Sphingosine 1-Phosphate Modulation in Inflammatory Bowel Diseases: Keeping Lymphocytes Out of the Intestine
Abstract
:1. Introduction
2. Methods
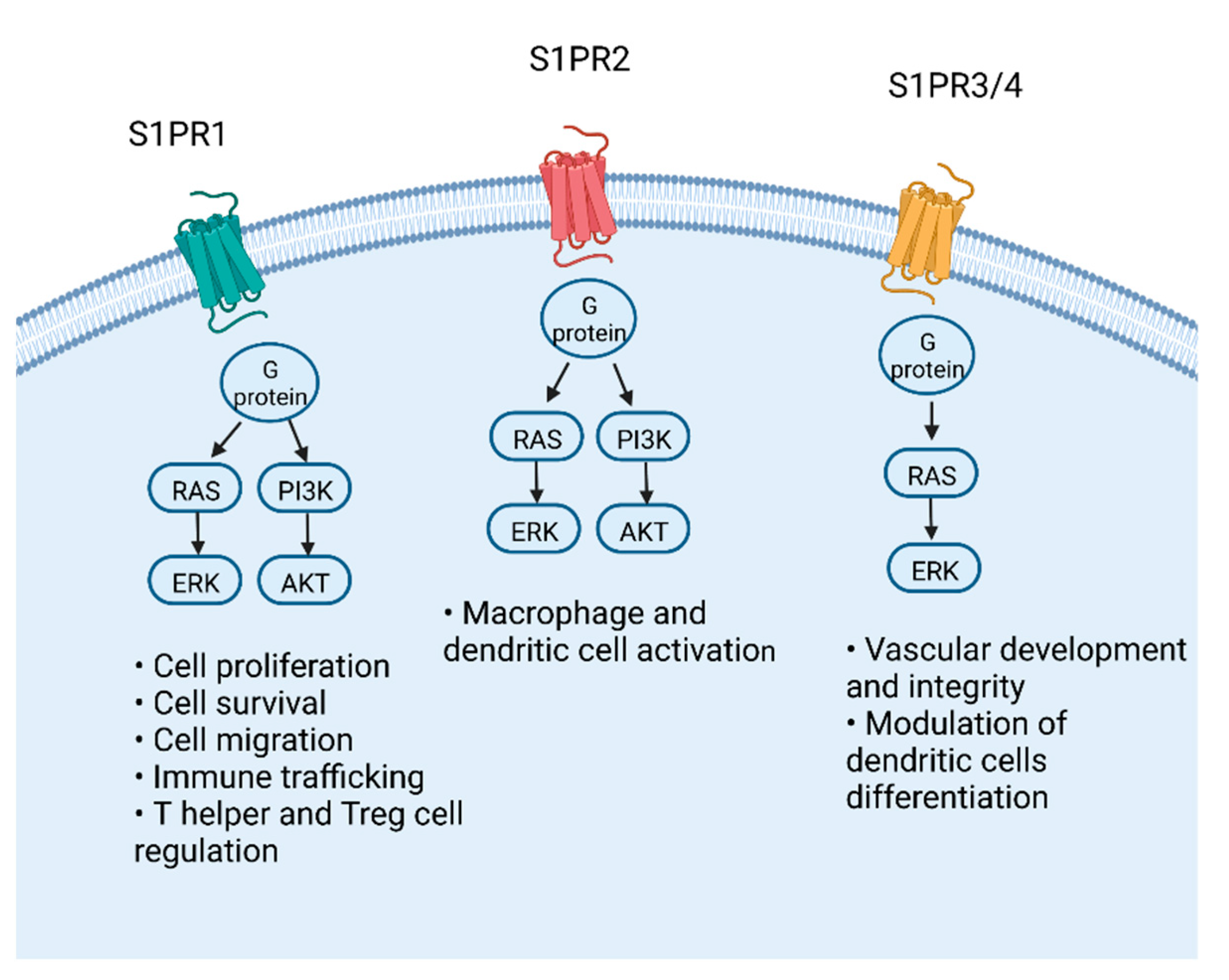
3. Molecular Pathway of S1P
4. S1P Modulators in IBD: From Clinical Trials to Real World
4.1. Fingolimod
4.2. Ozanimod
4.3. Etrasimod
4.4. Amiselimod
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Primers 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO guidelines on therapeutics in ulcerative colitis: Medical treatment. J. Crohns Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO guidelines on therapeutics in crohn’s disease: Medical treatment. J. Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Cholapranee, A.; Hazlewood, G.S.; Kaplan, G.G.; Peyrin-Biroulet, L.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment. Pharmacol. Ther. 2017, 45, 1291–1302. [Google Scholar] [CrossRef] [Green Version]
- Battat, R.; Duijvestein, M.; Guizzetti, L.; Choudhary, D.; Boland, B.S.; Dulai, P.S.; Parker, C.E.; Nguyen, T.M.; Singh, S.; Vande Casteele, N.; et al. Histologic Healing Rates of Medical Therapies for Ulcerative Colitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2019, 114, 733–745. [Google Scholar] [CrossRef]
- Paschos, P.; Katsoula, A.; Salanti, G.; Giouleme, O.; Athanasiadou, E.; Tsapas, A. Systematic review with network meta-analysis: The impact of medical interventions for moderate-to-severe ulcerative colitis on health-related quality of life. Aliment. Pharmacol. Ther. 2018, 48, 1174–1185. [Google Scholar] [CrossRef]
- Hossain, A.; Lördal, M.; Olsson, A.E.; Storlåhls, A.; Aleman, S.; Eberhardson, M.; Befrits, R. Sustained clinical benefit, improved quality of life, and reduced intestinal surgery from maintenance infliximab treatment in inflammatory bowel disease. Scand. J. Gastroenterol. 2020, 55, 178–183. [Google Scholar] [CrossRef]
- Stidham, R.W.; Lee, T.C.H.; Higgins, P.D.R.; Deshpande, A.R.; Sussman, D.A.; Singal, A.G.; Saini, S.D.; Vijan, S.; Waljee, A.K. Systematic review with network meta-analysis: The efficacy of anti-TNF agents for the treatment of Crohn’s disease. Aliment. Pharmacol. Ther. 2014, 39, 1349–1362. [Google Scholar] [CrossRef]
- Singh, S.; George, J.; Boland, B.S.; Vande Casteele, N.; Sandborn, W.J. Primary Non-Response to Tumor Necrosis Factor Antagonists is Associated with Inferior Response to Second-line Biologics in Patients with Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis. J. Crohns Colitis 2018, 12, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Chaparro, M. Primary Failure to an Anti-TNF Agent in Inflammatory Bowel Disease: Switch (to a Second Anti-TNF Agent) or Swap (for Another Mechanism of Action)? J. Clin. Med. 2021, 10, 5318. [Google Scholar] [CrossRef] [PubMed]
- Park, K.T.; Ehrlich, O.G.; Allen, J.I.; Meadows, P.; Szigethy, E.M.; Henrichsen, K.; Kim, S.C.; Lawton, R.C.; Murphy, S.M.; Regueiro, M.; et al. The cost of inflammatory bowel disease: An initiative from the crohn’s & colitis foundation. Inflamm. Bowel Dis. 2020, 26, 1118. [Google Scholar]
- Privitera, G.; Pugliese, D.; Lopetuso, L.R.; Scaldaferri, F.; Neri, M.; Guidi, L.; Gasbarrini, A.; Armuzzi, A. Novel trends with biologics in inflammatory bowel disease: Sequential and combined approaches. Therap. Adv. Gastroenterol. 2021, 14, 17562848211006668. [Google Scholar] [CrossRef] [PubMed]
- Petti, L.; Rizzo, G.; Rubbino, F.; Elangovan, S.; Colombo, P.; Restelli, S.; Piontini, A.; Arena, V.; Carvello, M.; Romano, B.; et al. Unveiling role of sphingosine-1-phosphate receptor 2 as a brake of epithelial stem cell proliferation and a tumor suppressor in colorectal cancer. J. Exp. Clin. Cancer Res. 2020, 39, 253. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Christopher, R.; Behan, D.; Lassen, C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun. Rev. 2017, 16, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Vetrano, S.; Salas, A.; Nayeri, S.; Duijvestein, M.; Vande Casteele, N. Sphingosine 1-phosphate modulation and immune cell trafficking in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 351–366. [Google Scholar] [CrossRef]
- Mayzent [Prescribing Information]. Available online: https://www.novartis.us/sites/www.novartis.us/files/mayzent.pdf (accessed on 7 June 2022).
- Gilenya [Prescribing Information]. Available online: https://www.novartis.us/sites/www.novartis.us/files/gilenya.pdf (accessed on 7 June 2022).
- Zeposia [Prescribing Information]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209899s000lbl.pdf (accessed on 7 June 2022).
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef]
- Cyster, J.G.; Schwab, S.R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 2012, 30, 69–94. [Google Scholar] [CrossRef]
- Schwab, S.R.; Pereira, J.P.; Matloubian, M.; Xu, Y.; Huang, Y.; Cyster, J.G. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 2005, 309, 1735–1739. [Google Scholar] [CrossRef]
- Sensken, S.-C.; Bode, C.; Nagarajan, M.; Peest, U.; Pabst, O.; Gräler, M.H. Redistribution of sphingosine 1-phosphate by sphingosine kinase 2 contributes to lymphopenia. J. Immunol. 2010, 184, 4133–4142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.; Dev, K.K. The structure and function of the S1P1 receptor. Trends Pharmacol. Sci. 2013, 34, 401–412. [Google Scholar] [CrossRef]
- Lo, C.G.; Xu, Y.; Proia, R.L.; Cyster, J.G. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 2005, 201, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Czeloth, N.; Bernhardt, G.; Hofmann, F.; Genth, H.; Förster, R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J. Immunol. 2005, 175, 2960–2967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhry, B.Z.; Cohen, J.A.; Conway, D.S. Sphingosine 1-Phosphate Receptor Modulators for the Treatment of Multiple Sclerosis. Neurotherapeutics 2017, 14, 859–873. [Google Scholar] [CrossRef]
- Cohen, J.A.; Barkhof, F.; Comi, G.; Hartung, H.-P.; Khatri, B.O.; Montalban, X.; Pelletier, J.; Capra, R.; Gallo, P.; Izquierdo, G.; et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 402–415. [Google Scholar] [CrossRef]
- Kappos, L.; Radue, E.-W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, T.; Ito, T.; Kishi, D.; Kai, Y.; Tamagawa, H.; Nezu, R.; Kiyono, H.; Matsuda, H. Therapeutic effects of a new lymphocyte homing reagent FTY720 in interleukin-10 gene-deficient mice with colitis. Inflamm. Bowel Dis. 2004, 10, 182–192. [Google Scholar] [CrossRef]
- Daniel, C.; Sartory, N.; Zahn, N.; Geisslinger, G.; Radeke, H.H.; Stein, J.M. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J. Immunol. 2007, 178, 2458–2468. [Google Scholar] [CrossRef] [Green Version]
- Parigi, T.L.; Roda, G.; Argollo, M.; Gilardi, D.; Danese, S. Is there a role for therapeutic sphingolipids in inflammatory bowel disease? Expert Rev. Gastroenterol. Hepatol. 2020, 14, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Vargas, W.S.; Perumal, J.S. Fingolimod and cardiac risk: Latest findings and clinical implications. Ther. Adv. Drug Saf. 2013, 4, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Scott, F.L.; Clemons, B.; Brooks, J.; Brahmachary, E.; Powell, R.; Dedman, H.; Desale, H.G.; Timony, G.A.; Martinborough, E.; Rosen, H.; et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br. J. Pharmacol. 2016, 173, 1778–1792. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.A.; Comi, G.; Selmaj, K.W.; Bar-Or, A.; Arnold, D.L.; Steinman, L.; Hartung, H.P.; Montalban, X.; Kubala Havrdová, E.; Cree, B.A.C.; et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): A multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019, 18, 1021–1033. [Google Scholar] [CrossRef]
- Comi, G.; Kappos, L.; Selmaj, K.W.; Bar-Or, A.; Arnold, D.L.; Steinman, L.; Hartung, H.P.; Montalban, X.; Kubala Havrdová, E.; Cree, B.A.C.; et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): A multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019, 18, 1009–1020. [Google Scholar] [CrossRef]
- Gilardi, D.; Gabbiadini, R.; Allocca, M.; Correale, C.; Fiorino, G.; Furfaro, F.; Zilli, A.; Peyrin-Biroulet, L.; Danese, S. PK, PD, and interactions: The new scenario with JAK inhibitors and S1P receptor modulators, two classes of small molecule drugs, in IBD. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 797–806. [Google Scholar] [CrossRef]
- Sndborn, W.J.; Feagan, B.G.; Wolf, D.C.; D’Haens, G.; Vermeire, S.; Hanauer, S.B.; Ghosh, S.; Smith, H.; Cravets, M.; Frohna, P.A.; et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N. Engl. J. Med. 2016, 374, 1754–1762. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; D’Haens, G.; Wolf, D.C.; Jovanovic, I.; Hanauer, S.B.; Ghosh, S.; Petersen, A.; Hua, S.Y.; Lee, J.H.; et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2021, 385, 1280–1291. [Google Scholar] [CrossRef]
- Danese, S.; Colombel, J.F.; Ponich, T.; Jovanovic, I.; Bossuyt, P.; Longman, R.; Alekseeva, O.; Petersen, A.; Chitkara, D.; Marta, C.; et al. Long-term use of ozanimod in patients with moderately to severely active Ulcerative Colitis. In Proceedings of the ECCO Congress 2022—DOP44, Virtual, 16–19 February 2022. [Google Scholar]
- Feagan, B.G.; Sandborn, W.J.; Danese, S.; Wolf, D.C.; Liu, W.J.; Hua, S.Y.; Minton, N.; Olson, A.; D’Haens, G. Ozanimod induction therapy for patients with moderate to severe Crohn’s disease: A single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol. Hepatol. 2020, 5, 819–828. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Hanauer, S.; Vermeire, S.; Ghosh, S.; Liu, W.J.; Petersen, A.; Charles, L.; Huang, V.; Usiskin, K.; et al. Long-Term Efficacy and Safety of Ozanimod in Moderately to Severely Active Ulcerative Colitis: Results from the Open-Label Extension of the Randomized, Phase 2 TOUCHSTONE Study. J. Crohns Colitis 2021, 15, 1120–1129. [Google Scholar] [CrossRef]
- Wolf, D.; Colombel, J.F.; Ponich, T.P.; Jovanovich, I.; Bossuyt, P.; Longman, R.; Alekseeva, O.; Petersen, A.; Chitkara, D.; Marta, C.; et al. Long-term use of ozanimod in patients with moderately to severely active ulcerative colitis—DDW ePoster—Tu1458. Gastroenterology 2022, 162, S-969–S-970. [Google Scholar] [CrossRef]
- Armuzzi, A.; Cross, R.K.; Lichtenstein, G.; Danese, S.; Vermeire, S.; Zhou, W.; Sands, B.E.; Dignass, A.; Irving, P. Long-term cardiac safety of ozanimod in phase 3 clinical program of Ulcerative Colitis and relapsing multiple sclerosis. In Proceedings of the ECCO Congress 2022—DOP45, Virtual, 16–19 February 2022. [Google Scholar]
- Cohen, N.A.; Choi, D.; Choden, T.; Cleveland, N.K.; Cohen, R.D.; Rubin, D.T. Ozanimod in the Treatment of Ulcerative Colitis: Initial Real-World Data from a Large Tertiary Center. Clin. Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Axelrad, J.; Ahmad, H.A.; Sandborn, W.J.; Feagan, B.G.; D’Haens, G. Early mucosal healing at week 10 with ozanimod predicts clinical outcomes at week 52: Post hoc analysis of the phase 3 True North clinical trial [ECCO abstract P431]. J. Crohns Colitis 2022, 16 (Suppl. 1), i415. [Google Scholar] [CrossRef]
- Schreiber, S.; Morgan, M.; Christopher, L. Etrasimod [APD334], a Potent, Selective, Oral S1P Receptor Modulator with Preclinical Autoimmune Disease-Modifying Activity Exhibits Favorable PK/PD Properties in Healthy Volunteers. In Proceedings of the Advances in Inflammatory Bowel Diseases (AIBD), Orlando, FL, USA, 8–10 December 2016. [Google Scholar]
- Peyrin-Biroulet, L.; Adams, J.; Turner, S.; Trokan, L.; Panes, J. Safety and immune modulatory properties of etrasimod [APD334], a next-generation oral, selective sphingosine 1-phosphate receptor [S1PR] modulator, in healthy volunteers. In Proceedings of the ECCO Congress, Vienna, Austria, 14–17 February 2018. [Google Scholar]
- Sandborn, W.J.; Peyrin-Biroulet, L.; Zhang, J.; Chiorean, M.; Vermeire, S.; Lee, S.D.; Kühbacher, T.; Yacyshyn, B.; Cabell, C.H.; Naik, S.U.; et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology 2020, 158, 550–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeire, S.; Chiorean, M.; Panés, J.; Peyrin-Biroulet, L.; Zhang, J.; Sands, B.E.; Lazin, K.; Klassen, P.; Naik, S.U.; Cabell, C.H.; et al. Long-term Safety and Efficacy of Etrasimod for Ulcerative Colitis: Results from the Open-label Extension of the OASIS Study. J. Crohns Colitis 2021, 15, 950–959. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Sands, B.E.; Feagan, B.G.; D’Haens, G.R.; Danese, S.; Hanauer, S.; Peyrin-Biroulet, L.; Zhang, J.; Chiorean, M.; Vermeire, S.; et al. Etrasimod 2mg once daily as treatment for moderately to severely active ulcerative colitis: Results from the phase 3 ELEVATE UC 52 and ELEVATE UC 12 trials—Digestive Disease Week (DDW) 2022—Abstract 968a. Gastroenterology 2022, 162, S-1395. [Google Scholar] [CrossRef]
- Pérez-Jeldres, T.; Alvarez-Lobos, M.; Rivera-Nieves, J. Targeting Sphingosine-1-Phosphate Signaling in Immune-Mediated Diseases: Beyond Multiple Sclerosis. Drugs 2021, 81, 985–1002. [Google Scholar] [CrossRef]
- McGinley, M.P.; Cohen, J.A. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet 2021, 398, 1184–1194. [Google Scholar] [CrossRef]
- Harada, T.; Wilbraham, D.; de La Borderie, G.; Inoue, S.; Bush, J.; Camm, A.J. Cardiac effects of amiselimod compared with fingolimod and placebo: Results of a randomised, parallel-group, phase I study in healthy subjects. Br. J. Clin. Pharmacol. 2017, 83, 1011–1027. [Google Scholar] [CrossRef]
- Sugahara, K.; Maeda, Y.; Shimano, K.; Mogami, A.; Kataoka, H.; Ogawa, K.; Hikida, K.; Kumagai, H.; Asayama, M.; Yamamoto, T.; et al. Amiselimod, a novel sphingosine 1-phosphate receptor-1 modulator, has potent therapeutic efficacy for autoimmune diseases, with low bradycardia risk. Br. J. Pharmacol. 2017, 174, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Shimano, K.; Maeda, Y.; Kataoka, H.; Murase, M.; Mochizuki, S.; Utsumi, H.; Oshita, K.; Sugahara, K. Amiselimod (MT-1303), a novel sphingosine 1-phosphate receptor-1 functional antagonist, inhibits progress of chronic colitis induced by transfer of CD4+CD45RBhigh T cells. PLoS ONE 2019, 14, e0226154. [Google Scholar] [CrossRef]
- D’Haens, G.; Danese, S.; Davies, M.; Watanabe, M.; Hibi, T. A Phase II, Multicentre, Randomised, Double-Blind, Placebo-Controlled Study to Evaluate Safety, Tolerability and Efficacy of Amiselimod in Patients with Moderate to Severe Active Crohn’s Disease. J. Crohns Colitis 2022, 16, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Bausch Health Picks Up IBD Drug Amiselimod Rejected by Biogen. Available online: https://www.thepharmaletter.com/article/bausch-health-picks-up-ibd-drug-amiselimod-rejected-by-biogen (accessed on 7 June 2022).
- Sigmund, B.; Axelrad, J.; Pondel, M.; Osterman, M.T.; Ahmad, H.A.; Memaj, A.; Regueiro, M.; Armuzzi, A.; Afzali, A. Rapidity of ozanimod-induced symptomatic response and remission in patients with moderately to severely active Ulcerative Colitis: Results from the induction period of True North—ECCO 2022—DOP43. In Proceedings of the ECCO Congress, Virtual, 16–19 February 2022. [Google Scholar]
- Jung, B.; Obinata, H.; Galvani, S.; Mendelson, K.; Ding, B.S.; Skoura, A.; Kinzel, B.; Brinkmann, V.; Rafii, S.; Evans, T.; et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell 2012, 23, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.J.; Thangada, S.; Claffey, K.P.; Ancellin, N.; Liu, C.H.; Volpi, M.; Sha’afi, R.I.; Hla, T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999, 99, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Greenspon, J.; Li, R.; Xiao, L.; Rao, J.N.; Sun, R.; Strauch, E.D.; Shea-Donohue, T.; Wang, J.Y.; Turner, D.J. Sphingosine-1-phosphate regulates the expression of adherens junction protein E-cadherin and enhances intestinal epithelial cell barrier function. Dig. Dis. Sci. 2011, 56, 1342–1353. [Google Scholar] [CrossRef] [Green Version]
- Privitera, G.; Pugliese, D.; Onali, S.; Petito, V.; Scaldaferri, F.; Gasbarrini, A.; Danese, S.; Armuzzi, A. Combination therapy in inflammatory bowel disease—From traditional immunosuppressors towards the new paradigm of dual targeted therapy. Autoimmun. Rev. 2021, 20, 102832. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Dignass, A.; Irving, P.; Chiorean, M.; Long, M.; Eren, D.; Ahmad, H.A.; Osterman, M.T.; Petersen, A.; Elegbe, A.; et al. P316 Ozanimod is an efficacious oral therapy after 5-ASA failure in immunomodulator- and biologic-naive patients with ulcerative colitis: Post hoc analysis from True North—ECCO Congress 2022—P316. In Proceedings of the ECCO Congress, Virtual, 16–19 February 2022. [Google Scholar]
- Dubinsky, M.; Betts, K.; Eren, D.; Tang, W.; Yin, L.; Gupte-Singh, K. Comparative efficacy and safety of ozanimod and ustekinumab in patients with moderately to severely active ulcerative colitis—DDW ePoster—Su1500. Gastroenterology 2022, 162, S-602. [Google Scholar] [CrossRef]
| Molecule | Pharmacological Mechanism | Administration | Development |
|---|---|---|---|
| Fingolimod | non-selective, S1PR1-3-4-5 | Oral | Never tested in humans |
| Ozanimod | S1PR1, S1PR4 and S1PR5 | Oral | UC: FDA and EMA approved CD: Phase II/III recruiting |
| Etrasimod | S1PR1 and S1PR5 | Oral | UC: Phase III completed CD: Phase III recruiting |
| Amiselimod (MT-1303) | S1PR1 | Oral | CD: Phase II completed |
| Molecule | Efficacy in CD | Efficacy in UC | Safety in CD | Safety in UC |
|---|---|---|---|---|
| Ozanimod | 39.1% and 56.5% of the patients reached clinical remission (CDAI score <150) and clinical response (CDAI decrease of ≥100 from baseline), respectively | Clinical remission at 8 weeks achieved in 16% and 14% of the patients in the 1 mg and 0.5 mg group Endoscopic healing * observed in 34% and in 28% of the patients in the 1 mg and 0.5 mg group. Clinical remission rates were significantly higher compared to placebo during induction (18.4% vs. 6.0%, p < 0.001) and maintenance (37.0% vs. 18.5%, p < 0.001). At 94 weeks 34% of the whole study population and 55% of the responders continued to maintain clinical response. | In the STEPSTONE trial, no cases of bradycardia/arrhythmia occurred in the treated CD patients | Transient dose-dependent heart rate reduction at induction. Infections’ rate in ozanimod-treated UC patients was comparable with placebo during induction (9.9% vs. 10.7%) and higher during maintenance (23% vs. 11.9%). |
| Etrasimod | ongoing phase II/III study | Clinical remission assessed by 33.0% after 12 weeks of treatment (2 mg/day). Endoscopic improvement at week 12 was significantly higher as compared with placebo (41.8% vs. 17.8%; p= 0.003). Steroid-free clinical remission observed in 22% of patients at end of treatment (week 46/52) | ongoing phase II/III study | One patient with a second-degree atrioventricular block type 1 and two patients with first-degree atrioventricular block. Neither treatment-related serious infections nor infections of severity grade ≥3 were observed. Two cases of herpes zoster were reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Buono, A.; Gabbiadini, R.; Alfarone, L.; Solitano, V.; Repici, A.; Vetrano, S.; Spinelli, A.; Armuzzi, A. Sphingosine 1-Phosphate Modulation in Inflammatory Bowel Diseases: Keeping Lymphocytes Out of the Intestine. Biomedicines 2022, 10, 1735. https://doi.org/10.3390/biomedicines10071735
Dal Buono A, Gabbiadini R, Alfarone L, Solitano V, Repici A, Vetrano S, Spinelli A, Armuzzi A. Sphingosine 1-Phosphate Modulation in Inflammatory Bowel Diseases: Keeping Lymphocytes Out of the Intestine. Biomedicines. 2022; 10(7):1735. https://doi.org/10.3390/biomedicines10071735
Chicago/Turabian StyleDal Buono, Arianna, Roberto Gabbiadini, Ludovico Alfarone, Virginia Solitano, Alessandro Repici, Stefania Vetrano, Antonino Spinelli, and Alessandro Armuzzi. 2022. "Sphingosine 1-Phosphate Modulation in Inflammatory Bowel Diseases: Keeping Lymphocytes Out of the Intestine" Biomedicines 10, no. 7: 1735. https://doi.org/10.3390/biomedicines10071735
APA StyleDal Buono, A., Gabbiadini, R., Alfarone, L., Solitano, V., Repici, A., Vetrano, S., Spinelli, A., & Armuzzi, A. (2022). Sphingosine 1-Phosphate Modulation in Inflammatory Bowel Diseases: Keeping Lymphocytes Out of the Intestine. Biomedicines, 10(7), 1735. https://doi.org/10.3390/biomedicines10071735







