Stem Cell-Based Therapy: A Promising Treatment for Diabetic Foot Ulcer
Abstract
1. Introduction
2. Diabetes Mellitus and Its Associated Diseases
2.1. Peripheral Polyneuropathy
2.2. Peripheral Vascular Disease
2.3. Macroangiopathy
2.4. Microangiopathy
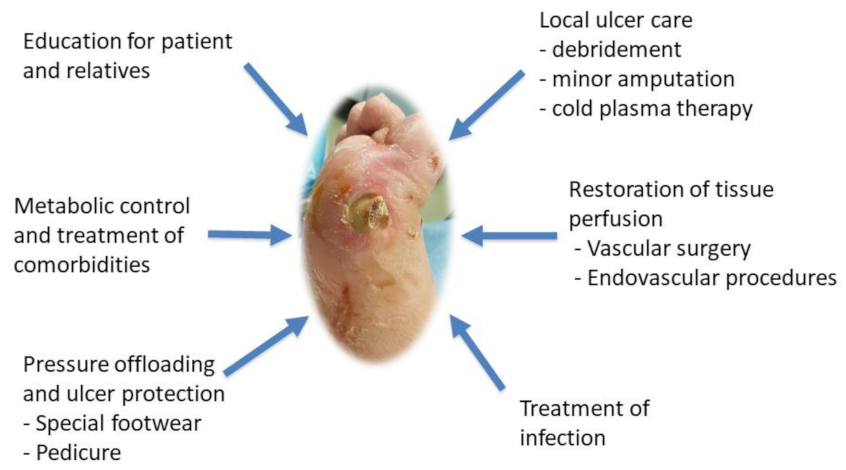
3. Treatment Therapies for Diabetic Foot Ulcers
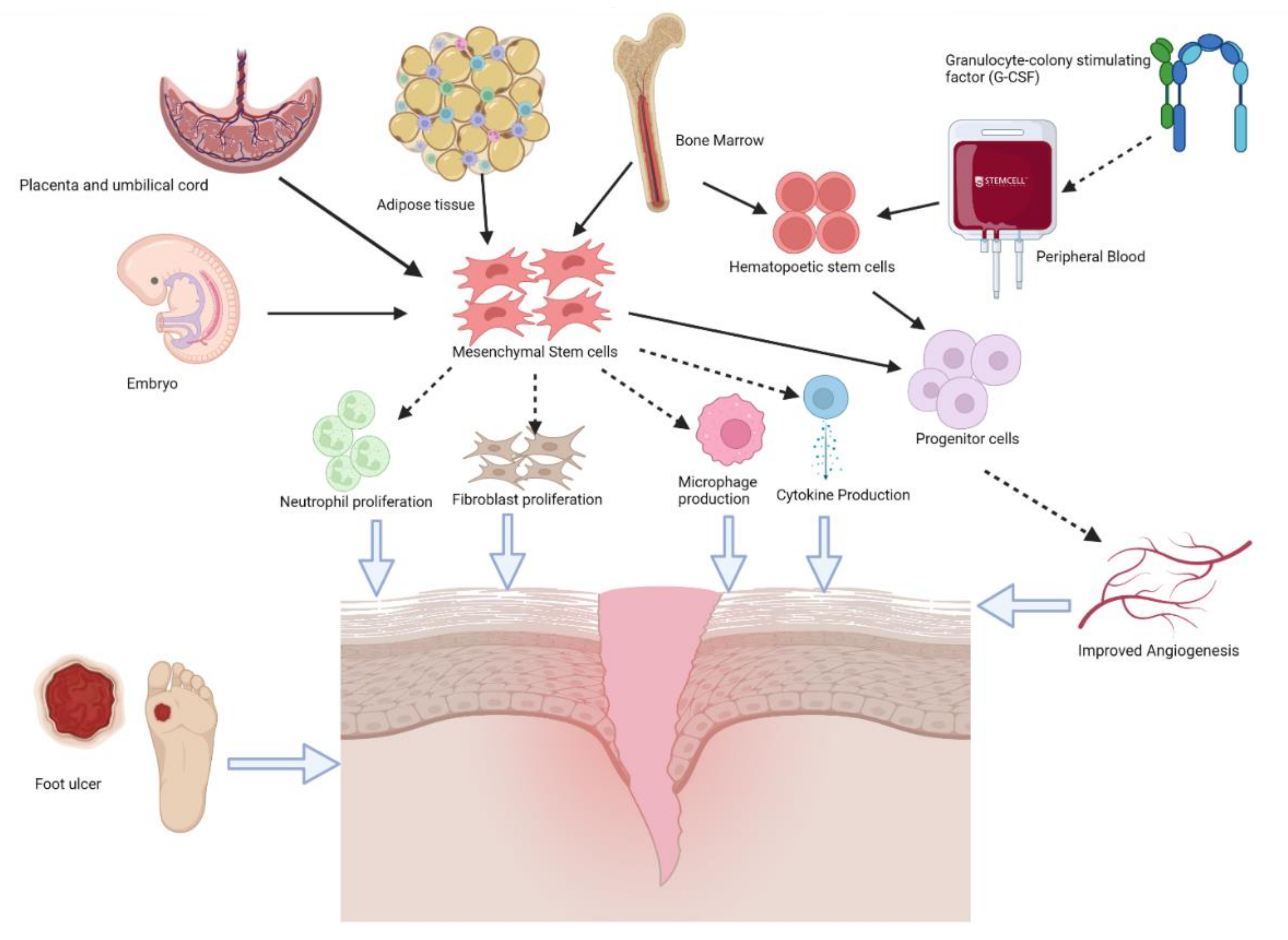
4. Stem Cell Therapy
4.1. Autologous Stem Cells
4.1.1. Bone Marrow-Derived Stem Cells
4.1.2. Peripheral Blood Stem Cells and Granulocyte Colony-Stimulating Factor
4.1.3. Adipose-Derived Mesenchymal Stem Cells
4.2. Allogeneic Stem Cells
4.2.1. Human Umbilical Cord Mesenchymal Stem Cells
4.2.2. Placental-Derived Mesenchymal Stem Cells
4.2.3. Embryonic Stem Cells
4.3. Routes of Administration
5. Cell Secretome: A Promising Therapeutic Alternative in Wound Healing
6. Bionanomaterials: A Modality for Stem Cell-Based Therapy Application
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Diabetes. 2022. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 23 February 2022).
- Bassi, R.; Trevisani, A.; Tezza, S.; Ben Nasr, M.; Gatti, F.; Vergani, A.; Farina, A.; Fiorina, P. Regenerative Therapies for Diabetic Microangiopathy. Exp. Diabetes Res. 2012, 2012, 11. [Google Scholar] [CrossRef]
- Hasheminasabgorji, E.; Jha, J.C. Dyslipidemia, Diabetes and Atherosclerosis: Role of Inflammation and ROS-Redox-Sensitive Factors. Biomedicines 2021, 9, 1602. [Google Scholar] [CrossRef] [PubMed]
- Christian Rask-Madsen, G.L.K. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Joshua, A.; Beckman, M.A.C. Vascular Complications of Diabetes. Circ. Res. 2016, 118, 1771–1785. [Google Scholar]
- Agata Sasor, B.O. Microangiopathy is Common in Submucosal Vessels of the Colon in Patients with Diabetes Mellitus. Rev. Diabet. Stud. 2014, 11, 175–180. [Google Scholar] [CrossRef][Green Version]
- Senneville, É.; Lipsky, B.A.; Abbas, Z.G.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; van Asten, S.A.; et al. Diagnosis ofinfection in thefoot in diabetes: A systematic review. Diabetes/Metab. Res. Rev. 2020, 36, e3281. [Google Scholar] [CrossRef]
- Banu, A.; Hassan, M.M.N.; Rajkumar, J.; Srinivasa, S. Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation: A prospective study. Australas. Med. J. 2015, 8, 280–285. [Google Scholar] [CrossRef]
- Kleopatra Alexiadou, J.D. Management of Diabetic Foot Ulcers. Diabetes Ther. 2012, 3, 4. [Google Scholar] [CrossRef]
- Lazzarini, P.A.; Jarl, G.; Gooday, C.; Viswanathan, V.; Caravaggi, C.F.; Armstrong, D.G.; Bus, S.A. Effectiveness of offloading interventions to heal foot ulcers in persons with diabetes: A systematic review. Diabetes/Metab. Res. Rev. 2020, 36, e3275. [Google Scholar] [CrossRef]
- van Netten, J.J.; Raspovic, A.; Lavery, L.A.; Monteiro-Soares, M.; Rasmussen, A.; Sacco, I.C.; Bus, S.A. Prevention of foot ulcers in the at-risk patient with diabetes: A systematic review. Diabetes/Metab. Res. Rev. 2020, 36, e3270. [Google Scholar] [CrossRef]
- Gorecka, J.; Gao, X.; Fereydooni, A.; Dash, B.C.; Luo, J.; Lee, S.R.; Taniguchi, R.; Hsia, H.C.; Qyang, Y.; Dardik, A. Induced pluripotent stem cell-derived smooth muscle cells increase angiogenesis and accelerate diabetic wound healing. Regen. Med. 2020, 15, 1277–1293. [Google Scholar] [CrossRef]
- Rijken, P.M.; Dekker, J.; Dekker, E.; Lankhorst, G.J.; Bakker, K.; Dooren, J.; Rauwerda, J.A. Clinical and functional correlates of foot pain in diabetic patients. Disabil. Rehabil. 1998, 20, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Pintos, L.M.R.; Villegas-Rivera, G.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G.; Muñoz, E.G.C. Diabetic Polyneuropathy in Type 2 Diabetes Mellitus: Inflammation, Oxidative Stress, and Mitochondrial Function. J. Diabetes Res. 2016, 2016, 3425617. [Google Scholar]
- Diabetic Neuropathy. 2022. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/diabetes/diabetic-neuropathy-nerve-problems (accessed on 24 February 2022).
- Boulton, A.J.; Malik, R.A.; Arezzo, J.C.; Sosenko, J.M. Diabetic Somatic Neuropathies. Diabetes Care 2004, 27, 1458–1486. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Gupta, A.; Krishna, S.; Kulothungan, V.; Sharma, T. Prevalence and risk factors for diabetic microvascular complications in newly diagnosed type II diabetes mellitus. Sankara Nethralaya Diabetic Retinopathy Epidemiology And Molecular Genetic Study (SN-DREAMS, report 27). J. Diabetes Its Complicat. 2012, 26, 123–128. [Google Scholar] [CrossRef]
- Peripheral Vascular Disease. 2022. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/peripheral-vascular-disease (accessed on 24 February 2022).
- Giorgi, A. Peripheral Vascular Disease. 2018. Available online: https://www.healthline.com/health/peripheral-vascular-disease#prevention (accessed on 24 February 2022).
- Huysman, F.; Mathieu, C. Diabetes and Peripheral Vascular Disease. Acta Chir. Belg. 2009, 109, 587–594. [Google Scholar] [CrossRef]
- Kirana, S.; Stratmann, B.; Prante, C.; Prohaska, W.; Koerperich, H.; Lammers, D.; Gastens, M.H.; Quast, T.; Negrean, M.; Stirban, O.A.; et al. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int. J. Clin. Pract. 2012, 66, 384–393. [Google Scholar] [CrossRef]
- Tresierra-Ayala, M.Á.; Rojas, A.G. Association between peripheral arterial disease and diabetic foot ulcers in patients with diabetes mellitus type 2. Med. Univ. 2017, 19, 123–126. [Google Scholar] [CrossRef]
- Mohler, E.R. Therapy Insight: Peripheral arterial disease and diabetes—From pathogenesis to treatment guidelines. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 151–162. [Google Scholar] [CrossRef]
- Association, A.D. Standards of Medical Care in Diabetes—2011. Diabetes Care 2011, 34, S11. [Google Scholar] [CrossRef]
- Schaper, N.C.; Andros, G.; Apelqvist, J.; Bakker, K.; Lammer, J.; Lepantalo, M.; Mills, J.; Reekers, J.; Shearman, C.P.; Zierler, R.E.; et al. Diagnosis and treatment of peripheral arterial disease in diabetic patients with a foot ulcer. A progress report of the International Working Group on the Diabetic Foot. Diabetes/Metab. Res. Rev. 2012, 28, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Schaper, N.C.; Andros, G.; Apelqvist, J.; Bakker, K.; Lammer, J.; Lepäntalo, M.; Mills, J.; Reekers, J.; Shearman, C.P.; Zierler, R.E.; et al. Specific guidelines for the diagnosis and treatment of peripheral arterial disease in a patient with diabetes and ulceration of the foot 2011. Diabetes/Metab. Res. Rev. 2012, 28, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [PubMed]
- Minar, E. Critical limb ischaemia. Hamostaseologie 2009, 29, 102–109. [Google Scholar] [PubMed]
- Chen, Y.-C.; Bui, A.V.; Diesch, J.; Manasseh, R.; Hausding, C.; Rivera, J.; Haviv, I.; Agrotis, A.; Htun, N.M.; Jowett, J.; et al. A Novel Mouse Model of Atherosclerotic Plaque Instability for Drug Testing and Mechanistic/Therapeutic Discoveries Using Gene and MicroRNA Expression Profiling. Circ. Res. 2013, 113, 252–265. [Google Scholar] [CrossRef]
- Madonna, R.; Pieragostino, D.; Balistreri, C.R.; Rossi, C.; Geng, Y.-J.; Del Boccio, P.; De Caterina, R. Diabetic macroangiopathy: Pathogenetic insights and novel therapeutic approaches with focus on high glucose-mediated vascular damage. Vasc. Pharmacol. 2018, 107, 27–34. [Google Scholar] [CrossRef]
- Rosalinda Madonna, R.D.C. Cellular and molecular mechanisms of vascular injury in diabetes—Part I: Pathways of vascular disease in diabetes. Vasc. Pharmacol. 2011, 54, 68–74. [Google Scholar] [CrossRef]
- Fadini, G.P. A reappraisal of the role of circulating (progenitor) cells in the pathobiology of diabetic complications. Diabetologia 2014, 57, 4–15. [Google Scholar] [CrossRef][Green Version]
- Moon, J.H.; Chae, M.K.; Kim, K.J.; Kim, H.M.; Cha, B.-S.; Lee, H.C.; Kim, Y.J.; Lee, B.-W. Decreased Endothelial Progenitor Cells and Increased Serum Glycated Albumin Are Independently Correlated with Plaque-Forming Carotid Artery Atherosclerosis in Type 2 Diabetes Patients Without Documented Ischemic Disease. Circ. J. 2012, 76, 2273–2279. [Google Scholar] [CrossRef]
- DiPersio, J.F. Diabetic Stem-Cell “Mobilopathy”. N. Engl. J. Med. 2011, 356, 2536–2538. [Google Scholar] [CrossRef]
- Madonna, R.; Geng, Y.J.; De Caterina, R. Adipose Tissue–Derived Stem Cells Characterization and Potential for Cardiovascular Repair. Atheroscler. Thromb. Vasc. Biol. 2009, 29, 1723–1729. [Google Scholar] [CrossRef]
- De Pascale, M.R.; Bruzzese, G.; Crimi, E.; Grimaldi, V.; Liguori, A.; Brongo, S.; Barbieri, M.; Picascia, A.; Schiano, C.; Sommese, L.; et al. Severe Type 2 Diabetes Induces Reversible Modifications of Endothelial Progenitor Cells Which are Ameliorate by Glycemic Control. Int. J. Stem Cells 2016, 9, 137–144. [Google Scholar] [CrossRef][Green Version]
- Yue, W.-S.; Lau, K.-K.; Siu, C.-W.; Wang, M.; Yan, G.-H.; Yiu, K.-H.; Tse, H.-F. Impact of glycemic control on circulating endothelial progenitor cells and arterial stiffness in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2011, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Sartore, S.; Agostini, C.; Avogaro, A. Significance of Endothelial Progenitor Cells in Subjects with Diabetes. Diabetes Care 2007, 30, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Mancuso, P.; Bertolini, F.; de Kreutzenberg, S.; Avogaro, A. Amelioration of Glucose Control Mobilizes Circulating Pericyte Progenitor Cells in Type 2 Diabetic Patients with Microangiopathy. Exp. Diabetes Res. 2012, 2012, 274363. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.W. Metabolic and storage diseases. In Weedon's Skin Pathology; Elsevier Limited: Amsterdam, The Netherlands, 2021; pp. 593–616. [Google Scholar]
- Coppelli, A.; Abbruzzese, L.; Goretti, C.; Iacopi, E.; Riitano, N.; Piaggesi, A. Does Microangiopathy Contribute to the Pathogenesis of the Diabetic Foot Syndrome? Diabet. Foot Syndr. Front. Diabetes 2018, 26, 70–82. [Google Scholar]
- Bakker, K.; Apelqvist, J.; Schaper, N.C. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes/Metab. Res. Rev. 2012, 28, 225–231. [Google Scholar] [CrossRef]
- International, W. International Best Practice Guidelines: Wound Management in Diabetic Foot U. Wounds Int. 2013, 1, 1–27. [Google Scholar]
- Centre for Clinical Practice at NICE. Diabetic Foot Problems: Inpatient Management of Diabetic Foot Problems; NHS: London, UK, 2011. [Google Scholar]
- Graffy, J.; Eaton, S.; Sturt, J.; Chadwick, P. Personalized care planning for diabetes: Policy lessons from systematic reviews of consultation and self-management interventions. Prim. Health Care Res. Dev. 2009, 10, 210–222. [Google Scholar] [CrossRef]
- Frykberg, R.G. Diabetic Foot Ulcers: Pathogenesis and Management. Am. Fam. Physician 2002, 66, 1655–1662. [Google Scholar]
- Boulton, A.J.M. What you can’t feel can hurt you. J. Vasc. Surg. 2010, 52, 28S–30S. [Google Scholar] [CrossRef] [PubMed]
- Apelqvist, J. Diagnostics and treatment of the diabetic foot. Endocrine 2012, 41, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M.; Foster, A.V.M.; Vowden, P. Wound bed preparation for diabetic foot ulcer. In Position Document: Wound Bed Preparation in Practice; MEP Ltd.: London, UK, 2004. [Google Scholar]
- Peter, R.; Cavanagh, S.A.B. Off-loading the diabetic foot for ulcer prevention and healing. J. Vasc. Surg. 2010, 52, 37S–43S. [Google Scholar]
- Armstrong, D.G.; Lavery, L.A.; Nixon, B.P.; Boulton, A.J. It’s Not What You Put On, but What You Take Off: Techniques for Debriding and Off-Loading the Diabetic Foot Wound. Clin. Infect. Dis. 2004, 39, S92–S99. [Google Scholar] [CrossRef] [PubMed]
- SIGN. Management of Diabetes a National Clinical Guideline (SIGN 116); SIGN: Edinburgh, UK, 2010. [Google Scholar]
- Yu, Q.; Qiao, G.-H.; Wang, M.; Yu, L.; Sun, Y.; Shi, H.; Ma, T.-L. Stem Cell-Based Therapy for Diabetic Foot Ulcers. Front. Cell Dev. Biol. 2022, 10, 812262. [Google Scholar] [CrossRef] [PubMed]
- Regranex. Regranex (Becaplermin) Labeling. 2008. Available online: https://www.fda.gov/media/76010 (accessed on 1 March 2022).
- Qin, H.L.; Zhu, X.H.; Zhang, B.; Zhou, L.; Wang, W.Y. Clinical Evaluation of Human Umbilical Cord Mesenchymal Stem Cell Transplantation After Angioplasty for Diabetic Foot. Exp. Clin. Endocrinol. Diabetes 2016, 124, 497–503. [Google Scholar] [CrossRef]
- Houlind, K. Surgical revascularization and reconstruction proceduresin diabetic foot ulceration. Diabetes/Metab. Res. Rev. 2020, 36, e3256. [Google Scholar] [CrossRef]
- A Hart, C.; Tsui, J.; Khanna, A.; Abraham, D.J.; Baker, D.M. Stem cells of the lower limb: Their role and potential in management of critical limb ischemia. Exp. Biol. Med. 2013, 238, 1118–1126. [Google Scholar] [CrossRef]
- Yang, M.; Sheng, L.; Zhang, T.R.; Li, Q. Stem Cell Therapy for Lower Extremity Diabetic Ulcers: Where Do We Stand? BioMed Res. Int. 2013, 2013, 462179. [Google Scholar] [CrossRef]
- Chiang, K.J.; Chiu, L.C.; Kang, Y.N.; Chen, C. Autologous Stem Cell Therapy for Chronic Lower Extremity Wounds: A Meta-Analysis of Randomized Controlled Trials. Cells 2021, 10, 3307. [Google Scholar] [CrossRef]
- Kalka, C.; Masuda, H.; Takahashi, T.; Kalka-Moll, W.M.; Silver, M.; Kearney, M.; Li, T.; Isner, J.M.; Asahara, T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kalka, C.; Masuda, H.; Chen, D.; Silver, M.; Kearney, M.; Magner, M.; Isner, J.M.; Asahara, T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999, 5, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Raposio, E.; Bertozzi, N.; Grignaffini, E.; Simonacci, F.; Grieco, M.P. Adipose-derived Stem Cells for Treatment of Chronic Cutaneous Ulcers in Patients with Critical Limb Ischemia: A Pilot Study. J. Regen. Med. 2016, 5, 1000128. [Google Scholar] [CrossRef]
- Dai, J.; Jiang, C.; Chen, H.; Chai, Y. Treatment of Diabetic Foot with Autologous Stem Cells: A MetaAnalysis of Randomized Studies. Stem Cell Int. 2020, 2020, 6748530. [Google Scholar] [CrossRef]
- Wahid, S.F.A.; Ismail, N.A.; Jamaludin, W.F.W.; Muhamad, N.A.; Hamid, M.K.A.A.; Harunarashid, H.; Lai, N.M. Autologous cells derived from different sources and administered using different regimens for ‘no-option’ critical lower limb ischaemia patients (Review). Cochrane Database Syst. Rev. 2018, 8, CD010747. [Google Scholar] [CrossRef] [PubMed]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H.; et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef]
- Rigato, M.; Monami, M.; Fadini, G.P. Autologous Cell Therapy for Peripheral Arterial Disease Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ. Res. 2017, 120, 1326–1340. [Google Scholar] [CrossRef] [PubMed]
- Carstens, M.H.; Quintana, F.J.; Calderwood, S.T.; Sevilla, J.P.; Ríos, A.B.; Rivera, C.M.; Calero, D.W.; Zelaya, M.L.; Garcia, N.; Bertram, K.A.; et al. Treatment of chronic diabetic foot ulcers with adipose-derived stromal vascular fraction cell injections: Safety and evidence of efficacy at 1 year. Stem Cells Transl. Med. 2021, 10, 1138–1147. [Google Scholar] [CrossRef]
- Lingen, M.W. Role of Leukocytes and Endothelial Cells in the Development of Angiogenesis in Inflammation and Wound Healing. Arch. Pathol. Lab. Med. 2001, 125, 67–71. [Google Scholar] [CrossRef]
- Reinhard Gillitzer, M.G. Chemokines in cutaneous wound healing. J. Leukoc. Biol. 2001, 69, 513–521. [Google Scholar]
- Huang, P.; Li, S.; Han, M.; Xiao, Z.; Yang, R.; Han, Z.C. Autologous Transplantation of Granulocyte Colony–Stimulating Factor—Mobilized Peripheral Blood Mononuclear Cells Improves Critical Limb Ischemia in Diabetes. Diabetes Care 2005, 28, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, S.N.; Berger, A.; Hwang, L.; Pastar, I.; Warren, S.M.; Chen, W. The role of stem cells in the treatment of diabetic foot ulcers. Diabetes Res. Clin. Pract. 2012, 96, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Evangelos, V.; Badiavas, V.F. Treatment of Chronic Wounds With Bone Marrow-Derived Cells. Arch. Dermatol. 2003, 139, 510–516. [Google Scholar]
- Jain, P.; Perakath, B.; Jesudason, M.R.; Nayak, S. The Effect of Autologous Bone Marrow-Derived Cells on Healing Chronic Lower Extremity Wounds: Results of a Randomized Controlled Study. Ostomy Wound Manag. 2011, 57, 38–44. [Google Scholar]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous Bone Marrow–Derived Cultured Mesenchymal Stem Cells Delivered in a Fibrin Spray Accelerate Healing in Murine and Human Cutaneous Wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef]
- Dash, N.R.; Dash, S.; Routray, P.; Mohapatra, S.; Mohapatra, P.C. Targeting Nonhealing Ulcers of Lower Extremity in Human through Autologous Bone Marrow-Derived Mesenchymal Stem Cells. Rejuvenation Res. 2009, 12, 359–366. [Google Scholar] [CrossRef]
- Wu, Q.; Lei, X.; Chen, L.; Zheng, Y.; Huang, H.; Qian, C.; Liang, Z. Autologous platelet-rich gel combined with in vitro amplification of bone marrow mesenchymal stem cell transplantation to treat the diabetic foot ulcer: A case report. Ann. Transl. Med. 2018, 6, 307. [Google Scholar] [CrossRef]
- Prochazka, V.; Gumulec, J.; Chmelova, J.; Klement, P.; Klement, G.L.; Jonszta, T.; Czerny, D.; Krajca, J. Autologous Bone Marrow Stem Cell Transplantation in Patients with End-Stage Chronical Critical Limb Ischemia and Diabetic Foot. Vnitr. Lek. 2009, 55, 173–178. [Google Scholar]
- Amann, B.; Luedemann, C.; Ratei, R.; Schmidt-Lucke, J.A. Autologous Bone Marrow Cell Transplantation Increases Leg Perfusion and Reduces Amputations in Patients with Advanced Critical Limb Ischemia Due to Peripheral Artery Disease. Cell Transplant. 2009, 18, 371–380. [Google Scholar] [CrossRef]
- Matoba, S.; Tatsumi, T.; Murohara, T.; Imaizumi, T.; Katsuda, Y.; Ito, M.; Saito, Y.; Uemura, S.; Suzuki, H.; Fukumoto, S.; et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am. Heart J. 2008, 156, 1010–1018. [Google Scholar] [CrossRef]
- Lu, D.; Chen, B.; Liang, Z.; Deng, W.; Jiang, Y.; Li, S.; Xu, J.; Wu, Q.; Zhang, Z.; Xie, B.; et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res. Clin. Pract. 2011, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Scatena, A.; Petruzzi, P.; Maioli, F.; Lucaroni, F.; Ambrosone, C.; Ventoruzzo, G.; Liistro, F.; Tacconi, D.; Di Filippi, M.; Attempati, N.; et al. Autologous Peripheral Blood Mononuclear Cells for Limb Salvage in Diabetic Foot Patients with No-Option Critical Limb Ischemia. J. Clin. Med. 2021, 10, 2213. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Wu, Y.X.; Wang, H.M.; Xu, Y.F.; Lü, X.; Zhang, Y.B.; Wang, F.; Zhang, Y. Autologous peripheral blood stem cells transplantation in treatment of 62 cases of lower extremity ischemic disorder. Zhonghua Nei Ke Za Zhi 2005, 44, 95–98. [Google Scholar] [PubMed]
- Gough, A.; Clapperton, M.; Rolando, N.; Foster, A.V.; Philpott-Howard, J.; Edmonds, M.E. Randomised placebo-controlled trial of granulocyte-colony stimulating factor in diabetic foot infection. Lancet 1997, 350, 855–859. [Google Scholar] [CrossRef]
- Sato, T.; Yamauchi, N.; Kobayashi, D.; Sato, Y.; Mochizuki, C.; Hori, C.; Watanabe, N.; Niitsu, Y. Granulocyte colony stimulating factor (G-CSF) producing renal cell carcinoma. Rinsho Ketsueki 1997, 38, 1189–1193. [Google Scholar]
- Nelson, S.; Heyder, A.M.; Stone, J.; Bergeron, M.G.; Daugherty, S.; Peterson, G.; Fotheringham, N.; Welch, W.; Milwee, S.; Root, R.; et al. A Randomized Controlled Trial of Filgrastim for the Treatment of Hospitalized Patients with Multilobar Pneumonia. J. Infect. Dis. 2000, 182, 970–973. [Google Scholar] [CrossRef][Green Version]
- Dale, D.C.; Liles, W.C.; Summer, W.R.; Nelson, S. Granulocyte Colony-Stimulating Factor: Role and Relationships in Infectious Diseases. J. Infect. Dis. 1995, 172, 1061–1075. [Google Scholar] [CrossRef]
- Hartung, T. Granulocyte colony-stimulating factor: Its potential role in infectious disease. AIDS 1999, 13, S3–S9. [Google Scholar]
- Cruciani, M.; A Lipsky, B.; Mengoli, C.; de Lalla, F. Granulocyte-colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Collab. 2011, 8, 1–36. [Google Scholar] [CrossRef]
- Finnerty, C.C.; Przkora, R.; Herndon, D.N.; Jeschke, M.G. Cytokine expression profile over time in burned mice. Cytokine 2009, 45, 20–25. [Google Scholar] [CrossRef]
- Lopes, L.; Setia, O.; Aurshina, A.; Liu, S.; Hu, H.; Isaji, T.; Liu, H.; Wang, T.; Ono, S.; Guo, X.; et al. Stem cell therapy for diabetic foot ulcers: A review of preclinical and clinical research. Stem Cell Res. Ther. 2018, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.-Y.; Park, I.-H.; Song, Y.-S.; Joo, H.-W.; Lee, Y.; Shin, J.-H.; Kim, K.-S.; Kim, H. Local Injection of Granulocyte-Colony Stimulating Factor Accelerates Wound Healing in a Rat Excisional Wound Model. Korean Tissue Eng. Regen. Med. Soc. 2016, 13, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Ma, J.; Li, S.; Liu, W. Applicability of adipose-derived mesenchymal stem cells in treatment of patients with type 2 diabetes. Stem Cell Res. Ther. 2019, 10, 274. [Google Scholar] [CrossRef]
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.W.; Xian, C.J. Adipose-derived stem cells for wound healing. J. Cell. Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef] [PubMed]
- Kokai, L.E.; Marra, K.; Rubin, J.P. Adipose stem cells: Biology and clinical applications for tissue repair and regeneration. Transl. Res. 2014, 136, 399–408. [Google Scholar] [CrossRef]
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Cifone, M.G.; Cinque, B.; Giuliani, M. Methods of Isolation, Characterization and Expansion of Human Adipose-Derived Stem Cells (ASCs): An Overview. Int. J. Mol. Sci. 2018, 19, 1897. [Google Scholar] [CrossRef]
- GadElkarim, M.; Abushouk, A.I.; Ghanem, E.; Hamaad, A.M.; Saad, A.M.; Abdel-Daim, M.M. Adipose-derived stem cells: Effectiveness and advances in delivery in diabetic wound healing. Biomed. Pharmacother. 2018, 107, 625–633. [Google Scholar] [CrossRef]
- Álvaro-Afonso, F.J.; Sanz-Corbalán, I.; Lázaro-Martínez, J.L.; Kakagia, D.; Papanas, N. Adipose-Derived Mesenchymal Stem Cells in the Treatment of Diabetic Foot Ulcers: A Review of Preclinical and Clinical Studies. Angiology 2020, 71, 853–863. [Google Scholar] [CrossRef]
- Cianfarani, F.; Toietta, G.; Di Rocco, G.; Cesareo, E.; Zambruno, G.; Odorisio, T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013, 21, 545–553. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, Y.H.; Jun, Y.J.; Yoo, G.; Rhie, J.W. The effect of diabetes on the wound healing potential of adipose-tissue derived stem cells. Int. Wound J. 2016, 13, 33–41. [Google Scholar] [CrossRef]
- Rennert, R.C.; Sorkin, M.; Januszyk, M.; Duscher, D.; Kosaraju, R.; Chung, M.T.; Lennon, J.; Radiya-Dixit, A.; Raghvendra, S.; Maan, Z.N.; et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res. Ther. 2014, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Fromer, M.W.; Chang, S.; Hagaman, A.L.; Koko, K.R.; Nolan, R.S.; Zhang, P.; Brown, S.A.; Carpenter, J.P.; Caputo, F.J. The endothelial cell secretome as a novel treatment to prime adipose-derived stem cells for improved wound healing in diabetes. J. Vasc. Surg. 2018, 68, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Tobita, M.; Tajima, S.; Mizuno, H. Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: Stem cell transplantation methods that enhance stemness. Stem Cell Res. Ther. 2015, 6, 215. [Google Scholar] [CrossRef] [PubMed]
- Louwen, F.; Ritter, A.; Kreis, N.N.; Yuan, J. Insight into the development of obesity: Functional alterations of adipose-derived mesenchymal stem cells. Obes. Rev. 2018, 19, 888–904. [Google Scholar] [CrossRef]
- Caso, G.; McNurlan, M.A.; Mileva, I.; Zemlyak, A.; Mynarcik, D.C.; Gelato, M.C. Peripheral fat loss and decline in adipogenesis in older humans. Metabolism 2013, 62, 337–340. [Google Scholar] [CrossRef]
- Tchkonia, T.; Morbeck, D.E.; Von Zglinicki, T.; Van Deursen, J.; Lustgarten, J.; Scrable, H.; Khosla, S.; Jensen, M.D.; Kirkland, J.L. Fat tissue, aging, and cellular senescence. Aging Cell 2010, 9, 667–684. [Google Scholar] [CrossRef]
- Khalil, C.; Chaker, D.; Salameh, R.; Germanos, Y.-E.; El Kayem, E.; Nader, F.; Habbouche, J.; Chemaly, R.; Azar, A.; Ibrahim, A. Autologous Adipose-Derived Mesenchymal Stem Cells Embedded in Platelet-Rich Fibrin in Diabetic Foot Ulcers. Open J. Regen. Med. 2021, 10, 19–30. [Google Scholar] [CrossRef]
- Gonzalez, A.C.D.O.; Costa, T.F.; Andrade, Z.D.A.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Shih, D.T.-B.; Tseng, C.-P.; Hsieh, T.-B.; Lee, D.-C.; Hwang, S.-M. Disparate Mesenchyme-Lineage Tendencies in Mesenchymal Stem Cells from Human Bone Marrow and Umbilical Cord Blood. Stem Cells 2006, 24, 679–685. [Google Scholar] [CrossRef]
- Honma, T.; Honmou, O.; Iihoshi, S.; Harada, K.; Houkin, K.; Hamada, H.; Kocsis, J. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp. Neurol. 2006, 199, 56–66. [Google Scholar] [CrossRef]
- Tang, J.; Xie, Q.; Pan, G.; Wang, J.; Wang, M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur. J. Cardio-Thorac. Surg. 2006, 30, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaldi, A.; Al-Sabti, H.; Galipeau, J.; Lachapelle, K. Therapeutic angiogenesis using autologous bone marrow stromal cells: Improved blood flow in a chronic limb ischemia model. Ann. Thorac. Surg. 2003, 75, 204–209. [Google Scholar] [CrossRef]
- Ian Rogers, R.F.C. Umbilical cord blood stem cells. Best Pract. Res. Clin. Obstet. Gynecol. 2004, 18, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Xv, Y.; Lin, Z.; Endo, Y.; Xue, H.; Hu, Y.; Hu, L.; Chen, L.; Cao, F.; Zhou, W.; et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Accelerate Diabetic Wound Healing via Ameliorating Oxidative Stress and Promoting Angiogenesis. Front. Bioeng. Biotechnol. 2022, 10, 829868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, Y.; Liu, Y.; Li, X.; Tang, L.; Duan, M.; Li, J.; Zhang, G. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Res. Ther. 2021, 12, 434. [Google Scholar] [CrossRef]
- Wen, Z.; Mai, Z.; Zhu, X.; Wu, T.; Chen, Y.; Geng, D.; Wang, J. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 36. [Google Scholar] [CrossRef]
- Xue, C.; Shen, Y.; Li, X.; Li, B.; Zhao, S.; Gu, J.; Chen, Y.; Ma, B.; Wei, J.; Han, Q.; et al. Exosomes Derived from Hypoxia-Treated Human Adipose Mesenchymal Stem Cells Enhance Angiogenesis through the PKA Signaling Pathway. Stem Cells Dev. 2018, 27, 456–465. [Google Scholar] [CrossRef]
- Mihu, C.M.; Mihu, D.; Costin, N.; Ciucă, D.R.; Suşman, S.; Ciortea, R. Isolation and characterization of stem cells from the placenta and the umbilical cord. Rom. J. Morphol. Embryol. 2008, 49, 441–446. [Google Scholar]
- Fukuchi, Y.; Nakajima, H.; Sugiyama, D.; Hirose, I.; Kitamura, T.; Tsuji, K.; Fukuchi, Y. Human Placenta-Derived Cells Have Mesenchymal Stem/Progenitor Cell Potential. Stem Cells 2004, 22, 649–658. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Liu, Y.; Luo, B.; Xie, N.; Tan, T.; Song, L.; Erli, P.; Luo, M. Implantation of placenta-derived mesenchymal stem cells accelerates murine dermal wound closure through immunomodulation. Am. J. Transl. Res. 2016, 8, 4912–4921. [Google Scholar]
- Zeng, X.; Tang, Y.; Hu, K.; Jiao, W.; Ying, L.; Zhu, L.; Liu, J.; Xu, J. Three-week topical treatment with placenta-derived mesenchymal stem cells hydrogel in a patient with diabetic foot ulcer A case report. Medicine 2017, 96, e9212. [Google Scholar] [CrossRef] [PubMed]
- Du, W.J.; Chi, Y.; Yang, Z.X.; Li, Z.J.; Cui, J.J.; Song, B.Q.; Li, X.; Yang, S.G.; Han, Z.B.; Han, Z.C. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res. Ther. 2016, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Meamar, R.; Ghasemi-Mobarakeh, L.; Norouzi, M.-R.; Siavash, M.; Hamblin, M.R.; Fesharaki, M. Improved wound healing of diabetic foot ulcers using human placenta-derived mesenchymal stem cells in gelatin electrospun nanofibrous scaffolds plus a platelet-rich plasma gel: A randomized clinical trial. Int. Immunopharmacol. 2021, 101 Pt B, 108282. [Google Scholar] [CrossRef]
- DaVanzo, J.; Hartzman, A.; Surfield, C.; Dobson, A. Cryopreserved placental membrane allograft reduces the likelihood of developing a new or recurring foot ulcer and all-cause mortality in diabetic patients, when compared to other cellular- and tissue-based products. Adv. Wound Care 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Moraci, M.; Armenia, E.; Orabona, C.; Sergio, R.; De Sena, G.; Capuozzo, V.; Barbarisi, M.; Rosso, F.; Giordano, G.; et al. Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J. Surg. Res. 2013, 185, 36–44. [Google Scholar] [CrossRef]
- Xu, S.M.; Liang, T. Clinical observation of the application of autologous peripheral blood stem cell transplantation for the treatment of diabetic foot gangrene. Exp. Ther. Med. 2016, 11, 283–288. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, Z.; Chen, K.; Yang, W.; Wan, X.; Zeng, P.; He, H.; Luo, Y.; Xiao, Q.; Mo, Z. Combined Transplantation of Mesenchymal Stem Cells and Endothelial Colony-Forming Cells Accelerates Refractory Diabetic Foot Ulcer Healing. Stem Cells Int. 2020, 2020, 8863649. [Google Scholar] [CrossRef]
- Uzun, E.; Güney, A.; Gönen, Z.B.; Özkul, Y.; Kafadar, I.H.; Günay, M.; Mutlu, M. Intralesional allogeneic adipose-derived stem cells application in chronic diabetic foot ulcer: Phase I/2 safety study. Foot Ankle Surg. 2021, 27, 636–642. [Google Scholar] [CrossRef]
- Bogliotti, Y.S.; Wu, J.; Vilarino, M.; Okamura, D.; Soto, D.A.; Zhong, C.; Sakurai, M.; Sampaio, R.V.; Suzuki, K.; Belmonte, J.C.I.; et al. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc. Natl. Acad. Sci. USA 2018, 115, 2090–2095. [Google Scholar] [CrossRef]
- Lerou, P. Embryonic stem cell derivation from human embryos. Methods Mol. Biol. 2011, 767, 31–35. [Google Scholar]
- Chiao, E.; Kmet, M.; Behr, B.; Baker, J. Derivation of human embryonic stem cells in standard and chemically defined conditions. Methods Cell Biol. 2008, 86, 1–14. [Google Scholar]
- Cho, S.-W.; Moon, S.-H.; Lee, S.-H.; Kang, S.-W.; Kim, J.; Lim, J.M.; Kim, H.-S.; Kim, B.-S.; Chung, H.M. Improvement of Postnatal Neovascularization by Human Embryonic Stem Cell–Derived Endothelial-Like Cell Transplantation in a Mouse Model of Hindlimb Ischemia. Circulation 2007, 116, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-B.; Choi, J.; Cho, S.-B.; Chung, J.-Y.; Moon, E.-S.; Kim, N.-S.; Han, H.-J. Topical Embryonic Stem Cells Enhance Wound Healing in Diabetic Rats. J. Orthop. Res. 2011, 29, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Loretelli, C.; Ben Nasr, M.; Giatsidis, G.; Bassi, R.; Lancerotto, L.; D’Addio, F.; Valderrama-Vasquez, A.; Scherer, S.S.; Salvatore, L.; Madaghiele, M.; et al. Embryonic stem cell extracts improve wound healing in diabetic mice. Acta Diabetol. 2020, 57, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Y.; Lu, D.B.; Chen, B. Progress in stem cell therapy for the diabetic foot. Diabetes Res. Clin. Pract. 2012, 97, 43–50. [Google Scholar] [CrossRef]
- Assi, R.; Foster, T.R.; He, H.; Stamati, K.; Bai, H.; Huang, Y.; Hyder, F.; Rothman, D.; Shu, C.; Homer-Vanniasinkam, S.; et al. Delivery of mesenchymal stem cells in biomimetic engineered scaffolds promotes healing of diabetic ulcers. Regen. Med. 2016, 11, 245–260. [Google Scholar] [CrossRef]
- Chen, J.S.; Wong, V.W.; Gurtner, G.C. Therapeutic potential of bone marrow-derived mesenchymal stem cells for cutaneous wound healing. Front. Immunol. 2012, 3, 192. [Google Scholar] [CrossRef]
- Critser, P.; Kreger, S.; Voytik-Harbin, S.; Yoder, M. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc. Res. 2010, 80, 23–30. [Google Scholar] [CrossRef]
- Gower, R.M.; Shea, L.D. Biomaterial Scaffolds for Controlled, Localized Gene Delivery of Regenerative Factors. Adv. Wound Care 2013, 2, 100–106. [Google Scholar] [CrossRef]
- Mudera, V.; Morgan, M.; Cheema, U.; Nazhat, S.; Brown, R. Ultra-rapid engineered collagen constructs tested in an in vivo nursery site. J. Tissue Eng. Regen. Med. 2007, 1, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Comerota, A.J.; Link, A.; Douville, J.; Burchardt, E.R. Upper extremity ischemia treated with tissue repair cells from adult bone marrow. J. Vasc. Surg. 2010, 52, 723–729. [Google Scholar] [CrossRef][Green Version]
- Held, M.; Rahmanian-Schwarz, A.; Schiefer, J.; Rath, R.; Werner, J.-O.; Rahmanian, S.; Schaller, H.-E.; Petersen, W. A Novel Collagen-Gelatin Scaffold for the Treatment of Deep Dermal Wounds-An Evaluation in a Minipig Model. Dermatol. Surg. 2016, 42, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-N.; Rhie, J.W.; Kwon, H.; Jun, Y.J.; Seo, J.-W.; Yoo, G.; Oh, D.Y.; Ahn, S.T.; Woo, J.; Oh, J. In vivo cartilage formation using chondrogenic-differentiated human adipose-derived mesenchymal stem cells mixed with fibrin glue. J. Craniofacial Surg. 2010, 21, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Mills, S.J.; Cowin, A.J. Mesenchymal Stem Cell Secretome as an Emerging Cell-Free Alternative for Improving Wound Repair. Int. J. Mol. Sci. 2020, 21, 7038. [Google Scholar] [CrossRef] [PubMed]
- Alrubaiy, L.; Al-Rubaiy, K.K. Skin substitutes: A brief review of types and clinical applications. Oman Med. J. 2009, 24, 4–6. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Hong, Q.; Zhang, C.-Y.; Yang, Y.-J.; Cai, G.-Y.; Chen, X.-M. miRNAs in stem cell-derived extracellular vesicles for acute kidney injury treatment: Comprehensive review of preclinical studies. Stem Cell Res. Ther. 2019, 10, 281. [Google Scholar] [CrossRef]
- Arnold, I.; Caplan, J.E.D. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar]
- Vieira, N.M.; Zucconi, E.; Bueno, C.R.; Secco, M.; Suzuki, M.F.; Bartolini, P.; Vainzof, M.; Zatz, M. Human multipotent mesenchymal stromal cells from distinct sources show different in vivo potential to differentiate into muscle cells when injected in dystrophic mice. Stem Cells Rev. Rep. 2010, 6, 560–566. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine Factors of Mesenchymal Stem Cells Recruit Macrophages and Endothelial Lineage Cells and Enhance Wound Healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef]
- Park, S.-R.; Kim, J.-W.; Jun, H.-S.; Roh, J.Y.; Lee, H.-Y.; Hong, I.-S. Stem Cell Secretome and Its Effect on Cellular Mechanisms Relevant to Wound Healing. Mol. Ther. 2018, 26, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Methot, D.; Poppa, V.; Fujio, Y.; Walsh, K.; Murry, C.E. Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. J. Mol. Cell. Cardiol. 2001, 33, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Wahlberg, B.; Ghuman, H.; Liu, J.R.; Modo, M. Ex vivo biomechanical characterization of syringe-needle ejections for intracerebral cell delivery. Sci. Rep. 2018, 8, 9194. [Google Scholar] [CrossRef] [PubMed]
- de Mayo, T.; Conget, P.; Becerra-Bayona, S.; Sossa, C.L.; Galvis, V.; Arango-Rodríguez, M.L. The role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic mice. PLoS ONE 2017, 12, e0177533. [Google Scholar] [CrossRef]
- Bellu, E.; Medici, S.; Coradduzza, D.; Cruciani, S.; Amler, E.; Maioli, M. Nanomaterials in Skin Regeneration and Rejuvenation. Int. J. Mol. Sci. 2021, 22, 7095. [Google Scholar] [CrossRef]
- Trommer, H.; Neubert, R.H.H. Overcoming the Stratum Corneum: The Modulation of Skin Penetration. Ski. Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef]
- Zhu, C.; Cao, R.; Zhang, Y.; Chen, R. Metallic Ions Encapsulated in Electrospun Nanofiber for Antibacterial and Angiogenesis Function to Promote Wound Repair. Front. Cell Dev. Biol. 2021, 9, 660571. [Google Scholar] [CrossRef]
- Daňková, J.; Buzgo, M.; Vejpravová, J.; Kubíčková, S.; Sovková, V.; Vysloužilová, L.; Mantlíková, A.; Amler, E.; Nečas, A. Highly efficient mesenchymal stem cell proliferation on poly-ε-caprolactone nanofibers with embedded magnetic nanoparticles. Int. J. Nanomed. 2015, 10, 7307–7317. [Google Scholar] [CrossRef]
- Başaran, D.D.A.; Gündüz, U.; Tezcaner, A.; Keskin, D. Topical delivery of heparin from PLGA nanoparticles entrapped in nanofibers of sericin/gelatin scaffolds for wound healing. Int. J. Pharm. 2021, 597, 120207. [Google Scholar] [CrossRef]
- Graça, M.; de Melo-Diogo, D.; Correia, I.; Moreira, A. Electrospun Asymmetric Membranes as Promising Wound Dressings: A Review. Pharmaceutics 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
| Reference | Study Design | Cell Type | Administration Route | Outcome | Follow-Up |
|---|---|---|---|---|---|
| Kirana et al. 2012 [21] | 24 patients with DFU 2 treatment groups: - Bone marrow mononuclear cells (BMCs) - Tissue repair cells | Autologous BMMSC | - Intramuscular injection - Intraarterial | - Both groups had improvement in wound healing without a significant difference. - Improvement in TcPO2 was detected in both groups. | 45 weeks |
| Marino et al. 2013 [124] | 20 patients with PAD with chronic ulcers of the lower limb - 10 patients treated with AMSCs extracted by celution method | Autologous AMSCs | Local perilesional injection | - Six out of ten had complete healing. - Closure of the ulcer was observed. | 90 days |
| Qin et al. 2016 [55] | 53 patients - 2 groups: control group, experimental group - Both groups received angioplasty; those in the experimental group also received HUCMSCs | Allogeneic HUCMSCs | - Intraarterial infusion - Intramuscular | Experimental group had significant improvement in: - Skin temperature - Ankle-brachial pressure index - Transcutaneous oxygen tension- Claudication distance | 1–3 months |
| Xu and Liang 2016 [125] | 127 patients were treated with - Granulocyte colony-stimulating factor (G-CSF). - Extracted PBSC suspension | - G-CSF - Autologous PBSCs | Injection into the ischemic lower extremities at multiple points around the embolized blood vessels | Ischemic area of the patients was improved significantly. | 4 weeks |
| Zeng et al. 2017 [120] | 57-year old patient with DFU - PDMSC hydrogel | Allogeneic PDMSCs | Topical | - Healing of foot ulcer was observed. - Walking foot function was well preserved. | 6 months |
| Wu et al. 2018 [76] | A 54-year-old patient with DFU. Received standard treatment including debridement, dressing, and continuous negative pressure suction followed by autologous platelet-rich gel (APG) and BMMSC transplantation | Autologous BMMSCs | Local perilesional injection | Significant improvement of wound and complete healing was detected. | 30 days |
| Zhao et al. 2020 [126] | 12 patients with DFU | - Allogeneic umbilical cord mesenchymal stem cells (UCMSCs) - Umbilical cord blood-derived endothelial colony-forming cells (ECFCs) | Local injection | - Accelerated healing in wounds treated with combination therapy was observed. - Wound size reduction was detected. | 1–4 weeks |
| Scatena et al. 2021 [81] | 76 no-option critical ischemia (NO-CLI) patients DFUs - All patients treated with the same standard care (control group); - 38 patients were also treated with autologous PBMNC implants | Autologous PBMNC | - Intramuscular - Local peri -lesional injection | - Four out 38 amputations (10.5%) in the PBMNC group were done. - 15 out of 38 amputations (39.5%) in the control group (p = 0.0037) were done. - At 2 years follow-up, 80% of the PBMNC group was still alive vs. only 20% of the control group (p = 0.000). - 33 patients healed (86.6%) in the PBMNC group. - One patient healed in the control group. | 2 years |
| Carstens et al. 2021 [67] | 63 patients with type 2 diabetes with chronic DFU—all amputation candidates - Treated with 30 × 106 SVF cells | Autologous adipose-derived stromal vascular fraction (SVF) | - Paravascular injection (pedal arteries) - Local perilesional injection | - At 6 months, 59 of the 63 subjects enrolled were evaluable for closure. - Fifty of the evaluable subjects achieved closure at 12 months (93%; confidence interval = 0.813–0.976). - The remaining four evaluable subjects had wound closure of ≥85%. | 6–12 months |
| Chiang et al. 2021 [59] | Meta-analysis: - Authors assessed randomized controlled trials (RCTs), and extracted data on complete healing rate, amputation rate, and outcomes regarding peripheral circulation. - Extracted data pooled using a random-effects model - A total of 28 RCTs were eligible | Autologous stem cell therapy (ASCT) | Intramuscular | - ASCT significantly improved complete wound healing rate as compared with standard treatment for lower extremity chronic wounds (LECWs). - ASCT could promote the healing of LECWs. | 1–5 months |
| Khalil et al. 2021 [106] | 10 patients with an open DFU wound 2 groups: - Group A: injected with PRF alone - Group B: injected with AMSC embedded in PRF | Autologous AMSCs | Topical | Group B had better healing index than group A. | 2–4 months |
| Uzun et al. 2021 [127] | 20 patients with DFU 2 groups: AMSCs, standard wound care | Allogeneic adipose-derived mesenchymal stem cells | Local intra -lesional injection | Treatment group had better and faster wound healing compared to control. | 48 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hage, R.; Knippschild, U.; Arnold, T.; Hinterseher, I. Stem Cell-Based Therapy: A Promising Treatment for Diabetic Foot Ulcer. Biomedicines 2022, 10, 1507. https://doi.org/10.3390/biomedicines10071507
El Hage R, Knippschild U, Arnold T, Hinterseher I. Stem Cell-Based Therapy: A Promising Treatment for Diabetic Foot Ulcer. Biomedicines. 2022; 10(7):1507. https://doi.org/10.3390/biomedicines10071507
Chicago/Turabian StyleEl Hage, Racha, Uwe Knippschild, Tobias Arnold, and Irene Hinterseher. 2022. "Stem Cell-Based Therapy: A Promising Treatment for Diabetic Foot Ulcer" Biomedicines 10, no. 7: 1507. https://doi.org/10.3390/biomedicines10071507
APA StyleEl Hage, R., Knippschild, U., Arnold, T., & Hinterseher, I. (2022). Stem Cell-Based Therapy: A Promising Treatment for Diabetic Foot Ulcer. Biomedicines, 10(7), 1507. https://doi.org/10.3390/biomedicines10071507







