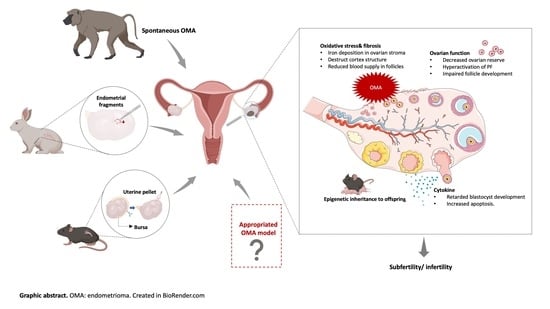
What We Have Learned from Animal Models to Understand the Etiology and Pathology of Endometrioma-Related Infertility
Abstract
1. Introduction
2. Methodology
3. Animal Models of Endometriosis-Associated Infertility and OMA-Related Infertility
3.1. Endometriosis Models and Infertility
3.1.1. Non-Human Primates
3.1.2. Rodents
3.1.3. Organoids
3.2. OMA Models for Infertility
3.2.1. The Current OMA Animal Models
3.2.2. The Urge to Establish a Proper OMA Animal Models
4. Pathology and Underlying Mechanisms of OMA-Related Infertility Based on OMA Models
4.1. Periovarian Adhesions
4.2. Ovarian Function
4.3. Oxidative Stress
5. Potential Therapeutics
5.1. Management of Periovarian Adhesions
5.2. Antioxidants
5.3. Restoration of Ovarian Function
6. Summary and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Exacoustos, C.; De Felice, G.; Pizzo, A.; Morosetti, G.; Lazzeri, L.; Centini, G.; Piccione, E.; Zupi, E. Isolated Ovarian Endometrioma: A History Between Myth and Reality. J. Minim. Invasive Gynecol. 2018, 25, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Fuldeore, M.; Yang, H.; Du, E.X.; Soliman, A.M.; Wu, E.Q.; Winkel, C. Healthcare utilization and costs in women diagnosed with endometriosis before and after diagnosis: A longitudinal analysis of claims databases. Fertil. Steril. 2015, 103, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.A.S.; Wright, K.N.; Lin, X.; Abbasi, F.; Haro, M.; Sun, J.; Hernandez, L.; Orr, N.L.; Hong, J.; Choi-Kuaea, Y.; et al. A cellular and molecular portrait of endometriosis subtypes. bioRxiv 2021, arXiv:2021.05.20.445037. [Google Scholar]
- Redwine, D.B. Age-related evolution in color appearance of endometriosis. Fertil. Steril. 1987, 48, 1062–1063. [Google Scholar] [CrossRef]
- Harirchian, P.; Gashaw, I.; Lipskind, S.T.; Braundmeier, A.G.; Hastings, J.M.; Olson, M.R.; Fazleabas, A.T. Lesion kinetics in a non-human primate model of endometriosis. Hum. Reprod. 2012, 27, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Holt, V.L.; Weiss, N.S. Recommendations for the Design of Epidemiologic Studies of Endometriosis. Epidemiology 2000, 11, 654–659. [Google Scholar] [CrossRef]
- Adamson, G.D.; Pasta, D.J. Endometriosis fertility index: The new, validated endometriosis staging system. Fertil. Steril. 2010, 94, 1609–1615. [Google Scholar] [CrossRef]
- Dai, Y.; Li, X.; Shi, J.; Leng, J. A review of the risk factors, genetics and treatment of endometriosis in Chinese women: A comparative update. Reprod. Health 2018, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Bulletti, C.; Coccia, M.E.; Battistoni, S.; Borini, A. Endometriosis and infertility. Fertil. Steril. 2006, 86 (Suppl. S5), S156–S160. [Google Scholar] [CrossRef]
- Kajiyama, H.; Suzuki, S.; Yoshihara, M.; Tamauchi, S.; Yoshikawa, N.; Niimi, K.; Shibata, K.; Kikkawa, F. Endometriosis and cancer. Free Radic. Biol. Med. 2019, 133, 186–192. [Google Scholar] [CrossRef]
- Filip, L.; Duică, F.; Prădatu, A.; Crețoiu, D.; Suciu, N.; Crețoiu, S.M.; Predescu, D.V.; Varlas, V.N.; Voinea, S.C. Endometriosis Associated Infertility: A Critical Review and Analysis on Etiopathogenesis and Therapeutic Approaches. Medicina 2020, 56, 460. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Barnhart, K. Managing endometriosis-associated infertility. Clin. Obstet. Gynecol. 2011, 54, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.G.; Fedorkow, D.M.; Collins, J.A. A quantitative overview of controlled trials in endometriosis-associated infertility. Fertil. Steril. 1993, 59, 963–970. [Google Scholar] [CrossRef]
- Naples, J.D.; Batt, R.E.; Sadigh, H. Spontaneous abortion rate in patients with endometriosis. Obstet. Gynecol. 1981, 57, 509–512. [Google Scholar]
- Groll, M. Endometriosis and spontaneous abortion. Fertil. Steril. 1984, 41, 933–935. [Google Scholar] [CrossRef]
- De Ziegler, D.; Borghese, B.; Chapron, C. Endometriosis and infertility: Pathophysiology and management. Lancet 2010, 376, 730–738. [Google Scholar] [CrossRef]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian aging: Mechanisms and clinical consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef]
- Hickey, M.; Ballard, K.; Farquhar, C. Endometriosis. BMJ 2014, 348, g1752. [Google Scholar] [CrossRef]
- Hung, S.W.; Zhang, R.; Tan, Z.; Chung, J.P.W.; Zhang, T.; Wang, C.C. Pharmaceuticals targeting signaling pathways of endometriosis as potential new medical treatment: A review. Med. Res. Rev. 2021, 41, 2489–2564. [Google Scholar] [CrossRef]
- Muzii, L.; Di Tucci, C.; Achilli, C.; Di Donato, V.; Musella, A.; Palaia, I.; Panici, P.B. Continuous versus cyclic oral contraceptives after laparoscopic excision of ovarian endometriomas: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2016, 214, 203–211. [Google Scholar] [CrossRef]
- Smith, M.R.; Lee, W.C.; Brandman, J.; Wang, Q.; Botteman, M.; Pashos, C.L. Gonadotropin-releasing hormone agonists and fracture risk: A claims-based cohort study of men with nonmetastatic prostate cancer. J. Clin. Oncol. 2005, 23, 7897–7903. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.; Brown, J.; Collins, J.J.; Farquhar, C.; Fedorkow, D.M.; Vandekerckhove, P. Ovulation suppression for endometriosis. Cochrane Database Syst. Rev. 2007, 2007, Cd000155. [Google Scholar]
- Ahmad, G.; Gent, D.; Henderson, D.; O’Flynn, H.; Phillips, K.; Watson, A. Laparoscopic entry techniques. Cochrane Database Syst. Rev. 2015, 8, Cd006583. [Google Scholar] [CrossRef] [PubMed]
- Muzii, L.; Di Tucci, C.; Di Feliciantonio, M.; Marchetti, C.; Perniola, G.; Panici, P.B. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 2190–2198. [Google Scholar] [CrossRef]
- Seyhan, A.; Ata, B.; Uncu, G. The Impact of Endometriosis and Its Treatment on Ovarian Reserve. Semin. Reprod. Med. 2015, 33, 422–428. [Google Scholar] [CrossRef]
- Goodman, L.R.; Goldberg, J.M.; Flyckt, R.L.; Gupta, M.; Harwalker, J.; Falcone, T. Effect of surgery on ovarian reserve in women with endometriomas, endometriosis and controls. Am. J. Obstet. Gynecol. 2016, 215, 589.e1–589.e6. [Google Scholar] [CrossRef]
- Mehdizadeh Kashi, A.; Chaichian, S.; Ariana, S.; Fazaeli, M.; Moradi, Y.; Rashidi, M.; Najmi, Z. The impact of laparoscopic cystectomy on ovarian reserve in patients with unilateral and bilateral endometrioma. Int. J. Gynaecol. Obstet. 2017, 136, 200–204. [Google Scholar] [CrossRef]
- Donnez, J.; Nisolle, M.; Gillet, N.; Smets, M.; Bassil, S.; Casanas-Roux, F. Large ovarian endometriomas. Hum. Reprod. 1996, 11, 641–646. [Google Scholar] [CrossRef]
- Rajab, T.K.; Ahmad, U.N.; Kelly, E. Implications of late complications from adhesions for preoperative informed consent. J. R. Soc. Med. 2010, 103, 317–321. [Google Scholar] [CrossRef]
- Hui, Y.; Zhao, S.; Gu, J.; Hang, C. Analysis of factors related to fertility after endometriosis combined with infertility laparoscopic surgery. Medicine 2020, 99, e20132. [Google Scholar] [CrossRef]
- Bourdon, M.; Raad, J.; Dahan, Y.; Marcellin, L.; Maignien, C.; Even, M.; Pocate-Cheriet, K.; Lamau, M.C.; Santulli, P.; Chapron, C. Endometriosis and ART: A prior history of surgery for OMA is associated with a poor ovarian response to hyperstimulation. PLoS ONE 2018, 13, e0202399. [Google Scholar]
- Coccia, M.E.; Rizzello, F.; Cammilli, F.; Bracco, G.L.; Scarselli, G. Endometriosis and infertility Surgery and ART: An integrated approach for successful management. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 138, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, M.; Dunselman, G.; Li, T.C.; Cheong, Y. The impact of endometrioma on IVF/ICSI outcomes: A systematic review and meta-analysis. Hum. Reprod. Update 2015, 21, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Dunselman, G.A.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [PubMed]
- Zaliska, O.; Stasiv, K.; Maksymovych, N.; Hrynkiv, Y. The trends of assisted reproductive technologies and cost for ovarian stimulation protocols in Ukraine. Pharmacia 2020, 67, 269–277. [Google Scholar] [CrossRef]
- Shreffler, K.M.; Johnson, D.R.; Scheuble, L.K. Ethical Problems with Infertility Treatments: Attitudes and Explanations. Soc. Sci. J. 2010, 47, 731–746. [Google Scholar] [CrossRef]
- Malvezzi, H.; Marengo, E.B.; Podgaec, S.; Piccinato, C.A. Endometriosis: Current challenges in modeling a multifactorial disease of unknown etiology. J. Transl. Med. 2020, 18, 311. [Google Scholar]
- Tanbo, T.; Fedorcsak, P. Endometriosis-associated infertility: Aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 2017, 96, 659–667. [Google Scholar]
- Burns, K.A.; Pearson, A.M.; Slack, J.L.; Por, E.D.; Scribner, A.N.; Eti, N.A.; Burney, R.O. Endometriosis in the Mouse: Challenges and Progress Toward a ’Best Fit’ Murine Model. Front. Physiol. 2021, 12, 806574. [Google Scholar]
- Sampson, J.A. Heterotopic or misplaced endometrial tissue. Am. J. Obstet. Gynecol. 1925, 10, 649–664. [Google Scholar] [CrossRef]
- Braundmeier, A.G.; Fazleabas, A.T. The non-human primate model of endometriosis: Research and implications for fecundity. Mol. Hum. Reprod. 2009, 15, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [PubMed]
- Zhang, X.-L.; Pang, W.; Hu, X.-T.; Li, J.-L.; Yao, Y.-G.; Zheng, Y.-T. Experimental primates and non-human primate (NHP) models of human diseases in China: Current status and progress. Dongwuxue Yanjiu 2014, 35, 447–464. [Google Scholar] [PubMed]
- D’Hooghe, T.M.; Kyama, C.M.; Chai, D.; Fassbender, A.; Vodolazkaia, A.; Bokor, A.; Mwenda, J.M. Nonhuman primate models for translational research in endometriosis. Reprod. Sci. 2009, 16, 152–161. [Google Scholar] [CrossRef]
- Ami, Y.; Suzaki, Y.; Goto, N. Endometriosis in cynomolgus monkeys retired from breeding. J. Vet. Med. Sci. 1993, 55, 7–11. [Google Scholar] [CrossRef]
- Nishimoto-Kakiuchi, A.; Netsu, S.; Okabayashi, S.; Taniguchi, K.; Tanimura, H.; Kato, A.; Suzuki, M.; Sankai, T.; Konno, R. Spontaneous endometriosis in cynomolgus monkeys as a clinically relevant experimental model. Hum. Reprod. 2018, 33, 1228–1236. [Google Scholar] [CrossRef]
- Simitsidellis, I.; Gibson, D.A.; Saunders, P.T.K. Animal models of endometriosis: Replicating the aetiology and symptoms of the human disorder. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 257–269. [Google Scholar] [CrossRef]
- Yamanaka, A.; Kimura, F.; Takebayashi, A.; Kita, N.; Takahashi, K.; Murakami, T. Primate model research for endometriosis. Tohoku J. Exp. Med. 2012, 226, 95–99. [Google Scholar] [CrossRef][Green Version]
- D’Hooghe, T.M.; Bambra, C.S.; Raeymaekers, B.M.; De Jonge, I.; Lauweryns, J.M.; Koninckx, P.R. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis). Am. J. Obstet. Gynecol. 1995, 173, 125–134. [Google Scholar] [CrossRef]
- Fazleabas, A.T.; Brudney, A.; Gurates, B.; Chai, D.; Bulun, S. A modified baboon model for endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 308–317. [Google Scholar]
- Grümmer, R. Animal models in endometriosis research. Hum. Reprod. Update 2006, 12, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Story, L.; Kennedy, S. Animal studies in endometriosis: A review. ILAR J. 2004, 45, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Sharpe-Timms, K.L. Using rats as a research model for the study of endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Tirado-González, I.; Barrientos, G.; Tariverdian, N.; Arck, P.C.; García, M.G.; Klapp, B.F.; Blois, S.M. Endometriosis research: Animal models for the study of a complex disease. J. Reprod. Immunol. 2010, 86, 141–147. [Google Scholar] [CrossRef]
- Dinulescu, D.M.; Ince, T.A.; Quade, B.J.; Shafer, S.A.; Crowley, D.; Jacks, T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat. Med. 2005, 11, 63–70. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Mokshagundam, S.; Herington, J.L.; Ding, T.; Osteen, K.G. Rodent Models of Experimental Endometriosis: Identifying Mechanisms of Disease and Therapeutic Targets. Curr. Womens Health Rev. 2018, 14, 173–188. [Google Scholar] [CrossRef]
- Martinez, J.; Bisbal, V.; Marin, N.; Cano, A.; Gómez, R. Noninvasive Monitoring of Lesion Size in a Heterologous Mouse Model of Endometriosis. J. Vis. Exp. 2019, 144, e58358. [Google Scholar] [CrossRef]
- Tejada, M.A.; Santos-Llamas, A.I.; Escriva, L.; Tarin, J.J.; Cano, A.; Fernández-Ramírez, M.J.; Nunez-Badinez, P.; De Leo, B.; Saunders, P.T.K.; Vidal, V.; et al. Identification of Altered Evoked and Non-Evoked Responses in a Heterologous Mouse Model of Endometriosis-Associated Pain. Biomedicines 2022, 10, 501. [Google Scholar] [CrossRef]
- Nowak, N.M.; Fischer, O.M.; Gust, T.C.; Fuhrmann, U.; Habenicht, U.F.; Schmidt, A. Intraperitoneal inflammation decreases endometriosis in a mouse model. Hum. Reprod. 2008, 23, 2466–2474. [Google Scholar] [CrossRef]
- Berkley, K.J.; Dmitrieva, N.; Curtis, K.S.; Papka, R.E. Innervation of ectopic endometrium in a rat model of endometriosis. Proc. Natl. Acad. Sci. USA 2004, 101, 11094–11098. [Google Scholar] [CrossRef]
- Schenken, R.S.; Asch, R.H. Surgical induction of endometriosis in the rabbit: Effects on fertility and concentrations of peritoneal fluid prostaglandins. Fertil. Steril. 1980, 34, 581–587. [Google Scholar] [CrossRef]
- Vernon, M.W.; Wilson, E.A. Studies on the surgical induction of endometriosis in the rat. Fertil. Steril. 1985, 44, 684–694. [Google Scholar] [CrossRef]
- Moon, C.E.; Bertero, M.C.; Curry, T.E.; London, S.N.; Muse, K.N.; Sharpe, K.L.; Vernon, M.W. The presence of luteinized unruptured follicle syndrome and altered folliculogenesis in rats with surgically induced endometriosis. Am. J. Obstet. Gynecol. 1993, 169, 676–682. [Google Scholar] [CrossRef]
- Pal, A.K.; Biswas, S.; Goswami, S.K.; Kabir, S.N. Effect of pelvic endometrial implants on overall reproductive functions of female rats. Biol. Reprod. 1999, 60, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Stilley, J.A.; Woods-Marshall, R.; Sutovsky, M.; Sutovsky, P.; Sharpe-Timms, K.L. Reduced fecundity in female rats with surgically induced endometriosis and in their daughters: A potential role for tissue inhibitors of metalloproteinase 1. Biol. Reprod. 2009, 80, 649–656. [Google Scholar] [PubMed]
- Huch, M.; Gehart, H.; van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.; Ellis, E.; van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Heidari-Khoei, H.; Esfandiari, F.; Hajari, M.A.; Ghorbaninejad, Z.; Piryaei, A.; Baharvand, H. Organoid technology in female reproductive biomedicine. Reprod. Biol. Endocrinol. 2020, 18, 64. [Google Scholar]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrova-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 2017, 19, 568–577. [Google Scholar]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017, 144, 1775–1786. [Google Scholar]
- Luddi, A.; Pavone, V.; Semplici, B.; Governini, L.; Criscuoli, M.; Paccagnini, E.; Gentile, M.; Morgante, G.; Leo, V.; Belmonte, G.; et al. Organoids of Human Endometrium: A Powerful In Vitro Model for the Endometrium-Embryo Cross-Talk at the Implantation Site. Cells 2020, 9, 1121. [Google Scholar] [CrossRef]
- Betjes, M.A.; Zheng, X.; Kok, R.N.U.; van Zon, J.S.; Tans, S.J. Cell Tracking for Organoids: Lessons From Developmental Biology. Front. Cell Dev. Biol. 2021, 9, 675013. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.D. Classic pages in obstetrics and gynecology. Aberrant portions of the müllerian duct found in an ovary: William Wood Russell Johns Hopkins Hospital Bulletin, vol. 10, pp. 8–10, 1899. Am. J. Obstet. Gynecol. 1979, 134, 225–226. [Google Scholar] [PubMed]
- Kaplan, C.R.; Eddy, C.A.; Olive, D.L.; Schenken, R.S. Effect of ovarian endometriosis on ovulation in rabbits. Am. J. Obstet. Gynecol. 1989, 160, 40–44. [Google Scholar] [CrossRef]
- Su, H.-W.; Yi, Y.-C.; Wei, T.-Y.; Chang, T.-C.; Cheng, C.-M. Detection of ovulation, a review of currently available methods. Bioeng. Transl. Med. 2017, 2, 238–246. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M. Clinical relevance of the baboon as a model for the study of endometriosis. Fertil. Steril. 1997, 68, 613–625. [Google Scholar] [CrossRef]
- D’Hooghe, T.M.; Bambra, C.S.; Cornillie, F.J.; Isahakia, M.; Koninckx, P.R. Prevalence and laparoscopic appearance of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus). Biol. Reprod. 1991, 45, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Dick, E.J., Jr.; Hubbard, G.B.; Martin, L.J.; Leland, M.M. Record review of baboons with histologically confirmed endometriosis in a large established colony. J. Med. Primatol. 2003, 32, 39–47. [Google Scholar] [CrossRef]
- D’Hooghe, T.M.; Bambra, C.S.; Isahakia, M.; Koninckx, P.R. Evolution of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) over a 12-month period. Fertil. Steril. 1992, 58, 409–412. [Google Scholar]
- D’Hooghe, T.M.; Bambra, C.S.; Raeymaekers, B.M.; Riday, A.M.; Suleman, M.A.; Koninckx, P.R. The cycle pregnancy rate is normal in baboons with stage I endometriosis but decreased in primates with stage II and stage III-IV disease. Fertil. Steril. 1996, 66, 809–813. [Google Scholar] [CrossRef]
- Onaciu, A.; Munteanu, R.; Munteanu, V.C.; Gulei, D.; Raduly, L.; Feder, R.-I.; Pirlog, R.; Atanasov, A.G.; Korban, S.S.; Irimie, A.; et al. Spontaneous and Induced Animal Models for Cancer Research. Diagnostics 2020, 10, 660. [Google Scholar]
- Francia, G.; Cruz-Munoz, W.; Man, S.; Xu, P.; Kerbel, R.S. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat. Rev. Cancer 2011, 11, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Nakamura, T.; Motooka, Y.; Ito, F.; Jiang, L.; Akatsuka, S.; Iwase, A.; Kajiyama, H.; Kikkawa, F.; Toyokuni, S. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice. Redox. Biol. 2020, 37, 101726. [Google Scholar] [CrossRef] [PubMed]
- Motohara, T.; Masuko, S.; Ishimoto, T.; Yae, T.; Onishi, N.; Muraguchi, T.; Hirao, A.; Matsuzaki, Y.; Tashiro, H.; Katabuchi, H.; et al. Transient depletion of p53 followed by transduction of c-Myc and K-Ras converts ovarian stem-like cells into tumor-initiating cells. Carcinogenesis 2011, 32, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Vigano, P.; Parazzini, F.; Stoppelli, S.; Giambattista, E.; Vercellini, P. Association between endometriosis and cancer: A comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol. Oncol. 2006, 101, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Grümmer, R. Translational animal models to study endometriosis-associated infertility. Semin. Reprod. Med. 2013, 31, 125–132. [Google Scholar] [CrossRef]
- Fauser, B.C.; Van Heusden, A.M. Manipulation of human ovarian function: Physiological concepts and clinical consequences. Endocr. Rev. 1997, 18, 71–106. [Google Scholar]
- Kawamara, K.; Kelsey, T.; Hiraike, O. Editorial: Ovarian Ageing: Pathophysiology and Recent Development of Maintaining Ovarian Reserve. Front. Endocrinol. 2020, 11, 591764. [Google Scholar] [CrossRef]
- Hsueh, A.J.; Kawamura, K.; Cheng, Y.; Fauser, B.C. Intraovarian control of early folliculogenesis. Endocr. Rev. 2015, 36, 1–24. [Google Scholar]
- Titus, S.; Li, F.; Stobezki, R.; Akula, K.; Unsal, E.; Jeong, K.; Dickler, M.; Robson, M.; Moy, F.; Goswami, S.; et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci. Transl. Med. 2013, 5, 172ra21. [Google Scholar] [CrossRef]
- Kitajima, M.; Defrère, S.; Dolmans, M.M.; Colette, S.; Squifflet, J.; Van Langendonckt, A.; Donnez, J. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil. Steril. 2011, 96, 685–691. [Google Scholar] [CrossRef]
- Mutlu, M.F.; Erdem, A. Evaluation of ovarian reserve in infertile patients. J. Turk. Ger. Gynecol. Assoc. 2012, 13, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Infantino, M.; Benedetti, F.; Arnoldi, M.; Calanna, G.; Ragni, G. The presence of ovarian endometriomas is associated with a reduced responsiveness to gonadotropins. Fertil. Steril. 2006, 86, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.A.; de Jong, F.H.; Laven, J.S.; Themmen, A.P. Anti-Müllerian hormone: A new marker for ovarian function. Reproduction 2006, 131, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Weenen, C.; Laven, J.S.; Von Bergh, A.R.; Cranfield, M.; Groome, N.P.; Visser, J.A.; Kramer, P.; Fauser, B.C.; Themmen, A.P. Anti-Müllerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol. Hum. Reprod. 2004, 10, 77–83. [Google Scholar] [CrossRef]
- Pacchiarotti, A.; Frati, P.; Milazzo, G.N.; Catalano, A.; Gentile, V.; Moscarini, M. Evaluation of serum anti-Mullerian hormone levels to assess the ovarian reserve in women with severe endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 172, 62–64. [Google Scholar] [CrossRef]
- Visser, J.A.; Themmen, A.P. Anti-Müllerian hormone and folliculogenesis. Mol. Cell. Endocrinol. 2005, 234, 81–86. [Google Scholar] [CrossRef]
- Adriaens, I.; Cortvrindt, R.; Smitz, J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum. Reprod. 2004, 19, 398–408. [Google Scholar] [CrossRef]
- Casarini, L.; Crépieux, P. Molecular Mechanisms of Action of FSH. Front. Endocrinol. 2019, 10, 305. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Y.; Zhang, C.; Wang, Y.; Zhu, H.-L. Antral follicle count is reduced in the presence of endometriosis: A systematic review and meta-analysis. Reprod. BioMed. Online 2021, 42, 237–247. [Google Scholar] [CrossRef]
- Kitajima, M.; Dolmans, M.M.; Donnez, O.; Masuzaki, H.; Soares, M.; Donnez, J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014, 101, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Koga, K.; Satake, E.; Makabe, T.; Taguchi, A.; Miyashita, M.; Takamura, M.; Harada, M.; Hirata, T.; Hirota, Y.; et al. Endometriosis Triggers Excessive Activation of Primordial Follicles via PI3K-PTEN-Akt-Foxo3 Pathway. J. Clin. Endocrinol. Metab. 2019, 104, 5547–5554. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Wang, Z.B.; Feng, H.L.; Miao, Y.L.; Wang, Q.; Yu, Y.; Wei, Y.C.; Yan, J.; Wang, W.H.; Shen, W.; et al. The root of reduced fertility in aged women and possible therapentic options: Current status and future perspects. Mol. Asp. Med. 2014, 38, 54–85. [Google Scholar] [CrossRef] [PubMed]
- Da Broi, M.G.; Jordão, A.A., Jr.; Ferriani, R.A.; Navarro, P.A. Oocyte oxidative DNA damage may be involved in minimal/mild endometriosis-related infertility. Mol. Reprod. Dev. 2018, 85, 128–136. [Google Scholar] [CrossRef]
- Lin, X.; Dai, Y.; Tong, X.; Xu, W.; Huang, Q.; Jin, X.; Li, C.; Zhou, F.; Zhou, H.; Lin, X.; et al. Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility. Redox. Biol. 2020, 30, 101431. [Google Scholar] [CrossRef]
- Notarstefano, V.; Gioacchini, G.; Byrne, H.; Zacà, C.; Sereni, E.; Vaccari, L.; Borini, A.; Carnevali, O.; Giorgini, E. Vibrational characterization of granulosa cells from patients affected by unilateral ovarian endometriosis: New insights from infrared and Raman microspectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 212, 206–214. [Google Scholar] [CrossRef]
- Jiang, D.; Nie, X. Effect of endometrioma and its surgical excision on fertility (Review). Exp. Ther. Med. 2020, 20, 114. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Lian, X.; Li, F.; Wang, C.; Zhu, F.; Qiu, Y.; Chen, Y. Therapeutic target database update 2022: Facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res. 2022, 50, D1398–D1407. [Google Scholar] [CrossRef]
- Al-Jabri, S.; Tulandi, T. Management and prevention of pelvic adhesions. Semin. Reprod. Med. 2011, 29, 130–137. [Google Scholar] [CrossRef]
- Henry-Suchet, J.; Tesquier, L. Role of laparoscopy in the management of pelvic adhesions and pelvic sepsis. Baillieres Clin. Obstet. Gynaecol. 1994, 8, 759–772. [Google Scholar] [CrossRef]
- Matés, J.M.; Pérez-Gómez, C.; Núñez de Castro, I. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Alkadi, H. A Review on Free Radicals and Antioxidants. Infect. Disord. Drug Targets 2020, 20, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, H.; Wang, Z.; Zhang, C.; Bian, Y.; Liu, X.; Zhang, C.; Zhang, X.; Zhao, Y. Quercetin promotes in vitro maturation of oocytes from humans and aged mice. Cell Death Dis. 2020, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Xuan, M.F.; Luo, Z.B.; Wang, J.X.; Jin, S.S.; Yin, X.J.; Kang, J.D. Baicalin improves IVM of pig oocytes and subsequent preimplantation embryo development by inhibiting apoptosis. Reprod. Fertil. Dev. 2019, 31, 983–992. [Google Scholar] [CrossRef]
- Wang, H.; Jo, Y.J.; Oh, J.S.; Kim, N.H. Quercetin delays postovulatory aging of mouse oocytes by regulating SIRT expression and MPF activity. Oncotarget 2017, 8, 38631–38641. [Google Scholar] [CrossRef]
- Wallace, D.C.; Fan, W.; Procaccio, V. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 2010, 5, 297–348. [Google Scholar] [CrossRef]
- Fissore, R.A.; Kurokawa, M.; Knott, J.; Zhang, M.; Smyth, J. Mechanisms underlying oocyte activation and postovulatory ageing. Reproduction 2002, 124, 745–754. [Google Scholar] [CrossRef]
- Guo, Q.; Xuan, M.F.; Luo, Z.B.; Wang, J.X.; Han, S.Z.; Ri, M.H.; Choe, Y.G.; Hwang, K.M.; Yin, X.J.; Kang, J.D. Baicalin improves the in vitro developmental capacity of pig embryos by inhibiting apoptosis, regulating mitochondrial activity and activating sonic hedgehog signaling. Mol. Hum. Reprod. 2019, 25, 538–549. [Google Scholar] [CrossRef]
- Giorgi, V.S.I.; Ferriani, R.A.; Navarro, P.A. Follicular Fluid from Infertile Women with Mild Endometriosis Impairs In Vitro Bovine Embryo Development: Potential Role of Oxidative Stress. Rev. Bras. Ginecol. Obstet. 2021, 43, 119–125. [Google Scholar] [CrossRef]
- Song, C.; Peng, W.; Yin, S.; Zhao, J.; Fu, B.; Zhang, J.; Mao, T.; Wu, H.; Zhang, Y. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci. Rep. 2016, 6, 35165. [Google Scholar] [CrossRef]
- Cayci, T.; Akgul, E.O.; Kurt, Y.G.; Ceyhan, T.S.; Aydin, I.; Onguru, O.; Yaman, H.; Cakir, E.; Yasar, M.; Bilgi, C.; et al. The levels of nitric oxide and asymmetric dimethylarginine in the rat endometriosis model. J. Obstet. Gynaecol. Res. 2011, 37, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Song, H.; Shi, G. Anti-TNF-alpha treatment for pelvic pain associated with endometriosis. Cochrane Database Syst. Rev. 2010, 3, Cd008088. [Google Scholar]
- Kwak-Kim, J.Y.; Chung-Bang, H.S.; Ng, S.C.; Ntrivalas, E.I.; Mangubat, C.P.; Beaman, K.D.; Beer, A.E.; Gilman-Sachs, A. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum. Reprod. 2003, 18, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, Q.; Chen, Y.; Wang, X.; Ran, Z.; Fang, F.; Xiong, J.; Liu, G.; Li, X.; Yang, L.; et al. Melatonin delays ovarian aging in mice by slowing down the exhaustion of ovarian reserve. Commun. Biol. 2021, 4, 534. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.Y.; Derbyshire, E.R. Tafenoquine: A Step toward Malaria Elimination. Biochemistry 2020, 59, 911–920. [Google Scholar] [CrossRef]
- Frampton, J.E. Tafenoquine: First Global Approval. Drugs 2018, 78, 1517–1523. [Google Scholar] [CrossRef]
- Golstein, P.; Kroemer, G. Cell death by necrosis: Towards a molecular definition. Trends Biochem. Sci. 2007, 32, 37–43. [Google Scholar] [CrossRef]
- Smirlis, D.; Duszenko, M.; Ruiz, A.J.; Scoulica, E.; Bastien, P.; Fasel, N.; Soteriadou, K. Targeting essential pathways in trypanosomatids gives insights into protozoan mechanisms of cell death. Parasit. Vectors 2010, 3, 107. [Google Scholar] [CrossRef]
- Santo, E.E.; Stroeken, P.; Sluis, P.V.; Koster, J.; Versteeg, R.; Westerhout, E.M. FOXO3a is a major target of inactivation by PI3K/AKT signaling in aggressive neuroblastoma. Cancer Res. 2013, 73, 2189–2198. [Google Scholar] [CrossRef]
- ArQule Inc. Vevorisertib (ARQ 751) (4440-001) as a Single Agent or in Combination with Other Anti-Cancer Agents, in Solid Tumors With PIK3CA/AKT/PTEN Mutations; ArQule Inc.: Burlington, MA, USA, 2022. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Hughes, B. 2009 FDA drug approvals. Nat. Rev. Drug Discov. 2010, 9, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Elixir Medical Corporation. Post Marketing Study of the Elixir DeSyne® Novolimus Eluting Coronary Stent System and the Desyne® BD Novolimus Eluting Coronary Stent System in the Treatment of Patients with de Novo Native Coronary Artery Lesions; Elixir Medical Corporation: Milpitas, CA, USA, 2021. [Google Scholar]
- Mullard, A. 2018 FDA Drug Approvals. Nat. Rev. Drug Discov. 2019, 18, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Rask-Andersen, M.; Zhang, J.; Fabbro, D.; Schiöth, H.B. Advances in kinase targeting: Current clinical use and clinical trials. Trends Pharmacol. Sci. 2014, 35, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Southan, C.; Sharman, J.L.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Alexander, S.P.; Buneman, O.P.; Davenport, A.P.; McGrath, J.C.; Peters, J.A.; et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: Towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res. 2016, 44, D1054–D1068. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf (accessed on 2 May 2022).
- CardioVascular Research Foundation. Triple Versus Dual Antiplatelet Therapy after ABT578-Eluting Stent (DECLARELONG). Available online: https://www.clinicaltrials.gov/ct2/show/NCT00589927 (accessed on 31 May 2022).
- Moridi, I.; Chen, A.; Tal, O.; Tal, R. The Association between Vitamin D and Anti-Müllerian Hormone: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1567. [Google Scholar] [CrossRef]
- Messinis, I.E.; Messini, C.I.; Dafopoulos, K. Novel aspects of the endocrinology of the menstrual cycle. Reprod. Biomed. Online 2014, 28, 714–722. [Google Scholar] [CrossRef]
| Type | Species/Sources | Year | Method | Fertility Parameters | Limitations | Ref | |
|---|---|---|---|---|---|---|---|
| In vivo | NHP | 2003 | Baboon | Spontaneously | Total pregnancies | 1. Low successful rate of spontaneous model; 2. Long period for disease manifestation; 3. Maintenance issues; 4. Ethical concerns; 5. High cost | [46,77] |
| Rodent | 1989 | Rabbit | Place endometrial tissue in ovaries after incision | Ovulation points | 1. Do not develop endometriosis spontaneously 2. Autologous transplantation limited 3. Long generation time; 4. Long estrus cycle 5. Not available for gene modification | [61,73] | |
| 2020 | Mouse | Uterine tissue pellet was placed to ovaries after removing ovarian bursa | FSHR, pup numbers | 1. Species differences between murine and human; 2. Successful rate of OMA model cannot reach 100%. 3. Lesions was not exclusively located in ovaries. | [82] |
| Targets | Drug Name 1 | Approved | Disease |
|---|---|---|---|
| FSHR | Follitropin beta | Y | Female infertility |
| FSHR | Menotropins | Y | Female infertility |
| FSHR | Urofollitropin | Y | Female infertility |
| ROS | Tafenoquine | Y | Plasmodium vivax malaria |
| Electron transport complex III (Complex III) | Tafenoquine | Y | Plasmodium vivax malaria |
| PI3K/AKT/mTOR pathway | Sirolimus | Y | Organ transplant rejection |
| Serine/threonine-protein kinase mTOR (mTOR) | Everolimus | Y | Renal cell carcinoma |
| Serine/threonine-protein kinase mTOR (mTOR) | Novolimus | Y | Artery stenosis |
| Serine/threonine-protein kinase mTOR (mTOR) | PF-04449913 | Y | Chronic myelomonocytic leukaemia |
| Serine/threonine-protein kinase mTOR (mTOR) | Sirolimus | Y | Organ transplant rejection |
| Serine/threonine-protein kinase mTOR (mTOR) | Temsirolimus | Y | Renal cell carcinoma |
| Serine/threonine-protein kinase mTOR (mTOR) | Zotarolimus | Y | Solid tumour/cancer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Hung, S.-W.; Zheng, X.; Wang, C.-C.; Chung, J.P.-W.; Zhang, T. What We Have Learned from Animal Models to Understand the Etiology and Pathology of Endometrioma-Related Infertility. Biomedicines 2022, 10, 1483. https://doi.org/10.3390/biomedicines10071483
Tan Z, Hung S-W, Zheng X, Wang C-C, Chung JP-W, Zhang T. What We Have Learned from Animal Models to Understand the Etiology and Pathology of Endometrioma-Related Infertility. Biomedicines. 2022; 10(7):1483. https://doi.org/10.3390/biomedicines10071483
Chicago/Turabian StyleTan, Zhouyurong, Sze-Wan Hung, Xu Zheng, Chi-Chiu Wang, Jacqueline Pui-Wah Chung, and Tao Zhang. 2022. "What We Have Learned from Animal Models to Understand the Etiology and Pathology of Endometrioma-Related Infertility" Biomedicines 10, no. 7: 1483. https://doi.org/10.3390/biomedicines10071483
APA StyleTan, Z., Hung, S.-W., Zheng, X., Wang, C.-C., Chung, J. P.-W., & Zhang, T. (2022). What We Have Learned from Animal Models to Understand the Etiology and Pathology of Endometrioma-Related Infertility. Biomedicines, 10(7), 1483. https://doi.org/10.3390/biomedicines10071483