. Silencing of Src by siRNA inhibits laryngeal carcinoma growth through the Src/PI-3 K/Akt pathway in vitro and in vivo. Tumour Biol. 2014, 35, 9009–9014. [Google Scholar] [CrossRef]
- Dong, L.B.; Li, G.Q.; Tian, Z.H.; Wang, Z.M.; Xu, K. Expressions of Src homology 2 domain-containing phosphatase and its clinical significance in laryngeal carcinoma. Genet. Mol. Res. 2013, 12, 4207–4212. [Google Scholar] [CrossRef]
- Porcheri, C.; Meisel, C.T.; Mitsiadis, T. Multifactorial Contribution of Notch Signaling in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 1520. [Google Scholar] [CrossRef]
- Zou, Y.; Fang, F.; Ding, Y.J.; Dai, M.Y.; Yi, X.; Chen, C.; Tao, Z.Z.; Chen, S.M. Notch 2 signaling contributes to cell growth, anti-apoptosis and metastasis in laryngeal squamous cell carcinoma. Mol. Med. Rep. 2016, 14, 3517–3524. [Google Scholar] [CrossRef]
- Dai, M.Y.; Fang, F.; Zou, Y.; Yi, X.; Ding, Y.J.; Chen, C.; Tao, Z.Z.; Chen, S.M. Downregulation of Notch1 induces apoptosis and inhibits cell proliferation and metastasis in laryngeal squamous cell carcinoma. Oncol. Rep. 2015, 34, 3111–3119. [Google Scholar] [CrossRef]
- Papanikolaou, V.; Chrysovergis, A.; Mastronikolis, S.; Tsiambas, E.; Ragos, V.; Peschos, D.; Spyropoulou, D.; Pantos, P.; Niotis, A.; Mastronikolis, N.; et al. Impact of K-Ras Over-expression in Laryngeal Squamous Cell Carcinoma. In Vivo 2021, 35, 1611–1615. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, D.; Liu, M.; Sun, Y.; Tian, L. Downregulation of miR-21 modulates Ras expression to promote apoptosis and suppress invasion of Laryngeal squamous cell carcinoma. Eur. J. Cancer 2010, 46, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Legerstee, K.; Houtsmuller, A.B. A Layered View on Focal Adhesions. Biology 2021, 10, 1189. [Google Scholar] [CrossRef] [PubMed]
- Shams, H.; Hoffman, B.D.; Mofrad, M.R.K. The “Stressful” Life of Cell Adhesion Molecules: On the Mechanosensitivity of Integrin Adhesome. J. Biomech. Eng. 2018, 140, 1–7. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Li, M.; Wu, X.; Setrerrahmane, S.; Xu, H. Integrins as attractive targets for cancer therapeutics. Acta Pharm. Sin. B 2021, 11, 2726–2737. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Kan, X.; Li, Y.; Liu, M.; Lu, J.G. Elevated expression of integrin αv and β5 subunit in laryngeal squamous-cell carcinoma associated with lymphatic metastasis and angiogenesis. Pathol. Res. Pract. 2013, 209, 105–109. [Google Scholar] [CrossRef]
- Dong, X.; Luo, Z.; Liu, T.; Chai, J.; Ke, Q.; Shen, L. Identification of Integrin β1 as a Novel PAG1-Interacting Protein Involved in the Inherent Radioresistance of Human Laryngeal Carcinoma. J. Cancer 2018, 9, 4128–4138. [Google Scholar] [CrossRef]
- Lu, J.G.; Li, Y.; Li, L.; Kan, X. Overexpression of osteopontin and integrin αv in laryngeal and hypopharyngeal carcinomas associated with differentiation and metastasis. J. Cancer Res. Clin. Oncol. 2011, 137, 1613–1618. [Google Scholar] [CrossRef]
- Lu, J.G.; Sun, Y.N.; Wang, C.; Jin, D.J.; Liu, M. Role of the alpha v-integrin subunit in cell proliferation, apoptosis and tumor metastasis of laryngeal and hypopharyngeal squamous cell carcinomas: A clinical and in vitro investigation. Eur. Arch. Otorhinolaryngol. 2009, 266, 89–96. [Google Scholar] [CrossRef]
- Vitolo, D.; Ciocci, L.; Ferrauti, P.; Cicerone, E.; Gallo, A.; De Vincentiis, M.; Baroni, C.D. alpha5 integrin distribution and TGFbeta1 gene expression in supraglottic carcinoma: Their role in neoplastic local invasion and metastasis. Head Neck 2000, 22, 48–56. [Google Scholar] [CrossRef]
- Pan, G.; Liu, Y.; Shang, L.; Zhou, F.; Yang, S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun. 2021, 41, 199–217. [Google Scholar] [CrossRef] [PubMed]
- MusaviShenas, M.H.; Eghbal-Fard, S.; Mehrisofiani, V.; Abd Yazdani, N.; Rahbar Farzam, O.; Marofi, F.; Yousefi, M. MicroRNAs and signaling networks involved in epithelial-mesenchymal transition. J. Cell Physiol. 2019, 234, 5775–5785. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef]
- Hill, L.; Browne, G.; Tulchinsky, E. ZEB/miR-200 feedback loop: At the crossroads of signal transduction in cancer. Int. J. Cancer 2013, 132, 745–754. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bracken, C.P.; Smith, E.; Bert, A.G.; Wright, J.A.; Roslan, S.; Morris, M.; Wyatt, L.; Farshid, G.; Lim, Y.-Y.; et al. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol. Biol. Cell 2011, 22, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.Y.; Yu, C.C.; Liao, Y.W.; Hsieh, P.L.; Ohiro, Y.; Chu, P.M.; Huang, Y.C.; Yu, C.H.; Tsai, L.L. miR-10b regulated by Twist maintains myofibroblasts activities in oral submucous fibrosis. J. Formos. Med. Assoc. 2020, 119, 1167–1173. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.; Wong, G.; Earle, C.; Krueger, K.; Spevak, C.C. Hyaluronan-CD44 interaction promotes c-Src-mediated twist signaling, microRNA-10b expression, and RhoA/RhoC upregulation, leading to Rho-kinase-associated cytoskeleton activation and breast tumor cell invasion. J. Biol. Chem. 2010, 285, 36721–36735. [Google Scholar] [CrossRef]
- Wu, C.; Peng, S.; Sun, W.; Luo, M.; Su, B.; Liu, D.; Hu, G. Association of E-cadherin methylation with risk of nasopharyngeal cancer: A meta-analysis. Head Neck 2018, 40, 2538–2545. [Google Scholar] [CrossRef]
- Yang, C.X.; Sedhom, W.; Song, J.; Lu, S.L. The Role of MicroRNAs in Recurrence and Metastasis of Head and Neck Squamous Cell Carcinoma. Cancers 2019, 11, 395. [Google Scholar] [CrossRef]
- Chen, L.; Sun, D.Z.; Fu, Y.G.; Yang, P.Z.; Lv, H.Q.; Gao, Y.; Zhang, X.Y. Upregulation of microRNA-141 suppresses epithelial-mesenchymal transition and lymph node metastasis in laryngeal cancer through HOXC6-dependent TGF-β signaling pathway. Cell Signal. 2020, 66, 109444. [Google Scholar] [CrossRef]
- Yang, B.; Zang, J.; Yuan, W.; Jiang, X.; Zhang, F. The miR-136-5p/ROCK1 axis suppresses invasion and migration, and enhances cisplatin sensitivity in head and neck cancer cells. Exp. Ther. Med. 2021, 21, 317. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, J.; Xia, X.; Ye, B. Long non-coding RNA OIP5-AS1 serves as an oncogene in laryngeal squamous cell carcinoma by regulating miR-204-5p/ZEB1 axis. Naunyn-Schmiedebergs Arch. Pharm. 2020, 393, 2177–2184. [Google Scholar] [CrossRef]
- Zhang, F.; Cao, H. MicroRNA 143 3p suppresses cell growth and invasion in laryngeal squamous cell carcinoma via targeting the k Ras/Raf/MEK/ERK signaling pathway. Int. J. Oncol. 2019, 54, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, C.; Li, W.; Li, H.; Sang, J.; Zhao, Q.; Bo, Y.; Luo, H.; Zheng, X.; Lu, Y.; et al. Promoter Methylation-Regulated miR-145-5p Inhibits Laryngeal Squamous Cell Carcinoma Progression by Targeting FSCN1. Mol. Ther. 2019, 27, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Lu, Z.M.; Lin, Y.F.; Chen, L.S.; Luo, X.N.; Song, X.H.; Chen, S.H.; Wu, Y.L. miR-144-3p, a tumor suppressive microRNA targeting ETS-1 in laryngeal squamous cell carcinoma. Oncotarget 2016, 7, 11637–11650. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Y.; Zhang, C.; Qi, M.; Yao, M.; Sun, L.; Xu, X. MicroRNA-195-5p suppresses the proliferation, migration, invasion and epithelial-mesenchymal transition of laryngeal cancer cells in vitro by targeting E2F3. Exp. Ther. Med. 2021, 22, 1078. [Google Scholar] [CrossRef]
- Li, Y.; Tao, C.; Dai, L.; Cui, C.; Chen, C.; Wu, H.; Wei, Q.; Zhou, X. MicroRNA-625 inhibits cell invasion and epithelial-mesenchymal transition by targeting SOX4 in laryngeal squamous cell carcinoma. Biosci. Rep. 2019, 39, BSR20181882. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, C.; Chen, D.; Chen, S.; Zheng, H. MicroRNA-98-HMGA2-POSTN signal pathway reverses epithelial-to-mesenchymal transition in laryngeal squamous cell carcinoma. Biomed. Pharm. 2019, 117, 108998. [Google Scholar] [CrossRef]
- Tian, L.; Li, M.; Ge, J.; Guo, Y.; Sun, Y.; Liu, M.; Xiao, H. MiR-203 is downregulated in laryngeal squamous cell carcinoma and can suppress proliferation and induce apoptosis of tumours. Tumour Biol. 2014, 35, 5953–5963. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, J.; Wang, B.; Ren, J.C.; Su, W.; Zhang, T. MicroRNA-10b Triggers the Epithelial-Mesenchymal Transition (EMT) of Laryngeal Carcinoma Hep-2 Cells by Directly Targeting the E-cadherin. Appl. Biochem. Biotechnol. 2015, 176, 33–44. [Google Scholar] [CrossRef]
- Wang, B.; Lv, K.; Chen, W.; Zhao, J.; Luo, J.; Wu, J.; Li, Z.; Qin, H.; Wong, T.S.; Yang, W.; et al. miR-375 and miR-205 Regulate the Invasion and Migration of Laryngeal Squamous Cell Carcinoma Synergistically via AKT-Mediated EMT. Biomed. Res. Int. 2016, 2016, 9652789. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. MiRNA-21-5p Accelerates EMT and Inhibits Apoptosis of Laryngeal Carcinoma via Inhibiting KLF6 Expression. Biochem. Genet. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, W.; Zhang, C.; Wen, S.; Huangfu, H.; Kang, J.; Wang, B. Hsa-miR-301a-3p Acts as an Oncogene in Laryngeal Squamous Cell Carcinoma via Target Regulation of Smad4. J. Cancer 2015, 6, 1260–1275. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, J.; Karlsson, M.C. TGF-β-induced epithelial-mesenchymal transition: A link between cancer and inflammation. Semin. Cancer Biol. 2012, 22, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Neil, J.R.; Johnson, K.M.; Nemenoff, R.A.; Schiemann, W.P. Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-beta through a PGE2-dependent mechanisms. Carcinogenesis 2008, 29, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.J.; Wang, L.; Mo, T.T.; Wang, J.; Wang, M.G.; Li, X.P. Pepsin promotes IL-8 signaling-induced epithelial-mesenchymal transition in laryngeal carcinoma. Cancer Cell Int. 2019, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Ambati, R.; Gundamaraju, R. Exploring the Crosstalk between Inflammation and Epithelial-Mesenchymal Transition in Cancer. Mediat. Inflamm. 2021, 2021, 9918379. [Google Scholar] [CrossRef]
- Pezzuto, A.; Carico, E. Role of HIF-1 in Cancer Progression: Novel Insights. A Review. Curr. Mol. Med. 2018, 18, 343–351. [Google Scholar] [CrossRef]
- Yang, M.H.; Wu, K.J. TWIST activation by hypoxia inducible factor-1 (HIF-1): Implications in metastasis and development. Cell Cycle 2008, 7, 2090–2096. [Google Scholar] [CrossRef]
- Zuo, J.; Wen, J.; Lei, M.; Wen, M.; Li, S.; Lv, X.; Luo, Z.; Wen, G. Hypoxia promotes the invasion and metastasis of laryngeal cancer cells via EMT. Med. Oncol. 2016, 33, 15. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Zhou, S.H.; Lu, Z.J.; Fan, J.; Huang, Y.P. Inhibiting GLUT-1 expression and PI3K/Akt signaling using apigenin improves the radiosensitivity of laryngeal carcinoma in vivo. Oncol. Rep. 2015, 34, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhou, M.L.; Fan, J. Inhibition of GLUT-1 expression and the PI3K/Akt pathway to enhance the chemosensitivity of laryngeal carcinoma cells in vitro. OncoTargets Ther. 2018, 11, 7865–7872. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Lu, Y.F.; Hu, X.D.; Mao, J.Y.; Ji, X.X.; Yao, H.T.; Zhou, S.H. Expression of hypoxia inducible factor-1α and its significance in laryngeal carcinoma. J. Int. Med. Res. 2010, 38, 2040–2046. [Google Scholar] [CrossRef]
- Li, D.W.; Zhou, L.; Jin, B.; Xie, J.; Dong, P. Expression and significance of hypoxia-inducible factor-1α and survivin in laryngeal carcinoma tissue and cells. Otolaryngol. Head Neck Surg. 2013, 148, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhu, X.; Cui, K.; Mancuso, J.; Federley, R.; Fischer, K.; Teng, G.; Mittal, V.; Gao, D.; Zhao, H.; et al. In Vivo Visualization and Characterization of Epithelial-Mesenchymal Transition in Breast Tumors. Cancer Res. 2016, 76, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- Janani, G.; Pillai, M.M.; Selvakumar, R.; Bhattacharyya, A.; Sabarinath, C. An in vitro 3D model using collagen coated gelatin nanofibers for studying breast cancer metastasis. Biofabrication 2017, 9, 015016. [Google Scholar] [CrossRef] [PubMed]
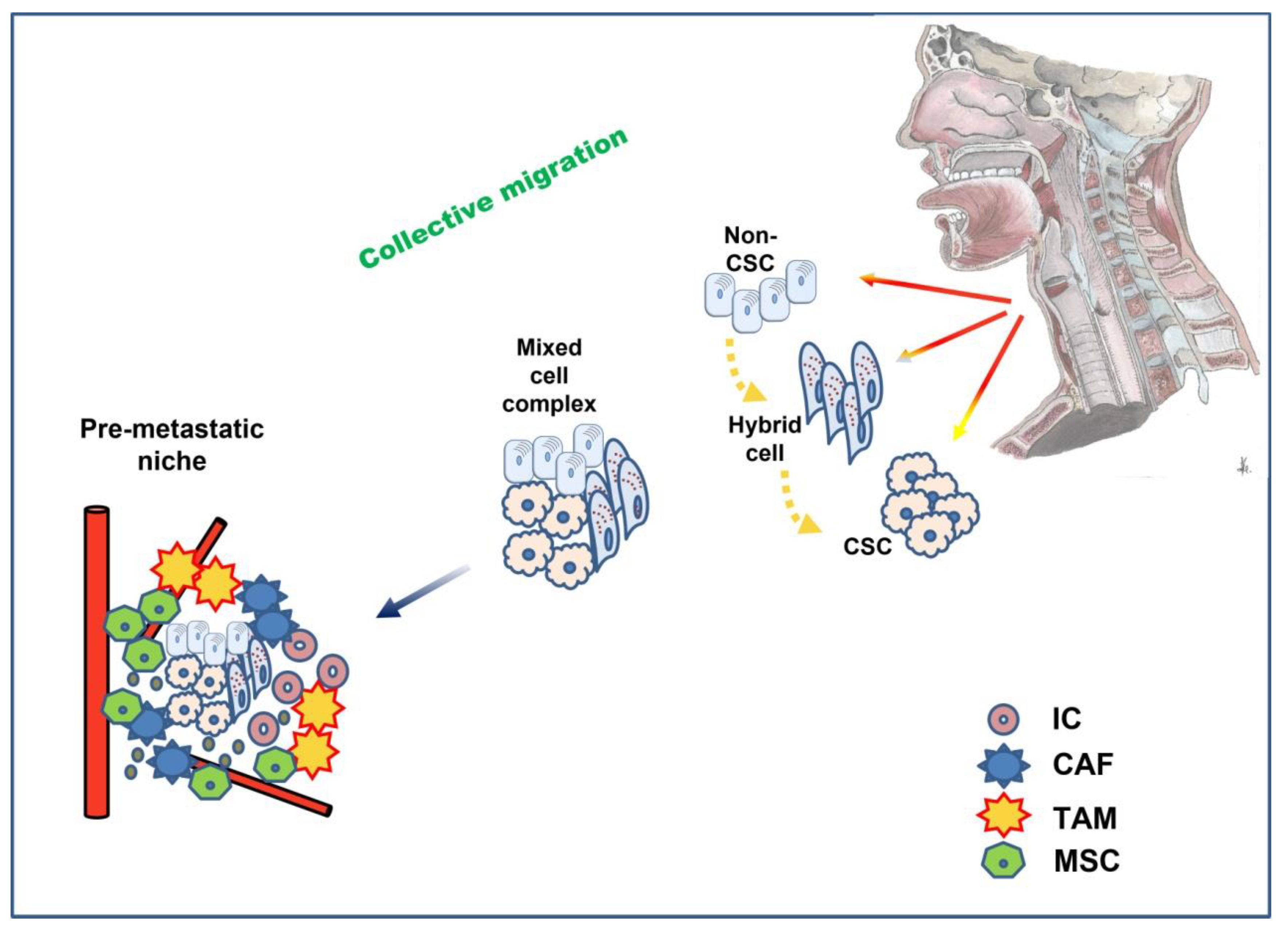
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Lu, M.; Ben-Jacob, E.; Onuchic, J.N.; Levine, H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front. Oncol. 2015, 5, 155. [Google Scholar] [CrossRef]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef]
- Barasch, J. Genes and proteins involved in mesenchymal to epithelial transition. Curr. Opin. Nephrol. Hypertens. 2001, 10, 429–436. [Google Scholar] [CrossRef]
- Sinha, D.; Saha, P.; Samanta, A.; Bishayee, A. Emerging Concepts of Hybrid Epithelial-to-Mesenchymal Transition in Cancer Progression. Biomolecules 2020, 10, 1561. [Google Scholar] [CrossRef]
- Saitoh, M. Involvement of partial EMT in cancer progression. J. Biochem. 2018, 164, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, S.; Sahoo, S.; Saha, A.; Chandran, S.; Majumdar, S.S.; Mandal, S.; Levine, H.; Jolly, M.K. Quantifying the Patterns of Metabolic Plasticity and Heterogeneity along the Epithelial-Hybrid-Mesenchymal Spectrum in Cancer. Biomolecules 2022, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 2021, 40, e108647. [Google Scholar] [CrossRef] [PubMed]
- Kisoda, S.; Mouri, Y.; Kitamura, N.; Yamamoto, T.; Miyoshi, K.; Kudo, Y. The role of partial-EMT in the progression of head and neck squamous cell carcinoma. J. Oral Biosci. 2022, 64, 176–182. [Google Scholar] [CrossRef]
- Pal, A.; Barrett, T.F.; Paolini, R.; Parikh, A.; Puram, S.V. Partial EMT in head and neck cancer biology: A spectrum instead of a switch. Oncogene 2021, 40, 5049–5065. [Google Scholar] [CrossRef]
- Kisoda, S.; Shao, W.; Fujiwara, N.; Mouri, Y.; Tsunematsu, T.; Jin, S.; Arakaki, R.; Ishimaru, N.; Kudo, Y. Prognostic value of partial EMT-related genes in head and neck squamous cell carcinoma by a bioinformatic analysis. Oral Dis. 2020, 26, 1149–1156, Online ahead of print. [Google Scholar] [CrossRef]
- Fan, L.; Wang, J.; Deng, P.; Wang, Y.; Zhang, A.; Yang, M.; Zeng, G. Foxhead box D1 promotes the partial epithelial-to-mesenchymal transition of laryngeal squamous cell carcinoma cells via transcriptionally activating the expression of zinc finger protein 532. Bioengineered 2022, 13, 3057–3069. [Google Scholar] [CrossRef]
- Liao, C.; Wang, Q.; An, J.; Long, Q.; Wang, H.; Xiang, M.; Xiang, M.; Zhao, Y.; Liu, Y.; Liu, J.; et al. Partial EMT in Squamous Cell Carcinoma: A Snapshot. Int. J. Biol. Sci. 2021, 17, 3036–3047. [Google Scholar] [CrossRef]
- Pastushenko, I.; Mauri, F.; Song, Y.; de Cock, F.; Meeusen, B.; Swedlund, B.; Impens, F.; Van Haver, D.; Opitz, M.; Thery, M.; et al. Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature 2021, 589, 448–455. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Steps in metastasis: An updated review. Med. Oncol. 2021, 38, 3. [Google Scholar] [CrossRef]
- Aggarwal, V.; Montoya, C.A.; Donnenberg, V.S.; Sant, S. Interplay between tumor microenvironment and partial EMT as the driver of tumor progression. iScience 2021, 24, 102113. [Google Scholar] [CrossRef] [PubMed]
- Labernadie, A.; Kato, T.; Brugués, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; González-Tarragó, V.; Elosegui-Artola, A.; Albertazzi, L.; et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 2017, 19, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Aiello, N.M.; Maddipati, R.; Norgard, R.J.; Balli, D.; Li, J.; Yuan, S.; Yamazoe, T.; Black, T.; Sahmoud, A.; Furth, E.E.; et al. EMT subtype influences epithelial plasticity and mode of cell migration. Dev. Cell 2018, 45, 681–695.e684. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Cao, M.; Liu, Y.; He, Y.; Du, Y.; Zhang, G.; Gao, F. Inducible formation of leadercells driven by CD44 switching gives rise to collective invasion and metastases in luminal breast carcinomas. Oncogene 2019, 38, 7113–7132. [Google Scholar] [CrossRef]
- Christofori, G. New signals from the invasive front. Nature 2006, 441, 444–450. [Google Scholar] [CrossRef]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- Forte, E.; Chimenti, I.; Rosa, P.; Angelini, F.; Pagano, F.; Calogero, A.; Giacomello, A.; Messina, E. EMT/MET at the Crossroad of Stemness, Regeneration and Oncogenesis: The Ying-Yang Equilibrium Recapitulated in Cell Spheroids. Cancers 2017, 9, 98. [Google Scholar] [CrossRef]
- Jolly, M.K.; Somarelli, J.A.; Sheth, M.; Biddle, A.; Tripathi, S.C.; Armstrong, A.J.; Hanash, S.M.; Bapat, S.A.; Ranagarajan, A.; Levine, H. Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacol. Ther. 2019, 194, 161–184. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef]
- Goetz, H.; Melendez-Alvarez, J.R.; Chen, L.; Tian, X.J. A plausible accelerating function of intermediate states in cancer metastasis. PLoS Comput. Biol. 2020, 16, e1007682. [Google Scholar] [CrossRef]
- Lowes, L.E.; Allan, A.L. Circulating Tumor Cells and Implications of the Epithelial-to-Mesenchymal Transition. Adv. Clin. Chem. 2018, 83, 121–181. [Google Scholar] [PubMed]
- Rizzo, M.I.; Ralli, M.; Nicolazzo, C.; Gradilone, A.; Carletti, R.; Di Gioia, C.; De Vincentiis, M.; Greco, A. Detection of circulating tumor cells in patients with laryngeal cancer using ScreenCell: Comparative pre- and post-operative analysis and association with prognosis. Oncol. Lett. 2020, 19, 4183–4188. [Google Scholar] [CrossRef]
- Kong, L.; Birkeland, A.C. Liquid Biopsies in Head and Neck Cancer: Current State and Future Challenges. Cancers 2021, 13, 1874. [Google Scholar] [CrossRef]
- Jung, A.R.; Jung, C.H.; Noh, J.K.; Lee, Y.C.; Eun, Y.G. Epithelial-mesenchymal transition gene signature is associated with prognosis and tumor microenvironment in head and neck squamous cell carcinoma. Sci. Rep. 2020, 10, 3652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hu, P.; Shen, H.; Yu, J.; Liu, Q.; Du, J. Prognostic role of Twist or Snail in various carcinomas: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2014, 44, 1072–1094. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wu, D.; Zou, J.; Chen, J.; Chen, L.; Chen, Y.; Ni, C.; Yuan, H. Prognostic impact of serum and tissue MMP-9 in non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 2016, 7, 18458–18468. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, Z.; Ma, J. Regulatory mechanisms and clinical significance of vimentin in breast cancer. Biomed. Pharm. 2021, 133, 111068. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Chen, S.; Jiang, J.; Qi, R.; Mi, X.; Zhang, X.; Xi, Y.; Zheng, H.; Hua, B. Prognostic significance of E-cadherin expression in prostatic carcinoma: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e19707. [Google Scholar] [CrossRef]
- Lv, F.; Du, Q.; Li, L.; Xi, X.; Liu, Q.; Li, W.; Liu, S. Eriodictyol inhibits glioblastoma migration and invasion by reversing EMT via downregulation of the P38 MAPK/GSK-3β/ZEB1 pathway. Eur. J. Pharm. 2021, 900, 174069. [Google Scholar] [CrossRef]
- Lamouille, S.; Subramanyam, D.; Blelloch, R.; Derynck, R. Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs. Curr. Opin. Cell Biol. 2013, 25, 200–207. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, S.; Zhan, Y.; Ke, J.; Wang, K.; Liang, Q.; Hou, Y.; Zhu, P.; Ao, W.; Wei, X.; et al. Dioscin Inhibits the Invasion and Migration of Hepatocellular Carcinoma HepG2 Cells by Reversing TGF-β1-Induced Epithelial-Mesenchymal Transition. Molecules 2019, 24, 2222. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Ren, Z.; Yang, X.; Yang, R.; Chen, Y.; Liu, Z.; Dai, Z.; Zhang, Y.; He, Y.; Zhang, C.; et al. Nerve growth factor (NGF)-TrkA axis in head and neck squamous cell carcinoma triggers EMT and confers resistance to the EGFR inhibitor erlotinib. Cancer Lett. 2020, 472, 81–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, W. Transforming Growth Factor β1 (TGF-β1)-Stimulated Integrin-Linked Kinase (ILK) Regulates Migration and Epithelial-Mesenchymal Transition (EMT) of Human Lens Epithelial Cells via Nuclear Factor κB (NF-κB). Med. Sci.Monit. 2018, 24, 7424–7430. [Google Scholar] [CrossRef]
- Kaşıkcı, E.; Aydemir, E.; Bayrak, Ö.F.; Şahin, F. Inhibition of Migration, Invasion and Drug Resistance of Pancreatic Adenocarcinoma Cells-Role of Snail, Slug and Twist and Small Molecule Inhibitors. Onco Targets Ther. 2020, 13, 5763–5777. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dai, F.; Zhang, Y.; Zheng, X.; Li, L.; Zhang, Y.; Cao, J.; Gao, W. miR-1207-5p suppresses laryngeal squamous cell carcinoma progression by downregulating SKA3 and inhibiting epithelial-mesenchymal transition. Mol. Ther. Oncolytics 2021, 22, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Yu, Y.; Xing, X.L.; Liu, R.Y. miR-183/TMSB4Y, a new potential signaling axis, involving in the progression of laryngeal cancer via modulating cell adhesion. J. Recept. Signal Transduct. Res. 2022, 42, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Wang, X.; Yin, W.H.; Niu, K.; Zhu, W.; Fang, N. MiR-199a-5p suppresses proliferation and invasion of human laryngeal cancer cells. Eur. Rev. Med. Pharm. Sci. 2020, 24, 12200–12207. [Google Scholar]
- Li, X.; Wu, P.; Tang, Y.; Fan, Y.; Liu, Y.; Fang, X.; Wang, W.; Zhao, S. Down-Regulation of MiR-181c-5p Promotes Epithelial-to-Mesenchymal Transition in Laryngeal Squamous Cell Carcinoma via Targeting SERPINE1. Front. Oncol. 2020, 10, 544476. [Google Scholar] [CrossRef]
- Wu, X.; Tan, Y.; Tang, X. Long Noncoding RNA MALAT1 Promotes Laryngocarcinoma Development by Targeting miR-708-5p/BRD4 Axis to Regulate YAP1-Mediated Epithelial-Mesenchymal Transition. Biomed. Res. Int. 2022, 2022, 8093949. [Google Scholar] [CrossRef]
- Yang, C.; Cao, H.; Yang, J.-W.; Wang, J.-T.; Yu, M.-M.; Wang, B.-S. The ETS1-LINC00278 negative feedback loop plays a role in COL4A1/COL4A2 regulation in laryngeal squamous cell carcinoma. Neoplasma 2022, 69, 841–858. [Google Scholar] [CrossRef]
- Du, R.; Liu, Z.; Hou, X.; Fu, G.; An, N.; Wang, L. Trichostatin A potentiates genistein-induced apoptosis and reverses EMT in HEp2 cells. Mol. Med. Rep. 2016, 13, 5045–5052. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, Y.; Lu, X.; Li, H.; Li, X. Dihydroartemisinin inhibits IL-6-induced epithelial-mesenchymal transition in laryngeal squamous cell carcinoma via the miR-130b-3p/STAT3/β-catenin signaling pathway. J. Int. Med. Res. 2021, 49, 3000605211009494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hou, J.; Wang, J.; Wang, J.; Gao, J.; Bai, Y. Brusatol inhibits laryngeal cancer cell proliferation and metastasis via abrogating JAK2/STAT3 signaling mediated epithelial-mesenchymal transition. Life Sci. 2021, 284, 119907. [Google Scholar] [CrossRef] [PubMed]
- Goulioumis, A.K.; Fuxe, J.; Varakis, J.; Repanti, M.; Goumas, P.; Papadaki, H. Estrogen receptor-beta expression in human laryngeal carcinoma: Correlation with the expression of epithelial-mesenchymal transition specific biomarkers. Oncol. Rep. 2009, 22, 1063–1068. [Google Scholar]
- Jolly, M.K.; Ware, K.E.; Gilja, S.; Somarelli, J.A.; Levine, H. EMT and MET: Necessary or permissive for metastasis? Mol. Oncol. 2017, 11, 755–769. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goulioumis, A.; Gyftopoulos, K. Epithelial-to-Mesenchymal Transition in Metastasis: Focus on Laryngeal Carcinoma. Biomedicines 2022, 10, 2148. https://doi.org/10.3390/biomedicines10092148
Goulioumis A, Gyftopoulos K. Epithelial-to-Mesenchymal Transition in Metastasis: Focus on Laryngeal Carcinoma. Biomedicines. 2022; 10(9):2148. https://doi.org/10.3390/biomedicines10092148
Chicago/Turabian StyleGoulioumis, Anastasios, and Kostis Gyftopoulos. 2022. "Epithelial-to-Mesenchymal Transition in Metastasis: Focus on Laryngeal Carcinoma" Biomedicines 10, no. 9: 2148. https://doi.org/10.3390/biomedicines10092148
APA StyleGoulioumis, A., & Gyftopoulos, K. (2022). Epithelial-to-Mesenchymal Transition in Metastasis: Focus on Laryngeal Carcinoma. Biomedicines, 10(9), 2148. https://doi.org/10.3390/biomedicines10092148






