Revertant Mosaicism in Genodermatoses: Natural Gene Therapy Right before Your Eyes
Abstract
:1. Genodermatoses
2. Mosaicism in Genodermatoses
3. Forward Mosaicism in Genodermatoses
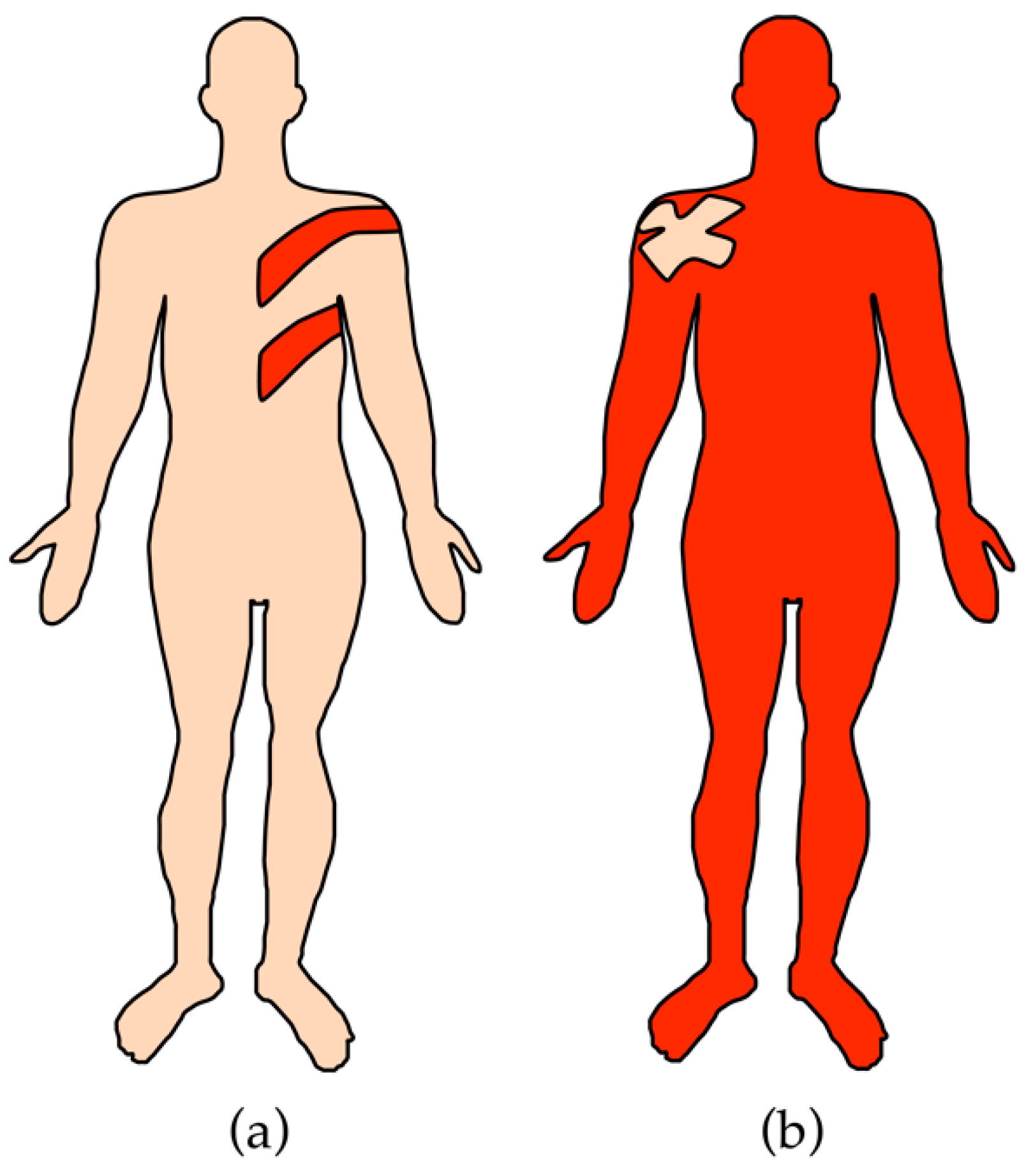
4. Revertant Mosaicism in Genodermatoses
| Disorder | Corrected Gene | Correction Mechanisms | Corrected Germline Mutations | Skin Layer | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Subtype (MIM) | Inheritance | Name (MIM) | Major Group | Specific Type | Consequence RNA-Level | Consequence Protein-Level | |||
| Epidermolysis bullosa | |||||||||
| EBS, severe (131760) | AD | KRT14 (148066) | Second-site mutation | Nucleotide insertion (1 Nt) | Disruption of reading frame Silencing dominant negative allele | Loss of expression of mutant protein | c.373C>T; p.Arg125Cys | EKCs | [76] |
| EBS, recessive (601001) | AR | KRT14 (148066) | Unknown | Unknown | Splice-modulating Generation of in-frame splice variant | Introduction of protein lacking 2 AA and carrying 1 missense AA. Non-functional. | c.526-2A>C (SS) | EKCs | [77] |
| JEB, intermediate (226650) | AR | COL17A1 (113811) | Gene conversion | N/A | Loss of heterozygosity for one mutant allele | Introduction of full-length protein, wildtype | c.1601delA; p.Asp534fs | EKCs | [26,75] |
| Back-mutation/ mitotic recombination | Nucleotide change | Nonsense to wildtype reversion | Introduction of full-length protein, wildtype | c.3676C>T; p.Arg1226* | EKCs | [75] | |||
| Second-site mutation | Nucleotide change | Nonsense to missense change | Introduction of full-length protein carrying 1 missense AA | c.3676C>T; p.Arg1226* | EKCs | [75] | |||
| Splice-modulating Restoration of reading frame | Introduction of full-length protein with 13 incorrect AA | c.4319dup; p.Gly1441fs | EKCs | [75] | |||||
| In-frame skipping of mutant exon | Introduction of shorter protein lacking AA of mutant exon | c.2237del; p.Gly746fs | EKCs | [73,79] | |||||
| c.3487G>T; p.Glu1163* | EKCs | [66] | |||||||
| Nucleotide insertion (2 Nt) | Restoration of reading frame | Introduction of full-length protein with 25 incorrect AA | c.3899_3900del; p.Ser1300fs | EKCs | [78] | ||||
| Intragenic genomic deletion | In-frame skipping of mutant exon | Introduction of shorter protein lacking AA of mutant exon | c.2237del; p.Gly746fs | EKCs | [73,79] | ||||
| In-frame deletion of multiple exons | Introduction of shorter protein lacking AA of mutant exon | c.2237del; p.Gly746fs | EKCs | [73,79] | |||||
| Intragenic genomic deletion and insertion | In-frame deletion of mutant exon | Introduction of shorter protein lacking AA of mutant exon | c.2237del; p.Gly746fs | EKCs | [73,79] | ||||
| LAMB3 (150310) | Second-site mutation | Nucleotide change | Splice-modulating. Increase in wild-type splicing pattern | Increased expression of full-length protein carrying 1 missense mutation | c.628G>A; p.Glu210Lys | EKCs | [74] | ||
| Splice modulating. Introduction of new, in-frame splice variant | Introduction of protein elongated by 22 AA | c.628G>A; p.Glu210Lys | EKCs | [74] | |||||
| Splice modulating. Increased expression of alternative in-frame splice variant | Increased expression of protein lacking 22 AA | c.628G>A; p.Glu210Lys | EKCs | [74] | |||||
| RDEB, severe (226600) | AR | COL7A1 (120120) | Intragenic cross-over (mitotic recombination) | N/A | Loss of heterozygosity of one mutant allele | Introduction of full-length protein, wildtype | c.7786del; p.Gly2596fs | EKCs | [72] |
| Second-site mutation | Nucleotide deletion (1 bp) | Restoration of reading frame | Introduction of full-length protein, wildtype | c.6527dup; p.Gly2177fs | EKCs | [71] | |||
| Nucleotide change | Splice-modulating. Restoration of splicing pattern towards wild-type | Increased expression of full-length protein, wildtype | c.2142A>G (SS) | EKCs | [86] | ||||
| c.425A>G (SS) | EKCs | [86] | |||||||
| Back mutation/mitotic recombination | N/A | Loss of heterozygosity of one mutant allele | Introduction of full-length protein, wildtype | c.884del; p.Gly296fs | EKCs | [86] | |||
| Introduction of full-length protein, wildtype, loss of expression of mutant protein | c.6176A>G; p.Glu2059Gly | EKCs | [86] | ||||||
| Mitotic recombination | N/A | Both variants on one allele | Introduction of full-length protein, wildtype | 425A>G (SS) | EKCs | [86] | |||
| c.1837C>T; p.Arg613* | EKCs | [86] | |||||||
| RDEB, intermediate (226600) | AR | COL7A1 (120120) | Mitotic recombination | N/A | Loss of heterozygosity of one mutant allele | Introduction of full-length protein, wildtype | c.425A>G | EKCs | [86] |
| Second-site mutation | Nucleotide change | Nonsense to missense change | Introduction of full-length protein with 1 missense AA | c.6508C>T; p.Gln2170* | EKCs | [68] | |||
| Intragenic cross-over (mitotic recombination) | N/A | Loss of heterozygosity of one mutant allele | Introduction of full-length protein, wildtype, loss of expression of mutant protein | c.6091G>A; p.Gly2031Ser | DFBs | [65] | |||
| Both variants on one allele | Introduction of full-length protein, wildtype, loss of expression of mutant protein | c.5932C>T; p.Arg1978*/ c.8029G>A; p.Gly2677Ser | EKCs | [64] | |||||
| DDEB (131750) | AD | COL7A1 (120120) | Back mutation/mitotic recombination | Nucleotide change | Loss of heterozygosity of mutant allele | Loss of expression of mutant protein | c.6127C>A; p.Gly2043Arg | EKCs | [86] |
| Kindler EB (173650) | AR | FERMT1 (607900) | Slipped mispairing | Nucleotide deletion (1 bp) | Loss of one mutant allele | Introduction of full-length protein, wildtype | c.676dup; p.Gln226fs | EKCs | [69,70] |
| c.456dup; p.Asp153fs | EKCs | [69,70] | |||||||
| Disorders of cornification | |||||||||
| Ichthyosis with confetti type 1, KRT10 (609165) | AD | KRT10 (148080) | Mitotic recombination | N/A | Loss of heterozygosity of mutant allele | Loss of expression of mutant protein | c.1369G>T (SS) | EKCs | [81] |
| c.1373del; p.Ser458fs | EKCs | [90] | |||||||
| c.1373+1G>A (SS) | EKCs | [81] | |||||||
| c.1374-2del (SS) | EKCs | [81] | |||||||
| c.1374-2A>G (SS) | EKCs | [81,88] | |||||||
| c.1374-1G>A (SS) | EKCs | [81] | |||||||
| c.1449dup; p.Gly484fs | EKCs | [81] | |||||||
| c.1546_1551delinsT; p.Gly516fs | EKCs | [91] | |||||||
| c.1560_1561del; p.Gly521fs | EKCs | [81] | |||||||
| Ichthyosis with confetti type 2, KRT1 (609165) | AD | KRT1 (139350) | Mitotic recombination | N/A | Loss of heterozygosity of mutant allele | Loss of expression of mutant protein | c.1865dup; p.Val623fs | EKCs | [80] |
| c.1759dup; p.Tyr587fs | [87] | ||||||||
| c.591+332_1129-34del; p.197_375del | [89] | ||||||||
| Loricrin keratoderma (604117) | AD | LOR (152445) | Mitotic recombination | N/A | Loss of heterozygosity of mutant allele | Loss of expression of mutant protein | c.545dup; p.Gly183fs | EKCs | [83] |
| Keratitis-ichthyosis-deafness syndrome (148210) | AD | GJB2 (121011) | Second-site mutation | Nucleotide change | Silencing dominant negative allele | Loss of expression of mutant protein | c.148G>A, p.Asp50Asn | EKCs | [82] |
| Pityriasis rubra pilaris (173200) | AD | CARD14 (607211) | Mitotic recombination | N/A | Loss of heterozygosity of mutant allele | Loss of expression of mutant protein | c.356T>C; p.Met119Thr | EKCs | [84] |
| c.407A>T; p.Gln136Leu | EKCs | [84] |
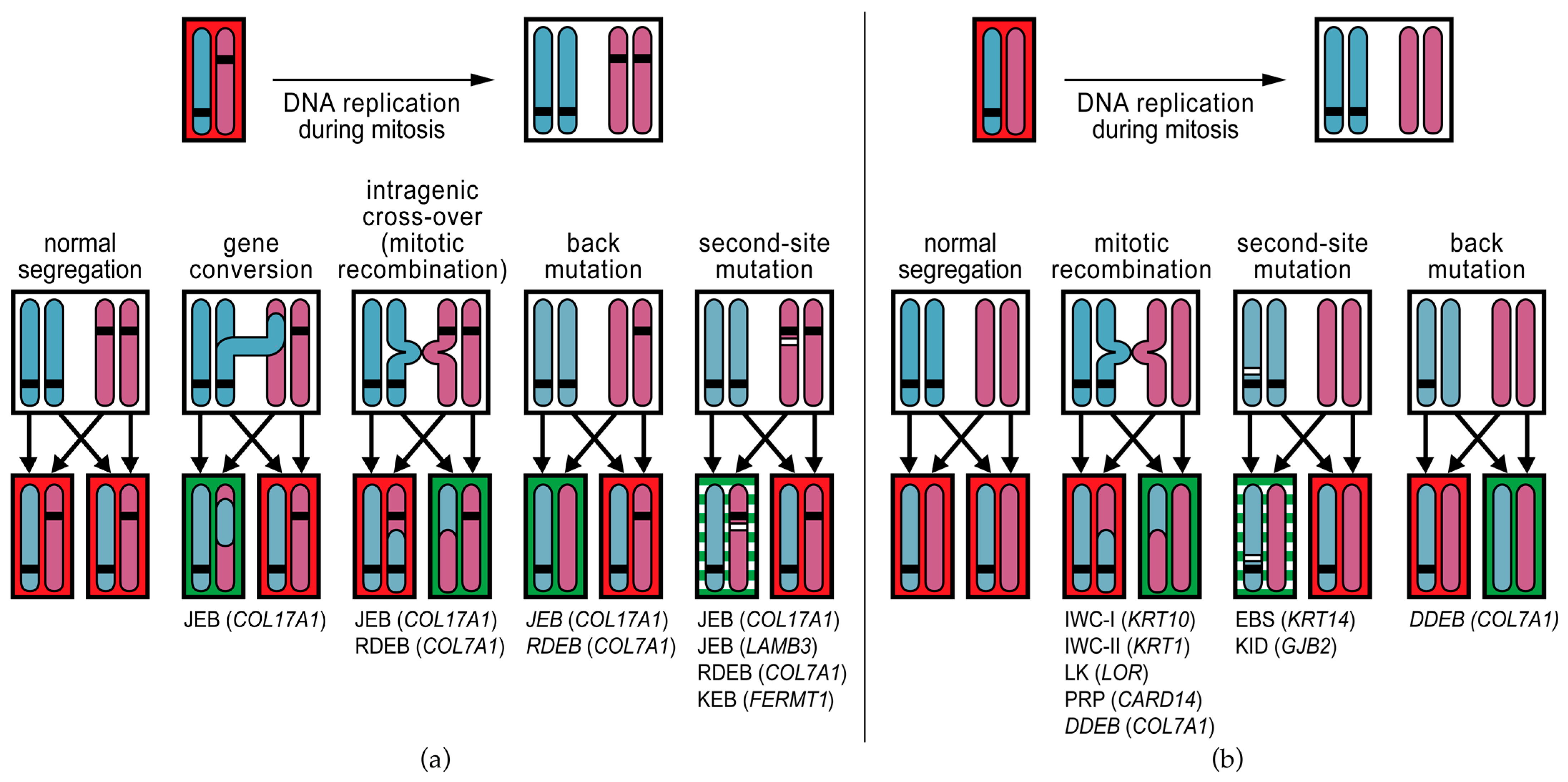
5. Molecular Mechanisms of Revertant Mosaicism in Genodermatoses
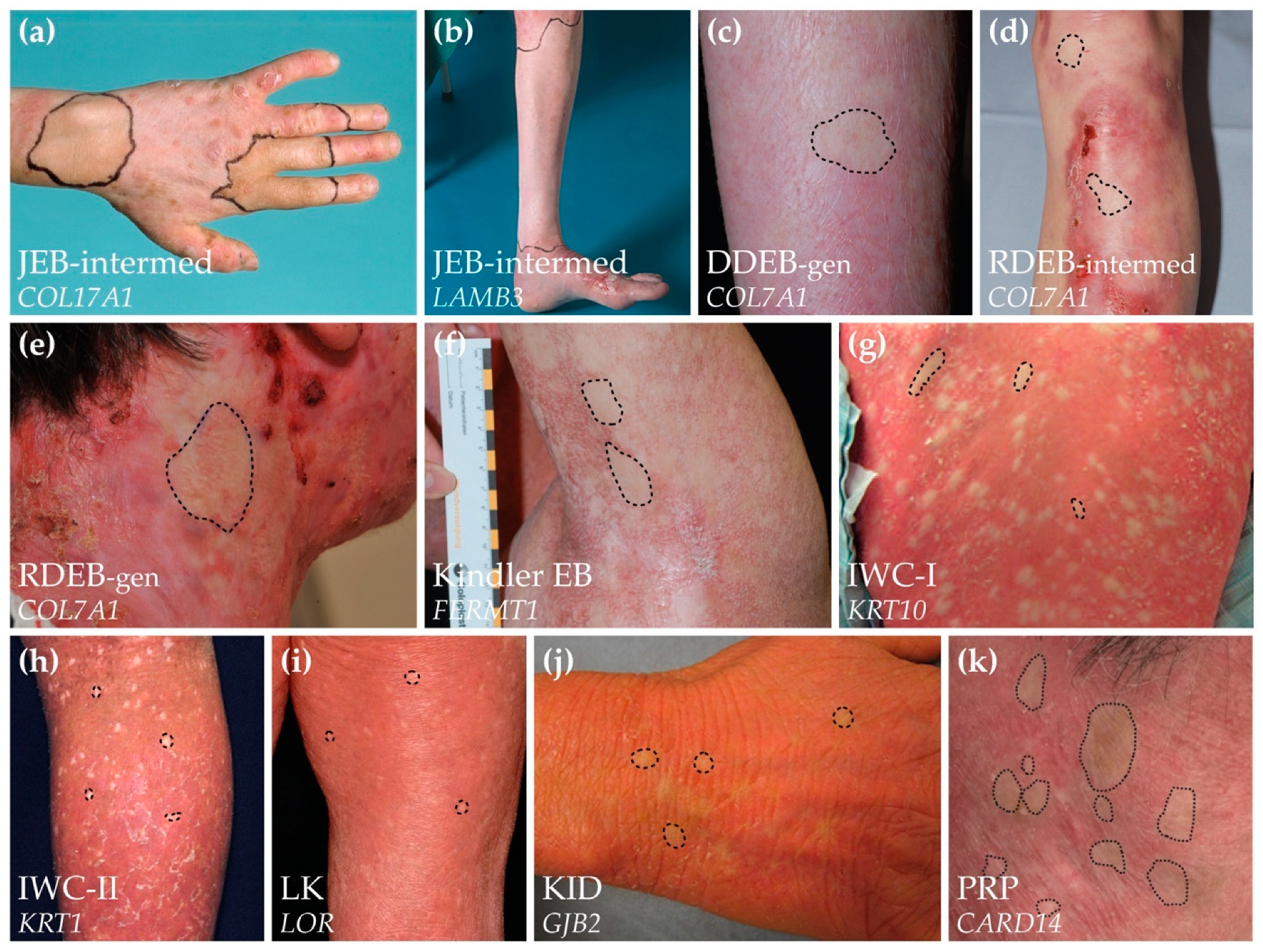
6. Clinical Recognition of Revertant Mosaicism in Genodermatoses
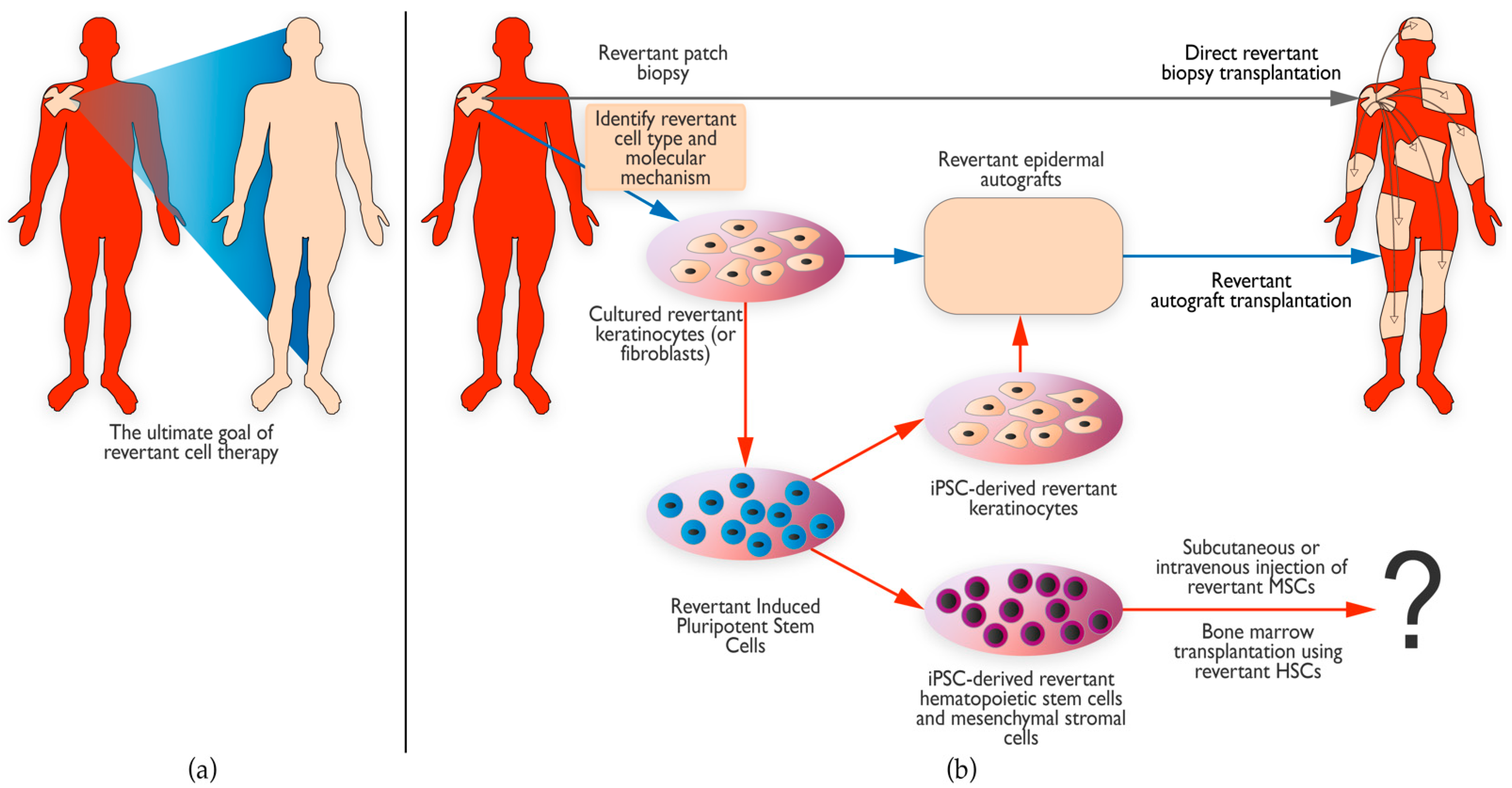
7. Revertant Mosaicism as Treatment for Genodermatoses
8. Concluding Remarks and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Has, C.; Bauer, J.W.; Bodemer, C.; Bolling, M.C.; Bruckner-Tuderman, L.; Diem, A.; Fine, J.D.; Heagerty, A.; Hovnanian, A.; Marinkovich, M.P.; et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br. J. Dermatol. 2020, 183, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.P.; Han, S.Y.; van der Zwaag, P.A.; Bolling, M.C.; Jongbloed, J.D.; Jonkman, M.F.; Getsios, S.; Paller, A.S.; Green, K.J. Insights from a desmoplakin mutation identified in lethal acantholytic epidermolysis bullosa. J. Investig. Dermatol. 2010, 130, 2680–2683. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, M.F.; Pasmooij, A.M.; Pasmans, S.G.; van den Berg, M.P.; Ter Horst, H.J.; Timmer, A.; Pas, H.H. Loss of desmoplakin tail causes lethal acantholytic epidermolysis bullosa. Am. J. Hum. Genet. 2005, 77, 653–660. [Google Scholar] [CrossRef]
- Kelsell, D.P.; Norgett, E.E.; Unsworth, H.; Teh, M.T.; Cullup, T.; Mein, C.A.; Dopping-Hepenstal, P.J.; Dale, B.A.; Tadini, G.; Fleckman, P.; et al. Mutations in ABCA12 underlie the severe congenital skin disease harlequin ichthyosis. Am. J. Hum. Genet. 2005, 76, 794–803. [Google Scholar] [CrossRef]
- Akiyama, M.; Sugiyama-Nakagiri, Y.; Sakai, K.; McMillan, J.R.; Goto, M.; Arita, K.; Tsuji-Abe, Y.; Tabata, N.; Matsuoka, K.; Sasaki, R.; et al. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J. Clin. Investig. 2005, 115, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Moulson, C.L.; Go, G.; Gardner, J.M.; van der Wal, A.C.; Smitt, J.H.; van Hagen, J.M.; Miner, J.H. Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J. Investig. Dermatol. 2005, 125, 913–919. [Google Scholar] [CrossRef]
- Navarro, C.L.; De Sandre-Giovannoli, A.; Bernard, R.; Boccaccio, I.; Boyer, A.; Geneviève, D.; Hadj-Rabia, S.; Gaudy-Marqueste, C.; Smitt, H.S.; Vabres, P.; et al. Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum. Mol. Genet. 2004, 13, 2493–2503. [Google Scholar] [CrossRef]
- Bohring, A.; Stamm, T.; Spaich, C.; Haase, C.; Spree, K.; Hehr, U.; Hoffmann, M.; Ledig, S.; Sel, S.; Wieacker, P.; et al. WNT10A mutations are a frequent cause of a broad spectrum of ectodermal dysplasias with sex-biased manifestation pattern in heterozygotes. Am. J. Hum. Genet. 2009, 85, 97–105. [Google Scholar] [CrossRef]
- Dharma, B.; Moss, C.; McGrath, J.A.; Mellerio, J.E.; Ilchyshyn, A. Dominant dystrophic epidermolysis bullosa presenting as familial nail dystrophy. Clin. Exp. Dermatol. 2001, 26, 93–96. [Google Scholar]
- Akiyama, M. ABCA12 mutations and autosomal recessive congenital ichthyosis: A review of genotype/phenotype correlations and of pathogenetic concepts. Hum. Mutat. 2010, 31, 1090–1096. [Google Scholar] [CrossRef]
- van den Akker, P.C.; van Essen, A.J.; Kraak, M.M.; Meijer, R.; Nijenhuis, M.; Meijer, G.; Hofstra, R.M.; Pas, H.H.; Scheffer, H.; Jonkman, M.F. Long-term follow-up of patients with recessive dystrophic epidermolysis bullosa in the Netherlands: Expansion of the mutation database and unusual phenotype-genotype correlations. J. Dermatol. Sci. 2009, 56, 9–18. [Google Scholar] [CrossRef]
- Oji, V.; Preil, M.L.; Kleinow, B.; Wehr, G.; Fischer, J.; Hennies, H.C.; Hausser, I.; Breitkreutz, D.; Aufenvenne, K.; Stieler, K.; et al. S1 guidelines for the diagnosis and treatment of ichthyoses-update. J. Dtsch. Dermatol. Ges. 2017, 15, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Happle, R. Mosaicism in Human Skin: Understanding Nevi, Nevoid Skin Disorders, and Cutaneous Neoplasia, 1st ed.; SpringerLink; Imprint; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Cheraghlou, S.; Lim, Y.; Choate, K.A. Mosaicism in genodermatoses. Clin. Dermatol. 2020, 38, 408–420. [Google Scholar] [CrossRef]
- Martínez-Glez, V.; Tenorio, J.; Nevado, J.; Gordo, G.; Rodríguez-Laguna, L.; Feito, M.; de Lucas, R.; Pérez-Jurado, L.A.; Ruiz Pérez, V.L.; Torrelo, A.; et al. A six-attribute classification of genetic mosaicism. Genet. Med. 2020, 22, 1743–1757. [Google Scholar] [CrossRef] [PubMed]
- Jafry, M.; Sidbury, R. RASopathies. Clin. Dermatol. 2020, 38, 455–461. [Google Scholar] [CrossRef]
- Ko, Y.; Lee, C.; Lee, H.; Lee, M.; Lee, J.S. Clinical application of next-generation sequencing for the diagnosis of segmental neurofibromatosis. J. Dermatol. Sci. 2017, 88, 370–372. [Google Scholar] [CrossRef]
- Weinstein, L.S.; Shenker, A.; Gejman, P.V.; Merino, M.J.; Friedman, E.; Spiegel, A.M. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N. Engl. J. Med. 1991, 325, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Mirzaa, G.; Timms, A.E.; Conti, V.; Boyle, E.A.; Girisha, K.M.; Martin, B.; Kircher, M.; Olds, C.; Juusola, J.; Collins, S.; et al. PIK3CA-associated developmental disorders exhibit distinct classes of mutations with variable expression and tissue distribution. JCI Insight. 2016, 1, e87623. [Google Scholar] [CrossRef]
- Kurek, K.C.; Luks, V.L.; Ayturk, U.M.; Alomari, A.I.; Fishman, S.J.; Spencer, S.A.; Mulliken, J.B.; Bowen, M.E.; Yamamoto, G.L.; Kozakewich, H.P.; et al. Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. Am. J. Hum. Genet. 2012, 90, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- van Leersum, F.S.; Seyger, M.M.B.; Theunissen, T.E.J.; Bongers, E.; Steijlen, P.M.; van Geel, M. Recessive mosaicism in ABCA12 causes blaschkoid congenital ichthyosiform erythroderma. Br. J. Dermatol. 2020, 182, 208–211. [Google Scholar] [CrossRef]
- Happle, R. The concept of type 2 segmental mosaicism, expanding from dermatology to general medicine. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Torrelo, A.; Hernández-Martín, A.; Bueno, E.; Colmenero, I.; Rivera, I.; Requena, L.; Happle, R.; González-Sarmiento, R. Molecular evidence of type 2 mosaicism in Gorlin syndrome. Br. J. Dermatol. 2013, 169, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Fölster-Holst, R.; Nellen, R.G.; Jensen, J.M.; Poblete-Gutiérrez, P.; Steijlen, P.M.; Schwarz, T.; Happle, R.; Van Geel, M.; Frank, J. Molecular genetic support for the rule of dichotomy in type 2 segmental Darier disease. Br. J. Dermatol. 2012, 166, 464–466. [Google Scholar] [CrossRef]
- Jonkman, M.F.; de Jong, M.C.; Heeres, K.; Pas, H.H.; van der Meer, J.B.; Owaribe, K.; Martinez de Velasco, A.M.; Niessen, C.M.; Sonnenberg, A. 180-kD bullous pemphigoid antigen (BP180) is deficient in generalized atrophic benign epidermolysis bullosa. J. Clin. Investig. 1995, 95, 1345–1352. [Google Scholar] [CrossRef]
- Jonkman, M.F.; Scheffer, H.; Stulp, R.; Pas, H.H.; Nijenhuis, M.; Heeres, K.; Owaribe, K.; Pulkkinen, L.; Uitto, J. Revertant mosaicism in epidermolysis bullosa caused by mitotic gene conversion. Cell 1997, 88, 543–551. [Google Scholar] [CrossRef]
- Tan, S.; Kermasson, L.; Hilcenko, C.; Kargas, V.; Traynor, D.; Boukerrou, A.Z.; Escudero-Urquijo, N.; Faille, A.; Bertrand, A.; Rossmann, M.; et al. Somatic genetic rescue of a germline ribosome assembly defect. Nat. Commun. 2021, 12, 5044. [Google Scholar] [CrossRef]
- Hoshino, A.; Yang, X.; Tanita, K.; Yoshida, K.; Ono, T.; Nishida, N.; Okuno, Y.; Kanzaki, T.; Goi, K.; Fujino, H.; et al. Modification of cellular and humoral immunity by somatically reverted T cells in X-linked lymphoproliferative syndrome type 1. J. Allergy Clin. Immunol. 2019, 143, 421–424.e411. [Google Scholar] [CrossRef]
- Garelli, E.; Quarello, P.; Giorgio, E.; Carando, A.; Menegatti, E.; Mancini, C.; Di Gregorio, E.; Crescenzio, N.; Palumbo, O.; Carella, M.; et al. Spontaneous remission in a Diamond-Blackfan anaemia patient due to a revertant uniparental disomy ablating a de novo RPS19 mutation. Br. J. Haematol. 2019, 185, 994–998. [Google Scholar] [CrossRef]
- Csillag, B.; Ilencikova, D.; Meissl, M.; Webersinke, G.; Laccone, F.; Narumi, S.; Haas, O.; Duba, H.C. Somatic mosaic monosomy 7 and UPD7q in a child with MIRAGE syndrome caused by a novel SAMD9 mutation. Pediatr. Blood Cancer 2019, 66, e27589. [Google Scholar] [CrossRef]
- Brigida, I.; Zoccolillo, M.; Cicalese, M.P.; Pfajfer, L.; Barzaghi, F.; Scala, S.; Oleaga-Quintas, C.; Álvarez-Álvarez, J.A.; Sereni, L.; Giannelli, S.; et al. T-cell defects in patients with ARPC1B germline mutations account for combined immunodeficiency. Blood 2018, 132, 2362–2374. [Google Scholar] [CrossRef]
- Asur, R.S.; Kimble, D.C.; Lach, F.P.; Jung, M.; Donovan, F.X.; Kamat, A.; Noonan, R.J.; Thomas, J.W.; Park, M.; Chines, P.; et al. Somatic mosaicism of an intragenic FANCB duplication in both fibroblast and peripheral blood cells observed in a Fanconi anemia patient leads to milder phenotype. Mol. Genet. Genomic. Med. 2018, 6, 77–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venugopal, P.; Moore, S.; Lawrence, D.M.; George, A.J.; Hannan, R.D.; Bray, S.C.; To, L.B.; D’Andrea, R.J.; Feng, J.; Tirimacco, A.; et al. Self-reverting mutations partially correct the blood phenotype in a Diamond Blackfan anemia patient. Haematologica 2017, 102, e506–e509. [Google Scholar] [CrossRef] [PubMed]
- Tesi, B.; Davidsson, J.; Voss, M.; Rahikkala, E.; Holmes, T.D.; Chiang, S.C.C.; Komulainen-Ebrahim, J.; Gorcenco, S.; Rundberg Nilsson, A.; Ripperger, T.; et al. Gain-of-function SAMD9L mutations cause a syndrome of cytopenia, immunodeficiency, MDS, and neurological symptoms. Blood 2017, 129, 2266–2279. [Google Scholar] [CrossRef]
- Marin, A.V.; Jiménez-Reinoso, A.; Briones, A.C.; Muñoz-Ruiz, M.; Aydogmus, C.; Pasick, L.J.; Couso, J.; Mazariegos, M.S.; Alvarez-Prado, A.F.; Blázquez-Moreno, A.; et al. Primary T-cell immunodeficiency with functional revertant somatic mosaicism in CD247. J. Allergy Clin. Immunol. 2017, 139, 347–349.e348. [Google Scholar] [CrossRef] [PubMed]
- Federico, C.; Dugo, K.; Bruno, F.; Longo, A.M.; Grillo, A.; Saccone, S. Somatic mosaicism with reversion to normality of a mutated transthyretin allele related to a familial amyloidotic polyneuropathy. Hum. Genet. 2017, 136, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.H.; Below, J.E.; Shimamura, A.; Keel, S.B.; Matsushita, M.; Wolff, J.; Sul, Y.; Bonkowski, E.; Castella, M.; Taniguchi, T.; et al. Ataxia-Pancytopenia Syndrome Is Caused by Missense Mutations in SAMD9L. Am. J. Hum. Genet. 2016, 98, 1146–1158. [Google Scholar] [CrossRef]
- McDermott, D.H.; Gao, J.L.; Liu, Q.; Siwicki, M.; Martens, C.; Jacobs, P.; Velez, D.; Yim, E.; Bryke, C.R.; Hsu, N.; et al. Chromothriptic cure of WHIM syndrome. Cell 2015, 160, 686–699. [Google Scholar] [CrossRef]
- Le Guen, T.; Touzot, F.; André-Schmutz, I.; Lagresle-Peyrou, C.; France, B.; Kermasson, L.; Lambert, N.; Picard, C.; Nitschke, P.; Carpentier, W.; et al. An in vivo genetic reversion highlights the crucial role of Myb-Like, SWIRM, and MPN domains 1 (MYSM1) in human hematopoiesis and lymphocyte differentiation. J. Allergy Clin. Immunol. 2015, 136, 1619–1626.e1615. [Google Scholar] [CrossRef]
- Fuchs, S.; Rensing-Ehl, A.; Pannicke, U.; Lorenz, M.R.; Fisch, P.; Jeelall, Y.; Rohr, J.; Speckmann, C.; Vraetz, T.; Farmand, S.; et al. Omenn syndrome associated with a functional reversion due to a somatic second-site mutation in CARD11 deficiency. Blood 2015, 126, 1658–1669. [Google Scholar] [CrossRef]
- Jing, H.; Zhang, Q.; Zhang, Y.; Hill, B.J.; Dove, C.G.; Gelfand, E.W.; Atkinson, T.P.; Uzel, G.; Matthews, H.F.; Mustillo, P.J.; et al. Somatic reversion in dedicator of cytokinesis 8 immunodeficiency modulates disease phenotype. J. Allergy Clin. Immunol. 2014, 133, 1667–1675. [Google Scholar] [CrossRef]
- Bayer, D.K.; Martinez, C.A.; Sorte, H.S.; Forbes, L.R.; Demmler-Harrison, G.J.; Hanson, I.C.; Pearson, N.M.; Noroski, L.M.; Zaki, S.R.; Bellini, W.J.; et al. Vaccine-associated varicella and rubella infections in severe combined immunodeficiency with isolated CD4 lymphocytopenia and mutations in IL7R detected by tandem whole exome sequencing and chromosomal microarray. Clin. Exp. Immunol. 2014, 178, 459–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, S.A.; Salzer, E.; Eibl, M.M.; Linder, A.; Geier, C.B.; Santos-Valente, E.; Garncarz, W.; Lion, T.; Ott, R.; Seelbach, C.; et al. Combined immunodeficiency evolving into predominant CD4+ lymphopenia caused by somatic chimerism in JAK3. J. Clin. Immunol. 2014, 34, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Jongmans, M.C.; Verwiel, E.T.; Heijdra, Y.; Vulliamy, T.; Kamping, E.J.; Hehir-Kwa, J.Y.; Bongers, E.M.; Pfundt, R.; van Emst, L.; van Leeuwen, F.N.; et al. Revertant somatic mosaicism by mitotic recombination in dyskeratosis congenita. Am. J. Hum. Genet. 2012, 90, 426–433. [Google Scholar] [CrossRef]
- Xia, B.; Dorsman, J.C.; Ameziane, N.; de Vries, Y.; Rooimans, M.A.; Sheng, Q.; Pals, G.; Errami, A.; Gluckman, E.; Llera, J.; et al. Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nat. Genet. 2007, 39, 159–161. [Google Scholar] [CrossRef]
- Tone, Y.; Wada, T.; Shibata, F.; Toma, T.; Hashida, Y.; Kasahara, Y.; Koizumi, S.; Yachie, A. Somatic revertant mosaicism in a patient with leukocyte adhesion deficiency type 1. Blood 2007, 109, 1182–1184. [Google Scholar] [CrossRef] [PubMed]
- Dorsman, J.C.; Levitus, M.; Rockx, D.; Rooimans, M.A.; Oostra, A.B.; Haitjema, A.; Bakker, S.T.; Steltenpool, J.; Schuler, D.; Mohan, S.; et al. Identification of the Fanconi anemia complementation group I gene, FANCI. Cell Oncol. 2007, 29, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Rieux-Laucat, F.; Hivroz, C.; Lim, A.; Mateo, V.; Pellier, I.; Selz, F.; Fischer, A.; Le Deist, F. Inherited and somatic CD3zeta mutations in a patient with T-cell deficiency. N. Engl. J. Med. 2006, 354, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Toma, T.; Okamoto, H.; Kasahara, Y.; Koizumi, S.; Agematsu, K.; Kimura, H.; Shimada, A.; Hayashi, Y.; Kato, M.; et al. Oligoclonal expansion of T lymphocytes with multiple second-site mutations leads to Omenn syndrome in a patient with RAG1-deficient severe combined immunodeficiency. Blood 2005, 106, 2099–2101. [Google Scholar] [CrossRef]
- Nishikomori, R.; Akutagawa, H.; Maruyama, K.; Nakata-Hizume, M.; Ohmori, K.; Mizuno, K.; Yachie, A.; Yasumi, T.; Kusunoki, T.; Heike, T.; et al. X-linked ectodermal dysplasia and immunodeficiency caused by reversion mosaicism of NEMO reveals a critical role for NEMO in human T-cell development and/or survival. Blood 2004, 103, 4565–4572. [Google Scholar] [CrossRef]
- Waisfisz, Q.; Morgan, N.V.; Savino, M.; de Winter, J.P.; van Berkel, C.G.; Hoatlin, M.E.; Ianzano, L.; Gibson, R.A.; Arwert, F.; Savoia, A.; et al. Spontaneous functional correction of homozygous fanconi anaemia alleles reveals novel mechanistic basis for reverse mosaicism. Nat. Genet. 1999, 22, 379–383. [Google Scholar] [CrossRef]
- Ariga, T.; Yamada, M.; Sakiyama, Y.; Tatsuzawa, O. A case of Wiskott-Aldrich syndrome with dual mutations in exon 10 of the WASP gene: An additional de novo one-base insertion, which restores frame shift due to an inherent one-base deletion, detected in the major population of the patient’s peripheral blood lymphocytes. Blood 1998, 92, 699–701. [Google Scholar] [PubMed]
- Lo Ten Foe, J.R.; Kwee, M.L.; Rooimans, M.A.; Oostra, A.B.; Veerman, A.J.; van Weel, M.; Pauli, R.M.; Shahidi, N.T.; Dokal, I.; Roberts, I.; et al. Somatic mosaicism in Fanconi anemia: Molecular basis and clinical significance. Eur. J. Hum. Genet. 1997, 5, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Stephan, V.; Wahn, V.; Le Deist, F.; Dirksen, U.; Broker, B.; Müller-Fleckenstein, I.; Horneff, G.; Schroten, H.; Fischer, A.; de Saint Basile, G. Atypical X-linked severe combined immunodeficiency due to possible spontaneous reversion of the genetic defect in T cells. N. Engl. J. Med. 1996, 335, 1563–1567. [Google Scholar] [CrossRef] [PubMed]
- Liehr, T.; Rautenstrauss, B.; Grehl, H.; Bathke, K.D.; Ekici, A.; Rauch, A.; Rott, H.D. Mosaicism for the Charcot-Marie-Tooth disease type 1A duplication suggests somatic reversion. Hum. Genet. 1996, 98, 22–28. [Google Scholar] [CrossRef]
- Hirschhorn, R.; Yang, D.R.; Puck, J.M.; Huie, M.L.; Jiang, C.K.; Kurlandsky, L.E. Spontaneous in vivo reversion to normal of an inherited mutation in a patient with adenosine deaminase deficiency. Nat. Genet. 1996, 13, 290–295. [Google Scholar] [CrossRef]
- Ellis, N.A.; Lennon, D.J.; Proytcheva, M.; Alhadeff, B.; Henderson, E.E.; German, J. Somatic intragenic recombination within the mutated locus BLM can correct the high sister-chromatid exchange phenotype of Bloom syndrome cells. Am. J. Hum. Genet. 1995, 57, 1019–1027. [Google Scholar]
- Kvittingen, E.A.; Rootwelt, H.; Brandtzaeg, P.; Bergan, A.; Berger, R. Hereditary tyrosinemia type I. Self-induced correction of the fumarylacetoacetase defect. J. Clin. Investig. 1993, 91, 1816–1821. [Google Scholar] [CrossRef]
- Brunner, H.G.; Jansen, G.; Nillesen, W.; Nelen, M.R.; de Die, C.E.; Höweler, C.J.; van Oost, B.A.; Wieringa, B.; Ropers, H.H.; Smeets, H.J. Brief report: Reverse mutation in myotonic dystrophy. N. Engl. J. Med. 1993, 328, 476–480. [Google Scholar] [CrossRef]
- Burrow, K.L.; Coovert, D.D.; Klein, C.J.; Bulman, D.E.; Kissel, J.T.; Rammohan, K.W.; Burghes, A.H.; Mendell, J.R. Dystrophin expression and somatic reversion in prednisone-treated and untreated Duchenne dystrophy. CIDD Study Group. Neurology 1991, 41, 661–666. [Google Scholar] [CrossRef]
- Yang, T.P.; Stout, J.T.; Konecki, D.S.; Patel, P.I.; Alford, R.L.; Caskey, C.T. Spontaneous reversion of novel Lesch-Nyhan mutation by HPRT gene rearrangement. Somat. Cell Mol. Genet. 1988, 14, 293–303. [Google Scholar] [CrossRef]
- German, J.; Schonberg, S.; Louie, E.; Chaganti, R.S. Bloom’s syndrome. IV. Sister-chromatid exchanges in lymphocytes. Am. J. Hum. Genet. 1977, 29, 248–255. [Google Scholar]
- Wang, Y.; Kitahata, H.; Kosumi, H.; Watanabe, M.; Fujimura, Y.; Takashima, S.; Osada, S.I.; Hirose, T.; Nishie, W.; Nagayama, M.; et al. Collagen XVII deficiency alters epidermal patterning. Lab. Investig. 2022, 102, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Natsuga, K.; Furuta, Y.; Takashima, S.; Nohara, T.; Kosumi, H.; Mai, Y.; Higashi, H.; Ujiie, H. Detection of revertant mosaicism in epidermolysis bullosa through Cas9-targeted long-read sequencing. Hum. Mutat. 2022, 43, 529–536. [Google Scholar] [CrossRef]
- Twaroski, K.; Eide, C.; Riddle, M.J.; Xia, L.; Lees, C.J.; Chen, W.; Mathews, W.; Keene, D.R.; McGrath, J.A.; Tolar, J. Revertant mosaic fibroblasts in recessive dystrophic epidermolysis bullosa. Br. J. Dermatol. 2019, 181, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, C.; Bremer, J.; Gostynski, A.; Wertheim-Tysarowska, K.; Wozniak, K.; Bal, J.; Jonkman, M.F.; Pasmooij, A.M. Amelioration of junctional epidermolysis bullosa due to exon skipping. Br. J. Dermatol. 2016, 174, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Tolar, J.; McGrath, J.A.; Xia, L.; Riddle, M.J.; Lees, C.J.; Eide, C.; Keene, D.R.; Liu, L.; Osborn, M.J.; Lund, T.C.; et al. Patient-specific naturally gene-reverted induced pluripotent stem cells in recessive dystrophic epidermolysis bullosa. J. Investig. Dermatol. 2014, 134, 1246–1254. [Google Scholar] [CrossRef]
- van den Akker, P.C.; Nijenhuis, M.; Meijer, G.; Hofstra, R.M.; Jonkman, M.F.; Pasmooij, A.M. Natural gene therapy in dystrophic epidermolysis bullosa. Arch. Dermatol. 2012, 148, 213–216. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; Moss, C.; Parsons, M.; Almaani, N.; McGrath, J.A. Revertant mosaicism in Kindler syndrome. J. Investig. Dermatol. 2012, 132, 730–732. [Google Scholar] [CrossRef]
- Kiritsi, D.; He, Y.; Pasmooij, A.M.; Onder, M.; Happle, R.; Jonkman, M.F.; Bruckner-Tuderman, L.; Has, C. Revertant mosaicism in a human skin fragility disorder results from slipped mispairing and mitotic recombination. J. Clin. Investig. 2012, 122, 1742–1746. [Google Scholar] [CrossRef]
- Pasmooij, A.M.; Garcia, M.; Escamez, M.J.; Nijenhuis, A.M.; Azon, A.; Cuadrado-Corrales, N.; Jonkman, M.F.; Del Rio, M. Revertant mosaicism due to a second-site mutation in COL7A1 in a patient with recessive dystrophic epidermolysis bullosa. J. Investig. Dermatol. 2010, 130, 2407–2411. [Google Scholar] [CrossRef]
- Almaani, N.; Nagy, N.; Liu, L.; Dopping-Hepenstal, P.J.; Lai-Cheong, J.E.; Clements, S.E.; Techanukul, T.; Tanaka, A.; Mellerio, J.E.; McGrath, J.A. Revertant mosaicism in recessive dystrophic epidermolysis bullosa. J. Investig. Dermatol. 2010, 130, 1937–1940. [Google Scholar] [CrossRef]
- Jonkman, M.F.; Pasmooij, A.M. Revertant mosaicism-patchwork in the skin. N. Engl. J. Med. 2009, 360, 1680–1682. [Google Scholar] [CrossRef] [PubMed]
- Pasmooij, A.M.; Pas, H.H.; Bolling, M.C.; Jonkman, M.F. Revertant mosaicism in junctional epidermolysis bullosa due to multiple correcting second-site mutations in LAMB3. J. Clin. Investig. 2007, 117, 1240–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasmooij, A.M.; Pas, H.H.; Deviaene, F.C.; Nijenhuis, M.; Jonkman, M.F. Multiple correcting COL17A1 mutations in patients with revertant mosaicism of epidermolysis bullosa. Am. J. Hum. Genet. 2005, 77, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.J.; Morley, S.M.; McLean, W.H. Novel mechanism of revertant mosaicism in Dowling-Meara epidermolysis bullosa simplex. J. Investig. Dermatol. 2004, 122, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Schuilenga-Hut, P.H.; Scheffer, H.; Pas, H.H.; Nijenhuis, M.; Buys, C.H.; Jonkman, M.F. Partial revertant mosaicism of keratin 14 in a patient with recessive epidermolysis bullosa simplex. J. Investig. Dermatol. 2002, 118, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Darling, T.N.; Yee, C.; Bauer, J.W.; Hintner, H.; Yancey, K.B. Revertant mosaicism: Partial correction of a germ-line mutation in COL17A1 by a frame-restoring mutation. J. Clin. Investig. 1999, 103, 1371–1377. [Google Scholar] [CrossRef]
- Pasmooij, A.M.; Nijenhuis, M.; Brander, R.; Jonkman, M.F. Natural gene therapy may occur in all patients with generalized non-Herlitz junctional epidermolysis bullosa with COL17A1 mutations. J. Investig. Dermatol. 2012, 132, 1374–1383. [Google Scholar] [CrossRef]
- Choate, K.A.; Lu, Y.; Zhou, J.; Elias, P.M.; Zaidi, S.; Paller, A.S.; Farhi, A.; Nelson-Williams, C.; Crumrine, D.; Milstone, L.M.; et al. Frequent somatic reversion of KRT1 mutations in ichthyosis with confetti. J. Clin. Investig. 2015, 125, 1703–1707. [Google Scholar] [CrossRef]
- Choate, K.A.; Lu, Y.; Zhou, J.; Choi, M.; Elias, P.M.; Farhi, A.; Nelson-Williams, C.; Crumrine, D.; Williams, M.L.; Nopper, A.J.; et al. Mitotic recombination in patients with ichthyosis causes reversion of dominant mutations in KRT10. Science 2010, 330, 94–97. [Google Scholar] [CrossRef]
- Gudmundsson, S.; Wilbe, M.; Ekvall, S.; Ameur, A.; Cahill, N.; Alexandrov, L.B.; Virtanen, M.; Hellström Pigg, M.; Vahlquist, A.; Törmä, H.; et al. Revertant mosaicism repairs skin lesions in a patient with keratitis-ichthyosis-deafness syndrome by second-site mutations in connexin 26. Hum. Mol. Genet. 2017, 26, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nomura, T.; Miyauchi, T.; Takeda, M.; Fujita, Y.; Nishie, W.; Akiyama, M.; Ishida-Yamamoto, A.; Shimizu, H. Somatic recombination underlies frequent revertant mosaicism in loricrin keratoderma. Life Sci. Alliance 2019, 2, e201800284. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Suzuki, S.; Takeda, M.; Peh, J.T.; Aiba, M.; Natsuga, K.; Fujita, Y.; Takeichi, T.; Sakamoto, T.; Akiyama, M.; et al. Altered replication stress response due to CARD14 mutations promotes recombination-induced revertant mosaicism. Am. J. Hum. Genet. 2021, 108, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, P.C.; Pasmooij, A.M.G.; Joenje, H.; Hofstra, R.M.W.; Te Meerman, G.J.; Jonkman, M.F. A “late-but-fitter revertant cell” explains the high frequency of revertant mosaicism in epidermolysis bullosa. PLoS ONE 2018, 13, e0192994. [Google Scholar] [CrossRef]
- Kiritsi, D.; Garcia, M.; Brander, R.; Has, C.; Meijer, R.; Jose Escámez, M.; Kohlhase, J.; van den Akker, P.C.; Scheffer, H.; Jonkman, M.F.; et al. Mechanisms of natural gene therapy in dystrophic epidermolysis bullosa. J. Investig. Dermatol. 2014, 134, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nomura, T.; Miyauchi, T.; Takeda, M.; Nakamura, H.; Shinkuma, S.; Fujita, Y.; Akiyama, M.; Shimizu, H. Revertant Mosaicism in Ichthyosis with Confetti Caused by a Frameshift Mutation in KRT1. J. Investig. Dermatol. 2016, 136, 2093–2095. [Google Scholar] [CrossRef]
- Nomura, T.; Suzuki, S.; Miyauchi, T.; Takeda, M.; Shinkuma, S.; Fujita, Y.; Nishie, W.; Akiyama, M.; Shimizu, H. Chromosomal inversions as a hidden disease-modifying factor for somatic recombination phenotypes. JCI Insight 2018, 3, 97595. [Google Scholar] [CrossRef]
- Takeichi, T.; Liu, L.; Abdul-Wahab, A.; McMillan, J.R.; Stone, K.L.; Akiyama, M.; Simpson, M.A.; Parsons, M.; Mellerio, J.E.; McGrath, J.A. Large Intragenic KRT1 Deletion Underlying Atypical Autosomal Dominant Keratinopathic Ichthyosis. J. Investig. Dermatol. 2016, 136, 2095–2098. [Google Scholar] [CrossRef]
- Lim, Y.H.; Qiu, J.; Saraceni, C.; Burrall, B.A.; Choate, K.A. Genetic Reversion via Mitotic Recombination in Ichthyosis with Confetti due to a KRT10 Polyalanine Frameshift Mutation. J. Investig. Dermatol. 2016, 136, 1725–1728. [Google Scholar] [CrossRef]
- Burger, B.; Spoerri, I.; Schubert, M.; Has, C.; Itin, P.H. Description of the natural course and clinical manifestations of ichthyosis with confetti caused by a novel KRT10 mutation. Br. J. Dermatol. 2012, 166, 434–439. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Martinez-Lopez, A.; Rodriguez-Tejero, A.; Salvador-Rodriguez, L.; García-Durá, E.; Molina-Leyva, A.; Arias-Santiago, S. Loricrin keratoderma: Description of a novel mutation, systematic review and meta-analysis between genotypic and phenotypic features. J. Dtsch. Dermatol. Ges. 2020, 18, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Diociaiuti, A.; El Hachem, M.; Castiglia, D.; Zambruno, G. Ichthyosis with confetti: Clinics, molecular genetics and management. Orphanet. J. Rare Dis. 2015, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Renz, P.; Imahorn, E.; Spoerri, I.; Aushev, M.; March, O.P.; Wariwoda, H.; Von Arb, S.; Volz, A.; Itin, P.H.; Reichelt, J.; et al. Arginine- but not alanine-rich carboxy-termini trigger nuclear translocation of mutant keratin 10 in ichthyosis with confetti. J. Cell Mol. Med. 2019, 23, 8442–8452. [Google Scholar] [CrossRef] [PubMed]
- Ishida-Yamamoto, A. Loricrin keratoderma: A novel disease entity characterized by nuclear accumulation of mutant loricrin. J. Dermatol. Sci. 2003, 31, 3–8. [Google Scholar] [CrossRef]
- Pasmooij, A.M.; Jonkman, M.F.; Uitto, J. Revertant mosaicism in heritable skin diseases: Mechanisms of natural gene therapy. Discov. Med. 2012, 14, 167–179. [Google Scholar]
- Gostyński, A.; Pasmooij, A.M.; Del Rio, M.; Diercks, G.F.; Pas, H.H.; Jonkman, M.F. Pigmentation and melanocyte supply to the epidermis depend on type XVII collagen. Exp. Dermatol. 2014, 23, 130–132. [Google Scholar] [CrossRef]
- Zhang, A.; Duchatelet, S.; Lakdawala, N.; Tower, R.L.; Diamond, C.; Marathe, K.; Hill, I.; Richard, G.; Diab, Y.; Kirkorian, A.Y.; et al. Targeted Inhibition of the Epidermal Growth Factor Receptor and Mammalian Target of Rapamycin Signaling Pathways in Olmsted Syndrome. JAMA Dermatol. 2020, 156, 196–200. [Google Scholar] [CrossRef]
- Luchsinger, I.; Knöpfel, N.; Theiler, M.; Bonnet des Claustres, M.; Barbieux, C.; Schwieger-Briel, A.; Brunner, C.; Donghi, D.; Buettcher, M.; Meier-Schiesser, B.; et al. Secukinumab Therapy for Netherton Syndrome. JAMA Dermatol. 2020, 156, 907–911. [Google Scholar] [CrossRef]
- Greco, C.; Leclerc-Mercier, S.; Chaumon, S.; Doz, F.; Hadj-Rabia, S.; Molina, T.; Boucheix, C.; Bodemer, C. Use of Epidermal Growth Factor Receptor Inhibitor Erlotinib to Treat Palmoplantar Keratoderma in Patients With Olmsted Syndrome Caused by TRPV3 Mutations. JAMA Dermatol. 2020, 156, 191–195. [Google Scholar] [CrossRef]
- Hernández-Martín, A.; Kennedy-Batalla, R.; Cañedo, E.; Bernaldo-de-Quirós, E.; Carazo-Gallego, B.; Vera, A.; Torrelo, A.; Noguera-Morel, L.; González-Sarmiento, R.; Bolling, M.; et al. Imbalance in T-Helper 17 Cells and Targeted Therapy in an Infant with SAM-like Syndrome. N. Engl. J. Med. 2019, 381, 2176–2178. [Google Scholar] [CrossRef]
- Venot, Q.; Blanc, T.; Rabia, S.H.; Berteloot, L.; Ladraa, S.; Duong, J.P.; Blanc, E.; Johnson, S.C.; Hoguin, C.; Boccara, O.; et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature 2018, 558, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Czarnowicki, T.; Renert-Yuval, Y.; Holland, K.; Huynh, T.; Sadlier, M.; McAleer, M.A.; Tran, G.; Geddes, G.C.; Irvine, A.D.; et al. The spectrum of manifestations in desmoplakin gene (DSP) spectrin repeat 6 domain mutations: Immunophenotyping and response to ustekinumab. J. Am. Acad. Dermatol. 2018, 78, 498–505.e492. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; He, H.; Leonard, A.; Malik, K.; Magidi, S.; Rangel, S.; Patel, K.; Ramsey, K.; Murphrey, M.; Song, T.; et al. The Major Orphan Forms of Ichthyosis Are Characterized by Systemic T-Cell Activation and Th-17/Tc-17/Th-22/Tc-22 Polarization in Blood. J. Investig. Dermatol. 2018, 138, 2157–2167. [Google Scholar] [CrossRef]
- Paller, A.S.; Renert-Yuval, Y.; Suprun, M.; Esaki, H.; Oliva, M.; Huynh, T.N.; Ungar, B.; Kunjravia, N.; Friedland, R.; Peng, X.; et al. An IL-17-dominant immune profile is shared across the major orphan forms of ichthyosis. J. Allergy Clin. Immunol. 2017, 139, 152–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, B. The technical risks of human gene editing. Hum. Reprod. 2019, 34, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Xafis, V.; Schaefer, G.O.; Labude, M.K.; Zhu, Y.; Holm, S.; Foo, R.S.; Lai, P.S.; Chadwick, R. Germline genome modification through novel political, ethical, and social lenses. PLoS Genet. 2021, 17, e1009741. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef]
- Has, C.; South, A.; Uitto, J. Molecular Therapeutics in Development for Epidermolysis Bullosa: Update 2020. Mol. Diagn. Ther. 2020, 24, 299–309. [Google Scholar] [CrossRef]
- Gurevich, I.; Agarwal, P.; Zhang, P.; Dolorito, J.A.; Oliver, S.; Liu, H.; Reitze, N.; Sarma, N.; Bagci, I.S.; Sridhar, K.; et al. In vivo topical gene therapy for recessive dystrophic epidermolysis bullosa: A phase 1 and 2 trial. Nat. Med. 2022, 28, 780–788. [Google Scholar] [CrossRef]
- Wang, X.; Alshehri, F.; Manzanares, D.; Li, Y.; He, Z.; Qiu, B.; Zeng, M.; Sigen, A.; Lara-Sáez, I.; Wang, W. Development of Minicircle Vectors Encoding COL7A1 Gene with Human Promoters for Non-Viral Gene Therapy for Recessive Dystrophic Epidermolysis Bullosa. Int. J. Mol. Sci. 2021, 22, 12774. [Google Scholar] [CrossRef]
- Bornert, O.; Hogervorst, M.; Nauroy, P.; Bischof, J.; Swildens, J.; Athanasiou, I.; Tufa, S.F.; Keene, D.R.; Kiritsi, D.; Hainzl, S.; et al. QR-313, an Antisense Oligonucleotide, Shows Therapeutic Efficacy for Treatment of Dominant and Recessive Dystrophic Epidermolysis Bullosa: A Preclinical Study. J. Investig. Dermatol. 2021, 141, 883–893.e886. [Google Scholar] [CrossRef] [PubMed]
- Lwin, S.M.; Syed, F.; Di, W.L.; Kadiyirire, T.; Liu, L.; Guy, A.; Petrova, A.; Abdul-Wahab, A.; Reid, F.; Phillips, R.; et al. Safety and early efficacy outcomes for lentiviral fibroblast gene therapy in recessive dystrophic epidermolysis bullosa. JCI Insight 2019, 4, e126243. [Google Scholar] [CrossRef] [PubMed]
- Marinkovich, M.P.; Tang, J.Y. Gene Therapy for Epidermolysis Bullosa. J. Investig. Dermatol. 2019, 139, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.W.; Koller, J.; Murauer, E.M.; De Rosa, L.; Enzo, E.; Carulli, S.; Bondanza, S.; Recchia, A.; Muss, W.; Diem, A.; et al. Closure of a Large Chronic Wound through Transplantation of Gene-Corrected Epidermal Stem Cells. J. Investig. Dermatol. 2017, 137, 778–781. [Google Scholar] [CrossRef] [Green Version]
- Siprashvili, Z.; Nguyen, N.T.; Gorell, E.S.; Loutit, K.; Khuu, P.; Furukawa, L.K.; Lorenz, H.P.; Leung, T.H.; Keene, D.R.; Rieger, K.E.; et al. Safety and Wound Outcomes Following Genetically Corrected Autologous Epidermal Grafts in Patients With Recessive Dystrophic Epidermolysis Bullosa. JAMA 2016, 316, 1808–1817. [Google Scholar] [CrossRef]
- Mavilio, F.; Pellegrini, G.; Ferrari, S.; Di Nunzio, F.; Di Iorio, E.; Recchia, A.; Maruggi, G.; Ferrari, G.; Provasi, E.; Bonini, C.; et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat. Med. 2006, 12, 1397–1402. [Google Scholar] [CrossRef]
- Hickerson, W.L.; Remmers, A.E.; Recker, D.P. Twenty-Five Years’ Experience and Beyond with Cultured Epidermal Autografts for Coverage of Large Burn Wounds in Adult and Pediatric Patients, 1989-2015. J. Burn Care Res. 2019, 40, 157–165. [Google Scholar] [CrossRef]
- Kueckelhaus, M.; Rothoeft, T.; De Rosa, L.; Yeni, B.; Ohmann, T.; Maier, C.; Eitner, L.; Metze, D.; Losi, L.; Secone Seconetti, A.; et al. Transgenic Epidermal Cultures for Junctional Epidermolysis Bullosa—5-Year Outcomes. N. Engl. J. Med. 2021, 385, 2264–2270. [Google Scholar] [CrossRef]
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.W.; Pellegrini, G.; De Rosa, L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D.; et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017, 551, 327–332. [Google Scholar] [CrossRef]
- Gostyński, A.; Pasmooij, A.M.; Jonkman, M.F. Successful therapeutic transplantation of revertant skin in epidermolysis bullosa. J. Am. Acad. Dermatol. 2014, 70, 98–101. [Google Scholar] [CrossRef]
- Fusumae, T.T.M.; Yashiro, K.; Kitahara, H.; Amagai, M.; Kubo, A. Replacing COL7A1-deficient epidermis over the entire body by autografting cultured revertant keratinocytes in severe recessive dystrophic epidermolysis bullosa. Society for Investigative Dermatology (SID) 2021 Virtual Meeting. J. Invest. Dermatol. 2021, 141, S28 (abstract 160). [Google Scholar]
- Kogut, I.; McCarthy, S.M.; Pavlova, M.; Astling, D.P.; Chen, X.; Jakimenko, A.; Jones, K.L.; Getahun, A.; Cambier, J.C.; Pasmooij, A.M.G.; et al. High-efficiency RNA-based reprogramming of human primary fibroblasts. Nat. Commun. 2018, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Tolar, J.; Xia, L.; Lees, C.J.; Riddle, M.; McElroy, A.; Keene, D.R.; Lund, T.C.; Osborn, M.J.; Marinkovich, M.P.; Blazar, B.R.; et al. Keratinocytes from induced pluripotent stem cells in junctional epidermolysis bullosa. J. Investig. Dermatol. 2013, 133, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Tolar, J.; Xia, L.; Riddle, M.J.; Lees, C.J.; Eide, C.R.; McElmurry, R.T.; Titeux, M.; Osborn, M.J.; Lund, T.C.; Hovnanian, A.; et al. Induced pluripotent stem cells from individuals with recessive dystrophic epidermolysis bullosa. J. Investig. Dermatol. 2011, 131, 848–856. [Google Scholar] [CrossRef]
- Umegaki-Arao, N.; Pasmooij, A.M.; Itoh, M.; Cerise, J.E.; Guo, Z.; Levy, B.; Gostyński, A.; Rothman, L.R.; Jonkman, M.F.; Christiano, A.M. Induced pluripotent stem cells from human revertant keratinocytes for the treatment of epidermolysis bullosa. Sci. Transl. Med. 2014, 6, 264ra164. [Google Scholar] [CrossRef]
- Wagner, J.E.; Ishida-Yamamoto, A.; McGrath, J.A.; Hordinsky, M.; Keene, D.R.; Woodley, D.T.; Chen, M.; Riddle, M.J.; Osborn, M.J.; Lund, T.; et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N. Engl. J. Med. 2010, 363, 629–639. [Google Scholar] [CrossRef]
- Deinsberger, J.; Reisinger, D.; Weber, B. Global trends in clinical trials involving pluripotent stem cells: A systematic multi-database analysis. NPJ Regen. Med. 2020, 5, 15. [Google Scholar] [CrossRef]
- Kim, J.Y.; Nam, Y.; Rim, Y.A.; Ju, J.H. Review of the Current Trends in Clinical Trials Involving Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2022, 18, 142–154. [Google Scholar] [CrossRef]
- Zhao, Z.; Anselmo, A.C.; Mitragotri, S. Viral vector-based gene therapies in the clinic. Bioeng. Transl. Med. 2022, 7, e10258. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Akker, P.C.; Bolling, M.C.; Pasmooij, A.M.G. Revertant Mosaicism in Genodermatoses: Natural Gene Therapy Right before Your Eyes. Biomedicines 2022, 10, 2118. https://doi.org/10.3390/biomedicines10092118
van den Akker PC, Bolling MC, Pasmooij AMG. Revertant Mosaicism in Genodermatoses: Natural Gene Therapy Right before Your Eyes. Biomedicines. 2022; 10(9):2118. https://doi.org/10.3390/biomedicines10092118
Chicago/Turabian Stylevan den Akker, Peter C., Maria C. Bolling, and Anna M. G. Pasmooij. 2022. "Revertant Mosaicism in Genodermatoses: Natural Gene Therapy Right before Your Eyes" Biomedicines 10, no. 9: 2118. https://doi.org/10.3390/biomedicines10092118
APA Stylevan den Akker, P. C., Bolling, M. C., & Pasmooij, A. M. G. (2022). Revertant Mosaicism in Genodermatoses: Natural Gene Therapy Right before Your Eyes. Biomedicines, 10(9), 2118. https://doi.org/10.3390/biomedicines10092118





