4-Aminopyridine Induces Nerve Growth Factor to Improve Skin Wound Healing and Tissue Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Wound Healing Assay
2.3. Histomorphometry Analysis
2.4. Immunofluorescence Staining of Tissue
2.5. Human Primary Cell Culture Experiments
2.6. Cell Viability Assay with 4-Aminopyridine
2.7. Cell Scratch Wound Healing Migration Assay
2.8. Tissue Protein Isolation and Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. 4-Aminopyridine (4-AP) Accelerates Wound Closure and Enhances Skin Regeneration
3.2. 4-AP Increases Keratinocyte Number and Epithelial Stem-Cell Markers in Healed Wounds
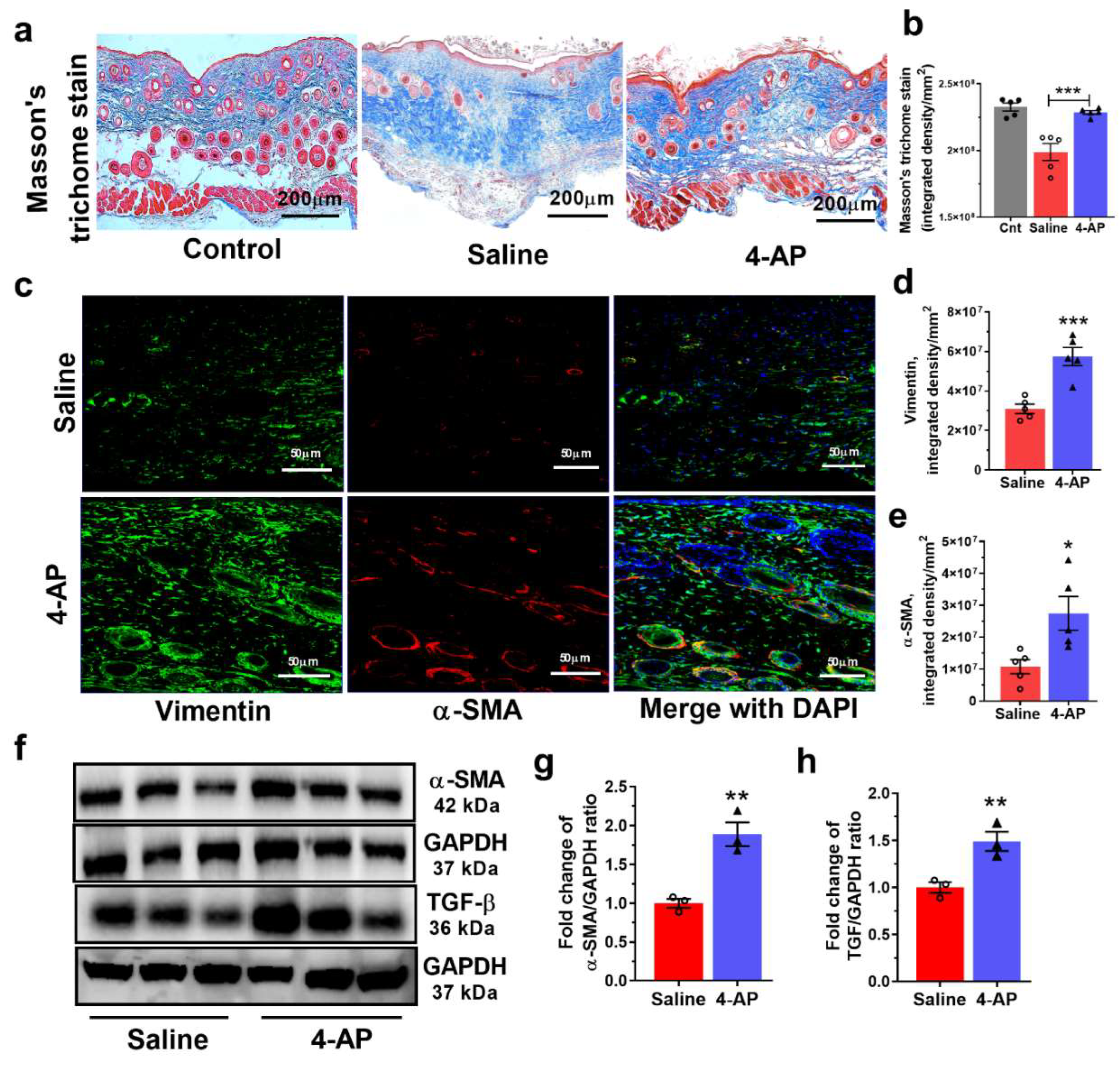
3.3. The 4-AP Treatment Promotes Increases in Fibroblasts, Myofibroblasts and Transforming Growth Factor-β (TGF-β)
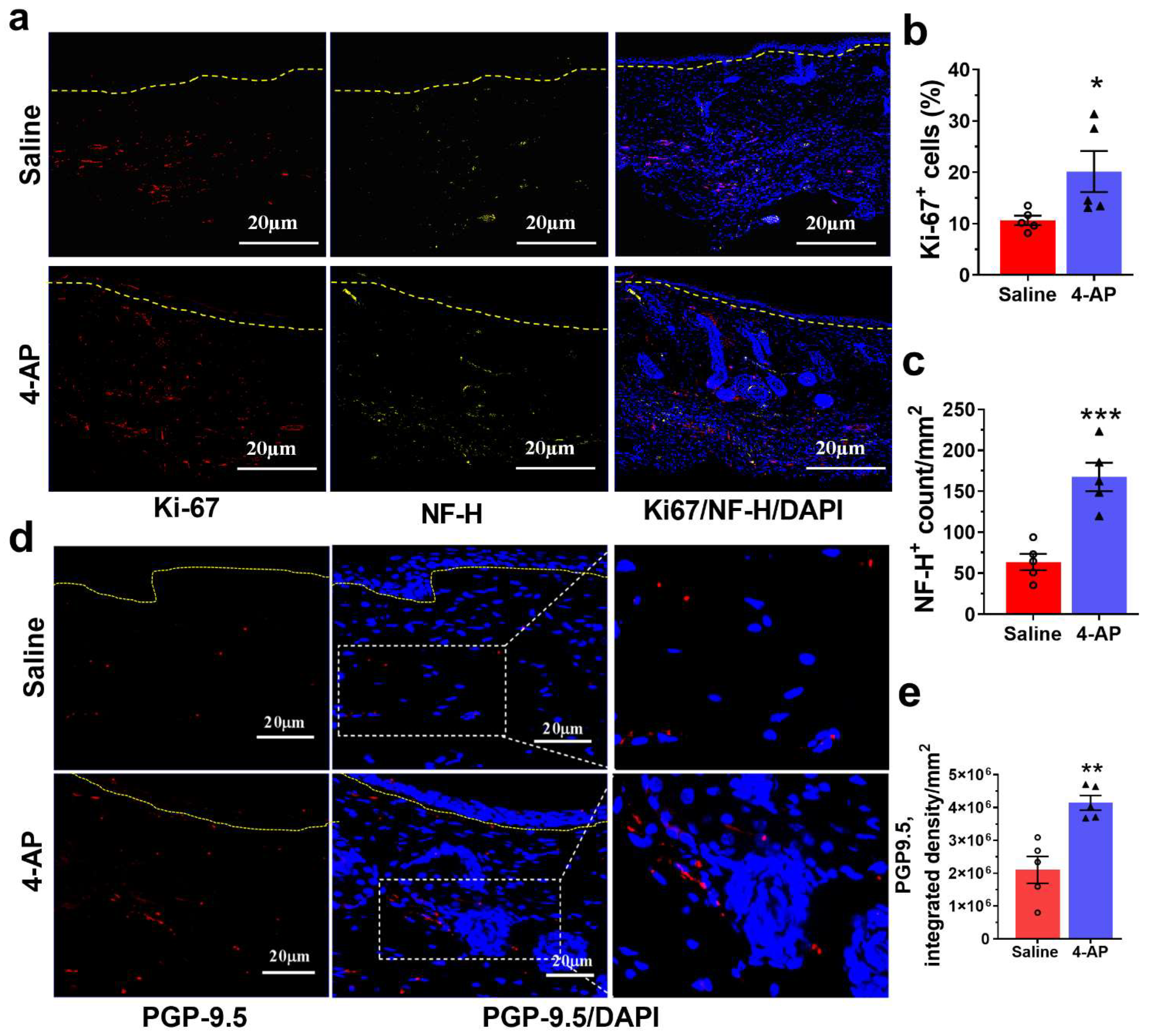
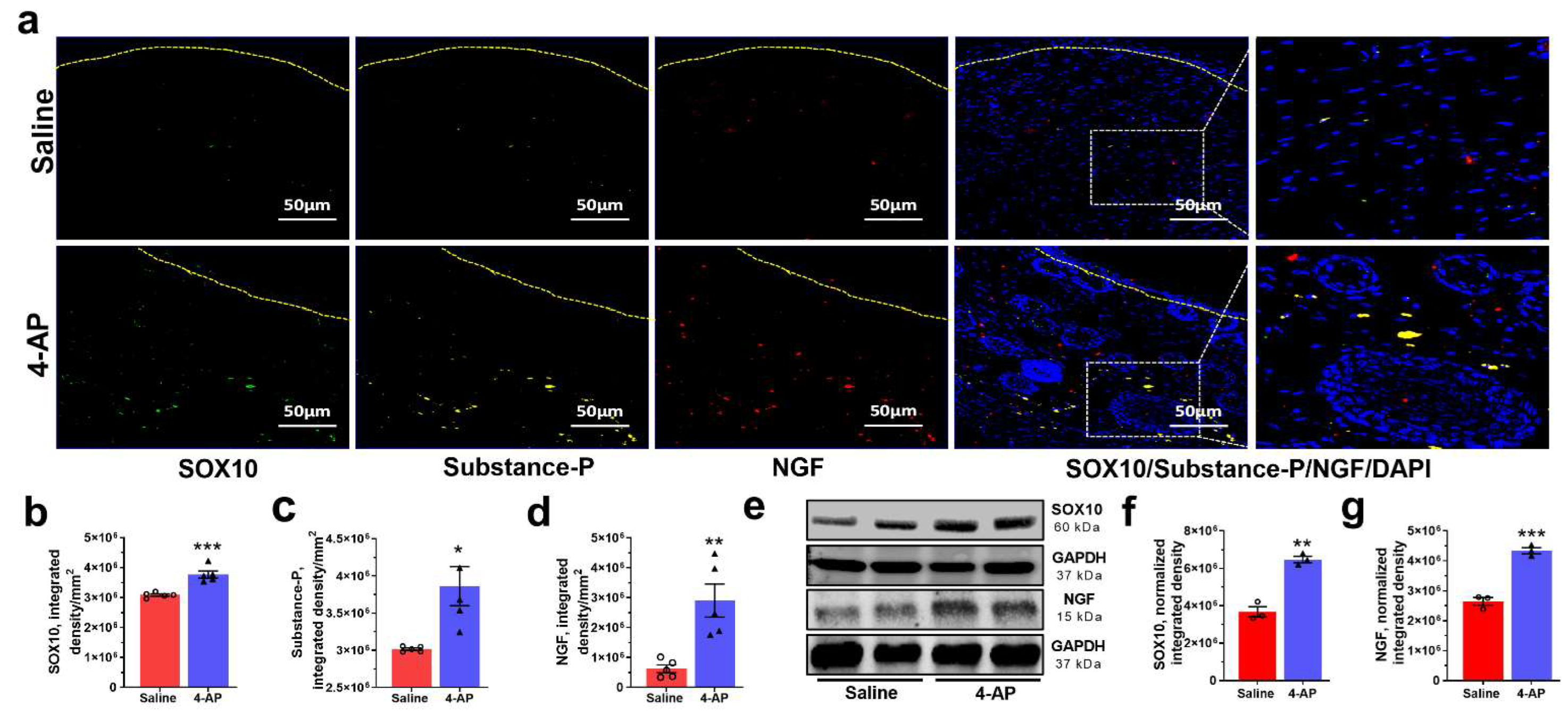
3.4. 4-AP Promotes Reinnervation and Neuropeptide Expression
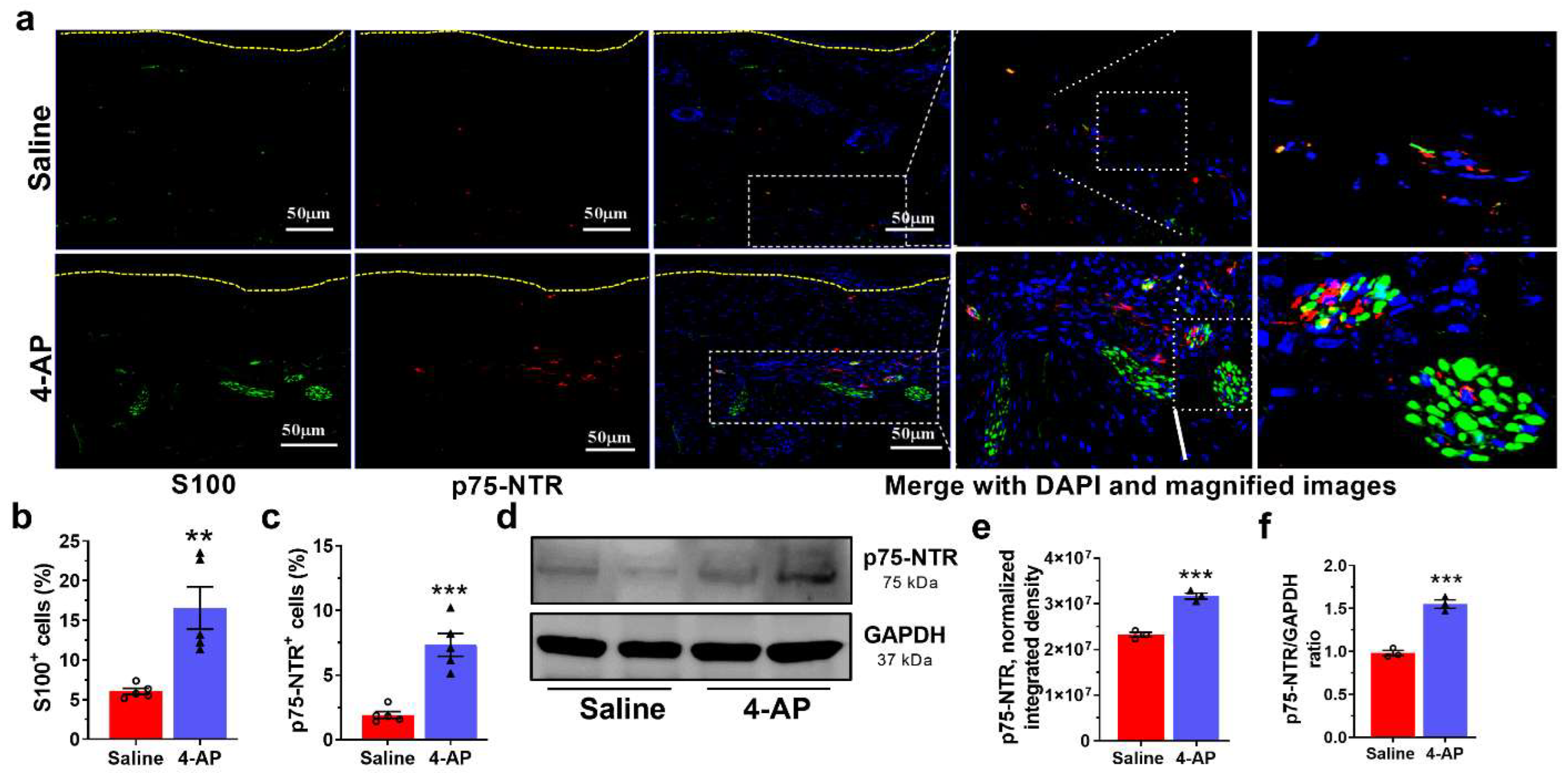
3.5. 4-AP Increases the Numbers of Schwann Cells (SC) and the Expression of Markers of an Early Differentiation State
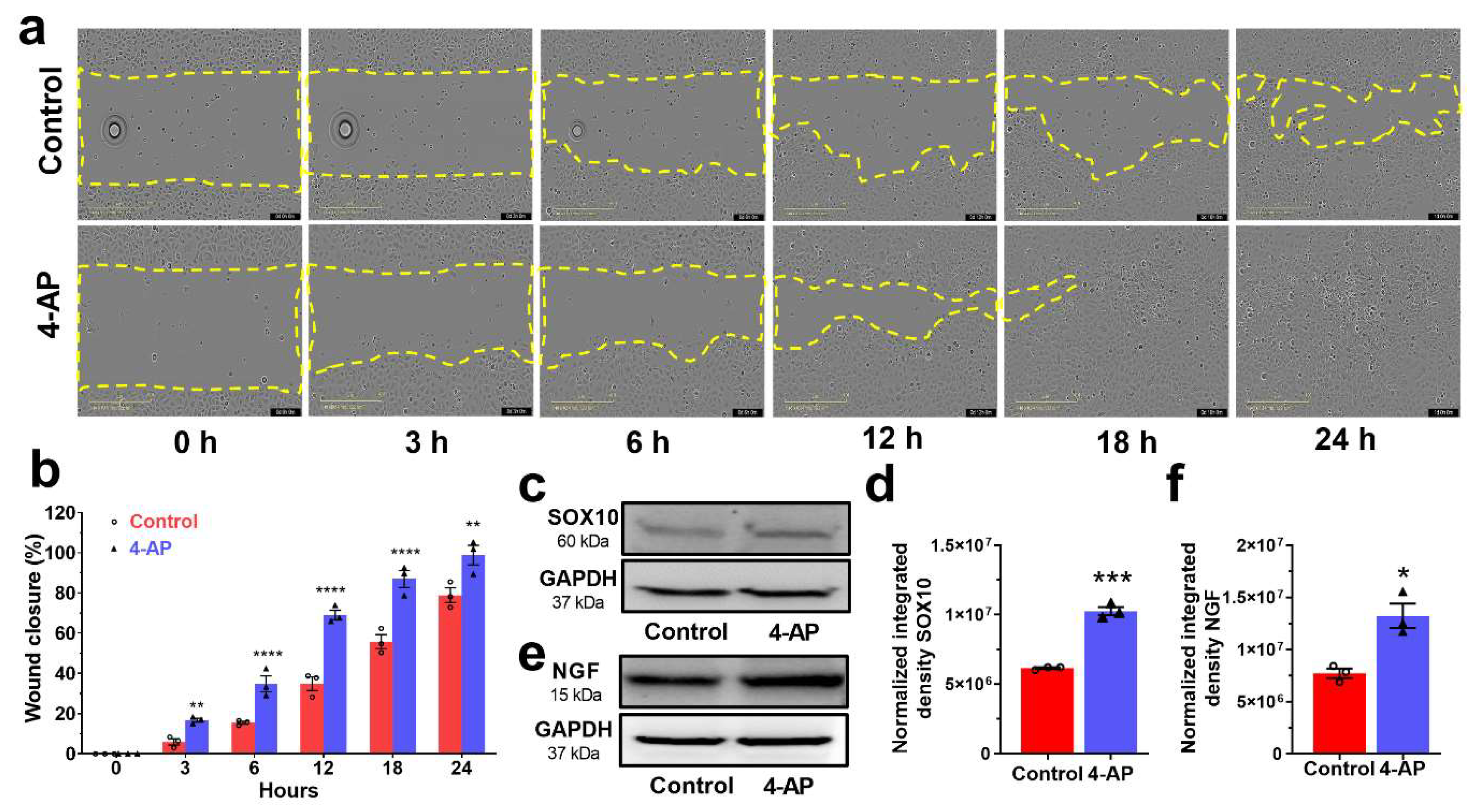
3.6. 4-AP Effectively Stimulates Proliferation and Migration in Primary Cultures of Human Skin-Derived Primary Cells In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parfejevs, V.; Debbache, J.; Shakhova, O.; Schaefer, S.M.; Glausch, M.; Wegner, M.; Suter, U.; Riekstina, U.; Werner, S.; Sommer, L. Injury-activated glial cells promote wound healing of the adult skin in mice. Nat. Commun. 2018, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Lebonvallet, N.; Laverdet, B.; Misery, L.; Desmouliere, A.; Girard, D. New insights into the roles of myofibroblasts and innervation during skin healing and innovative therapies to improve scar innervation. Exp. Dermatol. 2018, 27, 950–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Wang, J.; Chen, X.; Shi, Y.; Xie, J. Epidermal Stem Cells in Wound Healing and Regeneration. Stem Cells Int. 2020, 2020, 9148310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashrafi, M.; Baguneid, M.; Bayat, A. The Role of Neuromediators and Innervation in Cutaneous Wound Healing. Acta Derm. Venereol. 2016, 96, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.C.; Lin, B.B.; Hu, H.W.; Lin, C.; Jin, W.Y.; Zhang, F.B.; Zhu, Y.A.; Lu, C.J.; Wei, X.J.; Chen, R.J. NGF accelerates cutaneous wound healing by promoting the migration of dermal fibroblasts via the PI3K/Akt-Rac1-JNK and ERK pathways. Biomed. Res. Int. 2014, 2014, 547187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheret, J.; Lebonvallet, N.; Carre, J.L.; Misery, L.; Le Gall-Ianotto, C. Role of neuropeptides, neurotrophins, and neurohormones in skin wound healing. Wound Repair Regen. 2013, 21, 772–788. [Google Scholar] [CrossRef]
- Douglas, H.E. TGF-ß in wound healing: A review. J. Wound Care 2010, 19, 403–406. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Mojiri Forushani, H.; Mohammad Asgari, H. Wound healing potential of topical amlodipine in full thickness wound of rabbit. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e15638. [Google Scholar] [CrossRef] [Green Version]
- Ashkani-Esfahani, S.; Hosseinabadi, O.K.; Moezzi, P.; Moafpourian, Y.; Kardeh, S.; Rafiee, S.; Fatheazam, R.; Noorafshan, A.; Nadimi, E.; Mehrvarz, S.; et al. Verapamil, a Calcium-Channel Blocker, Improves the Wound Healing Process in Rats with Excisional Full-Thickness Skin Wounds Based on Stereological Parameters. Adv. Ski. Wound Care 2016, 29, 271–274. [Google Scholar] [CrossRef]
- Bhaskar, H.N.; Udupa, S.L.; Udupa, A.L. Effect of nifedipine and amlodipine on wound healing in rats. Indian J. Physiol. Pharmacol. 2004, 48, 111–114. [Google Scholar] [PubMed]
- Bagheri, M.; Jahromi, B.M.; Mirkhani, H.; Solhjou, Z.; Noorafshan, A.; Zamani, A.; Amirghofran, Z. Azelnidipine, a new calcium channel blocker, promotes skin wound healing in diabetic rats. J. Surg. Res. 2011, 169, e101–e107. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.C.; Li, H.; Clark, A.; Sundem, L.; Zuscik, M.; Noble, M.; Elfar, J. 4-Aminopyridine promotes functional recovery and remyelination in acute peripheral nerve injury. EMBO Mol. Med. 2016, 8, 1409–1420. [Google Scholar] [CrossRef] [Green Version]
- Yue, L.; Talukder, M.A.H.; Gurjar, A.; Lee, J.I.; Noble, M.; Dirksen, R.T.; Chakkalakal, J.; Elfar, J.C. 4-Aminopyridine attenuates muscle atrophy after sciatic nerve crush injury in mice. Muscle Nerve 2019, 60, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Noble, M.; Tseng, K.C.; Li, H.Y.; Elfar, J.C. 4-Aminopyridine as a Single Agent Diagnostic and Treatment for Severe Nerve Crush Injury. Mil. Med. 2019, 184, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Manoukian, O.S.; Arul, M.R.; Rudraiah, S.; Kalajzic, I.; Kumbar, S.G. Aligned microchannel polymer-nanotube composites for peripheral nerve regeneration: Small molecule drug delivery. J. Control. Release 2019, 296, 54–67. [Google Scholar] [CrossRef]
- Hayes, K.C. The use of 4-aminopyridine (fampridine) in demyelinating disorders. CNS Drug. Rev. 2004, 10, 295–316. [Google Scholar] [CrossRef]
- Hu, D.; Liu, J.; Keblesh, J.; Xiong, H. Involvement of the 4-aminopyridine-sensitive transient A-type K+ current in macrophage-induced neuronal injury. Eur. J. Neurosci. 2010, 31, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, D.P.; Chen, S.R.; Chen, J.; Hu, H.; Pan, H.L. Potentiation of high voltage-activated calcium channels by 4-aminopyridine depends on subunit composition. Mol. Pharmacol. 2014, 86, 760–772. [Google Scholar] [CrossRef] [Green Version]
- Bever, C.T.; Judge, S.I. Sustained-release fampridine for multiple sclerosis. Expert Opin Investig. Drugs 2009, 18, 1013–1024. [Google Scholar] [CrossRef]
- Chen, L.; Mirza, R.; Kwon, Y.; DiPietro, L.A.; Koh, T.J. The murine excisional wound model: Contraction revisited. Wound Repair Regen. 2015, 23, 874–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, C.J.; Chen, C.C.; Leu, Y.L.; Lin, M.W.; Chiu, M.M.; Wang, S.H. The effects of artocarpin on wound healing: In Vitro and in vivo studies. Sci. Rep. 2017, 7, 15599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, A.D.; Brown, T.R.; Cohen, J.A.; Krupp, L.B.; Schapiro, R.; Schwid, S.R.; Cohen, R.; Marinucci, L.N.; Blight, A.R.; Fampridine, M.S.F.S.G. Dose comparison trial of sustained-release fampridine in multiple sclerosis. Neurology 2008, 71, 1134–1141. [Google Scholar] [CrossRef]
- Suvik, A.; Effendy, A.W.M. The use of modified Masson’s trichrome staining in collagen evaluation in wound healing study. Malays. J. Vet. Res. 2016, 3, 39–47. [Google Scholar]
- Mcheik, J.N.; Barrault, C.; Pedretti, N.; Garnier, J.; Juchaux, F.; Levard, G.; Morel, F.; Lecron, J.C.; Bernard, F.X. Foreskin-isolated keratinocytes provide successful extemporaneous autologous paediatric skin grafts. J. Tissue Eng. Regen. M 2016, 10, 252–260. [Google Scholar] [CrossRef]
- Jagadeeshaprasad, M.G.; Govindappa, P.K.; Nelson, A.M.; Elfar, J.C. Isolation, Culture, and Characterization of Primary Schwann Cells, Keratinocytes, and Fibroblasts from Human Foreskin. J. Vis. Exp. 2022, 181, e63776. [Google Scholar] [CrossRef]
- Stratton, J.A.; Kumar, R.; Sinha, S.; Shah, P.; Stykel, M.; Shapira, Y.; Midha, R.; Biernaskie, J. Purification and Characterization of Schwann Cells from Adult Human Skin and Nerve. Eneuro 2017, 4, 307–0316. [Google Scholar] [CrossRef] [Green Version]
- Henrot, P.; Laurent, P.; Levionnois, E.; Leleu, D.; Pain, C.; Truchetet, M.E.; Cario, M. A Method for Isolating and Culturing Skin Cells: Application to Endothelial Cells, Fibroblasts, Keratinocytes, and Melanocytes From Punch Biopsies in Systemic Sclerosis Skin. Front. Immunol. 2020, 11, 566607. [Google Scholar] [CrossRef]
- Gostynska, N.; Pannella, M.; Rocco, M.L.; Giardino, L.; Aloe, L.; Calza, L. The pleiotropic molecule NGF regulates the in vitro properties of fibroblasts, keratinocytes, and endothelial cells: Implications for wound healing. Am. J. Physiol.-Cell Physiol. 2020, 318, C360–C371. [Google Scholar] [CrossRef]
- Riis, S.; Newman, R.; Ipek, H.; Andersen, J.I.; Kuninger, D.; Boucher, S.; Vemuri, M.C.; Pennisi, C.P.; Zachar, V.; Fink, T. Hypoxia enhances the wound-healing potential of adipose-derived stem cells in a novel human primary keratinocyte-based scratch assay. Int. J. Mol. Med. 2017, 39, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Bei, F.; Lee, H.H.C.; Liu, X.; Gunner, G.; Jin, H.; Ma, L.; Wang, C.; Hou, L.; Hensch, T.K.; Frank, E.; et al. Restoration of Visual Function by Enhancing Conduction in Regenerated Axons. Cell 2016, 164, 219–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, M.; Yang, Z.; Andl, T.; Cui, C.; Kim, N.; Millar, S.E.; Cotsarelis, G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007, 447, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.M.; Reddy, S.K.; Ratliff, T.S.; Hossain, M.Z.; Katseff, A.S.; Zhu, A.S.; Chang, E.; Resnik, S.R.; Page, C.; Kim, D.; et al. dsRNA Released by Tissue Damage Activates TLR3 to Drive Skin Regeneration. Cell Stem Cell 2015, 17, 139–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.; Paramio, J.M.; Bravo, A.; Ramirez, A.; Jorcano, J.L. The expression of keratin k10 in the basal layer of the epidermis inhibits cell proliferation and prevents skin tumorigenesis. J. Biol. Chem. 2002, 277, 19122–19130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, A.; Teh, M.T.; Mackenzie, I.C.; Waseem, A. Keratin k15 as a biomarker of epidermal stem cells. Int. J. Mol. Sci. 2013, 14, 19385–19398. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Coulombe, P.A. Keratin 17 modulates hair follicle cycling in a TNFalpha-dependent fashion. Genes Dev 2006, 20, 1353–1364. [Google Scholar] [CrossRef] [Green Version]
- Shwartz, Y.; Gonzalez-Celeiro, M.; Chen, C.L.; Pasolli, H.A.; Sheu, S.H.; Fan, S.M.; Shamsi, F.; Assaad, S.; Lin, E.T.; Zhang, B.; et al. Cell Types Promoting Goosebumps Form a Niche to Regulate Hair Follicle Stem Cells. Cell 2020, 182, 578–593.e19. [Google Scholar] [CrossRef]
- Dietrich, M.; Koska, V.; Hecker, C.; Gottle, P.; Hilla, A.M.; Heskamp, A.; Lepka, K.; Issberner, A.; Hallenberger, A.; Baksmeier, C.; et al. Protective effects of 4-aminopyridine in experimental optic neuritis and multiple sclerosis. Brain 2020, 143, 1127–1142. [Google Scholar] [CrossRef]
- Wallengren, J.; Chen, D.; Sundler, F. Neuropeptide-containing C-fibres and wound healing in rat skin. Neither capsaicin nor peripheral neurotomy affect the rate of healing. Br. J. Dermatol. 1999, 140, 400–408. [Google Scholar] [CrossRef]
- Johnston, A.P.; Yuzwa, S.A.; Carr, M.J.; Mahmud, N.; Storer, M.A.; Krause, M.P.; Jones, K.; Paul, S.; Kaplan, D.R.; Miller, F.D. Dedifferentiated Schwann Cell Precursors Secreting Paracrine Factors Are Required for Regeneration of the Mammalian Digit Tip. Cell Stem Cell 2016, 19, 433–448. [Google Scholar] [CrossRef] [Green Version]
- Peleshok, J.C.; Ribeiro-da-Silva, A. Neurotrophic factor changes in the rat thick skin following chronic constriction injury of the sciatic nerve. Mol. Pain 2012, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motohashi, T.; Kawamura, N.; Watanabe, N.; Kitagawa, D.; Goshima, N.; Kunisada, T. Sox10 Functions as an Inducer of the Direct Conversion of Keratinocytes Into Neural Crest Cells. Stem Cells Dev. 2020, 29, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Hoshikawa, S.; Ueno, T.; Hirata, M.; Saito, T.; Ikeda, T.; Kawaguchi, H.; Nakamura, K.; Tanaka, S.; Ogata, T. SOX10 transactivates S100B to suppress Schwann cell proliferation and to promote myelination. PLoS ONE 2014, 9, e115400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bremer, M.; Frob, F.; Kichko, T.; Reeh, P.; Tamm, E.R.; Suter, U.; Wegner, M. Sox10 is required for Schwann-cell homeostasis and myelin maintenance in the adult peripheral nerve. Glia 2011, 59, 1022–1032. [Google Scholar] [CrossRef]
- Matsuda, H.; Koyama, H.; Sato, H.; Sawada, J.; Itakura, A.; Tanaka, A.; Matsumoto, M.; Konno, K.; Ushio, H.; Matsuda, K. Role of nerve growth factor in cutaneous wound healing: Accelerating effects in normal and healing-impaired diabetic mice. J. Exp. Med. 1998, 187, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Wilfong, E.R.; Dey, R.D. Nerve growth factor and substance P regulation in nasal sensory neurons after toluene diisocyanate exposure. Am. J. Respir. Cell Mol. Biol. 2004, 30, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Paus, R.; Luftl, M.; Czarnetzki, B.M. Nerve Growth-Factor Modulates Keratinocyte Proliferation in Murine Skin Organ-Culture. Br. J. Dermatol. 1994, 130, 174–180. [Google Scholar] [CrossRef]
- Skoff, A.M.; Adler, J.E. Nerve growth factor regulates substance P in adult sensory neurons through both TrkA and p75 receptors. Exp. Neurol. 2006, 197, 430–436. [Google Scholar] [CrossRef]
- Kumar, S.; Tan, Y.Y.; Berthiaume, F. Neuropeptide Substance P Enhances Skin Wound Healing In Vitro and In Vivo under Hypoxia. Biomedicines 2021, 9, 222. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Grimaldi, M.; Atzori, M.; Ray, P.; Alkon, D.L. Mobilization of calcium from intracellular stores, potentiation of neurotransmitter-induced calcium transients, and capacitative calcium entry by 4-aminopyridine. J. Neurosci. 2001, 21, 3135–3143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.Z.; Li, D.P.; Chen, S.R.; Pan, H.L. Aminopyridines Potentiate Synaptic and Neuromuscular Transmission by Targeting the Voltage-activated Calcium Channel beta Subunit. J. Biol. Chem. 2009, 284, 36453–36461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, M.; Hartung, H.P.; Albrecht, P. Neuroprotective Properties of 4-Aminopyridine. Neurol.-Neuroimmunol. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Hansebout, R.R.; Blight, A.R.; Fawcett, S.; Reddy, K. 4-Aminopyridine in chronic spinal cord injury: A controlled, double-blind, crossover study in eight patients. J. Neurotrauma 1993, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lundh, H.; Nilsson, O.; Rosen, I. Effects of 4-aminopyridine in myasthenia gravis. J. Neurol. Neurosurg. Psychiatry 1979, 42, 171–175. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.G.; Talukder, M.A.H.; Yue, L.; Turpin, L.C.; Noble, M.; Elfar, J.C. Human equivalent dose of oral 4-aminopyridine differentiates nerve crush injury from transection injury and improves post-injury function in mice. Neural. Regen. Res. 2020, 15, 2098–2107. [Google Scholar]
- Kasatkina, L.A. 4-aminopyridine sequesters intracellular Ca2+ which triggers exocytosis in excitable and non-excitable cells. Sci. Rep. 2016, 6, 34749. [Google Scholar] [CrossRef] [Green Version]
- Strupp, M.; Teufel, J.; Zwergal, A.; Schniepp, R.; Khodakhah, K.; Feil, K. Aminopyridines for the treatment of neurologic disorders. Neurol. Clin. Pract. 2017, 7, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Disc. 2009, 8, 982–1001. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Das, P.; Kelangi, S.; Bei, M. Potassium channels as potential drug targets for limb wound repair and regeneration. Precis. Clin. Med. 2020, 3, 22–33. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagadeeshaprasad, M.G.; Govindappa, P.K.; Nelson, A.M.; Noble, M.D.; Elfar, J.C. 4-Aminopyridine Induces Nerve Growth Factor to Improve Skin Wound Healing and Tissue Regeneration. Biomedicines 2022, 10, 1649. https://doi.org/10.3390/biomedicines10071649
Jagadeeshaprasad MG, Govindappa PK, Nelson AM, Noble MD, Elfar JC. 4-Aminopyridine Induces Nerve Growth Factor to Improve Skin Wound Healing and Tissue Regeneration. Biomedicines. 2022; 10(7):1649. https://doi.org/10.3390/biomedicines10071649
Chicago/Turabian StyleJagadeeshaprasad, Mashanipalya G., Prem Kumar Govindappa, Amanda M. Nelson, Mark D. Noble, and John C. Elfar. 2022. "4-Aminopyridine Induces Nerve Growth Factor to Improve Skin Wound Healing and Tissue Regeneration" Biomedicines 10, no. 7: 1649. https://doi.org/10.3390/biomedicines10071649
APA StyleJagadeeshaprasad, M. G., Govindappa, P. K., Nelson, A. M., Noble, M. D., & Elfar, J. C. (2022). 4-Aminopyridine Induces Nerve Growth Factor to Improve Skin Wound Healing and Tissue Regeneration. Biomedicines, 10(7), 1649. https://doi.org/10.3390/biomedicines10071649





