Heparan Sulfate: A Regulator of White Adipocyte Differentiation and of Vascular/Adipocyte Interactions
Abstract
:1. Introduction
2. In Vivo Development of WAT
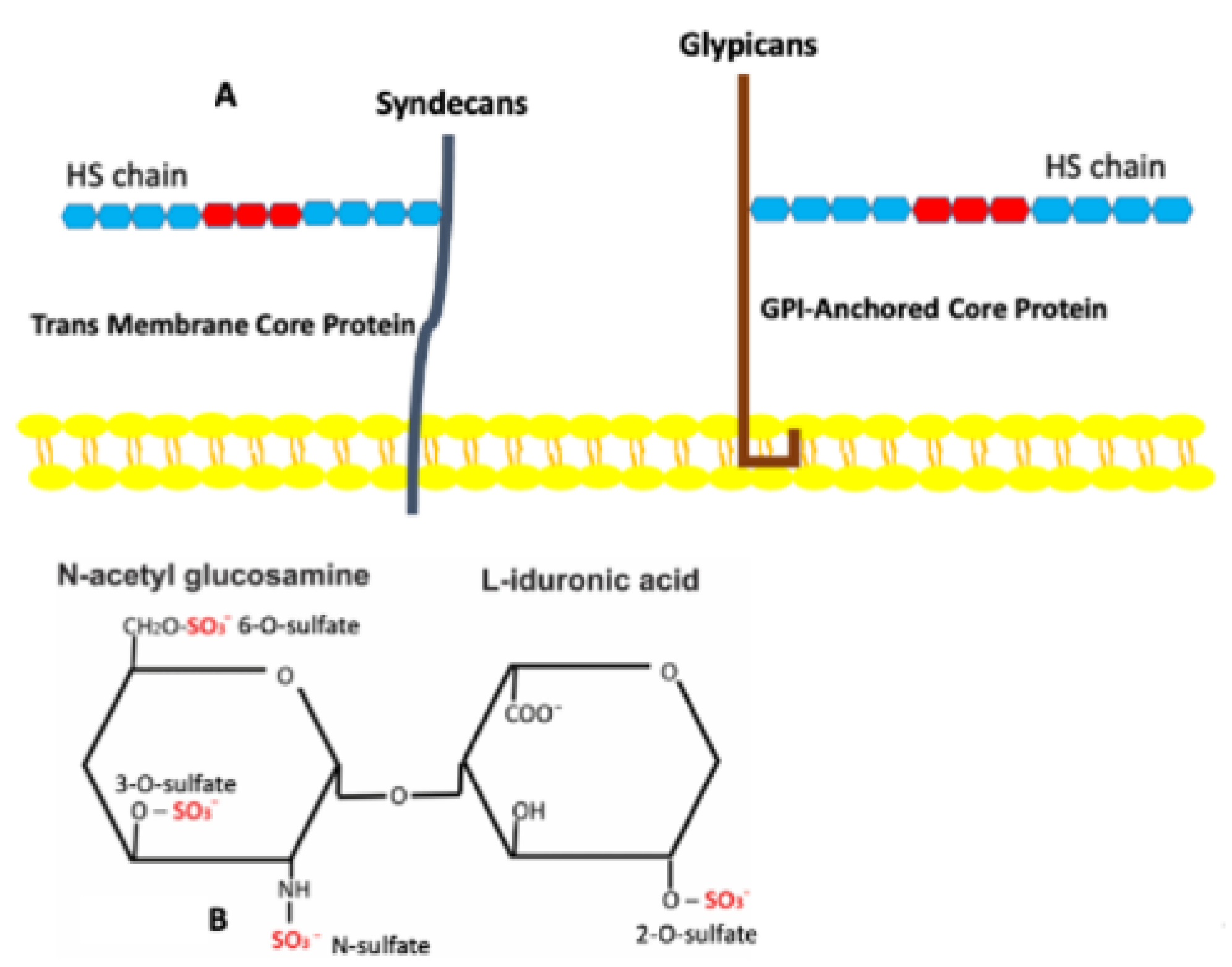
3. Heparan Sulfate Proteoglycans
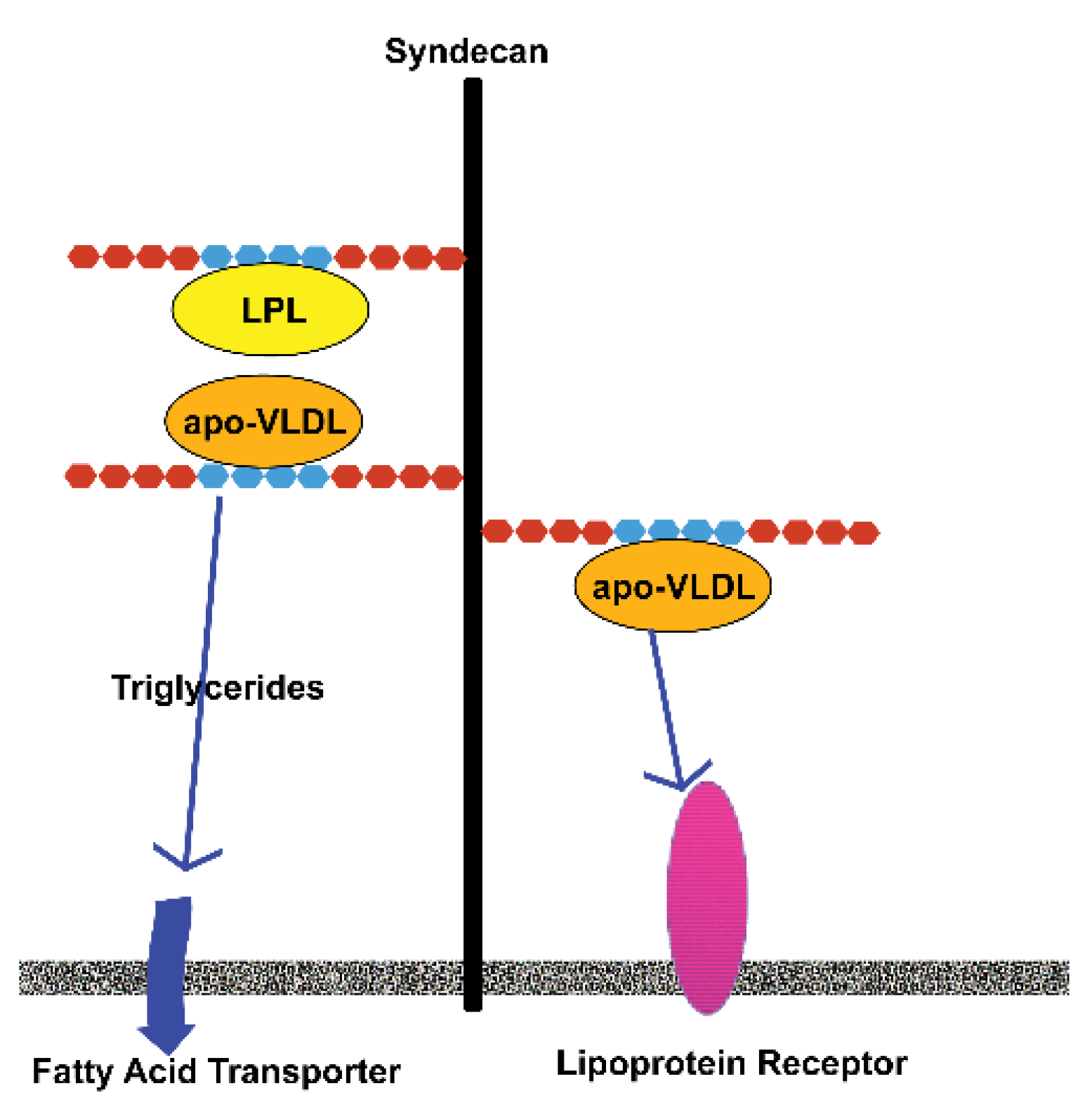
4. Sulfation of Heparan Sulfates and Its Role in Adipose Tissues
5. In Vitro Adipogenesis
6. Tissue Engineered Adipose Tissue
7. Discussion/Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gesta, S.; Tseng, Y.-H.; Kahn, C.R. Developmental origin of fat: Tracking obesity to its source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Gimble, J.M.; Lee, K.; Marra, K.G.; Rubin, J.P.; Yoo, J.J.; Vunjak-Novakovic, G.; Kaplan, D.L. Adipose tissue engineering for soft tissue regeneration. Tissue Eng. Part B 2010, 16, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Brunetti, L.; Leone, S. Adipokines: New potential therapeutic target for obesity and metabolic, rheumatic, and cardiovascular diseases. Front. Physiol. 2020, 11, 578966. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H. Secretory factors from human adipose tissue and their functional role. Proc. Nutr. Soc. 2005, 64, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Brakenhielm, E.; Wahlestedt, C.; Thyberg, J.; Cao, Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc. Natl. Acad. Sci. USA 2001, 98, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat. Rev. Drug Discov. 2010, 9, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hausman, G.J.; Wright, J.T.; Thomas, G.B. Vascular and cellular development in fetal adipose tissue: Lectin binding studies and immunocytochemistry for laminin and type IV collagen. Microvasc. Res. 1991, 41, 111–125. [Google Scholar] [CrossRef]
- Napolitano, L. The differentiation of white adipose cells. An electron microscope study. J. Cell Biol. 1963, 18, 663–679. [Google Scholar] [CrossRef]
- Smith, L.T.; Holbrook, K.A. Embryogenesis of the dermis in human skin. Pediatr. Dermatol. 1986, 3, 271–280. [Google Scholar] [CrossRef]
- Tavassoli, M. Ultrastructural development of bone marrow adipose cells. Acta Anat. 1976, 94, 65–77. [Google Scholar] [CrossRef]
- Schéele, S.; Nyström, A.; Ekblom, P. Laminin isoforms in development and disease. J. Mol. Med. 2007, 85, 825–836. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, J.; Chen, G.; Du, J.; Cai, H.; Chen, X.; Ye, G.; Luo, Y.; Zhang, L.; Duan, H.; et al. CD146 is a novel ANGPTL2 receptor that promotes obesity by manipulating lipid metabolism and energy expenditure. Adv. Sci. 2021, 8, 2004032. [Google Scholar] [CrossRef]
- Tang, W.; Zeve, D.; Suh, J.M.; Bosnakovski, D.; Kyba, M.; Hammer, R.E.; Tallquist, M.D.; Graff, J.M. White fat progenitor cells reside in the adipose vasculature. Science 2008, 322, 583–586. [Google Scholar] [CrossRef]
- Marinkovich, P.; Keene, D.R.; Rimbert, C.S.; Burgeson, R.E. Cellular origin of the dermal-epidermal basement membrane. Dev. Dyn. 1993, 197, 255–267. [Google Scholar] [CrossRef]
- Sillat, T.; Saat, R.; Pollanen, R.; Hukkanen, M.; Takagi, M.; Konttinen, Y.T. Basement membrane collagen type IV expression by human mesenchymal stem cells during adipogenic differentiation. J. Cell. Mol. Med. 2012, 16, 1485–1495. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Baber, M.A.; Traktuev, D.O.; March, K.L.; Caplan, A.I. The creation of an in vitro adipose tissue that contains a vascular-adipocyte complex. Biomaterials 2011, 32, 9667–9676. [Google Scholar] [CrossRef]
- Hassan, N.; Greve, B.; Sánchez, N.A.; Götte, M. Cell-surface heparan sulfate proteoglycans as multifunctional integrators of signaling in cancer. Cell. Signal. 2021, 77, 109822. [Google Scholar] [CrossRef]
- Iozzo, R.V. Heparan sulfate proteoglycans: Intricate molecules with intriguing functions. J. Clin. Investig. 2001, 108, 165–167. [Google Scholar] [CrossRef]
- Hiebert, L.M. Heparan sulfate proteoglycans in diabetes. Semin. Thromb. Hemost. 2021, 47, 261–273. [Google Scholar] [CrossRef]
- Gesta, S.; Blüher, M.; Yamamoto, Y.; Norris, A.W.; Berndt, J.; Kralisch, S.; Boucher, J.; Lewis, C.; Kahn, C.R. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. USA 2006, 103, 6676–6681. [Google Scholar] [CrossRef] [Green Version]
- Weng, X.; Maxwell-Warburton, S.; Hasib, A.; Ma, L.; Kang, L. The membrane receptor CD44, novel insights into metabolism. Trends Endocrinol. Metab. 2022, 33, 5318–5332. [Google Scholar] [CrossRef]
- Gómez-Gil, V.; Pascual, G.; Pérez-Köhler, B.; Cifuentes, A.; Buján, J.; Bellón, J.M. Involvement of transforming growth factor-β3 and betaglycan in the cytoarchitecture of postoperative omental adhesions. J. Surg. Res. 2014, 187, 699–711. [Google Scholar] [CrossRef]
- Bix, G.; Iozzo, R.V. Novel interactions of perlecan: Unraveling perlecan’s role in angiogenesis. Microsc. Res. Tech. 2008, 71, 339–348. [Google Scholar] [CrossRef]
- Rhodes, J.M.; Simons, M. The extracellular matrix and blood vessel formation: Not just a scaffold. J. Cell. Mol. Med. 2007, 11, 176–205. [Google Scholar] [CrossRef]
- Gallagher, J.T.; Turnbull, J.E.; Lyon, M. Patterns of sulfation in heparan sulfate: Polymorphism based on a common structural theme. Int. J. Biochem. 1992, 24, 553–560. [Google Scholar] [CrossRef]
- Langford, R.; Hurrion, E.; Dawson, P.A. Genetics and pathophysiology of mammalian sulfate biology. J. Genet. Genom. 2017, 44, 7–20. [Google Scholar] [CrossRef]
- Gallagher, J. Fell-Muir Lecture: Heparan sulphate and the art of cell regulation: A polymer chain conducts the protein orchestra. Int. J. Exp. Pathol. 2015, 96, 203–231. [Google Scholar] [CrossRef]
- Jenniskens, G.J.; Veerkamp, J.H.; Van Kuppevelt, T.H. Heparan sulfates in skeletal muscle development and physiology. J. Cell. Physiol. 2006, 206, 283–294. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Brejning, J.; Garcia, R.; Zhang, H.; Hayden, M.R.; Vilaró, S.; Gliemann, J. Segments in the C-terminal folding domain of lipoprotein lipase important for binding to the low density lipoprotein receptor-related protein and to heparan sulfate proteoglycans. J. Biol. Chem. 1997, 272, 5821–5827. [Google Scholar] [CrossRef]
- Sepuru, K.M.; Rajarathnam, K. Structural basis of chemokine interactions with heparan sulfate, chondroitin sulfate, and dermatan sulfate. J. Biol. Chem. 2019, 294, 15650–15661. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Loka, R.S.; Song, Z.; Vlodavsky, I.; Zhang, K.; Nguyen, H.N. Modulating heparanase activity: Tuning sulfation pattern and glycosidic linkages of oligosaccharides. J. Med. Chem. 2020, 63, 4227–4255. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, T.; Morita, M.; Shikida, T.; Karpati, A.; Kitano, H.; Nakamura, T.; Sugawara, A.; Yamaguchi, Y.; Yanai, K. Heparan sulfate in pancreatic β-cells contributes to normal glucose homeostasis by regulating insulin secretion. Biochem. Biophys. Res. Comm. 2018, 499, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Wilsie, L.C.; Chanchani, S.; Navaratna, D.; Orlando, R.A. Cell surface heparan sulfate proteoglycans contribute to intracellular lipid accumulation in adipocytes. Lipids Health Dis. 2005, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Pineau, N.; Carrino, D.A.; Caplan, A.I.; Breton, L. Biological evaluation of a new C-xylopyranoside derivative (C-Xyloside) and its role in glycosaminoglycan biosynthesis. Eur. J. Dermatol. 2011, 21, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Kolset, S.O.; Salmivirta, M. Cell surface heparan sulfate proteoglycans and lipoprotein metabolism. Cell. Mol. Life Sci. 1999, 56, 857–870. [Google Scholar] [CrossRef]
- Misra, K.B.; Kim, K.C.; Kim, S.; Cho, M.; Low, M.G.; Bensadoun, A. Purification and characterization of adipocyte heparan sulfate proteoglycans with affinity for lipoprotein lipase. J. Biol. Chem. 1994, 269, 23838–23844. [Google Scholar] [CrossRef]
- Parthasarathy, N.; Gotow, L.F.; Bottoms, J.D.; Obunike, J.C.; Naggi, A.; Casu, B.; Goldberg, I.J.; Wagner, W.D. Influence of glucose on production and N-sulfation of heparan sulfate in cultured adipocyte cells. Mol. Cell. Biochem. 2000, 213, 1–9. [Google Scholar] [CrossRef]
- Xi, G.; Solum, M.A.; Wai, C.; Maile, L.A.; Rosen, C.J.; Clemmons, D.R. The heparin-binding domains of IGFBP-2 mediate its inhibitory effect on preadipocyte differentiation and fat development in male mice. Endocrinology 2013, 154, 4146–4157. [Google Scholar] [CrossRef]
- Ussar, S.; Bezy, O.; Blüher, M.; Kahn, C.R. Glypican-4 enhances insulin signaling via interaction with the insulin receptor and serves as a novel adipokine. Diabetes 2012, 61, 2289–2298. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Morita, S.; Shimane, A.; Otsuka, R.; Mei, Y.; Irie, F.; Yamaguchi, Y.; Yanai, K.; Yoshikawa, T. Heparan sulfate promotes differentiation of white adipocytes to maintain insulin sensitivity and glucose homeostasis. J. Biol. Chem. 2021, 297, 101006. [Google Scholar] [CrossRef]
- Yamashita, Y.; Nakada, S.; Yoshihara, T.; Nara, T.; Furuya, N.; Miida, T.; Hattori, N.; Arikawa-Hirasasw, E. Perlecan, a heparan sulfate proteoglycan, regulates systemic metabolism with dynamic changes in adipose tissue and skeletal muscle. Sci. Rep. 2018, 8, 7766. [Google Scholar] [CrossRef]
- Christiaens, V.; Lijnen, H.R. Angiogenesis and development of adipose tissue. Mol. Cell. Endocrinol. 2010, 318, 2–9. [Google Scholar] [CrossRef]
- Van Wijk, X.M.R.; van Kuppevelt, T.H. Heparan sulfate in angiogenesis: A target for therapy. Angiogenesis 2014, 17, 443–462. [Google Scholar] [CrossRef]
- Kim, S.; Subramanian, V.; Abdel-Latif, A.; Lee, S. Role of heparin-binding epidermal growth factor-like growth factor in oxidative stress-associated metabolic diseases. Metab. Syndr. Relat. Disord. 2019, 18, 186–196. [Google Scholar] [CrossRef]
- Pessentheiner, A.R.; Ducasa, G.M.; Gordts, P.L.S.M. Proteoglycans in obesity-associated metabolic dysfunction and meta-inflammation. Front. Immunol. 2008, 11, 769. [Google Scholar] [CrossRef]
- Goldstein, B.J.; Scalia, R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J. Clin. Endocrinol. Metab. 2004, 89, 2563–2568. [Google Scholar] [CrossRef]
- Wiecek, A.; Adamczak, M.; Chudek, J. Adiponectin—An adipokines with unique metabolic properties. Nephrol. Dial. Transplant. 2007, 22, 981–988. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Maeda, K.; Kuriyama, H.; Okamoto, Y.; Kumada, M.; Hotta, K.; Nishida, M.; Takahashi, M.; et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB binding protein and regulates growth factor-induced common post receptor signal in vascular smooth muscle cell. Circulation 2002, 105, 2890–2893. [Google Scholar] [CrossRef]
- Martino, M.M.; Brkic, S.; Bovo, E.; Burger, M.; Schaefer, D.J.; Wolff, T.; Gurke, L.; Briquez, P.S.; Larsson, H.M.; Bianni-Barrera, R.; et al. Extracellular matrix and growth factor engineering for controlled angiogenesis in regenerative medicine. Front. Bioeng. Biotech. 2015, 3, 4. [Google Scholar] [CrossRef]
- Zoeller, J.J.; Whitelock, J.M.; Iozzo, R.V. Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol. 2009, 28, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Vashi, A.V.; Abberton, K.M.; Thomas, G.P.; Morrison, W.A.; O’Connor, A.J.; Cooper-White, J.J.; Thompson, E.W. Adipose tissue engineering based on the controlled release of fibroblast growth factor-2 in a collagen matrix. Tissue Eng. 2006, 12, 3035–3043. [Google Scholar] [CrossRef]
- Yoon, J.J.; Chung, H.J.; Lee, H.J.; Park, T.G. Heparin-immobilized biodegradable scaffolds for local and sustained release of angiogenic growth factor. J. Biomed. Mater. Res. 2006, 79A, 934–942. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Baber, M.A.; Caplan, A.I. Human dermal fibroblast subpopulations: Differential interactions with vascular endothelial cells in coculture: Non-soluble factors in the extracellular matrix influence interactions. Wound Repair Regen. 2008, 16, 300–309. [Google Scholar] [CrossRef]
- Aratani, Y.; Kitagawa, Y. Enhanced synthesis and secretion of type IV collagen and entactin during adipose conversion of 3T3-L1 cells and production of unorthodox laminin complex. J. Biol. Chem. 1988, 263, 16163–16189. [Google Scholar] [CrossRef]
- Sorrell, J.M. Vascular Adipose Complex. In Angiogenesis in Adipose Tissue; Cao, Y., Ed.; Springer: New York, NY, USA, 2013; pp. 53–73. [Google Scholar]
- Aubin, K.; Vincent, C.; Proulx, M.; Mayrand, D.; Fradette, J. Creating capillary networks within human engineered tissues: Impact of adipocytes and their secretory products. Acta Biomater. 2015, 11, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Huttala, O.; Palmroth, M.; Hemminki, P.; Toimela, T.; Heinonen, T.; Ylikomi, T.; Sarkanen, J.-R. Development of versatile human in vitro vascularized adipose tissue model with serum-free angiogenesis and natural adipogenesis induction. Basic Clin. Pharmacol. Toxicol. 2018, 123, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Ader, I.; Creff, J.; Leménager, H.; Achard, P.; Casteilla, L.; Sensebé, L.; Carrière, A.; Deschaseaux, F. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci. Rep. 2019, 9, 7250. [Google Scholar] [CrossRef] [PubMed]
- Proulx, M.; Mayrand, D.; Vincent, C.; Boisvert, A.; Aubin, K.; Trottier, V.; Fradette, J. Short-term post-implantation dynamics of in vitro engineered human microvascularized adipose tissues. Biomed. Mater. 2018, 13, 065013. [Google Scholar] [CrossRef]
- Vermette, M.; Trottier, V.; Ménard, V.; Saint-Pierre, L.; Roy, A.; Fradette, J. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials 2007, 28, 2850–2860. [Google Scholar] [CrossRef]
- Verseijden, F.; Posthumus-van Sluijs, S.J.; van Neck, J.W.; Hofer, S.O.; Hovius, S.E.; van Osch, G.J. Vascularization of prevascularized and non-prevascularized fibrin-based human adipose tissue constructs after implantation in nude mice. J. Tissue Eng. Regen. Med. 2012, 6, 169–178. [Google Scholar] [CrossRef]
- Frerich, B.; Winter, K.; Scheller, K.; Braumann, U.D. Comparison of different fabrication techniques for human adipose tissue engineering in severe combined immunodeficient mice. Artif. Organs 2011, 36, 227–237. [Google Scholar] [CrossRef]
- Krawiec, J.T.; Liao, H.-T.; Kwan, L.; D’Amore, A.; Weinbaum, J.S.; Rubin, P.; Wagner, W.R.; Vorp, D.A. Evaluation of the stromal vascular fraction of adipose tissue as the basis for a stem cell-based tissue-engineered vascular graft. J. Vasc. Surg. 2017, 66, 883–889. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Boyd, N.L. The adipose stromal vascular fraction as a complex cellular source for tissue engineering applications. Tissue Eng. B 2018, 24, 289–299. [Google Scholar] [CrossRef]
- Gomillion, C.T.; Burg, K.J.L. Stem cells and adipose tissue engineering. Biomaterials 2006, 27, 6052–6063. [Google Scholar] [CrossRef]
- Merfeld-Clauss, S.; Collahalli, N.; March, K.L.; Traktuev, D.O. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng. Part A 2010, 16, 2953–2966. [Google Scholar] [CrossRef]
- Traktuev, D.O.; Prater, D.N.; Merfeld-Clauss, S.; Sanjeevaiah, A.R.; Saadatzadeh, M.R.; Murphy, M.; Johnstone, B.H.; Ingram, D.A.; March, K.L. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ. Res. 2009, 104, 1410–1420. [Google Scholar] [CrossRef]
- Berthod, F.; Germain, L.; Tremblay, N.; Auger, F.A. Extracellular matrix deposition by fibroblasts is necessary to promote capillary-like tube formation in vitro. J. Cell. Physiol. 2006, 207, 491–498. [Google Scholar] [CrossRef]
- Borges, J.; Mueller, M.C.; Padron, T.; Tegtmeier, F.; Lang, E.M.; Stark, G.B. Engineered adipose tissue supplied by functional microvessels. Tissue Eng. 2003, 9, 1263–1270. [Google Scholar] [CrossRef]
- Rogal, J.; Roosz, J.; Teufel, C.; Cipriano, M.; Xu, R.; Eisler, W.; Weiss, M.; Schenke-Layland, K.; Loskill, P. Autologous human immunocompetent white adipose tissue-on-chip. Adv. Sci. 2022, 9, 2104451. [Google Scholar] [CrossRef]
- Aubin, K.; Safoine, M.; Proulx, M.; Audet-Casgrain, M.A.; Cote, J.F.; Tetu, F.A.; Roy, A.; Fradette, J. Characterization of in vitro engineered human adipose tissues: Relevant adipokine secretion and impact of TNF-alpha. PLoS ONE 2015, 10, e0137612. [Google Scholar] [CrossRef]
- Tran-Lundmark, K.; Tran, P.-K.; Paulsson-Berne, G.; Fridén, V.; Soininen, R. Heparan sulfate in perlecan promotes mouse atherosclerosis. Roles in lipid permeability, lipid retention, and smooth muscle cell proliferation. Circ. Res. 2008, 103, 43–52. [Google Scholar] [CrossRef]
- Huber, B.; Volz, A.-C.; Kluger, P.J. Understanding the effects of mature adipocytes and endothelial cells on fatty acid metabolism and vascular tone in physiological fatty tissue for vascularized adipose tissue engineering. Cell Tissue Res. 2015, 362, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Xu, D.; Kang, B.N.; Nussbacher, J.K.; Handel, T.M.; Ley, K.; Sriramarao, P.; Esko, J.D. Inactivation of heparan sulfate 2-O-sulfotransferase accentuates neutrophil infiltration during acute inflammation in mice. Blood 2012, 120, 1742–1751. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-A.; Kim, Y.; Hong, S.-H.; Lee, J.; Cho, Y.G.; Han, J.-Y.; Kim, Y.-H.; Han, J.; Shim, Y.M.; Lee, Y.-S.; et al. Epigenetic inactivation of heparan sulfate (glucosamine) 3-O-sulfotransferase 2 in lung cancer and its role in tumorigenesis. PLoS ONE 2013, 8, e79634. [Google Scholar]

| Proteoglycan | Present in Adipose Tissues | Location |
|---|---|---|
| Full-Time HSPGs | ||
| Syndecans 1-4 | Syndecans 1, 3, 4 | Cell Surface |
| Glypicans 1-6 | Glypican 4 | Cell Surface |
| Perlecan | Yes | Basement Membrane, Matrix |
| Agrin | Unknown | Matrix |
| Type XVII Collagen | Unknown | Matrix |
| Part-Time HSPGs | ||
| CD44 | Yes | Cell Surface |
| Betaglycan | Yes | Cell Surface |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorrell, J.M.; Caplan, A.I. Heparan Sulfate: A Regulator of White Adipocyte Differentiation and of Vascular/Adipocyte Interactions. Biomedicines 2022, 10, 2115. https://doi.org/10.3390/biomedicines10092115
Sorrell JM, Caplan AI. Heparan Sulfate: A Regulator of White Adipocyte Differentiation and of Vascular/Adipocyte Interactions. Biomedicines. 2022; 10(9):2115. https://doi.org/10.3390/biomedicines10092115
Chicago/Turabian StyleSorrell, J. Michael, and Arnold I. Caplan. 2022. "Heparan Sulfate: A Regulator of White Adipocyte Differentiation and of Vascular/Adipocyte Interactions" Biomedicines 10, no. 9: 2115. https://doi.org/10.3390/biomedicines10092115
APA StyleSorrell, J. M., & Caplan, A. I. (2022). Heparan Sulfate: A Regulator of White Adipocyte Differentiation and of Vascular/Adipocyte Interactions. Biomedicines, 10(9), 2115. https://doi.org/10.3390/biomedicines10092115





