Abstract
Background: Atrial fibroblasts activation causes atrial fibrosis, which is one major pathophysiological contributor to atrial fibrillation (AF) genesis. Klotho is a pleiotropic protein with remarkable cardiovascular effects, including anti-inflammatory, anti-oxidative, and anti-apoptotic effects. This study investigated whether Klotho can modulate the activity of human atrial fibroblasts and provides an anti-fibrotic effect. Methods: Cell migration assay and proliferation assay were used to investigate fibrogenesis activities in single human atrial fibroblasts with or without treatment of Klotho (10 and 100 pM, 48 h). Calcium fluorescence imaging, the whole-cell patch-clamp, and Western blotting were performed in human atrial fibroblasts treated with and without Klotho (100 pM, 48 h) to evaluate the store-operated calcium entry (SOCE), transient receptor potential (TRP) currents, and downstream signaling. Results: High dose of Klotho (100 pM, 48 h) significantly reduced the migration of human atrial fibroblasts without alternating their proliferation; in addition, treatment of Klotho (100 pM, 48 h) also decreased SOCE and TRP currents. In the presence of BI-749327 (a selective canonical TRP 6 channel inhibitor, 1 μM, 48 h), Klotho (100 pM, 48 h) could not inhibit fibroblast migration nor suppress the TRP currents. Klotho-treated fibroblasts (100 pM, 48 h) had lower phosphorylated phospholipase C (PLC) (p-PLCβ3 Ser537) expression than the control. The PLC inhibitor, U73122 (1 μM, 48 h), reduced the migration, decreased SOCE and TRP currents, and lowered p-PLCβ3 in atrial fibroblasts, similar to Klotho. In the presence of the U73122 (1 μM, 48 h), Klotho (100 pM, 48 h) could not further modulate the migration and collagen synthesis nor suppress the TRP currents in human atrial fibroblasts. Conclusions: Klotho inhibited pro-fibrotic activities and SOCE by inhibiting the PLC signaling and suppressing the TRP currents, which may provide a novel insight into atrial fibrosis and arrhythmogenesis.
1. Introduction
Atrial fibrillation (AF) is the most common clinical arrhythmia that raises the risk of major adverse cardiovascular events and has become a growing public health problem [1]. The pathophysiologic mechanisms of AF include ion channel dysfunction, abnormal calcium (Ca2+) homeostasis, structural remodeling, and autonomic neural dysregulation. Atrial fibrosis plays a significant role in both electrical and structural remodeling. Cardiac fibroblasts, which account for about 75% of total cardiac cells, can synthesize extracellular matrix (ECM) and modulate electrical characteristics of cardiomyocytes while they are coupling to cardiomyocytes [2]. These modulatory effects of fibroblasts coupling to cardiomyocytes include changes in pace-making function that generate abnormal spontaneous impulse formation and alternation of electrical conduction, which causes conduction barriers and slow conduction, which promote re-entry circuits [3].
Klotho, a type 1 single-pass transmembrane protein containing a large extracellular domain, is present in many organs, including the heart [4,5]. Soluble Klotho, the extracellular domain of Klotho cleaved and secreted into the blood, has multiple pleiotropic functions, including anti-oxidative, anti-inflammatory, and anti-apoptotic effects [5]. Haplodeficiency of Klotho gene increased collagen-1 expression and induced arterial stiffening [6]. Treatment of soluble Klotho suppressed myofibroblast proliferation and collagen production in cardiac myofibroblasts [7]. Chen et al. also reported that restoring serum soluble Klotho levels effectively suppressed inflammation and myofibroblastic transition in the aortic valve [8]. Several studies demonstrated that Klotho regulates transient receptor potential (TRP) channels to protect the cardiovascular system and maintain its integrity [5,9,10]. Additionally, Klotho was reported to suppress pulmonary and renal fibrosis [11,12,13]. However, knowledge of the role of Klotho in atrial fibrosis is limited.
TRP channels, consisting of 6-transmembrane polypeptide subunits that assemble as tetramers forming cation-permeable pores, are extensively expressed in the heart and are responsible for Ca2+-signaling in both cardiac fibroblasts [14,15,16]. There are six subfamilies of the TRP channel family, including TRPC (canonical), TRPM (melastatin), TRPV (vanilloid), TRPA (ankyrin), TRPP (polycystin), and TRPML (mucolipin) families [15]. Several studies demonstrated that TRP channels are associated with human cardiac health and disease [17]. Among these TRP subgroups, TRPC was extensively studied in the regulation of cardiac function, and previous studies demonstrated that TRPC6 is responsible for myofibroblast trans-differentiation and inhibition of TRPC6 ameliorates tissue fibrosis [18,19]. Xie et al. also reported that Klotho could protect against stress-induced cardiac remodeling by blocking exocytosis of TRPC6 channels [10]. However, it is not clear whether Klotho may modulate the activity of TRP channels, leading to its anti-fibrosis potential. Accordingly, this study aims to evaluate whether Klotho modulates atrial fibroblasts activity and study the underlying mechanisms.
2. Materials and Methods
2.1. Cell Cultures
The NHCF-A human atrial fibroblasts cell line was purchased from Lonza Research Laboratory (Morrisville, NC, USA), and all fibroblasts were seeded as monolayers on uncoated culture dishes containing Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific, Loughborough, UK), including 100 U/mL penicillin-streptomycin (Thermo Fisher Scientific, Loughborough, UK) and 10% fetal bovine serum (HyClone Laboratories, Logan, UT, USA), in a humidified atmosphere containing 5% CO2 at 37 °C as previously described [20]. Human atrial fibroblasts used in this study were obtained from 4–6 passages to avoid the potential variations in the cellular structure and function.
2.2. Cell Migration Assay
A wound-healing assay was used to analyze the migration of human atrial fibroblasts treated with or without Klotho in different concentrations (10 pM and 100 pM, 48 h, R&D Systems, Minneapolis, MN, USA) 6 h after a cell monolayer in a six-well plate was craped with a P200 pipette tip. To explore the role of TRPC6 and intracellular signaling underlying the effects of Klotho in fibroblasts activity, we evaluated the migration in control, Klotho 100 pM-treated, BI-749327 1 μM (selective TRPC6 inhibitor, MedChemExpress, Monmouth Junction, NJ, USA)-treated, combined Klotho and BI-749327-treated, U73122 1 μM (phospholipase C inhibitor, Abcam, Cambridge, UK)-treated and combined Klotho and U73122-treated human atrial fibroblasts for 48 h. Each gap area was assessed using the ImageJ software (National Institutes of Health, Bethesda, Maryland, USA). The net migration areas after 6 h were subtracted from that at the time of the initial scratches.
2.3. Cell Proliferation Assay
As previously described, we used a commercial MTS kit (Promega, Madison, WI, USA) to measure the proliferation of human atrial fibroblasts [21]. Human atrial fibroblasts were seeded onto a 96-well culture dish at a density of 3000 cells per well. After growing to 50% confluence, the fibroblasts were incubated in a serum-free medium with Klotho in different concentrations (10 pM and 100 pM) for 48 h. Cell growth was analyzed by the MTS reagent, which was added 4 h before spectrophotometric analyses were performed.
2.4. Patch-Clamp Experiments
We used the whole-cell patch-clamp technique to measure the ionic currents in human atrial fibroblasts with and without the administration of Klotho (100 pM, 48 h), BI-749327 (1 μM, 48 h), or U73122 (1 μM, 48 h) by using an Axopatch 1D amplifier (Axon Instruments, San Jose, CA, USA) at 35 ± 1 °C. The tip resistance of borosilicate glass electrodes containing pipette solution used in this study was 3–5 MΩ.
As described previously, the TRP current in human atrial fibroblasts was measured in the voltage-clamp configuration [22]. For recording TRP currents, the internal solution contained 120 mM CsCl, 9.4 mM NaCl, 1 mM MgCl2, 0.2 mM Na3GTP, buffered at 100 nM free Ca2+ with 10 mM BAPTA, and 10 mM HEPES (titrated with CsOH to pH 7.2), and the external solution contained 140 mM NaCl, 5 mM CsCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES (titrated with NaOH to pH 7.4). We depolarized the human atrial fibroblasts by using triangular voltage ramps from –120 mV to +120 mV with a 200 ms pulse duration (from a holding potential of –60 mV with a frequency of 1 Hz). Considering TRP to be a non-selective cation channel, peak inward (-110 mV) and peak outward (+110 mV) current density were used to analyze the current amplitude.
2.5. Measurement of Intracellular Ca2+ Imaging
The human atrial fibroblasts were loaded on 1 cm glass-bottom chamber slides with the Fura-2-acetoxymethyl ester (5 μmol/L, Life Technologies, Carlsbad, CA, USA) and Pluronic F-127 (20% solution in dimethyl sulfoxide; 2.5 μg/mL) in a Ca2+-free solution containing 120 mM NaCl, 5.4 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 10 mM glucose, 6 mM HEPES, and 8 mM taurine (PH 7.40) for 40 min at 36 °C. We used a Polychrome V monochromator (Till Photonics, Munich, Germany) mounted on an upright Leica DMI 3000B microscope (Leica Microsystems, Buffalo Grove, IL, USA) with dual-excitation wavelengths of 340 and 380 nm and an emission wavelength of 510 nm to acquire Fura-2 fluorescence images. The MetaFuor software version 7.7.6.0 (Molecular Devices, Sunnyvale, CA, USA) was used to analyze Fura-2 images and the relative level of intracellular Ca2+ was represented by the ratio of fluorescence due to excitation at 340 nm (F340) to 380 nm (F380). To measure Ca2+ entry, all fibroblasts were initially superfused in the Ca2+-free solution for 2 min, following thapsigargin (2.5 μM) application. After thapsigargin induced Ca2+ release from the endoplasmic reticulum (ER), the extracellular solution was changed from a Ca2+-free solution to a solution containing 2mmol/L Ca2+ to measure Ca2+ entry via store-operated channels activated by ER Ca2+-store depletion. The Ca2+ entry was represented by the change in intracellular Ca2+ from a Ca2+-free solution to a 2 mmol/L Ca2+ solution (ΔF340/F380).
2.6. Western Blot Analysis
Human atrial fibroblasts with and without the administration of Klotho (100 pM, 48 h) or U73122 (1 μM, 48 h) were homogenized and centrifuged in the buffer. We separated proteins electrophoretically on a polyacrylamide gel electrophoresis with 4% to 12% sodium dodecyl sulfate and electrophoretically transferred them to a polyvinylidene difluoride membrane that was equilibrated with sodium dodecyl sulfate as described previously [21]. We probed all blots with primary antibodies against procollagen type IA1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), procollagen type III (Abcam, Cambridge, UK), α-smooth muscle actin (α-SMA) (Abcam, Cambridge, UK), TRPC6 (Alomone Labs, Jerusalem, Israel), human PLC beta 3 (PLCβ3) (R&D Systems, Minneapolis, MN, USA), phospho-PLC beta 3 (Ser537) (pPLCβ3 Ser 537) (Cell Signaling Technology, Beverly, MA, USA); secondary antibodies were all conjugated with horseradish peroxidase. All bound antibodies were detected with an enhanced chemiluminescence detection system and analyzed using AlphaEaseFCTM software (Genetic Technologies, Miami, FL, USA). All targeted bands were normalized to the glyceraldehyde 3-phosphate dehydrogenase protein (GAPDH) (Sigma-Aldrich, Merck, Darmstadt, Germany) to confirm equal protein loading.
2.7. Statistical Analysis
Means and standard errors of the means were used to describe all continuous variables. We used an unpaired t-test, Mann–Whitney rank-sum test, or one-way analysis of variance (repeated measures or non-repeated measures) with Tukey’s post hoc test to compare human cardiac fibroblasts under different treatment conditions. Statistical significance was defined as a p-value < 0.05. Statistics analyses were carried out using SPSS Statistic 18.0 software (Chicago, IL, USA) and SigmaPlot 12 software (Systat Software Inc, San Jose, CA, USA).
3. Results
3.1. The Effects of Klotho on the Migration, Collagen Production, Myofibroblast Differentiation, and Proliferation of Human Atrial Fibroblasts
Compared with control human atrial fibroblasts, a high dose of Klotho (100 pM)-treated atrial fibroblasts exhibited lesser migratory capability and lower expression of procollagen type IA1 and procollagen type III (Figure 1A,B). However, control and Klotho (100 pM)-treated atrial fibroblasts had similar expression of α-SMA. In contrast, control and Klotho-treated (10 and 100 pM) human atrial fibroblasts had similar proliferation rates (Figure 1C).
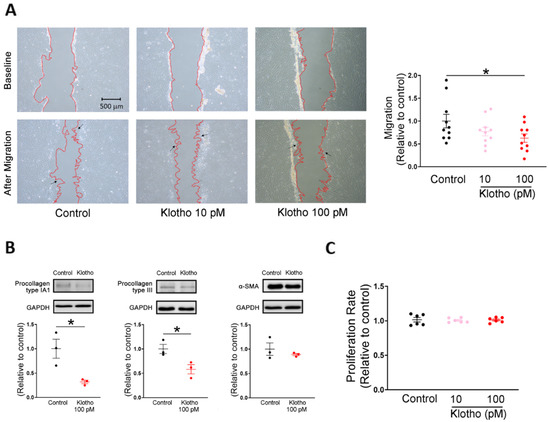
Figure 1.
Cell migration, collagen production, and proliferation capabilities in human atrial fibroblasts treated with and without Klotho. (A) Example photographs and average data (n = 10 from 3 independent experiments) showed that human atrial fibroblasts treated with Klotho at a high concentration (100 pM) exhibited less migratory capability than the control. Arrows indicate migrating fibroblasts in the wound space. (B) Example photographs and average data revealed that lower procollagen type IA1 expression (n = 3 from 3 independent experiments) and lower procollagen type III expression (n = 3 from 3 independent experiments) in human atrial fibroblasts treated with a high concentration of Klotho (100 pM). The expression of α-smooth muscle actin (α-SMA) was comparable in both groups (n = 3 from 3 independent experiments). GAPDH was used as a loading control. (C) Treatment with both lower and high concentrations of Klotho (10 and 100 pM) for 48 h had no significant effect on the proliferation rate of human atrial fibroblasts. (n = 6 from 3 independent experiments). GAPDH, glyceraldehyde 3-phosphate dehydrogenase protein. * p < 0.05.
3.2. Klotho Reduced the Store-Operated Ca2+ Entry (SOCE), TRP Currents, and Phosphorylation of PLC in Human Atrial Fibroblasts
Since store-operated Ca2+ entry (SOCE) plays an essential role in the cellular calcium signaling and modulates cell migration [23], we measured the intracellular calcium imaging and compared the difference in SOCE between fibroblasts with and without treatment of Klotho. Klotho-treated (100 pM) human atrial fibroblasts exhibited a lower ER calcium release and a lower store-operated Ca2+ entry (SOCE) than control fibroblasts (Figure 2). Since TRP channels critically control the Ca2+ influx and regulate the cardiac fibroblast function [24], we examined whether Klotho may modulate the TRP current. In this experiment, Klotho-treated (100 pM) human atrial fibroblasts had lower TRP currents than control fibroblasts (Figure 3A). Because previous studies demonstrated that Klotho inhibited the PLC signaling [25] and PLC modulates TRP channels and SOCE [26,27,28], we also performed Western blots to examine the effect of Klotho on PLC signaling. As shown in Figure 3B, Klotho-treated (100 pM) human atrial fibroblasts had similar expression of total PLCβ3 but less expression of pPLCβ3 Ser537 than control fibroblasts. TRPC6 channels have been shown to modulate fibroblast function and promote fibrosis [18,29]. Accordingly, we evaluated the role of TRPC6 in human atrial fibroblasts and found that BI-749327 (a selective TRPC6 inhibitor, 1 μM)-treated fibroblasts had smaller TRP currents than control fibroblasts. However, in the presence of Klotho (100 pM), fibroblasts with and without treatment of BI-749327 had similar TRP currents, which were smaller than control fibroblasts (Figure 3A). Moreover, Western blots showed that Klotho (100 pM)-treated and control fibroblasts had a similar expression of TRPC6 (Figure 3B). These findings suggest that Klotho might modulate TRPC6 function, leading to reduced atrial fibroblasts migration. We performed the migratory capability test of fibroblasts and found that Klotho (100 pM)-treated fibroblasts, BI-749327 (1 μM)-treated fibroblasts, and combined Klotho (100 pM) and BI-749327 (1 μM)-treated fibroblasts had lower migratory capability than control fibroblasts with a similar extent (Figure 3C,D).
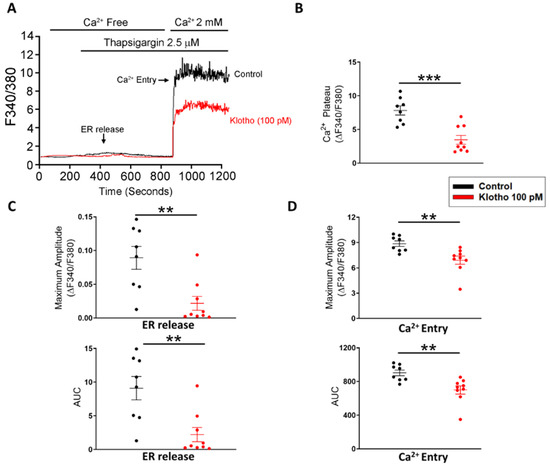
Figure 2.
Store-operated Ca2+ entry (SOCE) in human atrial fibroblasts with and without Klotho (100 pM). Representative tracings (A), average data of the Ca2+ plateaus (B), the endoplasmic reticulum (ER) release (C) and Ca2+ entry (D) in Fura-2 AM-loaded human atrial fibroblasts. Klotho (100 pM, 48 h)-treated fibroblasts (n = 8–9 from 4 independent experiments) had a lower ER release and a lower Ca2+ entry than control fibroblasts (n = 8 from 3 independent experiments). ER, endoplasmic reticulum. ** p < 0.01, *** p < 0.005.
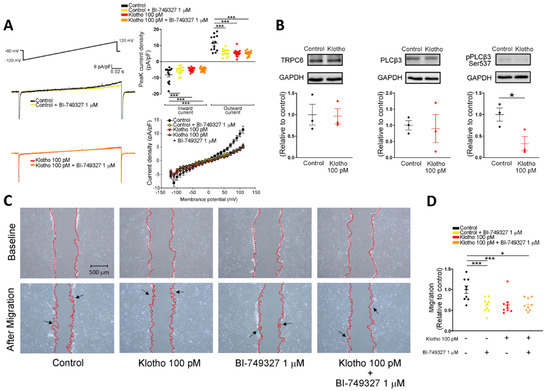
Figure 3.
Effects of Klotho (100 pM) and selective TRPC6 inhibitor (BI-749327, 1 μM) on ionic currents of transient receptor potential canonical channel (ITRPC) in human atrial fibroblasts. (A) The current tracings and I–V relationship of ITRP showed that BI-749327-treated fibroblasts (1 μM, 48 h, n = 13–15 from 4 independent experiments), Klotho-treated fibroblasts (100 pM, 48 h, n = 15 from 3 independent experiments), and combined Klotho and BI-749327 (n = 15 from 3 independent experiments) had similarly lower peak inward currents and similarly lower peak outward currents compared with the control (n = 13 from 4 independent experiments). (B) Western blots showed a similar expression of TRPC6 and total phospholipase C (PLC) between fibroblasts treated with Klotho (100 pM, 48 h) and control fibroblasts. However, the phosphorylated PLC (p-PLC) expression was lower in the fibroblasts treated with Klotho (100 pM, 48 h) (n = 3 from 3 independent experiments). (C,D) Example photographs and average data (n = 9 from 3 independent experiments) showed that human atrial fibroblasts treated with BI-749327 (1 μM, 48 h), Klotho (100 pM, 48 h), and combined Klotho and BI-749327 exhibited similarly less migratory capability than the control. Arrows indicate migrating fibroblasts in the wound space. GAPDH was used as a loading control. PLCβ3, phospholipase C beta 3; pPLCβ3, phosphorylated phospholipase C beta 3; TRPC6, transient receptor potential canonical channel 6. * p < 0.05, *** p < 0.005.
3.3. The PLC Inhibitor Suppressed the Fibroblasts Migration, Collagen Production, SOCE, and TRP Currents of Human Atrial Fibroblasts
As shown in Figure 4A, Klotho (100 pM)-treated fibroblasts, PLC inhibitor (U73122, 1 μM)-treated fibroblasts, and combined Klotho (100 pM) and PLC inhibitor (1 μM)-treated fibroblasts had lower migratory capability than control fibroblasts similarly. Western blots showed that Klotho (100 pM)-treated fibroblasts, PLC inhibitor (U73122, 1 μM)-treated fibroblasts, and combined Klotho (100 pM) and PLC inhibitor (1 μM)-treated fibroblasts had lower expression of procollagen IA1, procollagen III, and pPLCβ3 Ser 537 than the control. However, the expressions of α-SMA and total PLCβ3 were similar in the four groups (Figure 4B).
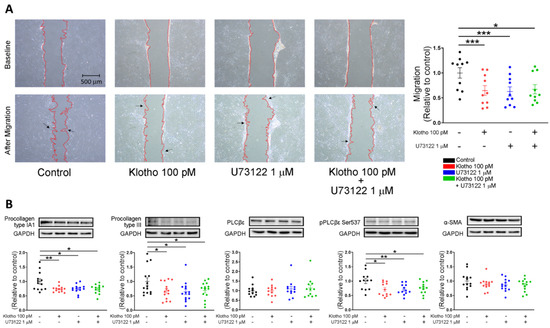
Figure 4.
Cell migration and collagen production in human atrial fibroblasts in 4 different groups (control; Klotho 100 pM, 48 h; U73122 1 μM, 48 h; Klotho 100 pM plus U73122 1 μM, 48 h). (A) Example photographs and average data (n = 9 from 3 independent experiments) showed the cell migration of human atrial fibroblasts in 4 different groups. Arrows indicate migrating fibroblasts in the wound space. (B) Example photographs and average data revealed the expression of procollagen type IA1, procollagen type III, total PLC, p-PLC, and α-SMA in human atrial fibroblasts in 4 groups (n = 12–14 from 3 independent experiments). GAPDH was used as a loading control. * p < 0.05, ** p < 0.01, *** p < 0.005.
Klotho (100 pM)-treated fibroblasts, PLC inhibitor (U73122, 1 μM)-treated fibroblasts, and combined Klotho (100 pM) and PLC inhibitor (1 μM)-treated fibroblasts had a lower ER release and a lower SOCE than control fibroblasts with a similar extent (Figure 5). Moreover, The Klotho (100 pM)-treated fibroblasts, PLC inhibitor (U73122, 1 μM)-treated fibroblasts, and combined Klotho (100 pM) and PLC inhibitor (1 μM)-treated fibroblasts had similarly lower TRP peak inward currents and lower TRP peak outward currents than control fibroblasts (Figure 6A–C). However, Western blots showed that fibroblasts in the four groups had similar expressions of TRPC6 (Figure 6D).
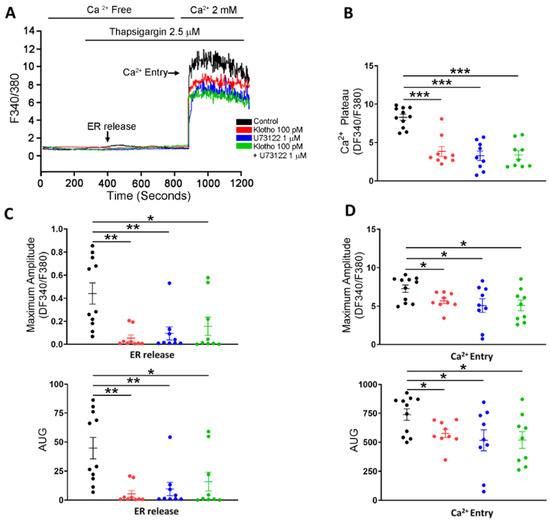
Figure 5.
SOCE in human atrial fibroblasts in 4 different groups (control; Klotho 100 pM, 48 h; U73122 1 μM, 48 h; Klotho 100 pM plus U73122 1 μM, 48 h). Representative tracings (A) and average data of the Ca2+ plateaus (B), the ER release (C), and the maximum amplitude of the Ca2+ entry (D) in Fura-2 AM-loaded human atrial fibroblasts (n = 9–11 from 4 independent experiments). Human atrial fibroblasts in the control group had a higher ER release and a higher Ca2+ entry than the other 3 groups. * p < 0.05, ** p < 0.01, *** p < 0.005.
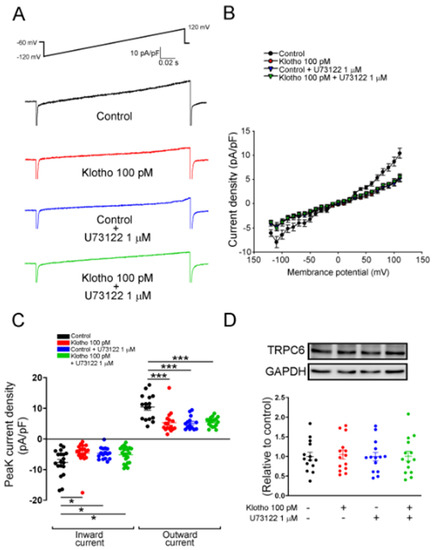
Figure 6.
ITRP of human atrial fibroblasts in 4 different groups (control; Klotho 100 pM, 48 h; U73122 1 μM, 48 h; Klotho 100 pM plus U73122 1 μM, 48 h). The current tracings (A) and I–V relationship (B) of ITRPC6 of human atrial fibroblasts in 4 groups (n = 16 from 5 independent experiments). (C) Fibroblasts in the control group had higher peak inward currents and outward currents than the other 3 groups (n = 14–18 from 5–7 independent experiments). (D) Western blots showed similar expression of TRPC6 among the four groups. (n = 14 from 3 independent experiments). GAPDH was used as a loading control. * p < 0.05, *** p < 0.005.
4. Discussion
4.1. Klotho Attenuates Pro-Fibrotic Effects in Human Atrial Fibroblasts
AF is the most common arrhythmia in clinical practice and contributes to cardiovascular adverse events such as heart failure and ischemic stroke [1]. The essential mechanisms of AF genesis include contractile, structural, and electrical remodeling of atrial tissues, and atrial fibrosis plays a crucial role in pathophysiological remodeling [30]. Atrial fibroblasts, one major component of atrial fibrosis, secrete various ECM, including procollagen type I and type III, and differentiate into myofibroblasts in response to pathological stimuli [31]. During atrial fibrosis, disorganized interstitial fibrosis changes the longitudinal conduction and the electronic coupling of myofibroblasts and cardiomyocytes, facilitating re-entrant arrhythmia [32]. In addition, fibrosis decelerates action potential propagation and enhances ectopic automaticity, contributing to arrhythmogenesis [3,33]. Klotho is a type 1 single-pass transmembrane protein with pleiotropic functions, including attenuating pro-fibrotic response [8,11,12,13,34,35,36]. Initially, Klotho was found to ameliorate renal fibrosis [37]; however, emerging evidence suggests that Klotho attenuates fibrosis in extra-renal tissues, such as pulmonary and cardiovascular systems [8,13]. In the present study, Klotho inhibited atrial fibroblasts migration in a dose-dependent manner and reduced procollagen synthesis without altering fibroblasts proliferation. These findings suggest that Klotho could suppress atrial fibrosis and potentially decrease fibrosis-associated conduction disturbance in atrial cardiomyocytes. Additionally, Klotho may be involved in another mechanism of protection from AF. Klotho can upregulate the expression of heat shock proteins, which protect from atrial tachycardia-induced remodeling and AF perpetuation [38,39]. The similarity in the α-SMA expression between control and Klotho-treated fibroblasts suggests that Klotho might not modulate the fibroblast activity via the regulation of myofibroblast differentiation. However, the cell culture environment with hard plastic dishes used in this study may not correlate well to the in vivo environment that may enhance myofibroblast differentiation with significant expression of α-SMA. Therefore, it is still not clear whether Klotho may potentially modulate fibroblast differentiation.
4.2. Klotho Modulates TRP Currents and Calcium Handling in Atrial Fibroblasts
Previous studies demonstrated that cytosolic Ca2+ is essential for the regulation of the activation of fibroblasts [40,41,42], and our previous study showed that Klotho modulates electrical activities and intracellular Ca2+ in pulmonary vein cardiomyocytes [43]. The present study revealed that Klotho (100 pM) significantly suppressed the thapsigargin-induced ER Ca2+ release and the subsequent Ca2+ entry. These findings exhibited an association between inhibition of intracellular Ca2+ through SOCE and suppression of fibroblast functions, such as collagen synthesis and migration, in fibroblasts treated with Klotho (100 pM).
Intracellular signaling of cardiac fibroblasts that govern fibroblast behavior is complex, among which Ca2+ signaling plays a vital role in cardiac fibrosis, including the synthesis of extracellular matrix proteins and migration [42]. Recently, SOCE and non-selective cation channels have been found to be critical to fibroblast Ca2+ signaling. Among numerous non-selective cation channels regulating the Ca2+ entry and subsequent extracellular signal-related kinase pathways [16,24,44], TRP channels activate atrial fibroblasts by modulating Ca2+ signaling and mediate fibrogenesis in AF [6,44]. TRPCs, one class of TRP channels, have been postulated to form both receptor-operated Ca2+ entry and SOCE [45]. Nikolova-Krstevski reported that TRPC6 channels expressed on the atrial endocardium modulate myocardial Ca2+ regulation in response to mechanical stretch, which contributes to the structure and electrical remodeling [46]. PLC signaling, critical in fibroblast activation, results in the generation of inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). Consequently, DAG activates protein kinase C, which promotes atrial fibrosis through modulating fibroblast function and stimulating collagen synthesis, and IP3 activates the IP3 receptor and induces rapid release of the luminal Ca2+ from the ER [47]. Depletion of ER Ca2+ activates the stromal interaction molecule that subsequently triggers SOCE through promoting Ca2+ release-activated Ca2+ channel protein 1, forming a complex with TRPCs, contributing to extracellular Ca2+ influx [48,49]. In addition, PLC-mediated DAG generation could activate TRPC6 and enables Ca2+ influx [50,51]. In our experiment, Klotho (100 pM) suppressed the TRP peak inward currents and peak outward currents significantly; however, the additional inhibitory effect was not observed in a selective TRPC6 inhibitor. These findings might imply that the inhibition of TRPC6 currents contributed to the main inhibitory effect of Klotho on the TRP current without changing the expression of TRPC6. Additionally, Klotho (100 pM) suppressed the pPLCβ3 (Ser537) expression while the total PLCβ3 expression remained unchanged. Our findings suggest that Klotho might modulate TRPC6 function by inhibiting PLC signaling.
4.3. Klotho Regulates the Intracellular Signaling in Atrial Fibroblasts
Klotho is a coreceptor of fibroblast growth factor (FGF) receptors, and it triggers signal propagation of FGFs, such as FGF19, FGF21, and FGF23 [52]. Loss of cardiac Klotho expression facilitates transforming growth factor-β1 pathway and aggravates cardiac fibrosis [53]. Klotho has also been shown to modulate intracellular calcium signaling and regulate ion channels on the plasma membrane, including Na+/K+-ATPase, TRPV5, TRPC6, and renal outer medullary potassium channel 1 [5,54]. A previous study demonstrated that soluble Klotho inhibited FGF23-stimulated PLC and phosphoinositide 3-kinase/Akt signaling [25]. In the present study, as compared to the control, Klotho (100 pM)-treated fibroblasts, U73122 (1 μM)-treated fibroblasts, and combined Klotho (100 pM) and U73122 (1 μM)-treated fibroblasts had lower fibroblasts migration, less procollagen IA1/III synthesis, and less phosphorylation of PLCβ3 (Ser537) with a similar extent. In addition, Klotho (100 pM)-treated fibroblasts, U73122 (1 μM)-treated fibroblasts, and combined Klotho (100 pM) and U73122 (1 μM)-treated fibroblasts had reduced thapsigargin-induced ER Ca2+ release and the subsequent extracellular Ca2+ entry to a similar extent. The above findings suggest no synergetic inhibitory effects of Klotho and U73122 on atrial fibroblasts. Thus, Klotho might exhibit its inhibitory effects on fibroblast migration, collagen synthesis, and SOCE by inhibiting the PLC signaling. Figure 7 summarizes the hypothesized mechanisms underlying the anti-fibrosis effects of Klotho.
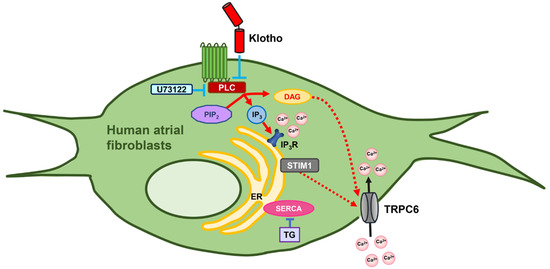
Figure 7.
Proposed mechanism of action of Klotho in the modulation of ion channels and intracellular calcium handling in human atrial fibroblasts. Klotho inhibits the activation of the PLC-IP3 signaling and suppresses thapsigargin-induced ER Ca2+ release, and subsequently attenuates SOCE by inhibition of TRPC6 currents. In addition, Klotho may also inhibit the PLC-DAG pathway and negatively modulate the inward currents of TRPC6. PIP2, phosphatidylinositol biphosphate; PLC, phospholipase C; DAG, diacylglycerol; IP3, inositol 1,4,5-triphosphate; IP3R, IP3 receptor; ER, endoplasmic reticulum; STIM1, stromal interaction molecule 1; SERCA, sarcoendoplasmic reticulum calcium transport ATPase; TG, thapsigargin.
4.4. Limitation
First, although we found that Klotho modulates PLC signaling and subsequent SOCE, the detailed mechanisms underlying Klotho’s regulation in human atrial fibroblasts are not fully elucidated. More experiments are mandatory to clarify the modulatory effects of Klotho on IP3R and following stromal interaction molecule activation. Second, human atrial fibroblasts from passages 4–6 were used in this study, and their fibroblast characteristics might be different from those in patients. Third, several conditions such as aging and heart failure could enhance cardiac fibrosis and AF genesis. Future experiments from pathological animal models are useful to confirm Klotho’s potential in reducing atrial fibrosis and AF prevention.
5. Conclusions
Klotho modulates the pro-fibrotic activities and intracellular calcium homeostasis in human atrial fibroblasts through its inhibitory effect on the PLC pathway and decreases SOCE by suppressing TRPC6 currents, which may provide a novel therapeutic insight into atrial fibrosis and AF genesis.
6. Highlights
- Klotho decreased migration and expressions of procollagen type IA1 and procollagen type III in human atrial fibroblasts.
- Klotho reduced TRP currents, mainly due to the decreased TRPC6 currents, and subsequently modulated fibroblasts activity.
- Klotho inhibited the PLC pathway and decreased thapsigargin-induced ER Ca2+ release and SOCE in human atrial fibroblasts.
Author Contributions
Conceptualization, Y.H. and Y.-J.C.; methodology, Y.H. and C.-C.C.; software, Y.H.; validation, Y.H., Y.-C.C. and C.-C.C.; formal analysis, Y.H.; investigation, Y.H.; Y.-C.C. and Y.-H.K.; resources, Y.H., W.-S.L., S.-A.C. and Y.-J.C.; data curation, Y.H.; writing—original draft preparation, Y.H.; writing—review and editing, Y.H.; C.-C.C. and Y.-J.C.; visualization, Y.H.; supervision, W.-S.L., S.-A.C. and Y.-J.C.; project administration, Y.H. and Y.-J.C.; funding acquisition, Y.H., W.-S.L., Y.-C.C. and Y.-J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Ministry of Science and Technology (MOST 105-2314-B-281-004-MY2, MOST 105-2314-B-038-059-MY3, MOST 108-2314-B-016-049, MOST 108-2314-B-016-049, MOST 109-2314-B-016-001-MY2, MOST 109-2314-B-038-124-MY3, MOST 110-2314-B-038-107-MY3, and MOST 110-2314-B-016-037-MY3), Taipei Medical University-Wan Fang Hospital (105-swf-02, 105-wf-eva-08, 105-wf-eva-14, 106-eva-02, 106-eva-06, and 106-swf-01), Tri-Service General Hospital (TSGH-C108-008-S01, TSGH-C02-108019, TSGH-C02-109019, TSGH-C01-110013, TSGH-C05-111040-03, and TSGH-E-111197), and the National Defense Medical Center, Taiwan (MAB-107-044).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the Tri-Service General Hospital, National Defense Medical Center for agreement of the study (TSGHIRB No.: B-110-42).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during the current study are included in this published article and are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank Hui-Yin Su for figure editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andrade, J.; Khairy, P.; Dobrev, D.; Nattel, S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014, 114, 1453–1468. [Google Scholar] [CrossRef]
- Kohl, P.; Noble, D. Mechanosensitive connective tissue: Potential influence on heart rhythm. Cardiovasc. Res. 1996, 32, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Yue, L.; Xie, J.; Nattel, S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc. Res. 2011, 89, 744–753. [Google Scholar] [CrossRef] [Green Version]
- Takeshita, K.; Fujimori, T.; Kurotaki, Y.; Honjo, H.; Tsujikawa, H.; Yasui, K.; Lee, J.K.; Kamiya, K.; Kitaichi, K.; Yamamoto, K.; et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation 2004, 109, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Dalton, G.D.; Xie, J.; An, S.W.; Huang, C.L. New insights into the mechanism of action of soluble klotho. Front. Endocrinol. 2017, 8, 323. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhou, X.; Sun, Z. Haplodeficiency of klotho gene causes arterial stiffening via upregulation of scleraxis expression and induction of autophagy. Hypertension 2015, 66, 1006–1013. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, Y.; McCoy, C.W.; Zhao, T.; Quarles, D.L.; Pi, M.; Bhattacharya, S.K.; King, G.; Sun, Y. Differential regulatory role of soluble klothos on cardiac fibrogenesis in hypertension. Am. J. Hypertens. 2016, 29, 1140–1147. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Fan, J.; Wang, S.; Sun, Z. Secreted klotho attenuates inflammation-associated aortic valve fibrosis in senescence-accelerated mice p1. Hypertension 2018, 71, 877–885. [Google Scholar] [CrossRef]
- Kusaba, T.; Okigaki, M.; Matui, A.; Murakami, M.; Ishikawa, K.; Kimura, T.; Sonomura, K.; Adachi, Y.; Shibuya, M.; Shirayama, T.; et al. Klotho is associated with vegf receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc. Natl. Acad. Sci. USA 2010, 107, 19308–19313. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Cha, S.K.; An, S.W.; Kuro, O.M.; Birnbaumer, L.; Huang, C.L. Cardioprotection by klotho through downregulation of trpc6 channels in the mouse heart. Nat. Commun. 2012, 3, 1238. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Nino, M.D.; Sanz, A.B.; Ortiz, A. Klotho to treat kidney fibrosis. J. Am. Soc. Nephrol. 2013, 24, 687–689. [Google Scholar] [CrossRef]
- Takenaka, T.; Inoue, T.; Miyazaki, T.; Kobori, H.; Nishiyama, A.; Ishii, N.; Hayashi, M.; Suzuki, H. Klotho ameliorates medullary fibrosis and pressure natriuresis in hypertensive rat kidneys. Hypertension 2018, 72, 1151–1159. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Shen, S.; Wang, Y.; Liu, L.; Wu, S.; Xu, W.; Zhao, W.; Lin, M.; Wu, J. Klotho antagonizes pulmonary fibrosis through suppressing pulmonary fibroblasts activation, migration, and extracellular matrix production: A therapeutic implication for idiopathic pulmonary fibrosis. Aging (Albany NY) 2020, 12, 5812–5831. [Google Scholar] [CrossRef]
- Dietrich, A.; Kalwa, H.; Fuchs, B.; Grimminger, F.; Weissmann, N.; Gudermann, T. In Vivo trpc functions in the cardiopulmonary vasculature. Cell Calcium 2007, 42, 233–244. [Google Scholar] [CrossRef]
- Clapham, D.E. Trp channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Du, J.; Xie, J.; Zhang, Z.; Tsujikawa, H.; Fusco, D.; Silverman, D.; Liang, B.; Yue, L. Trpm7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ. Res. 2010, 106, 992–1003. [Google Scholar] [CrossRef] [Green Version]
- Hof, T.; Chaigne, S.; Recalde, A.; Salle, L.; Brette, F.; Guinamard, R. Transient receptor potential channels in cardiac health and disease. Nat. Rev. Cardiol. 2019, 16, 344–360. [Google Scholar] [CrossRef]
- Davis, J.; Burr, A.R.; Davis, G.F.; Birnbaumer, L.; Molkentin, J.D. A trpc6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev. Cell 2012, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.L.; Xie, J.; An, S.W.; Oliver, N.; Barrezueta, N.X.; Lin, M.H.; Birnbaumer, L.; Huang, C.L. Inhibition of trpc6 channels ameliorates renal fibrosis and contributes to renal protection by soluble klotho. Kidney Int. 2017, 91, 830–841. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.C.; Lin, Y.K.; Chen, Y.C.; Kao, Y.H.; Lee, T.I.; Chen, Y.J. Vascular endothelial growth factor enhances profibrotic activities through modulation of calcium homeostasis in human atrial fibroblasts. Lab. Investig. 2020, 100, 285–296. [Google Scholar] [CrossRef]
- Chung, C.C.; Hsu, R.C.; Kao, Y.H.; Liou, J.P.; Lu, Y.Y.; Chen, Y.J. Androgen attenuates cardiac fibroblasts activations through modulations of transforming growth factor-beta and angiotensin ii signaling. Int. J. Cardiol. 2014, 176, 386–393. [Google Scholar] [CrossRef]
- Kuwahara, K.; Wang, Y.; McAnally, J.; Richardson, J.A.; Bassel-Duby, R.; Hill, J.A.; Olson, E.N. Trpc6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J. Clin. Investig. 2006, 116, 3114–3126. [Google Scholar] [CrossRef]
- Hammad, A.S.; Machaca, K. Store operated calcium entry in cell migration and cancer metastasis. Cells 2021, 10, 1246. [Google Scholar] [CrossRef]
- Harada, M.; Luo, X.; Qi, X.Y.; Tadevosyan, A.; Maguy, A.; Ordog, B.; Ledoux, J.; Kato, T.; Naud, P.; Voigt, N.; et al. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation 2012, 126, 2051–2064. [Google Scholar] [CrossRef]
- Xiao, Z.; King, G.; Mancarella, S.; Munkhsaikhan, U.; Cao, L.; Cai, C.; Quarles, L.D. Fgf23 expression is stimulated in transgenic alpha-klotho longevity mouse model. JCI Insight 2019, 4, e132820. [Google Scholar] [CrossRef] [Green Version]
- Itsuki, K.; Imai, Y.; Hase, H.; Okamura, Y.; Inoue, R.; Mori, M.X. Plc-mediated pi(4,5)p2 hydrolysis regulates activation and inactivation of trpc6/7 channels. J. Gen. Physiol. 2014, 143, 183–201. [Google Scholar] [CrossRef] [Green Version]
- Patterson, R.L.; van Rossum, D.B.; Nikolaidis, N.; Gill, D.L.; Snyder, S.H. Phospholipase c-gamma: Diverse roles in receptor-mediated calcium signaling. Trends Biochem. Sci. 2005, 30, 688–697. [Google Scholar] [CrossRef]
- Brechard, S.; Melchior, C.; Plancon, S.; Schenten, V.; Tschirhart, E.J. Store-operated Ca2+ channels formed by trpc1, trpc6 and orai1 and non-store-operated channels formed by trpc3 are involved in the regulation of nadph oxidase in hl-60 granulocytes. Cell Calcium 2008, 44, 492–506. [Google Scholar] [CrossRef]
- Hofmann, K.; Fiedler, S.; Vierkotten, S.; Weber, J.; Klee, S.; Jia, J.; Zwickenpflug, W.; Flockerzi, V.; Storch, U.; Yildirim, A.O.; et al. Classical transient receptor potential 6 (trpc6) channels support myofibroblast differentiation and development of experimental pulmonary fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Burstein, B.; Nattel, S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008, 51, 802–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Weber, K.T. Infarct scar: A dynamic tissue. Cardiovasc. Res. 2000, 46, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miragoli, M.; Salvarani, N.; Rohr, S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ. Res. 2007, 101, 755–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zununi Vahed, S.; Nikasa, P.; Ardalan, M. Klotho and renal fibrosis. Nephrourol. Mon. 2013, 5, 946–948. [Google Scholar] [CrossRef] [Green Version]
- Mencke, R.; Olauson, H.; Hillebrands, J.L. Effects of klotho on fibrosis and cancer: A renal focus on mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2017, 121, 85–100. [Google Scholar] [CrossRef]
- Maique, J.; Flores, B.; Shi, M.; Shepard, S.; Zhou, Z.; Yan, S.; Moe, O.W.; Hu, M.C. High phosphate induces and klotho attenuates kidney epithelial senescence and fibrosis. Front. Pharmacol. 2020, 11, 1273. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Zhou, D.; Tan, R.J.; Liu, Y. Loss of klotho contributes to kidney injury by derepression of wnt/beta-catenin signaling. J. Am. Soc. Nephrol. 2013, 24, 771–785. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Su, B.; Li, X.; Li, Y.; Zhao, J. Klotho overexpression suppresses apoptosis by regulating the hsp70/akt/bad pathway in h9c2(2-1) cells. Exp. Ther. Med. 2021, 21, 486. [Google Scholar] [CrossRef]
- Brundel, B.J.; Shiroshita-Takeshita, A.; Qi, X.; Yeh, Y.H.; Chartier, D.; van Gelder, I.C.; Henning, R.H.; Kampinga, H.H.; Nattel, S. Induction of heat shock response protects the heart against atrial fibrillation. Circ. Res. 2006, 99, 1394–1402. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, T.; Wu, J.; Yu, X.; Zheng, D.; Yang, F.; Li, T.; Wang, L.; Zhao, Y.; Dong, S.; et al. Calcium sensing receptor promotes cardiac fibroblast proliferation and extracellular matrix secretion. Cell Physiol. Biochem. 2014, 33, 557–568. [Google Scholar] [CrossRef]
- Chen, P.H.; Chung, C.C.; Lin, Y.F.; Kao, Y.H.; Chen, Y.J. Lithium reduces migration and collagen synthesis activity in human cardiac fibroblasts by inhibiting store-operated ca(2+) entry. Int. J. Mol. Sci. 2021, 22, 842. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Armillei, M.K.; Yu, A.S.; Liang, B.T.; Runnels, L.W.; Yue, L. Ca2+ signaling in cardiac fibroblasts and fibrosis-associated heart diseases. J. Cardiovasc. Dev. Dis. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, Y.; Chen, Y.C.; Huang, S.Y.; Lu, Y.Y.; Lin, Y.K.; Kao, Y.H.; Lin, W.S.; Chen, S.A.; Chen, Y.J. Klotho modulates electrical activity and calcium homeostasis in pulmonary vein cardiomyocytes via pi3k/akt signalling. Europace 2020, 22, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. Microrna-21 contributes to myocardial disease by stimulating map kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Boulay, G.; Brown, D.M.; Qin, N.; Jiang, M.; Dietrich, A.; Zhu, M.X.; Chen, Z.; Birnbaumer, M.; Mikoshiba, K.; Birnbaumer, L. Modulation of Ca2+ entry by polypeptides of the inositol 1,4,5-trisphosphate receptor (ip3r) that bind transient receptor potential (trp): Evidence for roles of trp and ip3r in store depletion-activated Ca2+ entry. Proc. Natl. Acad. Sci. USA 1999, 96, 14955–14960. [Google Scholar] [CrossRef] [Green Version]
- Nikolova-Krstevski, V.; Wagner, S.; Yu, Z.Y.; Cox, C.D.; Cvetkovska, J.; Hill, A.P.; Huttner, I.G.; Benson, V.; Werdich, A.A.; MacRae, C.; et al. Endocardial trpc-6 channels act as atrial mechanosensors and load-dependent modulators of endocardial/myocardial cross-talk. JACC Basic Transl. Sci. 2017, 2, 575–590. [Google Scholar] [CrossRef]
- Nattel, S.; Burstein, B.; Dobrev, D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ. Arrhythmia Electrophysiol. 2008, 1, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Gwathmey, J.K.; Xie, L.H. Role of transient receptor potential canonical channels in heart physiology and pathophysiology. Front. Cardiovasc. Med. 2020, 7, 24. [Google Scholar] [CrossRef]
- Yuan, J.P.; Zeng, W.; Huang, G.N.; Worley, P.F.; Muallem, S. Stim1 heteromultimerizes trpc channels to determine their function as store-operated channels. Nat. Cell Biol. 2007, 9, 636–645. [Google Scholar] [CrossRef]
- Weissmann, N.; Sydykov, A.; Kalwa, H.; Storch, U.; Fuchs, B.; Mederos y Schnitzler, M.; Brandes, R.P.; Grimminger, F.; Meissner, M.; Freichel, M.; et al. Activation of trpc6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat. Commun. 2012, 3, 649. [Google Scholar] [CrossRef]
- Asghar, M.Y.; Tornquist, K. Transient receptor potential canonical (trpc) channels as modulators of migration and invasion. Int. J. Mol. Sci. 2020, 21, 1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latko, M.; Czyrek, A.; Porebska, N.; Kucinska, M.; Otlewski, J.; Zakrzewska, M.; Opalinski, L. Cross-talk between fibroblast growth factor receptors and other cell surface proteins. Cells 2019, 8, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Zhu, L.J.; Waaga-Gasser, A.M.; Ding, Y.; Cao, M.; Jadhav, S.J.; Kirollos, S.; Shekar, P.S.; Padera, R.F.; Chang, Y.C.; et al. The axis of local cardiac endogenous klotho-tgf-beta1-wnt signaling mediates cardiac fibrosis in human. J. Mol. Cell Cardiol. 2019, 136, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imura, A.; Tsuji, Y.; Murata, M.; Maeda, R.; Kubota, K.; Iwano, A.; Obuse, C.; Togashi, K.; Tominaga, M.; Kita, N.; et al. Alpha-klotho as a regulator of calcium homeostasis. Science 2007, 316, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).