An Analysis of Microwave Ablation Parameters for Treatment of Liver Tumors from the 3D-IRCADb-01 Database
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamashita, T.; Wang, X.W. Cancer stem cells in the development of liver cancer. J. Clin. Investig. 2013, 123, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Willacy, H. Primary Liver Cancer. 2018. Available online: https://patient.info/cancer/primary-liver-cancer-leaflet (accessed on 8 February 2022).
- Pichard, G. Understanding Liver Cancer—The Basics. 2019. Available online: https://www.webmd.com/cancer/understanding-liver-cancer-basic-information (accessed on 10 February 2022).
- Crespo, M.; Leiva, M.; Sabio, G. Circadian Clock and Liver Cancer. Cancers 2021, 13, 3631. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, W.; Jia, Y.; Yu, Q.; Grau, G.E.; Peng, L.; Ran, Y.; Yang, Z.; Deng, H.; Lou, J. Single-cell clones of liver cancer stem cells have the potential of differentiating into different types of tumor cells. Cell Death Dis. 2013, 4, e857. [Google Scholar] [CrossRef] [PubMed]
- Gaba, R.C.; Zivin, S.P.; Dikopf, M.S.; Parvinian, A.; Casadaban, L.C.; Lu, Y.; Bui, J.T. Characteristics of Primary and Secondary Hepatic Malignancies Associated with Hepatopulmonary Shunting. Radiology 2014, 271, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Hydon, K.; Lodge, P. Primary and secondary liver tumours. InnovAiT 2016, 9, 477–482. [Google Scholar] [CrossRef]
- Heinrich, S.; Lang, H. Hepatic resection for primary and secondary liver malignancies. Innov. Surg. Sci. 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Markman, M. Liver Cancer Types. 2021. Available online: https://www.cancercenter.com/cancer-types/liver-cancer/types (accessed on 13 February 2022).
- Vogl, T.J.; Nour-Eldin, N.A.A.; Hammerstingl, R.M.; Panahi, B.; Naguib, N.N.N. Microwave Ablation (MWA): Basics, Technique and Results in Primary and Metastatic Liver Neoplasms—Review Article. Fortschr. Röntgenstr. 2017, 189, 1055–1066. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef]
- Hui, T.C.; Kwan, J.; Pua, U. Advanced Techniques in the Percutaneous Ablation of Liver Tumours. Diagnostics 2021, 11, 585. [Google Scholar] [CrossRef]
- Shira, K.; Ebata, T.; Oda, K.; Nishio, X.; Nagasaka, T.; Nimura, Y.; Nagino, M. Perineural Invasion Is a Prognostic Factor in Intrahepatic Cholangiocarcinoma. World J. Surg. 2008, 32, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Chen, H.Z.; Zhu, J.; Yang, Y.L.; Zhang, Y.H.; Huang, P.X.; Chen, Y.S.; Zhu, C.Y.; Yang, L.P.; Shen, K.; et al. Cancer survival in patients from a hospital-based cancer registry, China. J. Cancer. 2018, 9, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, J.; Zhang, Y.; Chen, Y.; Lu, J.; Zhu, Y.; Chen, H.; Shen, A.; Wang, G.; Groopman, J.D.; et al. Liver cancer mortality over six decades in an epidemic area: What we have learned. PeerJ 2021, 9, e10600. [Google Scholar] [CrossRef] [PubMed]
- Linn, Y.L.; Chee, M.Y.; Koh, Y.X.; Teo, J.T.; Cheow, P.C.; Chow, P.K.H.; Chan, C.Y.; Chung, A.Y.F.; Ooi, L.L.P.; Jgoh, B.K.P. Actual 10-year survivors and 10-year recurrence free survivors after primary liver resection for hepatocellular carcinoma in the 21st century: A single institution contemporary experience. J. Surg. Oncol. 2021, 123, 214–221. [Google Scholar] [CrossRef]
- Chen, J.G.; Zhu, J.; Zhang, Y.H.; Chen, Y.S.; Ding, L.L.; Chen, H.Z.; Shen, A.G.; Wang, G.R. Liver Cancer Survival: A Real World Observation of 45 Years with 32,556 Cases. J. Hepatocell. Carcinoma 2021, 8, 1023–1034. [Google Scholar] [CrossRef]
- Chen, J.G.; Zhang, Y.H.; Zhu, J.; Lu, J.H.; Wang, J.B.; Sun, Y.; Xue, X.F.; Lu, L.L.; Chen, Y.S.; Wu, Y.; et al. Early diagnosis and early treatment for liver cancer in Qidong: Survival of patients and effectiveness of screening. Chin. J. Oncol. 2017, 39, 946–951. [Google Scholar] [CrossRef]
- Hassanipour, S.; Mohammadzadeh, M.; Mansour-Ghanaei, F.; Fathalipour, M.; Joukar, F.; Salehiniya, H.; Abdzadeh, E.; Samadani, A.A.; Nikbakht, H.A.; Arab-Zozani, M. The Incidence of Hepatocellular Carcinoma in Iran from 1996 to 2016: A Systematic Review and Meta-analysis. J. Gastrointest. Cancer 2019, 50, 193–200. [Google Scholar] [CrossRef]
- Chen, J.G.; Zhang, Y.H.; Lu, L.L.; Chen, H.Z.; Shen, A.G.; Zhu, Y.R. Liver cancer screening in China: Practices and its extended questions. Hepatoma. Res. 2019, 5, 2. [Google Scholar] [CrossRef]
- Chong, C.C.N.; Lee, K.F.; Chu, C.M.; Chan, A.W.H.; Wong, J.; Chan, S.L.; Lok, H.T.; Fung, A.K.Y.; Fong, A.K.Y.; Cheung, Y.S.; et al. Microwave ablation provides better survival than liver resection for hepatocellular carcinoma in patients with borderline liver function: Application of ALBI score to patient selection. HPB 2018, 20, 546–554. [Google Scholar] [CrossRef]
- Liu, C.Y.; Chen, K.F.; Chen, P.J. Treatment of Liver Cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a021535. [Google Scholar] [CrossRef]
- Ong, S.L.; Gravante, G.; Metcalfe, M.S.; Strickland, A.D.; Dennison, A.R.; Lloyd, D.M. Efficacy and safety of microwave ablation for primary and secondary liver malignancies: A systematic review. Eur. J. Gastroenterol. Hepatol. 2009, 21, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Di Maso, M.; Muscatiello, N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int. J. Hyperth. 2016, 32, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Glassberg, M.B.; Ghosh, S.; Clymer, J.W.; Wright, G.W.J.; Ferko, N.; Amaral, J.F. Microwave ablation compared with hepatic resection for the treatment of hepatocellular carcinoma and liver metastases: A systematic review and meta-analysis. World J. Surg. Oncol. 2019, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Srinivas, A.N.; Kumar, D.P. Etiology of Hepatocellular Carcinoma: Special Focus on Fatty Liver Disease. Front. Oncol. 2020, 10, 601710. [Google Scholar] [CrossRef]
- Koulouris, A.; Tsagkaris, C.; Spyrou, V.; Pappa, E.; Troullinou, A.; Nikolaou, M. Hepatocellular Carcinoma: An Overview of the Changing Landscape of Treatment Options. J. Hepatocell. Carcinoma 2021, 8, 387–401. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burre, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Radiofrequency Ablation (RFA)/Microwave Ablation (MWA) of Liver Tumors. 2021. Available online: https://www.radiologyinfo.org/en/info/rfaliver (accessed on 14 February 2022).
- Ausania, F.; Borin, A.; Melendez, R.; del Rio, P.S.; Iglesias, A.; Bodenlle, P.; Paniagua, M.; Arias, M. Microwave ablation of colorectal liver metastases: Impact of a 10-mm safety margin on local recurrence in a tertiary care hospital. Ann. Hepatobiliary Pancreat. Surg. 2021, 25, 366–370. [Google Scholar] [CrossRef]
- Inchingolo, R.; Posa, A.; Mariappan, M.; Spiliopoulos, S. Locoregional treatments for hepatocellular carcinoma: Current evidence and future directions. World J. Gastroenterol. 2019, 25, 4614–4628. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J. Clin. Oncol. 2019, 37, 4012. [Google Scholar] [CrossRef]
- Crocetti, L.; Scalise, P.; Bozzi, E.; Campani, D.; Rossi, P.; Cervelli, R.; Bargellini, I.; Ghinolfi, D.; De Simone, P.; Cioni, R. Microwave Ablation of Very-Early- and Early-Stage HCC: Efficacy Evaluation by Correlation with Histology after Liver Transplantation. Cancers 2021, 13, 3420. [Google Scholar] [CrossRef]
- Primavesi, F.; Swierczynski, S.; Klieser, E.; Kiesslich, T.; Jäger, T.; Urbas, R.; Hutter, J.; Neureiter, D.; Öfner, D.; Stättner, S. Thermographic real-time-monitoring of surgical radiofrequency and microwave ablation in a perfused porcine liver model. Oncol. Lett. 2018, 15, 2913–2920. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, L.; Moser, M.A.J.; Zhang, W.; Zhang, B. A review of antenna designs for percutaneous microwave ablation. Phys. Med. 2021, 84, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Nagasawa, T.; Fujiwara, Y.; Sato, H.; Abe, T.; Kooka, Y.; Endo, K.; Oikawa, T.; Sawara, K.; Takikawa, Y. Comparing the Safety and Efficacy of Microwave Ablation Using Thermosphere™ Technology versus Radiofrequency Ablation for Hepatocellular Carcinoma: A Propensity Score-Matched Analysis. Cancers 2021, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Jiang, H.; Huang, X.; Zhou, Y.; Zhi, D.; Zhao, G.; Chen, Y.; Wang, L.; Qiu, B. A multi-slot coaxial microwave antenna for liver tumor ablation. Phys. Med. Biol. 2018, 6, 175011. [Google Scholar] [CrossRef]
- Andreozzi, A.; Iasiello, M.; Netti, P.A. A thermoporoelastic model for fluid transport in tumour tissues. J. R. Soc. Interface 2019, 16, 20190030. [Google Scholar] [CrossRef]
- Singh, S.; Melnik, R. Domain heterogeneity in radiofrequency therapies for pain relief: A computational study with coupled models. Bioengineering 2020, 7, 35. [Google Scholar] [CrossRef]
- Rubio, M.F.; López, G.D.; Perezgasga, F.V.; García, F.F.; Hernández, A.V.; Salas, L.L. Computer Modeling for Microwave Ablation in Breast Cancer Using a Coaxial Slot Antenna. Int. J. Thermophys. 2015, 36, 2687–2704. [Google Scholar] [CrossRef]
- Reinhardt, M.; Brandmaier, P.; Seider, D.; Kolesnik, M.; Jenniskens, S.; Sequeiros, R.B.; Eibisberger, M.; Voglreiterg, P.; Ronan Flanagan, R.; Mariappan, P.; et al. A prospective development study of software-guided radio-frequency ablation of primary and secondary liver tumors: Clinical intervention modelling, planning and proof for ablation cancer treatment (ClinicIMPPACT). Contemp. Clin. Trials Commun. 2017, 8, 25–32. [Google Scholar] [CrossRef]
- Radmilović-Radjenović, M.; Radjenović, D.; Radjenović, B. Finite element analysis of the effect of microwave ablation on the liver, lung, kidney, and bone malignant tissues. Europhys. Lett. 2021, 135, 3500. [Google Scholar] [CrossRef]
- Tucci, C.; Trujillo, M.; Berjano, E.; Iasiello, M.; Andreozzi, A.; Vanoli, G.P. Mathematical modeling of microwave liver ablation with a variable-porosity medium approach. Comput. Methods Progr. Biomed. 2022, 214, 106569. [Google Scholar] [CrossRef]
- Keangin, P.; Wessapan, T.; Rattanadecho, P. Analysis of heat transfer in deformed liver cancer modeling treated using a microwave coaxial antenna. Appl. Therm. Eng. 2011, 31, 3243–3254. [Google Scholar] [CrossRef]
- Wang, X.; Gao, H.; Wu, S.; Jiang, T.; Zhou, Z.; Bai, Y. Numerical evaluation of ablation zone under different tip temperatures during radiofrequency ablation. Math. Biosci. Eng. 2019, 16, 2514–2531. [Google Scholar] [CrossRef]
- Radmilović-Radjenović, M.; Sabo, M.; Prnova, M.; Šoltes, L.; Radjenović, B. Finite Element Analysis of the Microwave Ablation Method for Enhanced Lung Cancer Treatment. Cancers 2021, 13, 3500. [Google Scholar] [CrossRef] [PubMed]
- Radjenović, B.; Sabo, M.; Šoltes, L.; Prnova, M.; Čičak, P.; Radmilović-Radjenović, M. On Efficacy of Microwave Ablation in the Thermal Treatment of an Early-Stage Hepatocellular Carcinoma. Cancers 2021, 13, 5784. [Google Scholar] [CrossRef]
- Comsol Multiphysics. 1986–2020. Burlington (MA): COMSOL, Inc. Available online: https://www.comsol.com/comsol-multiphysics (accessed on 7 March 2022).
- Heat Transfer Modeling Software for Analyzing Thermal Effects. Burlington (MA): COMSOL, Inc. Available online: https://www.comsol.com/heat-transfer-module (accessed on 7 March 2022).
- 3D-IRCADb. Strasbourg (F): IrcadFrance. Available online: https://www.ircad.fr/research/data-sets/liver-segmentation-3d-ircadb-01/ (accessed on 7 March 2022).
- Ibitoye, A.Z.; Nwoye, E.O.; Aweda, A.M.; Oremosu, A.A.; Anunobi, C.C.; Akanmu, N.O. Microwave ablation of ex vivo bovine tissues using a dual slot antenna with a floating metallic sleeve. Int. J. Hyperth. 2016, 32, 923–930. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, D.S.; Bertram, J.M.; Converse, M.C.; O’Rourke, A.P.; Webster, J.G.; Hagness, S.C.; Will, J.A.; Mahvi, D.M. A floating sleeve antenna yields localized hepatic microwave ablation. IEEE Trans. Bio-Med. Eng. 2006, 53, 533–537. [Google Scholar] [CrossRef]
- Luyen, H.; Hagness, S.C.; Behdad, N. Reduced-Diameter Designs of Coax-Fed Microwave Ablation Antennas Equipped with Baluns. IEEE Antennas Wirel. Propag. Lett. 2017, 16, 1385–1388. [Google Scholar] [CrossRef]
- Cazacu, D.I. Modeling and Simulation of Microwave Ablation of Liver Tumors. Ph.D. Thesis, Jacobs University, Bremen, Germany, 2019. Available online: https://opus.jacobs-university.de/frontdoor/index/index/docId/887 (accessed on 25 February 2022).
- Tehrani, M.H.H.; Soltani, M.; Kashkooli, F.M.; Raahemifar, K. Use of microwave ablation for thermal treatment of solid tumors with different shapes and sizes—A computational approach. PLoS ONE 2020, 15, e0233219. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef]
- Cavagnaro, M.; Pinto, R.; Lopresto, V. Numerical models to evaluate the temperature increase induced by ex vivo microwave thermal ablation. Phys. Med. Biol. 2015, 60, 3287–3311. [Google Scholar] [CrossRef]
- Stauffer, P.R.; Rossetto, F.; Prakash, M.; Neuman, D.G.; Lee, T. Phantom and animal tissues for modelling the electrical properties of human liver. Int. J. Hyperth. 2003, 19, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P. Theoretical Modeling for Hepatic Microwave Ablation. Open Biomed. Eng. J. 2010, 4, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Diller, K.R.; Pearce, J.A. Issues in modeling thermal alterations in tissues. Ann. N. Y. Acad. Sci. 1999, 888, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Berjano, E.J. Theoretical modeling for radiofrequency ablation: State-of-the-art and challenges for the future. BioMed. Eng. OnLine 2006, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Converse, C.M.; Mahvi, D.M.; Webster, J.G. Expanding the Bioheat Equation to Include Tissue Internal Water Evaporation During Heating. IEEE Trans. Biomed. Eng. 2007, 54, 1382–1388. [Google Scholar] [CrossRef]
- Selmi, M.; Bajahzar, A.; Belmabrouk, H. Effects of target temperature on thermal damage during temperature-controlled MWA of liver tumor. Case Stud. Therm. Eng. 2022, 31, 101821. [Google Scholar] [CrossRef]
- Rozen, G.; Ptaszek, L.M.; Zilberman, I.; Douglas, V.; Heist, E.K.; Beeckler, C.; Altmann, A.; Ruskin, J.N.; Govari, A.; Mansour, M. Safety and efficacy of delivering high-power short-duration radiofrequency ablation lesions utilizing a novel temperature sensing technology. Europace 2018, 20, f444–f450. [Google Scholar] [CrossRef]
- Gorman, J.; Tan, W.; Abraham, J. Numerical Simulation of Microwave Ablation in the Human Liver. Processes 2022, 10, 361. [Google Scholar] [CrossRef]
- Wang, L.; Fan, J. Modeling bioheat transport at macroscale. J. Heat Transf. 2011, 133, 011010. [Google Scholar] [CrossRef]
- Andreozzi, A.; Iasiello, M.; Tucci, C. An overview of mathematical models and modulated-heating protocols for thermal ablation. Adv. Heat Transf. 2020, 52, 489–541. [Google Scholar] [CrossRef]
- Hristov, J. Bio-Heat Models Revisited—Concepts, Derivations, Nondimensalization and Fractionalization Approaches. Front. Phys. 2019, 7, 189. [Google Scholar] [CrossRef]

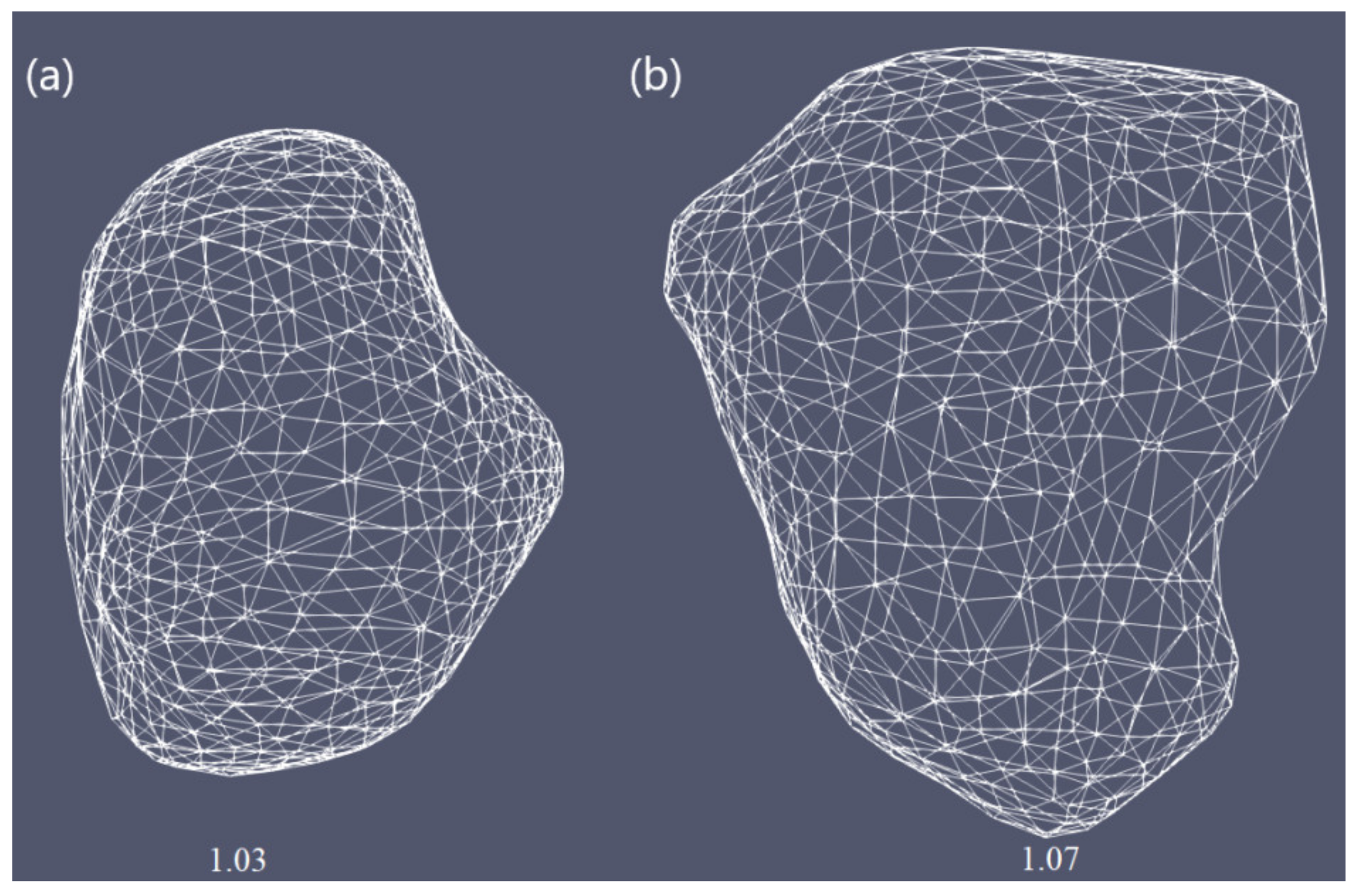

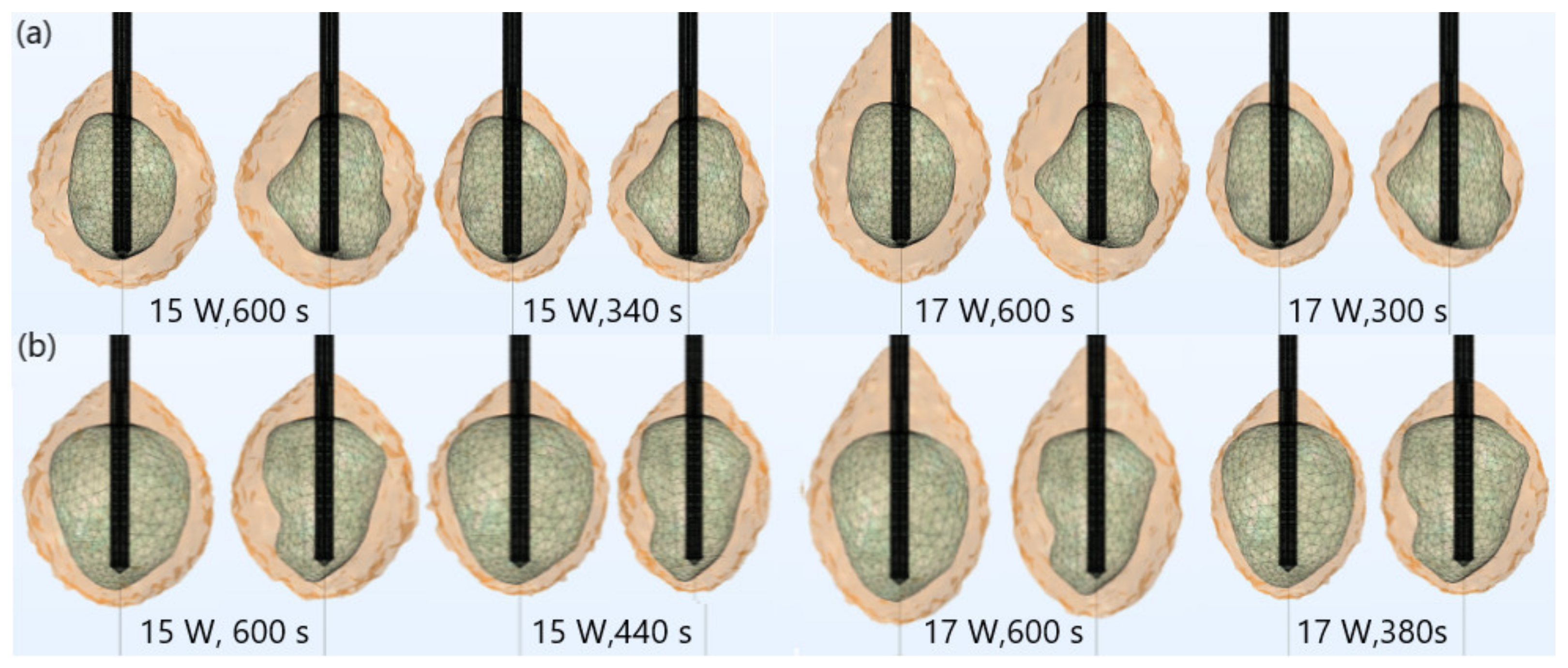
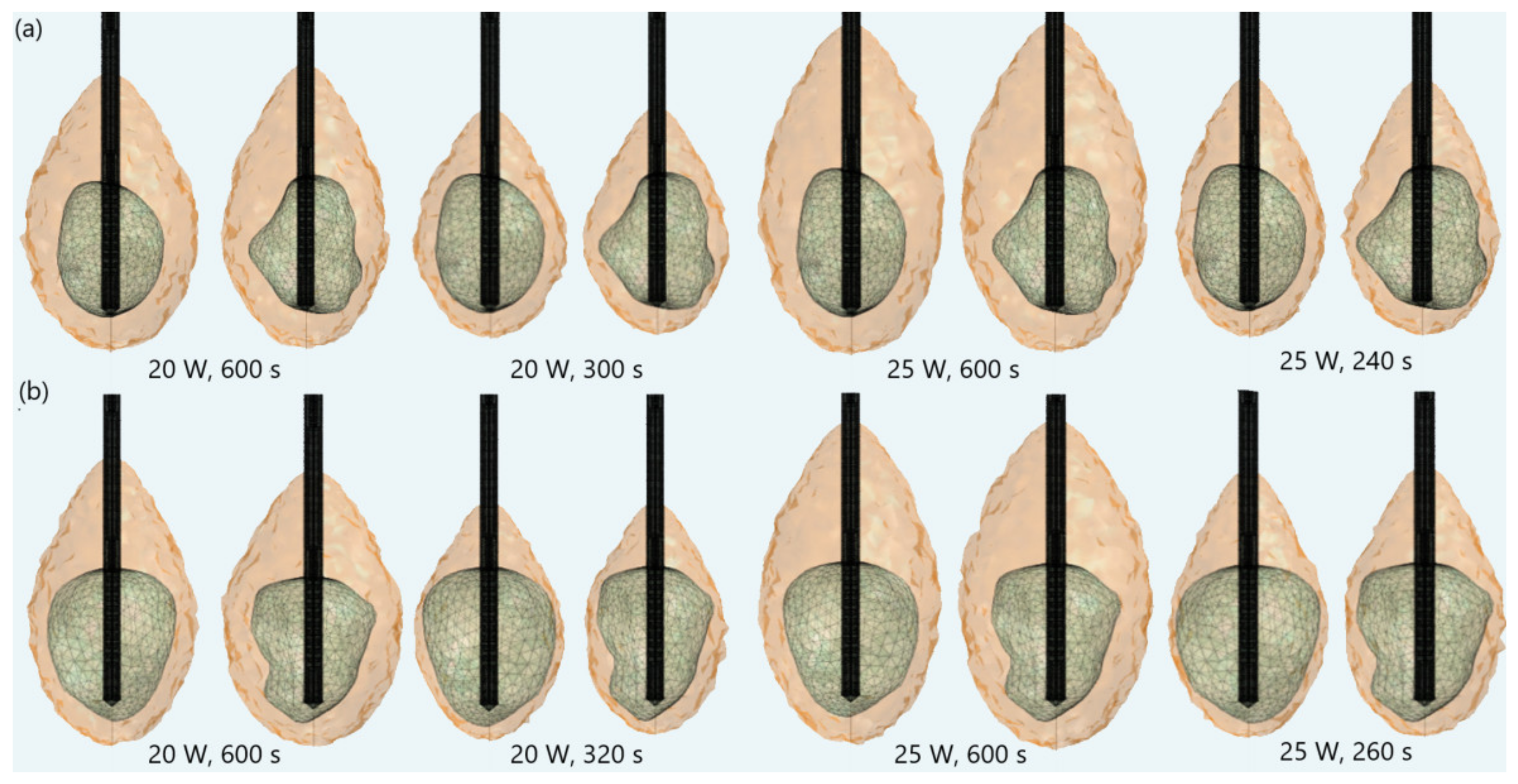

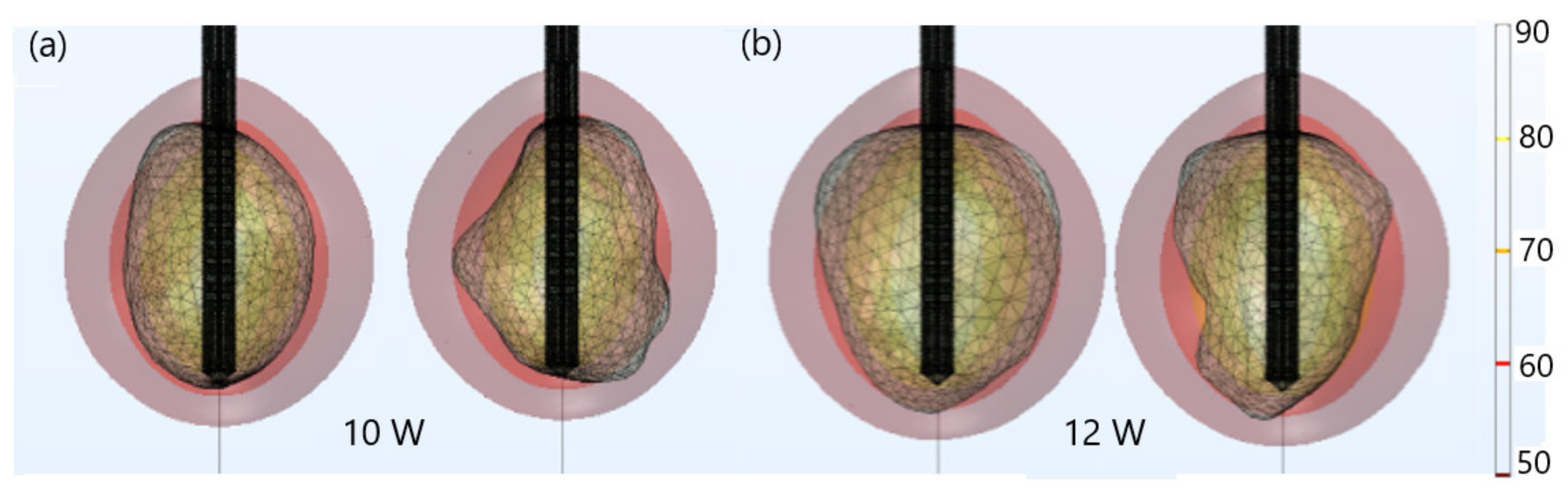
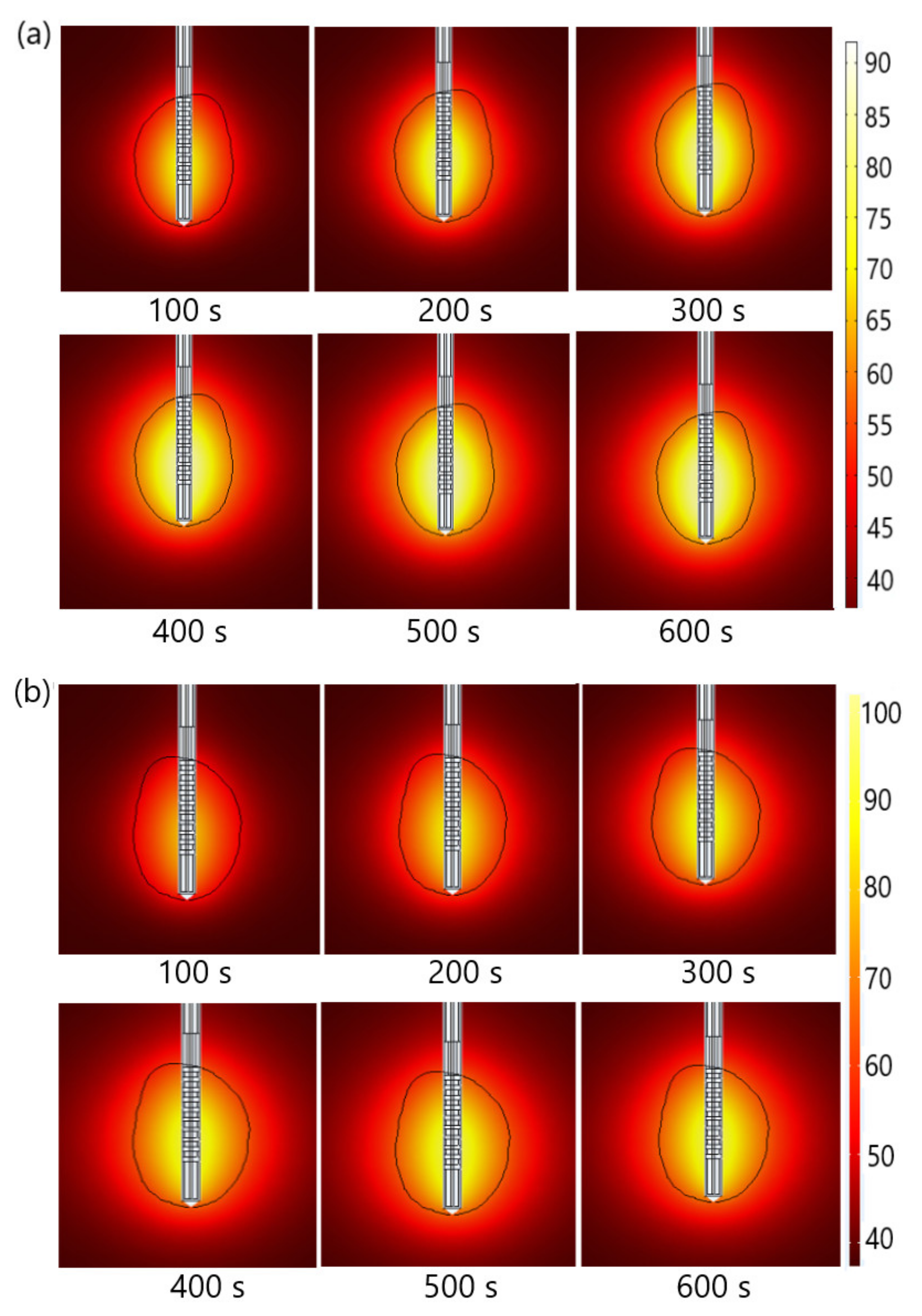
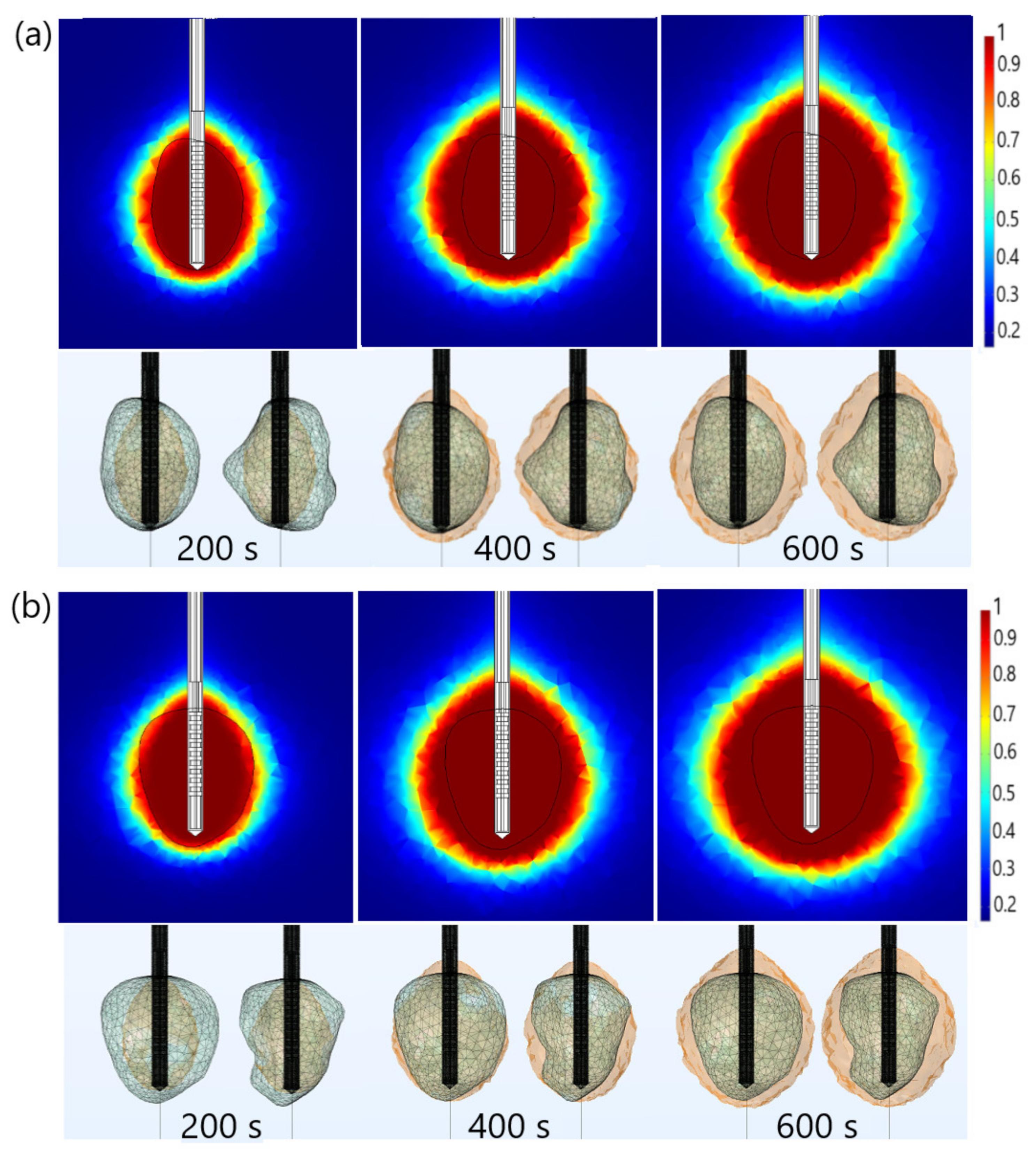
| Parameter | Value |
|---|---|
| Healthy tissue | |
| Density | 1079 kg/m3 |
| Relative permittivity | 44.3 |
| Electric conductivity | 1.8 S/m |
| Thermal conductivity | 0.52 W/m °C |
| Specific heat | 3540 J/kg °C |
| Tumoral tissue | |
| Density | 1040 kg/m3 |
| Relative permittivity | 54.8 |
| Electric conductivity | 2 S/m |
| Thermal conductivity | 0.57 W/m °C |
| Specific heat | 3960 J/kg °C |
| Blood | |
| Density | 1060 kg/m3 |
| Thermal conductivity | 0.5 W/m °C |
| Specific heat | 3600 J/kg °C |
| Temperature | 37 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radmilović-Radjenović, M.; Bošković, N.; Sabo, M.; Radjenović, B. An Analysis of Microwave Ablation Parameters for Treatment of Liver Tumors from the 3D-IRCADb-01 Database. Biomedicines 2022, 10, 1569. https://doi.org/10.3390/biomedicines10071569
Radmilović-Radjenović M, Bošković N, Sabo M, Radjenović B. An Analysis of Microwave Ablation Parameters for Treatment of Liver Tumors from the 3D-IRCADb-01 Database. Biomedicines. 2022; 10(7):1569. https://doi.org/10.3390/biomedicines10071569
Chicago/Turabian StyleRadmilović-Radjenović, Marija, Nikola Bošković, Martin Sabo, and Branislav Radjenović. 2022. "An Analysis of Microwave Ablation Parameters for Treatment of Liver Tumors from the 3D-IRCADb-01 Database" Biomedicines 10, no. 7: 1569. https://doi.org/10.3390/biomedicines10071569
APA StyleRadmilović-Radjenović, M., Bošković, N., Sabo, M., & Radjenović, B. (2022). An Analysis of Microwave Ablation Parameters for Treatment of Liver Tumors from the 3D-IRCADb-01 Database. Biomedicines, 10(7), 1569. https://doi.org/10.3390/biomedicines10071569






