Clinical Impact of Monoclonal Antibodies in the Treatment of High-Risk Patients with SARS-CoV-2 Breakthrough Infections: The ORCHESTRA Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Variables Description and Definition
2.3. Virological and Serological Analyses
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Description of the Study Population
3.2. Virological Characteristics
3.3. Clinical Outcomes
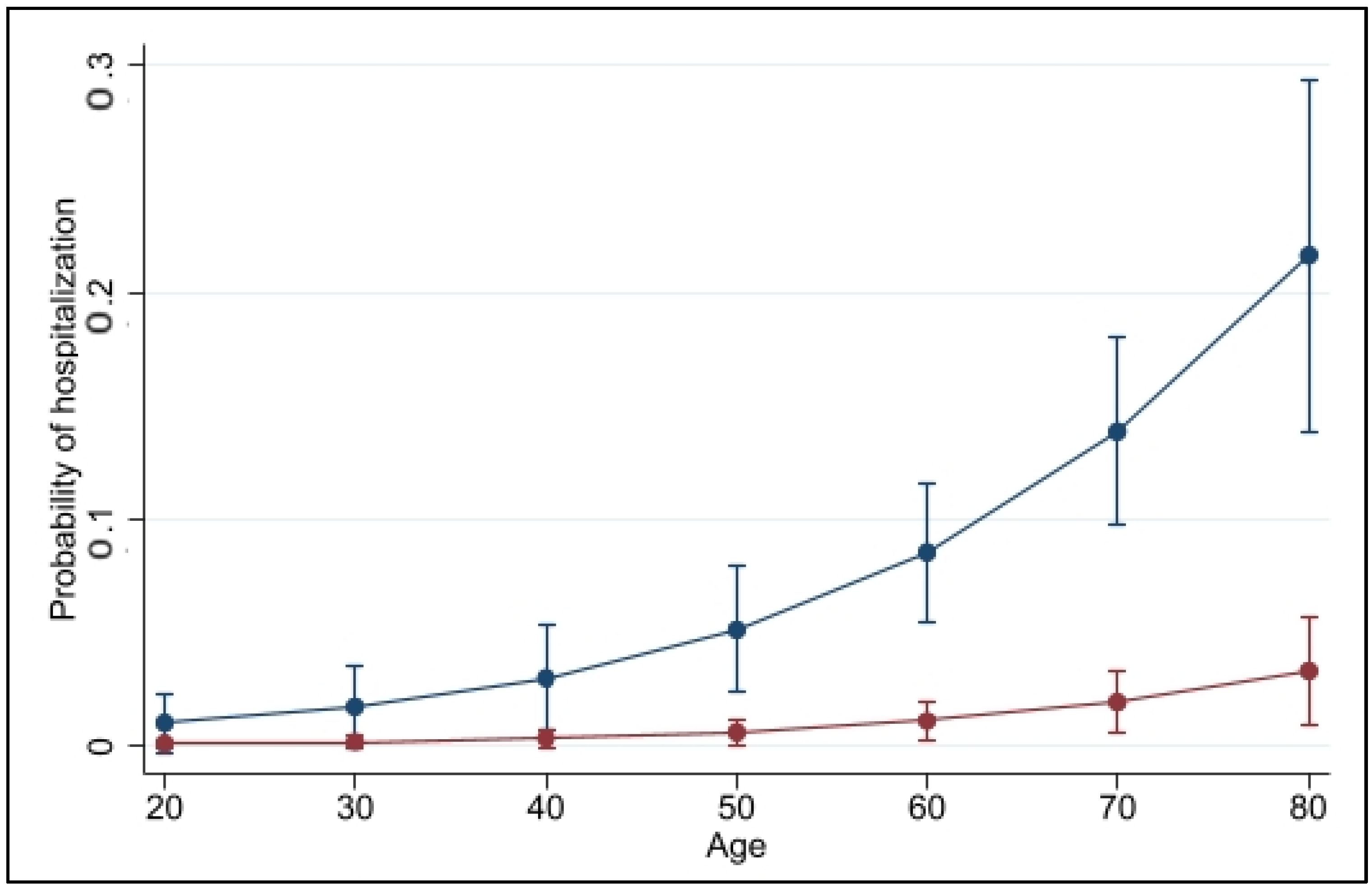
3.4. Predictors for COVID-19 Related Hospitalization
3.5. Serological Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swift, M.D.; Breeher, L.E.; Tande, A.J.; Tommaso, C.P.; Hainy, C.M.; Chu, H.; Murad, M.H.; Berbari, E.F.; Virk, A. Effectiveness of Messenger RNA Coronavirus Disease 2019 (COVID-19) Vaccines Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in a Cohort of Healthcare Personnel. Clin. Infect. Dis. 2021, 73, e1376–e1379. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Lipsitch, M.; Krammer, F.; Regev-Yochay, G.; Lustig, Y.; Balicer, R.D. SARS-CoV-2 breakthrough infections in vaccinated individuals: Measurement, causes and impact. Nat. Rev. Immunol. 2022, 22, 57–65. [Google Scholar] [CrossRef] [PubMed]
- François Watkins, L.K.; Mitruka, K.; Dorough, L.; Bressler, S.S.; Kugeler, K.J.; Sadigh, K.S.; Birhane, M.G.; Nolen, L.D.; Fischer, M. Characteristics of Reported Deaths Among Fully Vaccinated Persons with Coronavirus Disease 2019—United States, January–April 2021. Clin. Infect. Dis. 2022, ciac066. [Google Scholar] [CrossRef]
- Brosh-Nissimov, T.; Orenbuch-Harroch, E.; Chowers, M.; Elbaz, M.; Nesher, L.; Stein, M.; Maor, Y.; Cohen, R.; Hussein, K.; Weinberger, M.; et al. BNT162b2 vaccine breakthrough: Clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin. Microbiol. Infect. 2021, 27, 1652–1657. [Google Scholar] [CrossRef]
- Coburn, S.B.; Humes, E.; Lang, R.; Stewart, C.; Hogan, B.C.; Gebo, K.A.; Napravnik, S.; Edwards, J.K.; Browne, L.E.; Park, L.S.; et al. COVID-19 infections post-vaccination by HIV status in the United States. medRxiv, 2021; in press. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Valentina, S.; et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: A randomized clinical trial. JAMA 2021, 325, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef]
- Dougan, M.; Nirula, A.; Azizad, M.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; Hebert, C.; Perry, R.; Boscia, J.; Heller, B.; et al. Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19. N. Engl. J. Med. 2021, 385, 1382–1392. [Google Scholar] [CrossRef]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Takashita, E.; Kinoshita, N.; Yamayoshi, S.; Sakai-Tagawa, Y.; Fujisaki, S.; Ito, M.; Iwatsuki-Horimoto, K.; Chiba, S.; Halfmann, P.; Nagai, H.; et al. Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant. N. Engl. J. Med. 2022, 386, 995–998. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.K.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Public Health Investigations of COVID-19 Vaccine Breakthrough Cases. Case Investigation Protocol. Available online: https://www.cdc.gov/vaccines/covid-19/downloads/COVID-vaccine-breakthrough-case-investigations-Protocol.pdf (accessed on 27 May 2022).
- Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 27 May 2022).
- O’Toole, Á.; Hill, V.; Pybus, O.G.; Watts, A.; Bogoch, I.I.; Khan, K.; Messina, J.P.; Tegally, H.; Lessells, R.R.; Giandhari, J. COVID-19 Genomics UK (COG-UK) Consortium; Network for Genomic Surveillance in South Africa (NGS-SA); Brazil-UK CADDE Genomic Network; et al. Tracking the international spread of SARS-CoV-2 lineages B.1.1.7 and B.1.351/501Y-V2 with grinch. Wellcome Open Res. 2021, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Expert Committee on Biological Standardization: Seventy-Fourth Report. 2022. Available online: https://www.who.int/publications/i/item/9789240046870 (accessed on 27 May 2022).
- Wang, S.Y.; Juthani, P.V.; Borges, K.A.; Shallow, M.K.; Gupta, A.; Price, C.; Won, C.H.; Chun, H.J. Severe breakthrough COVID-19 cases in the SARS-CoV-2 delta (B.1.617.2) variant era. Lancet Microbe 2022, 3, e4–e5. [Google Scholar] [CrossRef]
- Wang, L.; Kaelber, D.C.; Xu, R.; Berger, N.A. COVID-19 breakthrough infections, hospitalizations and mortality in fully vaccinated patients with hematologic malignancies: A clarion call for maintaining mitigation and ramping-up research. Blood Rev. 2022, 54, 100931. [Google Scholar] [CrossRef]
- Razonable, R.R.; Ganesh, R.; Bierle, D.M. Clinical Prioritization of Antispike Monoclonal Antibody Treatment of Mild to Moderate COVID-19. Mayo Clin. Proc. 2022, 97, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Bierle, D.M.; Ganesh, R.; Tulledge-Scheitel, S.; Hanson, S.N.; Arndt, L.L.; Wilker, C.G.; Razonable, R.R. Monoclonal Antibody Treatment of Breakthrough COVID-19 in Fully Vaccinated Individuals with High-Risk Comorbidities. J. Infect. Dis. 2022, 225, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Moline, H.L.; Whitaker, M.; Deng, L.; Rhodes, J.C.; Milucky, J.; Pham, H.; Patel, K.; Anglin, O.; Reingold, A.; Chai, S.J.; et al. Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged ≥65 Years—COVID-NET, 13 States, February–April 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1088–1093. [Google Scholar] [CrossRef]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef]
- Alexander, J.L.; Kennedy, N.A.; Ibraheim, H.; Anandabaskaran, S.; Saifuddin, A.; Castro Seoane, R.; Liu, Z.; Nice, R.; Bewshea, C.; D’Mello, A.; et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): A multicentre, prospective, case-control study. Lancet Gastroenterol. Hepatol. 2022, 7, 342–352. [Google Scholar] [CrossRef]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Arbel, Y.S.; Scarfò, L.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Correlates of Protection Induced by Vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [Green Version]
- Bartleson, J.M.; Radenkovic, D.; Covarrubias, A.J.; Furman, D.; Winer, D.A.; Verdin, E. SARS-CoV-2, COVID-19 and the aging immune system. Nat. Aging 2021, 1, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Johannes, P.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- Mazzaferri, F.; Mirandola, M.; Savoldi, A.; De Nardo, P.; Morra, M.; Tebon, M.; Armellini, M.; De Luca, G.; Calandrino, L.; Sasset, L.; et al. Exploratory Data on the Clinical Efficacy of Monoclonal Antibodies against SARS-CoV-2 Omicron Variant of Concern. Available online: https://www.medrxiv.org/content/10.1101/2022.05.06.22274613v1 (accessed on 22 May 2022).

| Variables | All Patients (N = 847) | Primary Infection (N = 414) | Breakthrough Infection (N = 433) | p Value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, (years), median (Q1–Q3) | 63 (52–73) | 60 (50–71) | 66 (51–73) | <0.001 |
| Age Group, n (%) | ||||
| ≥65 years | 388 (45.8) | 156 (18.4) | 232 (27.4) | <0.001 |
| Gender, n (%) | 424 (50.1) | 219 (25.9) | 205 (24.2) | 0.106 |
| Male | ||||
| Body Mass Index ≥ 30 kg/m2 | 399 (47.1) | 233 (53.9) | 176 (40.7) | <0.001 |
| Clinical characteristics | ||||
| Underlying comorbidity Type, n% | ||||
| Chronic kidney disease | 53 (6.3) | 18 (2.1) | 35 (4.1) | 0.025 |
| Cardiovascular disease, any | 433 (52.3) | 209 (50.5) | 234 (54.1) | 0.300 |
| Hypertension alone | 244 (28.8) | 112 (27.1) | 132 (30.5) | 0.270 |
| Diabetes mellitus | 120 (14.2) | 56 (6.6) | 64 (7.6) | 0.601 |
| Neurological disease | 52 (6.1) | 28 (3.3) | 24 (2.8) | 0.459 |
| Chronic respiratory disease | 149 (17.6) | 62 (7.3) | 87 (10.3) | 0.051 |
| Immunocompromising condition | 180 (21.3) | 56 (6.6) | 124 (14.6) | <0.001 |
| Number, n (%) | ||||
| ≥2 underlying comorbidities | 410 (48.4) | 183 (44.2) | 227 (52.4) | 0.017 |
| Time from symptoms onset to infusion (days), median (Q1–Q3) | 3 (2–5) | 4 (2–5) | 3 (2–4) | 0.005 |
| Type of mAb regimen prescribed, n (%) | ||||
| Bamlanivimab | 43 (5.1) | 43 (5.1) | - | |
| Bamlanivimab-etesevimab | 384 (45.3) | 198 (23.4) | 186 (22.0) | <0.001 |
| Casirivimab-imdevimab | 315 (37.2) | 136 (16.1) | 179 (21.1) | |
| Sotrovimab | 105 (12.4) | 37 (4.4) | 68 (8.0) | |
| In-vitro active mAb, n (%) (n = 715) | ||||
| Yes | 614 (86.0) | 323 (52.6) | 291 (47.4) | |
| No | 101 (14.0) | 33 (32.7) | 68 (67.3) | 0.001 |
| Laboratory data | ||||
| Viral variant, NextClade, n (%) (n = 715) | ||||
| 20I (Alpha) | 153 (21.4) | 153 (43.0) | 0 (0) | |
| 21A, 21I, 21J (Delta) | 399 (55.9) | 149 (41.9) | 250 (69.6) | <0.001 |
| 21K, 21L (Omicron) | 163 (22.7) | 54 (15.1) | 109 (30.4) | |
| Baseline anti-SARS-CoV-2 serology (n = 547) | ||||
| Anti-spike, median BAU/mL (Q1–Q3) | NA | 1.26 (0.40–11.18) | 842 (294–1997) | <0.001 |
| Anti-RBD, median BAU/mL (Q1–Q3) | NA | 1.55 (0.48–8.19) | 1162 (398–3106) | <0.001 |
| Anti-NC, median BAU/mL (Q1–Q3) | NA | 1.03 (0.22–5.42) | 1.32 (0.10–5.70) | 0.06 |
| Clinical Outcomes | All Patients (N = 847) | Primary Infection (N = 414) | Breakthrough Infection (N = 433) | p Value |
|---|---|---|---|---|
| 28-day COVID-19-related hospitalization, n (%) | 50 (5.9) | 42 (10.1) | 8 (1.8) | <0.001 |
| Length of stay, median days (Q1–Q3) | 9 (6–14) | 9 (7–14) | 8 (5–12) | 0.339 |
| Need for ventilation, n (%) | 10 (1.1) | 9 (2.1) | 1 (0.2) | |
| Death, n (%) | 2 (0.2) | 1 | 1 |
| 28-day COVID-19 Related Hospitalization | ||||
|---|---|---|---|---|
| Variable | Bivariate | Multivariate | ||
| OR (95%CI) | p-Value | aOR (95%CI) | p-Value | |
| Age § | 1.05 (1.03–1.08) | <0.001 | 1.06 (1.03–1.08) | <0.001 |
| Gender (male) | 2.01 (1.10–3.68) | 0.022 | 1.97 (1.04–3.73) | 0.037 |
| Underlying comorbidities ≥ 2 | 1.79 (0.99–3.23) | 0.050 | ||
| Diabetes | 1.16 (0.53–2.54) | 0.702 | ||
| BMI ≥ 30 kg/m2 | 0.67 (0.37–1.21) | 0.186 | ||
| Any immunocompromising condition | 1.04 (0.52–2.09) | 0.894 | 2.35 (1.08–5.08) | 0.003 |
| Cardiovascular disease | 2.22 (1.19–4.13) | 0.012 | 1.49 (0.74–2.99) | 0.259 |
| Chronic respiratory disease | 1.34 (0.67–2.69) | 0.840 | ||
| Chronic kidney disease | 1.33 (0.45–3.83) | 0.601 | ||
| Time from symptoms onset to mAb infusion | 1.02 (0.99–1.03) | 0.083 | ||
| Breakthrough infection | 0.11 (0.08–0.35) | <0.001 | 0.12 (0.05–0.27) | <0.001 |
| In vitro active mAb | 0.86 (0.45–1.66) | 0.666 | 0.70 (0.35–1.41) | 0.320 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savoldi, A.; Morra, M.; Castelli, A.; Mirandola, M.; Berkell, M.; Smet, M.; Konnova, A.; Rossi, E.; Cataudella, S.; De Nardo, P.; et al. Clinical Impact of Monoclonal Antibodies in the Treatment of High-Risk Patients with SARS-CoV-2 Breakthrough Infections: The ORCHESTRA Prospective Cohort Study. Biomedicines 2022, 10, 2063. https://doi.org/10.3390/biomedicines10092063
Savoldi A, Morra M, Castelli A, Mirandola M, Berkell M, Smet M, Konnova A, Rossi E, Cataudella S, De Nardo P, et al. Clinical Impact of Monoclonal Antibodies in the Treatment of High-Risk Patients with SARS-CoV-2 Breakthrough Infections: The ORCHESTRA Prospective Cohort Study. Biomedicines. 2022; 10(9):2063. https://doi.org/10.3390/biomedicines10092063
Chicago/Turabian StyleSavoldi, Alessia, Matteo Morra, Alessandro Castelli, Massimo Mirandola, Matilda Berkell, Mathias Smet, Angelina Konnova, Elisa Rossi, Salvatore Cataudella, Pasquale De Nardo, and et al. 2022. "Clinical Impact of Monoclonal Antibodies in the Treatment of High-Risk Patients with SARS-CoV-2 Breakthrough Infections: The ORCHESTRA Prospective Cohort Study" Biomedicines 10, no. 9: 2063. https://doi.org/10.3390/biomedicines10092063
APA StyleSavoldi, A., Morra, M., Castelli, A., Mirandola, M., Berkell, M., Smet, M., Konnova, A., Rossi, E., Cataudella, S., De Nardo, P., Gentilotti, E., Gupta, A., Fasan, D., Gibbin, E., Puviani, F. C., Hasenauer, J., Gusinow, R., Tami, A., Kumar-Singh, S., ... Tacconelli, E. (2022). Clinical Impact of Monoclonal Antibodies in the Treatment of High-Risk Patients with SARS-CoV-2 Breakthrough Infections: The ORCHESTRA Prospective Cohort Study. Biomedicines, 10(9), 2063. https://doi.org/10.3390/biomedicines10092063






