Novel Chloro-Substituted Salicylanilide Derivatives and Their β-Cyclodextrin Complexes: Synthesis, Characterization, and Antibacterial Activity
Abstract
:1. Introduction
2. Materials and methods
2.1. Chemicals
2.2. Apparatus
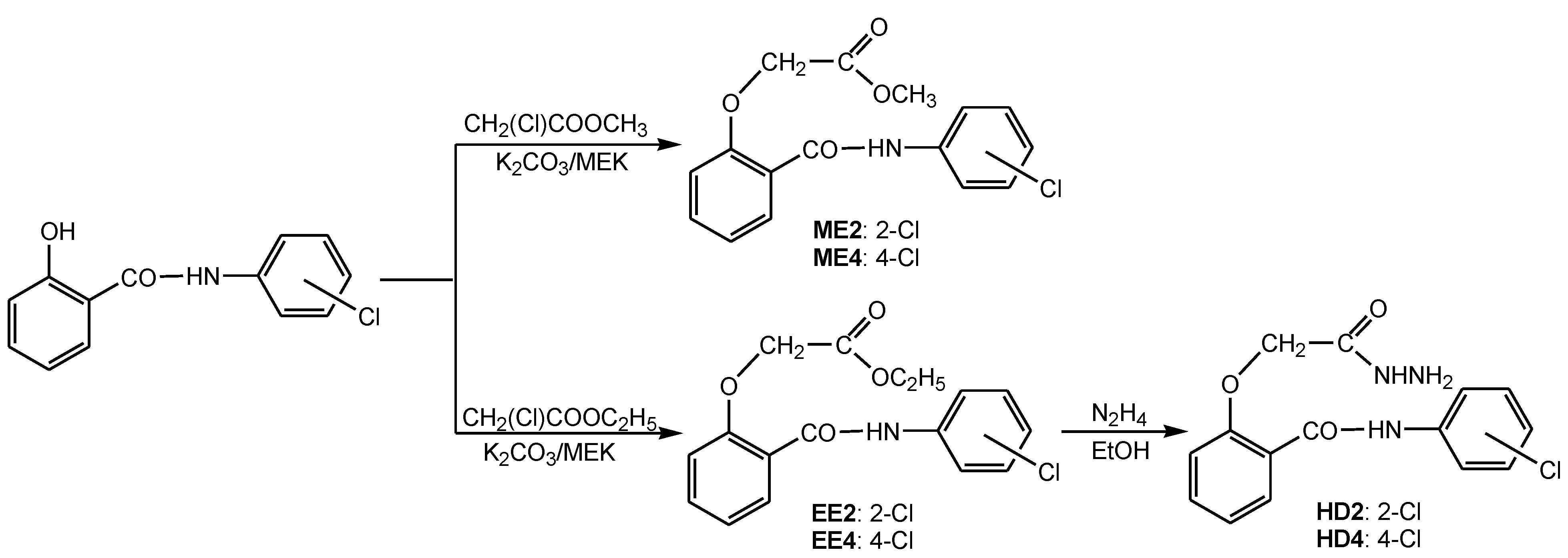
2.3. Microwave-Assisted Syntheses
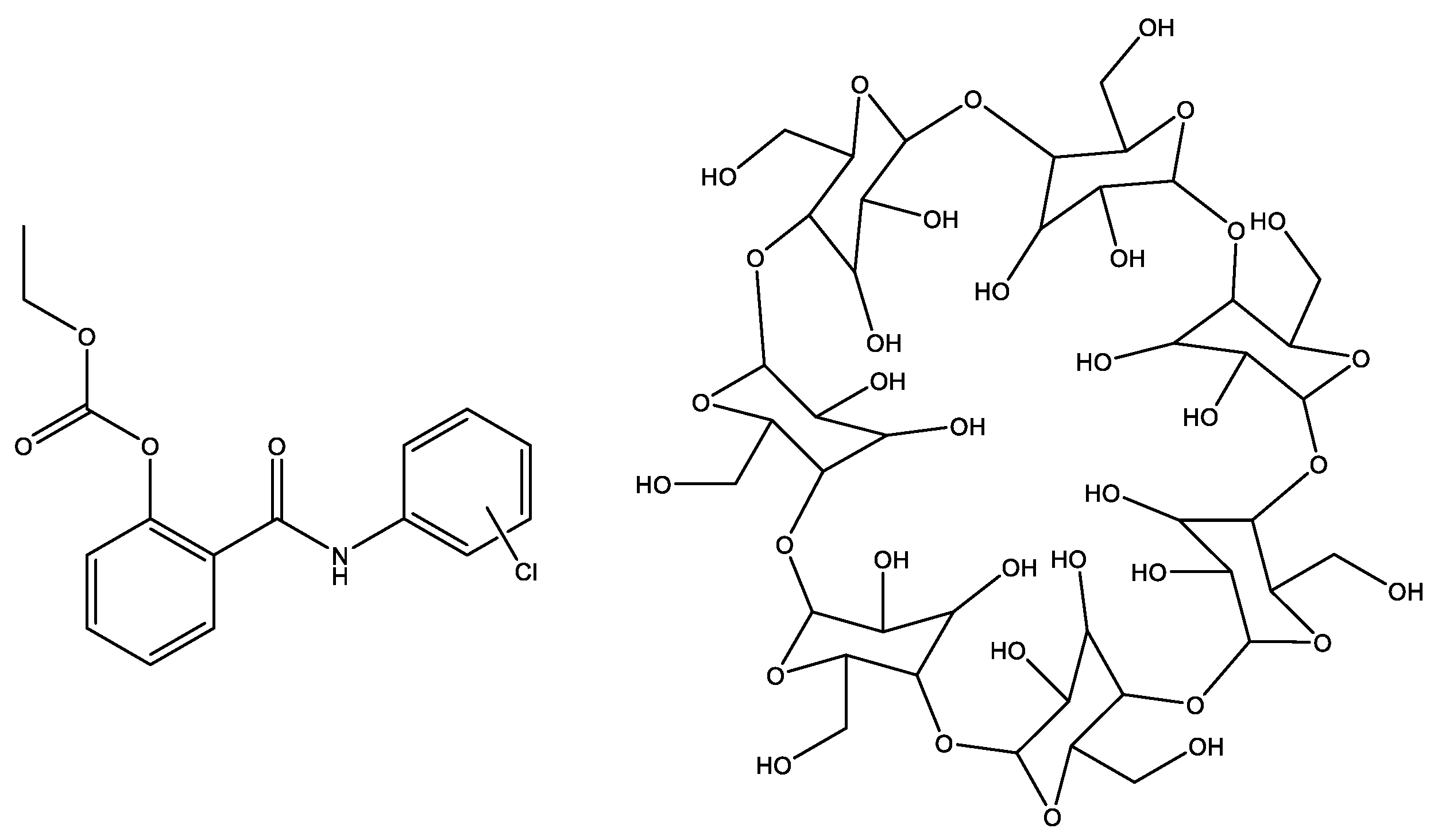
2.4. Obtaining the Inclusion Complexes
2.5. Obtaining the Physical Mixtures
2.6. Antibacterial Activity Evaluation
2.6.1. Test Compounds
2.6.2. Bacterial Strains
2.6.3. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3. Results and Discussion
3.1. Synthesis of the Chloro-Substituted Salicylanilide Derivatives
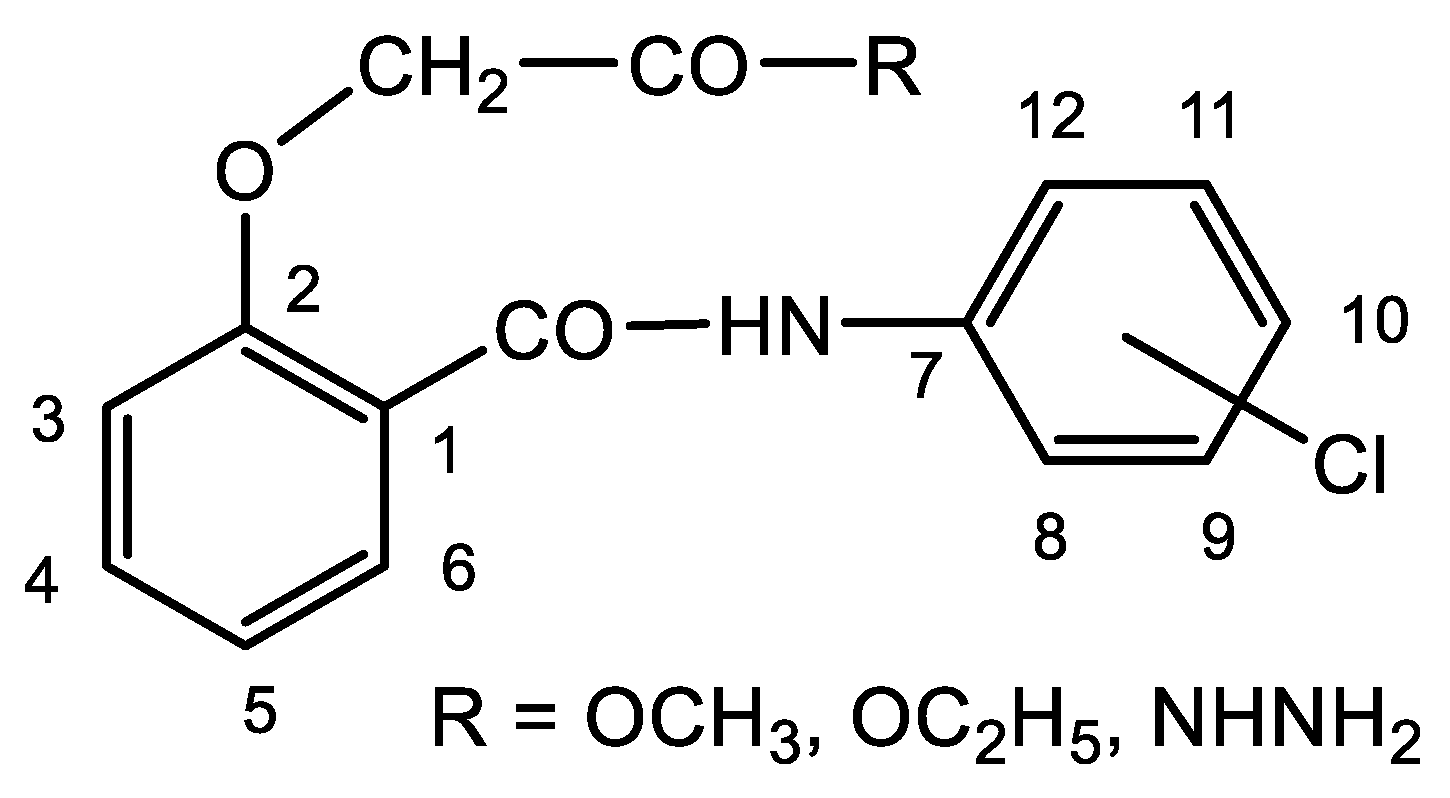
3.2. Characterization of the Chloro-Substituted Salicylanilide Derivatives
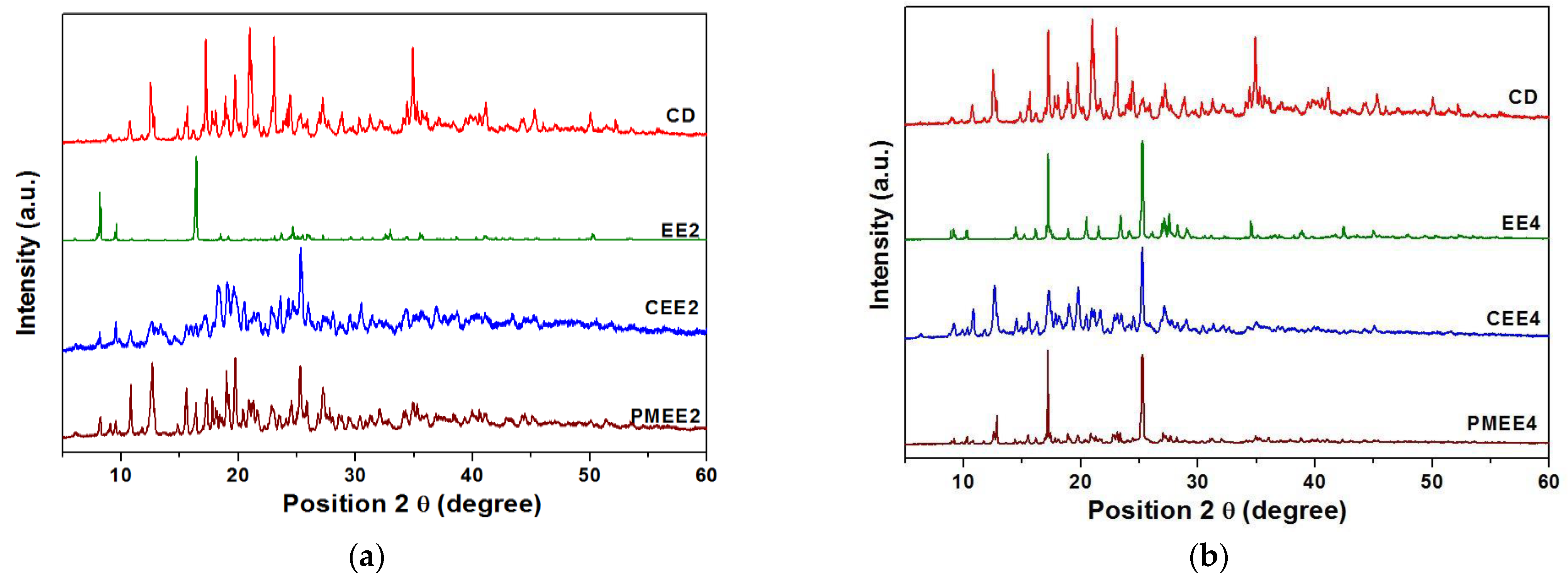
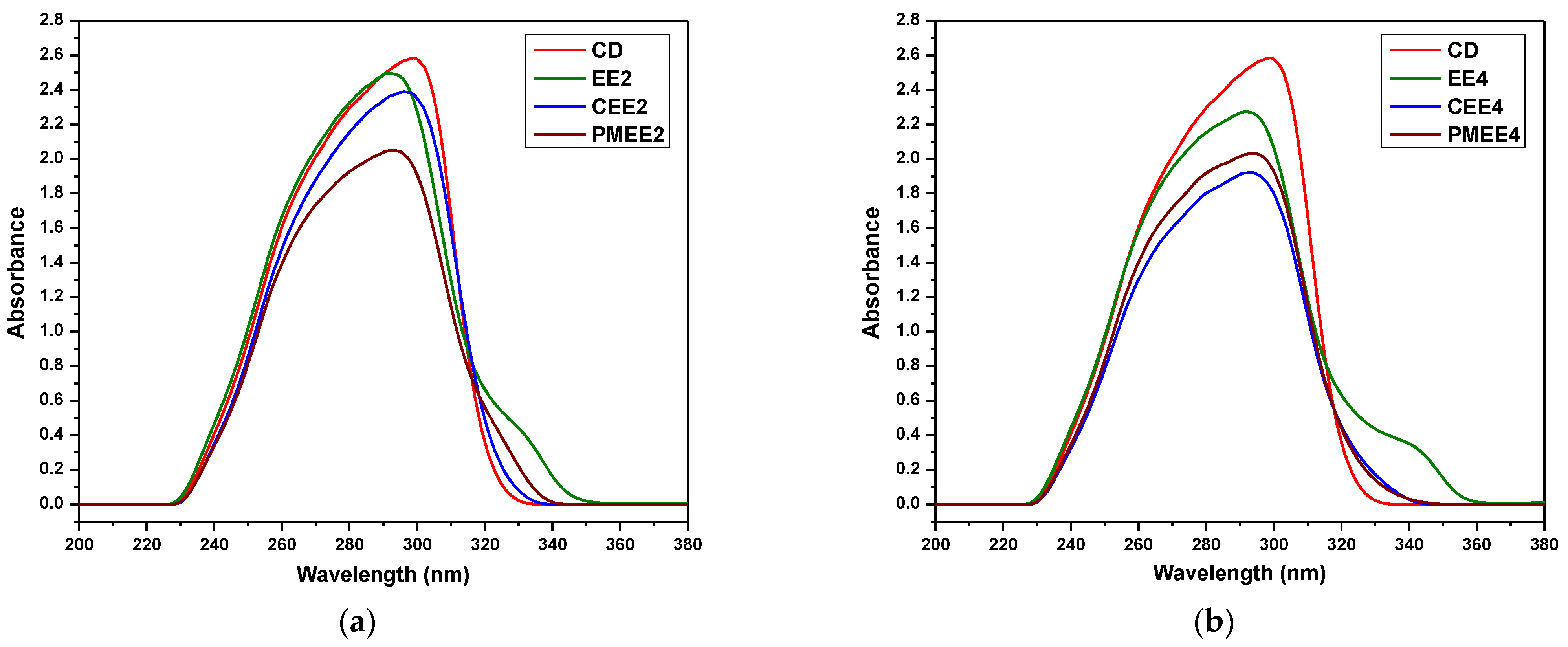
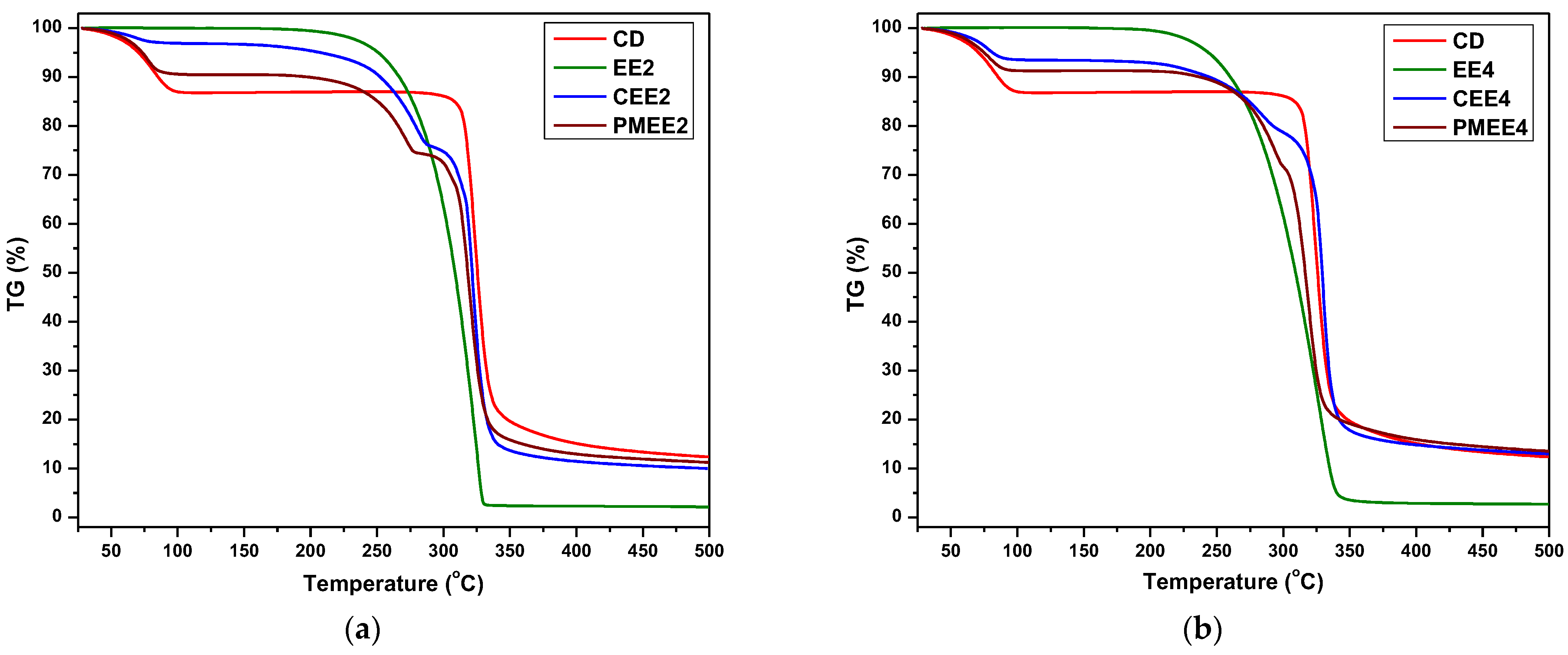
3.3. Characterization of the Inclusion Complexes
3.4. Antibacterial Activity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macielag, M.J.; Demers, J.P.; Fraga-Spano, S.A.; Hlasta, D.J.; Johnson, S.G.; Kanojia, R.M.; Russell, R.K.; Sui, Z.; Weidner-Wells, M.A.; Werblood, H.; et al. Substituted salicylanilides as inhibitors of two-component regulatory systems in bacteria. J. Med. Chem. 1998, 41, 2939–2945. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, R.; Sonawane, N.D.; Arumainayagam, D.; Verkman, A.S. Small molecules with antimicrobial activity against E. coli and P. aeruginosa identified by high-throughput screening. Br. J. Pharmacol. 2006, 149, 551–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paraskevopoulos, G.; Monteiro, S.; Vosátka, R.; Krátký, M.; Navrátilová, L.; Trejtnar, F.; Stolaríková, J.; Vinšová, J. Novel salicylanilides from 4,5-dihalogenated salicylic acids: Synthesis, antimicrobial activity and cytotoxicity. Bioorg. Med. Chem. 2017, 25, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Zhang, W.; Xiao, H.L.; Wang, P.; Li, Y.X. Synthesis and biological evaluation of a series of novel salicylanilides as inhibitors of EGFR protein tyrosine kinases. Chin. Chem. Lett. 2012, 23, 529–532. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšová, J. Salicylanilide ester prodrugs as potential antimicrobial agents-a review. Curr. Pharm. Des. 2011, 17, 3494–3505. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšová, J.; Novotná, E.; Mandíková, J.; Trejtnar, F.; Stolaříková, J. Antibacterial activity of salicylanilide 4-(trifluoromethyl)benzoates. Molecules 2013, 18, 3674–3688. [Google Scholar] [CrossRef] [Green Version]
- Vinšová, J.; Kozic, J.; Krátký, M.; Stolaříková, J.; Mandíková, J.; Trejtnar, F.; Buchta, V. Salicylanilide diethyl phosphates: Synthesis, antimicrobial activity and cytotoxicity. Bioorg. Med. Chem. 2014, 22, 728–737. [Google Scholar] [CrossRef]
- Fahmy, H.H.; El-Eraky, W. Synthesis and evaluation of the analgesic and antiinflammatory activities of O-substituted salicylamides. Arch. Pharm. Res. 2001, 24, 171–179. [Google Scholar] [CrossRef]
- Berillo, D.A.; Dyusebaeva, M.A. Synthesis of hydrazides of heterocyclic amines and their antimicrobial and spasmolytic activity. Saudi Pharm. J. 2022; in press. [Google Scholar] [CrossRef]
- Unissa, A.N.; Subbian, S.; Hanna, L.E.; Selvakumar, N. Overview on mechanisms of isoniazid action and resistance in Mycobacterium tuberculosis. Infect. Genet. Evol. 2016, 45, 474–492. [Google Scholar] [CrossRef]
- Vilchèze, C.; Jacobs Jr., W.R. Resistance to isoniazid and ethionamide in Mycobacterium tuberculosis: Genes, mutations, and causalities. Microbiol. Spectr. 2014, 2, MGM2–0014-2013. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: A review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Cyclodextrin as a magic switch in covalent and noncovalent anticancer drug release systems. Carbohydr. Polym. 2020, 242, 116401. [Google Scholar] [CrossRef]
- Minda, D.; Pavel, I.Z.; Borcan, F.; Coricovac, D.; Pinzaru, I.; Andrica, F.; Morgovan, C.; Nita, L.D.; Soica, C.; Muntean, D.; et al. Beneficial effects of a lupeol-cyclodextrin complex in a murine model of photochemical skin carcinoma. Rev. Chim. 2015, 66, 373–377. [Google Scholar]
- Antonio Cid-Samamed, A.; JarupornRakmai, J.; Juan Carlos Mejuto, J.C.; Jesus Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Sivakumar, K.; Parameswari, M. Salicylanilide/cyclodextrin inclusion complex: Preparation, characterization and molecular docking studies. SOJ Mater. Sci. Eng. 2015, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Ienașcu, I.M.C.; Ştefănuț, M.N.; Pascariu, C.; Popescu, I.M.; Căta, A.; Pop, R. Complexation of [2-(2-bromophenylcarbamoyl)phenoxy]acetic acid ethyl ester with β-cyclodextrin. Rev. Roum. Chim. 2019, 64, 849–857. [Google Scholar] [CrossRef]
- Sambasevam, K.P.; Mohamad, S.; Sarih, N.M.; Ismail, N.I. Synthesis and characterization of the inclusion complex of β-cyclodextrin and azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; M07Ed11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Muntean, D.; Ştefănuţ, M.N.; Căta, A.; Buda, V.; Danciu, C.; Bănică, R.; Pop, R.; Licker, M.; Ienaşcu, I.M.C. Symmetrical Antioxidant and Antibacterial Properties of Four Romanian Cruciferous Extracts. Symmetry 2021, 13, 893. [Google Scholar] [CrossRef]
- Ledeţi, I.; Bercean, V.; Alexa, A.; Şoica, C.; Şuta, L.; Dehelean, C.; Trandafirescu, C.; Muntean, D.; Licker, M.; Fuliaş, A. Preparation and Antibacterial Properties of Substituted 1,2,4-Triazoles. J. Chem. 2015, 2015, 879343. [Google Scholar] [CrossRef] [Green Version]
- Danciu, C.; Bojin, F.; Ambrus, R.; Muntean, D.; Soica, C.; Paunescu, V.; Cristea, M.; Pinzaru, I.; Dehelean, C. Physico-chemical and Biological Evaluation of Flavonols: Fisetin, Quercetin and Kaempferol Alone and Incorporated in beta Cyclodextrins. Anticancer Agents Med.Chem. 2017, 17, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Fecker, R.; Buda, V.; Alexa, E.; Avram, S.; Pavel, I.Z.; Muntean, D.; Cocan, I.; Watz, C.; Minda, D.; Dehelean, C.A.; et al. Phytochemical and biological screening of Oenothera Biennis L. hydroalcoholic extract. Biomolecules 2020, 10, 818. [Google Scholar] [CrossRef]
- Ienaşcu, I.M.C.; Obistioiu, D.; Popescu, I.; Ştefănuţ, M.N.; Osser, G.; Jurca, C.; Ciavoi, G.; Bechir, E.S.; Bechir, F.; Căta, A. In vitro testing of salicylanilide derivatives against some fungal and bacterial strains. Rev. Chim. 2019, 70, 1496–1499. [Google Scholar] [CrossRef]
- Ienașcu, I.M.C.; Balaeș, T.; Petre, C.V.; Pop, R.O.; Căta, A.; Ştefănuţ, M.N.; Albu, P.; Poenaru, M. Novel N-(2-bromo-phenyl)-2-hydroxy-benzamide Derivatives with Antifugal Activity. Rev. Chim. 2018, 69, 1876–1880. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.; Zhao, X.; Gao, Y.; Zhang, L.; Zhang, J.; Yu, L. Complex formation of ionic liquid surfactant and β-cyclodextrin. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 196–201. [Google Scholar] [CrossRef]
- Li, W.; Lu, B.; Sheng, A.; Yang, F.; Wang, Z. Spectroscopic and theoretical study on inclusion complexation of beta-cyclodextrin with permethrin. J. Mol. Struct. 2010, 981, 194–203. [Google Scholar] [CrossRef]
- Rusa, C.C.; Luca, C.; Tonelli, A.E. Polymer-cyclodextrin inclusion compounds. Toward new aspects of their inclusion mechanism. Macromolecules 2001, 34, 1318–1322. [Google Scholar] [CrossRef]
- Tang, B.; Chen, Z.-Z.; Zhang, N.; Zhang, J.; Wang, Y. Synthesis and characterization of a novel cross-linking complex of β-cyclodextrin-o-vanilinfurfuralhydrazone and highly selective spectrofluorimetric determination of trace gallium. Talanta 2006, 68, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Abderrahim, R.; Meganem, F. Spectroscopic studies of inclusion complex of β-cyclodextrin and benzidine diammonium dipicrate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 75, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Krátký, M.; Vinsová, J.; Buchta, V.; Horvati, K.; Bösze, S.; Stolaríková, J. New amino acid esters of salicylanilides active against MDR-TB and other microbes. Eur. J. Med. Chem. 2010, 45, 6106–6113. [Google Scholar] [CrossRef]
- Cunningham, M.W. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus mutans, caries and simulation models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Inoue, Y.; Suzuki, R.; Murata, I.; Nomura, H.; Isshiki, Y.; Kanamoto, I. Evaluation of antibacterial activity expression of the hinokitiol/cyclodextrin complex against bacteria. ACS Omega 2020, 5, 27180–27187. [Google Scholar] [CrossRef]


| Characteristic Vibrations | EE2 | EE4 | ||
|---|---|---|---|---|
| Free (cm−1) | Complexed (cm−1) | Free (cm−1) | Complexed (cm−1) | |
| Sk “1600” | 1592.14 | 1593.11 | 1593.11 | 1596.97 |
| γNH + νCN sec. amide | 1535.25 | 1536.21 | 1533.32 | 1535.25 |
| Sk “1500” | 1484.14, 1438.81 | 1482.21, 1439.78 | 1436.88 | 1440.74 |
| σCH3 aliphatic | 1391.56 | 1394.45 | 1392.52 | 1396.38 |
| σCH aromatic | 1378.06 | 1375.17 | 1361.66 | overlaped |
| νasCOC aliphatic | 1161.08 | 1156.26 | 1157.22 | 1155.29 |
| β-Cyclodextrin | Free (cm−1) | Complexed EE2 (cm−1) | Complexed EE4 (cm−1) |
|---|---|---|---|
| ν[OH] | 3396.45 | 3395.48 | 3398.38 |
| ν[CH2] | 2925.84 | 2928.74 | 2925.84 |
| ν[C–C] | 1157.22 | 1156.26 | 1155.29 |
| ν[O–H] | 1028.96 | 1029.93 | 1033.79 |
| Microbial Strains | Test Compounds | MIC (mg/mL, *) | MBC (mg/mL, *) |
|---|---|---|---|
| S. mutans ATCC 35668 | EE2 | 0.25 | 0.25 |
| EM2 | 0.25 | 0.25 | |
| HD2 | 0.5 | 0.5 | |
| CEE2 | 0.25 | 0.25 | |
| EE4 | NA | NA | |
| EM4 | 0.5 | 0.5 | |
| HD4 | 0.5 | 0.5 | |
| CEE4 | NA | NA | |
| S. pyogenes ATCC 19615 | EE2 | 0.125 | 0.125 |
| EM2 | 0.125 | 0.125 | |
| HD2 | 0.25 | 0.5 | |
| CEE2 | 0.125 | 0.125 | |
| EE4 | 1.0 | 1.0 | |
| EM4 | 0.5 | 0.5 | |
| HD4 | 0.5 | 0.5 | |
| CEE4 | NA | NA | |
| S. aureus ATCC 25923 | EE2 | 0.5 | 0.5 |
| EM2 | 0.5 | 0.5 | |
| HD2 | 1.0 | 1.0 | |
| CEE2 | 0.5 | 0.5 | |
| EE4 | 1.0 | 1.0 | |
| EM4 | 1.0 | 1.0 | |
| HD4 | 1.0 | 1.0 | |
| CEE4 | NA | NA | |
| E. coli ATCC 25922 | EE2 | NA | NA |
| EM2 | NA | NA | |
| HD2 | NA | NA | |
| CEE2 | NA | NA | |
| EE4 | NA | NA | |
| EM4 | NA | NA | |
| HD4 | NA | NA | |
| CEE4 | NA | NA | |
| P. aeruginosa ATCC 27853 | EE2 | NA | NA |
| EM2 | NA | NA | |
| HD2 | NA | NA | |
| CEE2 | NA | NA | |
| EE4 | NA | NA | |
| EM4 | NA | NA | |
| HD4 | NA | NA | |
| CEE4 | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ienașcu, I.M.C.; Căta, A.; Ştefănuț, M.N.; Popescu, I.; Rusu, G.; Sfîrloagă, P.; Ursu, D.; Moşoarcă, C.; Dabici, A.; Danciu, C.; et al. Novel Chloro-Substituted Salicylanilide Derivatives and Their β-Cyclodextrin Complexes: Synthesis, Characterization, and Antibacterial Activity. Biomedicines 2022, 10, 1740. https://doi.org/10.3390/biomedicines10071740
Ienașcu IMC, Căta A, Ştefănuț MN, Popescu I, Rusu G, Sfîrloagă P, Ursu D, Moşoarcă C, Dabici A, Danciu C, et al. Novel Chloro-Substituted Salicylanilide Derivatives and Their β-Cyclodextrin Complexes: Synthesis, Characterization, and Antibacterial Activity. Biomedicines. 2022; 10(7):1740. https://doi.org/10.3390/biomedicines10071740
Chicago/Turabian StyleIenașcu, Ioana Maria Carmen, Adina Căta, Mariana Nela Ştefănuț, Iuliana Popescu, Gerlinde Rusu, Paula Sfîrloagă, Daniel Ursu, Cristina Moşoarcă, Anamaria Dabici, Corina Danciu, and et al. 2022. "Novel Chloro-Substituted Salicylanilide Derivatives and Their β-Cyclodextrin Complexes: Synthesis, Characterization, and Antibacterial Activity" Biomedicines 10, no. 7: 1740. https://doi.org/10.3390/biomedicines10071740
APA StyleIenașcu, I. M. C., Căta, A., Ştefănuț, M. N., Popescu, I., Rusu, G., Sfîrloagă, P., Ursu, D., Moşoarcă, C., Dabici, A., Danciu, C., Muntean, D., & Pop, R. (2022). Novel Chloro-Substituted Salicylanilide Derivatives and Their β-Cyclodextrin Complexes: Synthesis, Characterization, and Antibacterial Activity. Biomedicines, 10(7), 1740. https://doi.org/10.3390/biomedicines10071740








