The Hypertensive Effect of Amphotericin B-Containing Liposomes (Abelcet) in Mice: Dissecting the Roles of C3a and C5a Anaphylatoxins, Macrophages and Thromboxane
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Liposomes, and ELISA Kits
2.2. Animals
2.3. Experimental Protocol
2.4. Statistical Analysis
3. Results
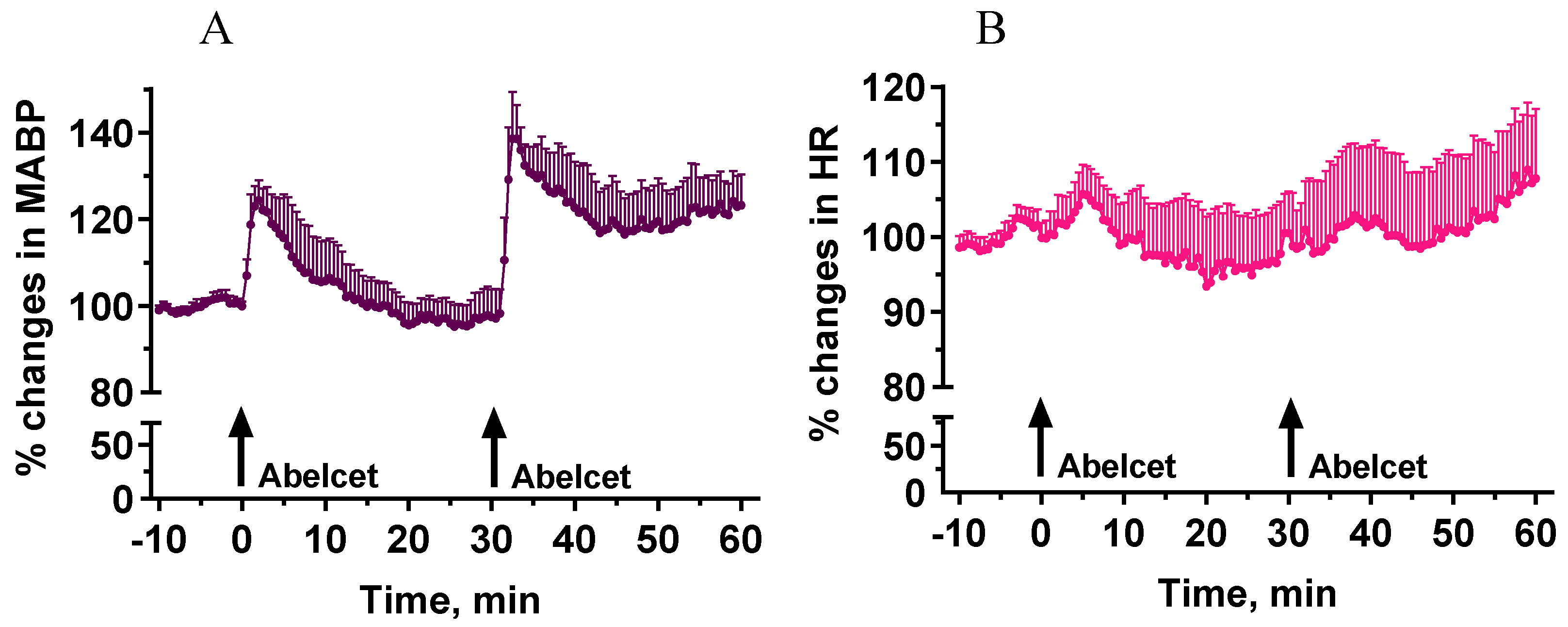
3.1. Effects of Two Subsequent Treatments with Abelcet
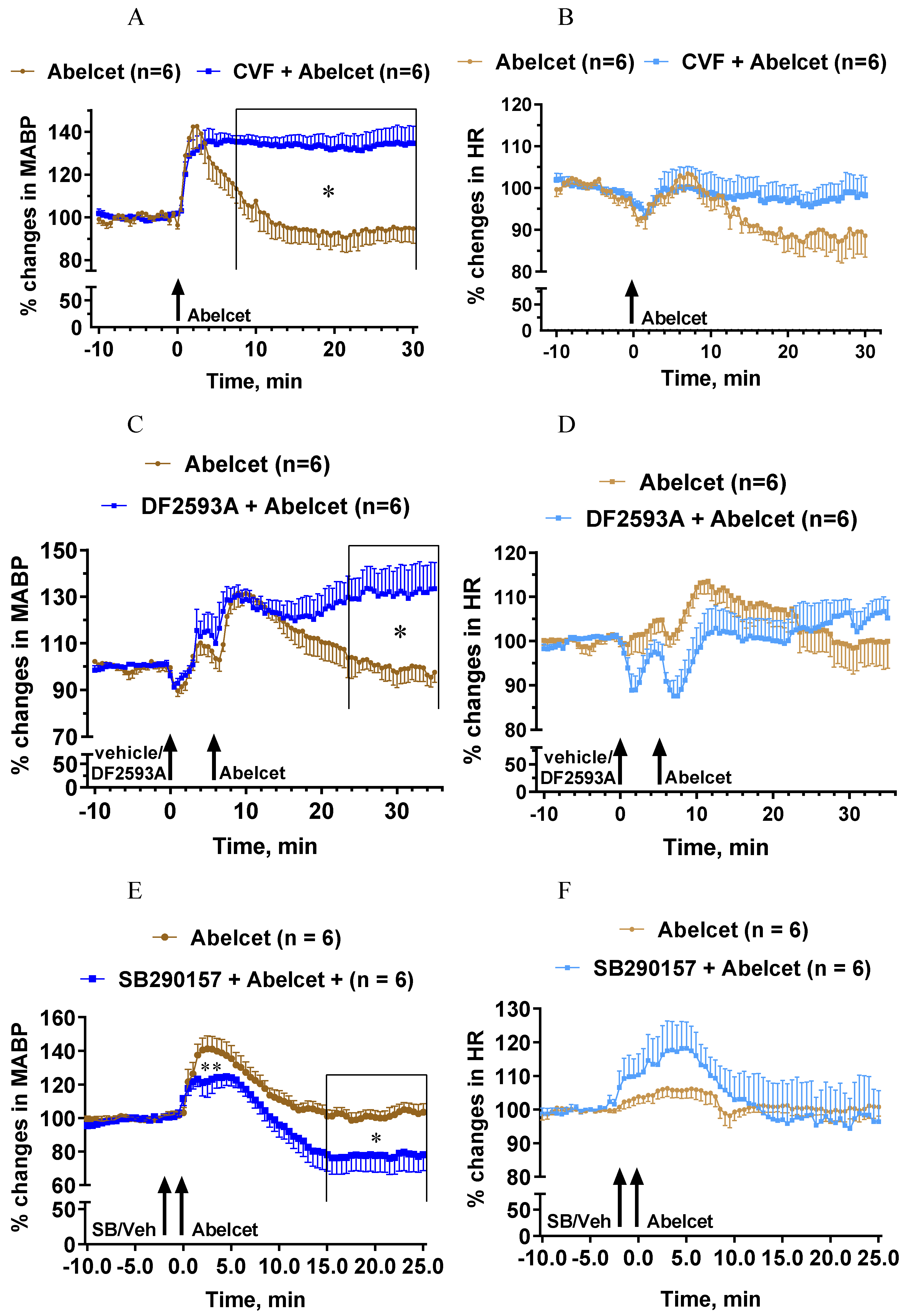
3.2. Effects of C Depletion and C3a and C5a Receptor Antagonists on Abelcet-Induced Hypertension
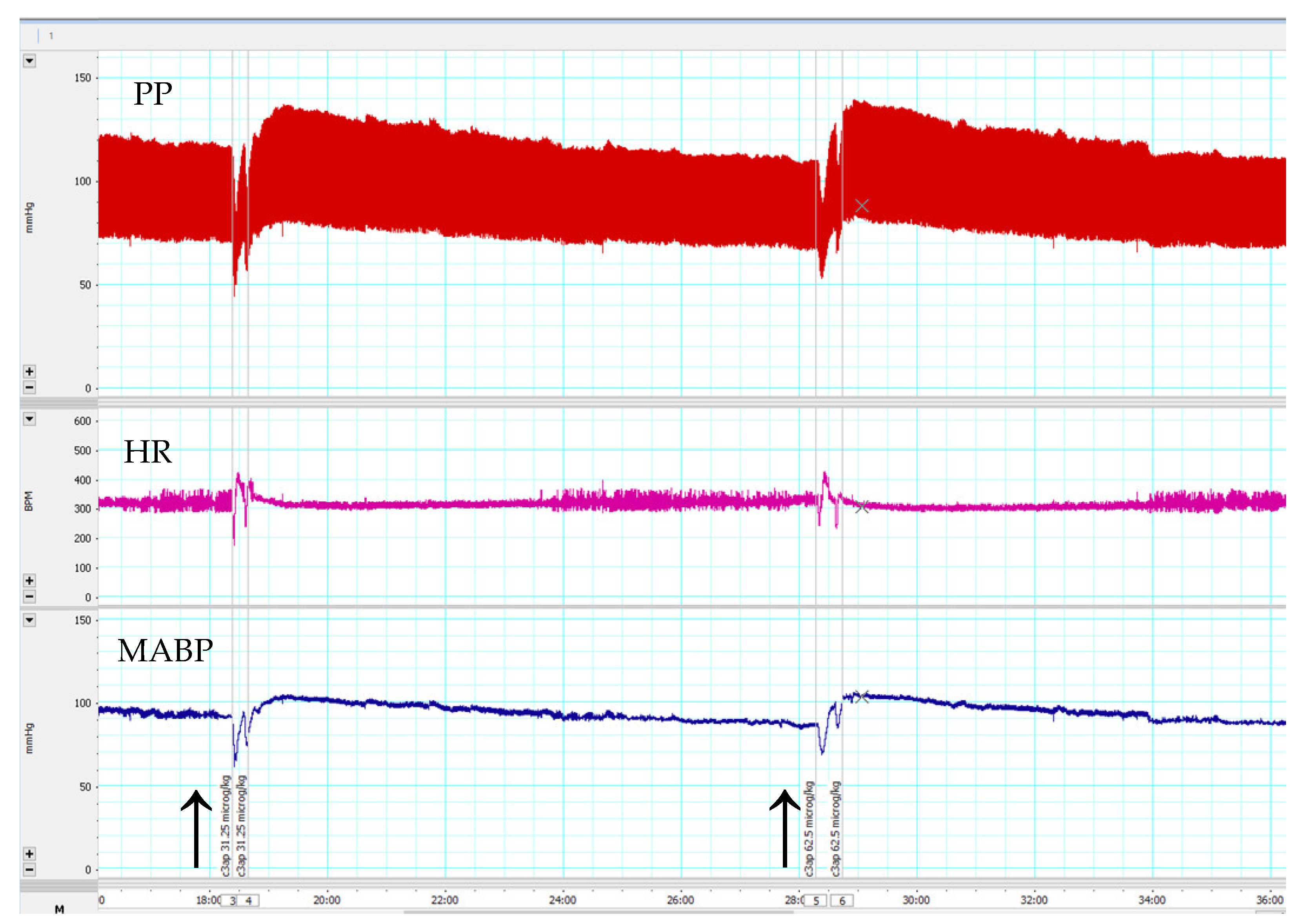
3.3. Effects of C3a Peptide Fragment (63–77) on Blood Pressure and Heart Rate
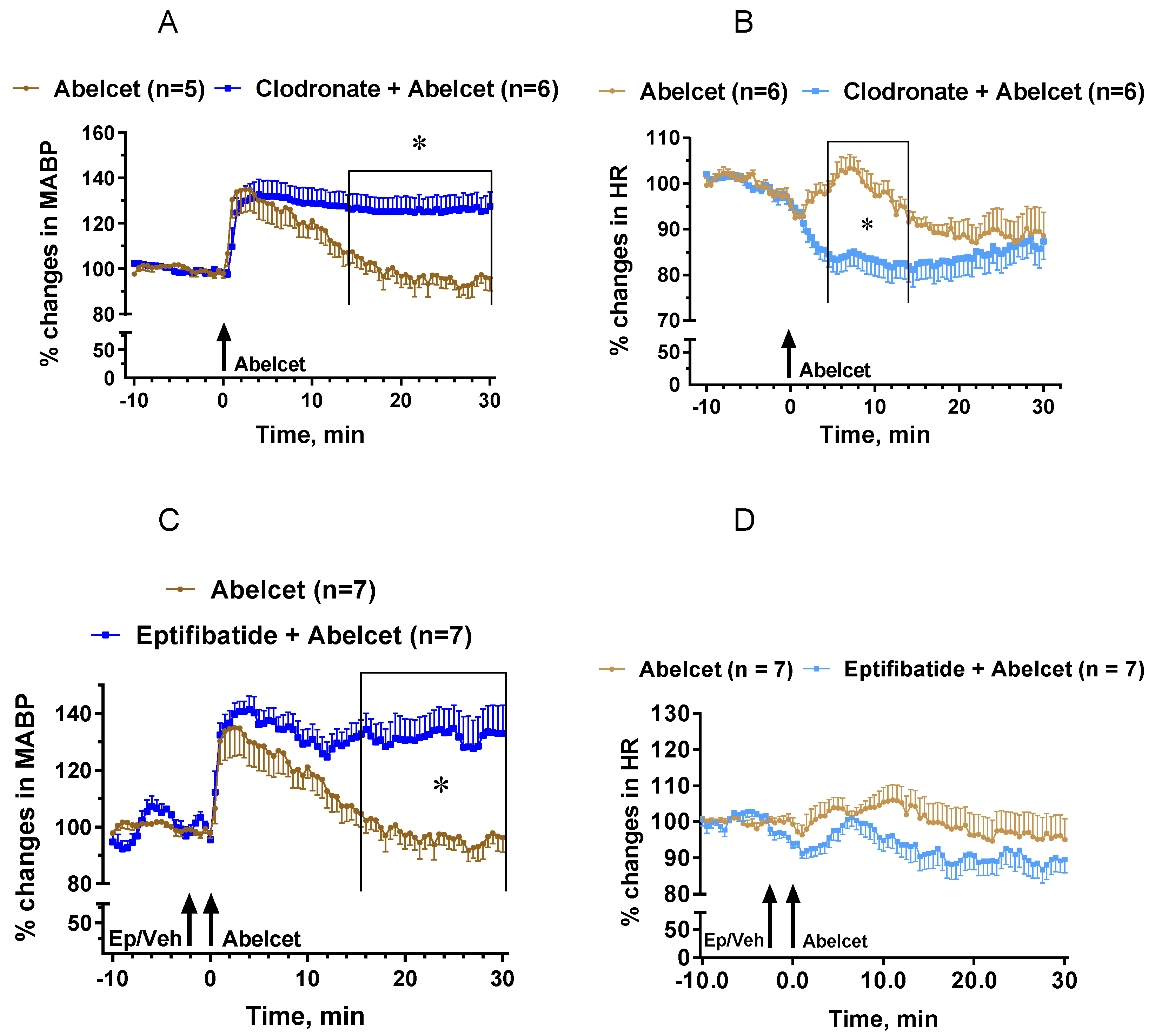
3.4. Effects of Macrophage Depletion and Platelet Inhibition
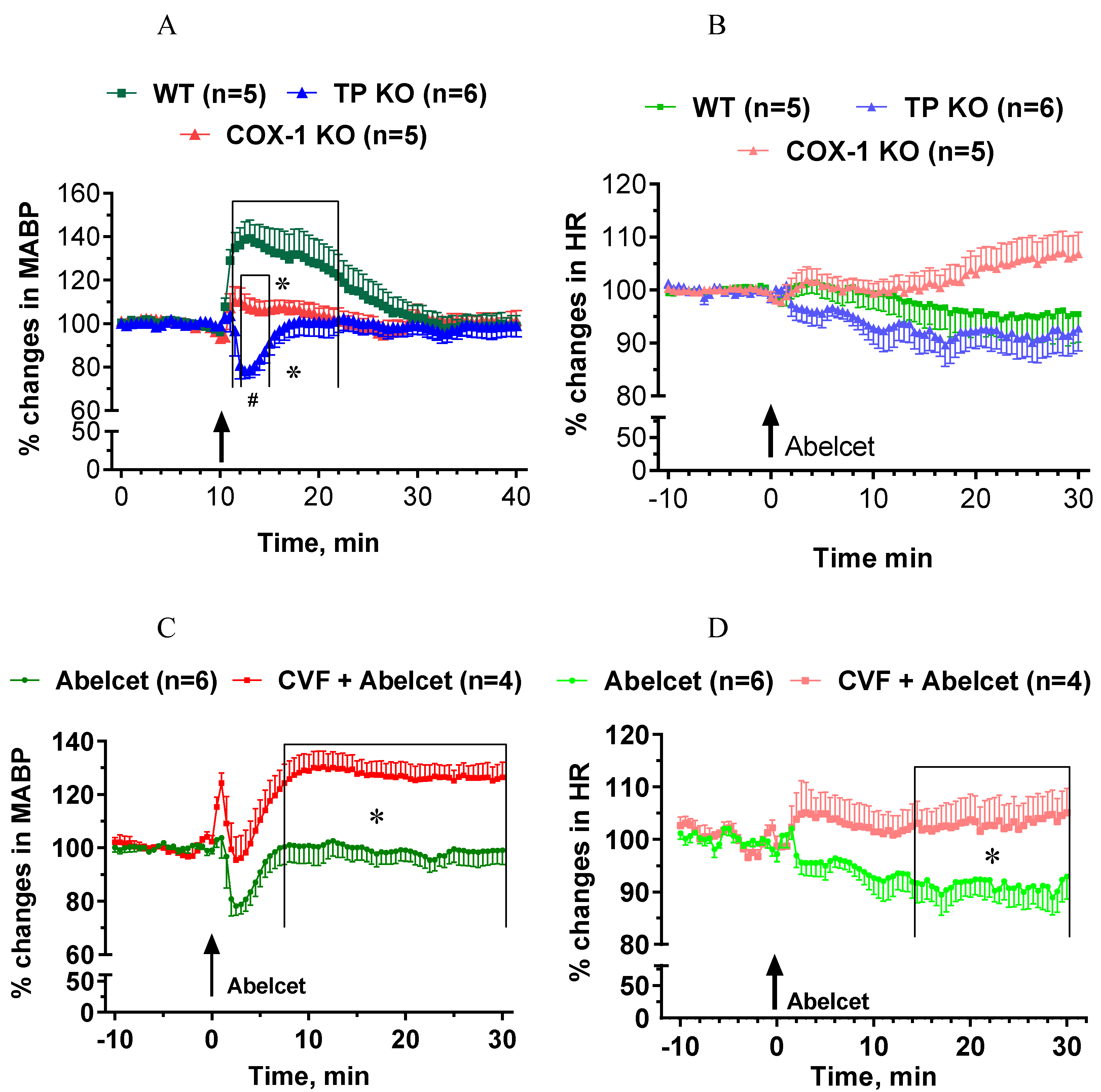
3.5. Effects of Abelcet in COX-1- and TP-Deficient Mice with and without Complement Depletion
4. Discussion
4.1. The Role of Complement Activation in Anaphylactoid Reaction in Mice
4.2. The Roles of Platelets and Macrophages
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szebeni, J.; Simberg, D.; González-Fernández, A.; Barenholz, Y.; Dobrovolskaia, M.A. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat. Nanotechnol. 2018, 13, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Őrfi, E.; Mészáros, T.; Hennies, M.; Fülöp, T.; Dézsi, L.; Nardocci, A.; Rosivall, L.; Hamar, P.; Neun, B.W.; Dobrovolskaia, M.A.; et al. Acute physiological changes caused by complement activators and amphotericin B-containing liposomes in mice. Int. J. Nanomed. 2019, 14, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Urbanics, R.; Bedőcs, P.; Szebeni, J. Lessons learned from the porcine CARPA model: Constant and variable responses to different nanomedicines and administration protocols. Eur. J. Nanomed. 2015, 7, 219. [Google Scholar] [CrossRef]
- Rossi, C.M.; Lenti, M.V.; Di Sabatino, A. Adult anaphylaxis: A state-of-the-art review. Eur. J. Intern. Med. 2022, 100, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Dézsi, L.; Rosivall, L.; Hamar, P.; Szebeni, J.; Szénási, G. Rodent models of complement activation-related pseudoallergy: Inducers, symptoms, inhibitors and reaction mechanisms. Eur. J. Nanomed. 2015, 7, 15. [Google Scholar] [CrossRef]
- Proctor, L.M.; Moore, T.A.; Monk, P.N.; Sanderson, S.D.; Taylor, S.M.; Woodruff, T.M. Complement factors C3a and C5a have distinct hemodynamic effects in the rat. Int. Immunopharmacol. 2009, 9, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Nag, O.; Awasthi, V. Biological Evaluation of Liposome-Encapsulated Hemoglobin Surface-Modified With a Novel PEGylated Nonphospholipid Amphiphile. Artif. Organs 2014, 38, 625–633. [Google Scholar] [CrossRef]
- Sakai, H.; Suzuki, Y.; Sou, K.; Kano, M. Cardiopulmonary hemodynamic responses to the small injection of hemoglobin vesicles (artificial oxygen carriers) in miniature pigs. J. Biomed. Mater. Res. Part A 2012, 100, 2668–2677. [Google Scholar] [CrossRef]
- Rabinovici, R.; Rudolph, A.S.; Vernick, J.; Feuerstein, G. Lyophilized liposome encapsulated hemoglobin: Evaluation of hemodynamic, biochemical, and hematologic responses. Crit. Care Med. 1994, 22, 480–485. [Google Scholar] [CrossRef]
- Chen, H. Role of thromboxane A2 signaling in endothelium-dependent contractions of arteries. Prostaglandins Other Lipid Mediat. 2018, 134, 32–37. [Google Scholar] [CrossRef]
- Damas, J.; Lagneaux, D. Dissociation between the effects of zymosan on the systemic and pulmonary vessels of the rat. J. Cereb. Blood Flow Metab. 1991, 104, 559–564. [Google Scholar] [CrossRef]
- Milosevits, G.; Mészáros, T.; Őrfi, E.; Bakos, T.; Garami, M.; Kovács, G.; Dézsi, L.; Hamar, P.; Győrffy, B.; Szabó, A.; et al. Complement-mediated hypersensitivity reactions to an amphotericin B-containing lipid complex (Abelcet) in pediatric patients and anesthetized rats: Benefits of slow infusion. Nanomed. Nanotechnol. Biol. Med. 2021, 34, 102366. [Google Scholar] [CrossRef] [PubMed]
- Borro, J.; Solé, A.; de la Torre, M.; Pastor, A.; Fernandez, R.; Saura, A.; Delgado, M.; Monte, E.; Gonzalez, D. Efficiency and Safety of Inhaled Amphotericin B Lipid Complex (Abelcet) in the Prophylaxis of Invasive Fungal Infections Following Lung Transplantation. Transplant. Proc. 2008, 40, 3090–3093. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.; Franklin, I. Adverse drug reactions associated with the administration of amphotericin B lipid complex (Abelcet). Bone Marrow Transplant. 1999, 23, 1325–1326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Furebring, M.; Oberg, G.; Sjölin, J. Side-effects of Amphotericin B lipid complex (Abelcet) in the Scandinavian population. Bone Marrow Transplant. 2000, 25, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.G. Depleting Macrophages In Vivo with Clodronate-Liposomes. Methods Mol. Biol. 2018, 1784, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Kerkovits, N.M.; Janovicz, A.; Ruisanchez, É.; Őrfi, E.; Gál, P.; Szénási, G.; Benyó, Z. Anaphylatoxin C3a induces vasoconstriction and hypertension mediated by thromboxane A 2 in mice. FASEB J. 2019, 33, lb510. [Google Scholar] [CrossRef]
- Xu, H.; Fang, B.; Du, S.; Wang, S.; Li, Q.; Jia, X.; Bao, C.; Ye, L.; Sui, X.; Qian, L.; et al. Endothelial cell prostaglandin E2 receptor EP4 is essential for blood pressure homeostasis. JCI Insight 2020, 5, e138505. [Google Scholar] [CrossRef]
- Song, W.-L.; Ricciotti, E.; Liang, X.; Grosser, T.; Grant, G.R.; Fitzgerald, G.A. Lipocalin-Like Prostaglandin D Synthase but Not Hemopoietic Prostaglandin D Synthase Deletion Causes Hypertension and Accelerates Thrombogenesis in Mice. J. Pharmacol. Exp. Ther. 2018, 367, 425–432. [Google Scholar] [CrossRef]
- Stæhr, M.; Madsen, K.; Vanhoutte, P.M.; Hansen, P.B.; Jensen, B.L.; Staehr, M. Disruption of COX-2 and eNOS does not confer protection from cardiovascular failure in lipopolysaccharide-treated conscious mice and isolated vascular rings. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R412–R420. [Google Scholar] [CrossRef]
- Viana, I.M.D.O.; Grenier, P.; Defrêne, J.; Barabé, F.; Lima, E.M.; Bertrand, N. Role of the complement cascade in the biological fate of liposomes in rodents. Nanoscale 2020, 12, 18875–18884. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, F.D.; Rothenberg, M.E.; Brandt, E.; Morris, S.C.; Strait, R.T. Molecular mechanisms of anaphylaxis: Lessons from studies with murine models. J. Allergy Clin. Immunol. 2005, 115, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Liu, Y.; Lu, X.; Liu, B.; Pan, Q.; Liu, Y.; Liu, Y.; Zhu, P.; Fu, N. Macrophages are the dominant effector cells responsible for IgG-mediated passive systemic anaphylaxis challenged by natural protein antigen in BALB/c and C57BL/6 mice. Cell. Immunol. 2014, 289, 97–105. [Google Scholar] [CrossRef]
- Wang, M.; Shibamoto, T.; Kuda, Y.; Tanida, M.; Zhang, T.; Song, J.; Kurata, Y. The responses of pulmonary and systemic circulation and airway to anaphylactic mediators in anesthetized BALB/c mice. Life Sci. 2016, 147, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Thomas, K.; Mersmann, S.; Skupski, J.; Margraf, A.; Tekath, T.; Jouvene, C.C.; Dalli, J.; Hidalgo, A.; Meuth, S.G.; et al. Platelets orchestrate the resolution of pulmonary inflammation in mice by T reg cell repositioning and macrophage education. J. Exp. Med. 2021, 218, e20201353. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, R.; Toyoda, E.; Maehara, M.; Wasai, S.; Omura, H.; Watanabe, M.; Sato, M. Effect of Platelet-Rich Plasma on M1/M2 Macrophage Polarization. Int. J. Mol. Sci. 2021, 22, 2336. [Google Scholar] [CrossRef]
- Li, Y.; Ryan, J.; Xu, F.; Vostal, J.G. Macrophage Depletion Mitigates Platelet Aggregate Formation in Splenic Marginal Zone and Alleviates LPS-Associated Thrombocytopenia in Rats. Front. Med. 2019, 6, 300. [Google Scholar] [CrossRef]
- Linke, B.; Schreiber, Y.; Picard-Willems, B.; Slattery, P.; Nüsing, R.M.; Harder, S.; Geisslinger, G.; Scholich, K. Activated Platelets Induce an Anti-Inflammatory Response of Monocytes/Macrophages through Cross-Regulation of PGE2 and Cytokines. Mediat. Inflamm. 2017, 2017, 1463216. [Google Scholar] [CrossRef]
- Ando, Y.; Oku, T.; Tsuji, T. Platelets attenuate production of cytokines and nitric oxide by macrophages in response to bacterial endotoxin. Platelets 2015, 27, 344–350. [Google Scholar] [CrossRef]
- Kral, J.B.; Schrottmaier, W.C.; Salzmann, M.; Assinger, A. Platelet interaction with innate immune cells. Transfus. Med. Hemother. 2016, 43, 78–88. [Google Scholar] [CrossRef]
- Ehrentraut, S.; Frede, S.; Stapel, H.; Mengden, T.; Grohé, C.; Fandrey, J.; Meyer, R.; Baumgarten, G. Antagonism of Lipopolysaccharide-Induced Blood Pressure Attenuation and Vascular Contractility. Arter. Thromb. Vasc. Biol. 2007, 27, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Bellou, A.; Lambert, H.; Gillois, P.; Montémont, C.; Gerard, P.; Vauthier, E.; Sainte-Laudy, J.; Longrois, D.; Guéant, J.L.; Mallié, J.P. Constitutive Nitric Oxide Synthase Inhibition Combined with Histamine and Serotonin Receptor Blockade Improves the Initial Ovalbumin-Induced Arterial Hypotension but Decreases the Survival Time in Brown Norway Rats Anaphylactic Shock. Shock 2003, 19, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Tacquard, C.; Oulehri, W.; Collange, O.; Garvey, L.H.; Nicoll, S.; Tuzin, N.; Geny, B.; Mertes, P.M. Treatment with a platelet-activating factor receptor antagonist improves hemodynamics and reduces epinephrine requirements, in a lethal rodent model of anaphylactic shock. Clin. Exp. Allergy 2019, 50, 383–390. [Google Scholar] [CrossRef]
- Nakamura, T.; Fujiwara, Y.; Yamada, R.; Fujii, W.; Hamabata, T.; Lee, M.Y.; Maeda, S.; Aritake, K.; Roers, A.; Sessa, W.; et al. Mast cell–derived prostaglandin D 2 attenuates anaphylactic reactions in mice. J. Allergy Clin. Immunol. 2017, 140, 630–632.e9. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Cheng, Y.; Smalera, I.; Jian, W.; Goldhahn, L.; FitzGerald, G.A.; Funk, C.D. Directed Vascular Expression of Human Cysteinyl Leukotriene 2 Receptor Modulates Endothelial Permeability and Systemic Blood Pressure. Circulation 2004, 110, 3360–3366. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.-I.; Kawasaki, T.; Kambayashi, J.-I.; Ariyoshi, H.; Shinoki, N.; Sakon, M.; Ikeda, Y.; Monden, M. The Release Mechanism of Platelet-Activating Factor during Shear-Stress Induced Platelet Aggregation. Biochem. Biophys. Res. Commun. 1997, 239, 101–105. [Google Scholar] [CrossRef]
- Giannattasio, G.; Lai, Y.; Granata, F.; Mounier, C.M.; Nallan, L.; Oslund, R.; Leslie, C.C.; Marone, G.; Lambeau, G.; Gelb, M.H.; et al. Expression of phospholipases A2 in primary human lung macrophages: Role of cytosolic phospholipase A2-α in arachidonic acid release and platelet activating factor synthesis. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2009, 1791, 92–102. [Google Scholar] [CrossRef]
- Berny-Lang, M.A.; Jakubowski, J.A.; Sugidachi, A.; Barnard, M.R.; Michelson, A.D.; FrelingerIII, A.L. P2Y 12 Receptor Blockade Augments Glycoprotein IIb-IIIa Antagonist Inhibition of Platelet Activation, Aggregation, and Procoagulant Activity. J. Am. Hear. Assoc. 2013, 2, e000026. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Őrfi, E.; Hricisák, L.; Dézsi, L.; Hamar, P.; Benyó, Z.; Szebeni, J.; Szénási, G. The Hypertensive Effect of Amphotericin B-Containing Liposomes (Abelcet) in Mice: Dissecting the Roles of C3a and C5a Anaphylatoxins, Macrophages and Thromboxane. Biomedicines 2022, 10, 1764. https://doi.org/10.3390/biomedicines10071764
Őrfi E, Hricisák L, Dézsi L, Hamar P, Benyó Z, Szebeni J, Szénási G. The Hypertensive Effect of Amphotericin B-Containing Liposomes (Abelcet) in Mice: Dissecting the Roles of C3a and C5a Anaphylatoxins, Macrophages and Thromboxane. Biomedicines. 2022; 10(7):1764. https://doi.org/10.3390/biomedicines10071764
Chicago/Turabian StyleŐrfi, Erik, László Hricisák, László Dézsi, Péter Hamar, Zoltán Benyó, János Szebeni, and Gábor Szénási. 2022. "The Hypertensive Effect of Amphotericin B-Containing Liposomes (Abelcet) in Mice: Dissecting the Roles of C3a and C5a Anaphylatoxins, Macrophages and Thromboxane" Biomedicines 10, no. 7: 1764. https://doi.org/10.3390/biomedicines10071764
APA StyleŐrfi, E., Hricisák, L., Dézsi, L., Hamar, P., Benyó, Z., Szebeni, J., & Szénási, G. (2022). The Hypertensive Effect of Amphotericin B-Containing Liposomes (Abelcet) in Mice: Dissecting the Roles of C3a and C5a Anaphylatoxins, Macrophages and Thromboxane. Biomedicines, 10(7), 1764. https://doi.org/10.3390/biomedicines10071764








