Risk of Malignancy in Patients with Asthma-COPD Overlap Compared to Patients with COPD without Asthma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- The Danish Register of Chronic Obstructive Pulmonary Disease (DrCOPD), which contains nationwide information on COPD outpatients since 2008 [27].
- The Danish Civil Registration System with unique personal identification of all Danish citizens [28].
- The Danish National Patient Registry holds information on all admissions to Danish hospitals and outpatient clinic visits [29].
- The Danish National Health Service Prescription Database holds information on all prescriptions dispensed in Danish pharmacies since 2004 including date of dispensation, formulation, strength, and quantity [30].
2.2. Statistical Analysis
2.3. Ethics
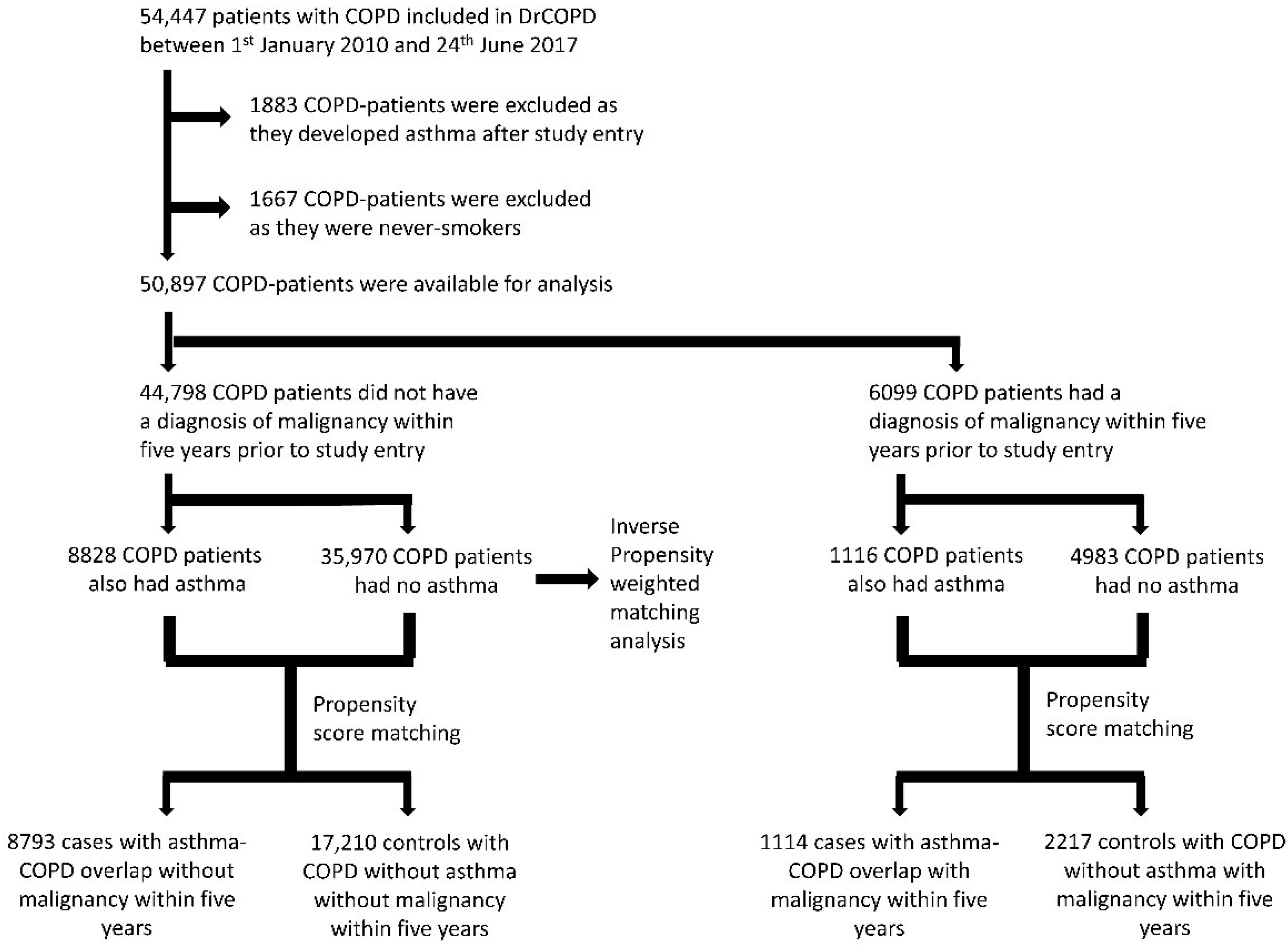
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- González-Pérez, A.; Fernández-Vidaurre, C.; Rueda, A.; Rivero, E.; Rodríguez, L.A.G. Cancer incidence in a general population of asthma patients. Pharmacoepidemiol. Drug Saf. 2006, 15, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R. The problem of tobacco smoking. BMJ 2004, 328, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.; Edwards, R. Tobacco smoking, harm reduction, and nicotine product regulation. Lancet 2008, 371, 441–445. [Google Scholar] [CrossRef]
- Neville, A.J.; Kuruvilla, M.S. Lung cancer. BMJ Clin. Evid. 2010, 2010, 1504. [Google Scholar]
- Takemura, M.; Matsumoto, H.; Niimi, A.; Ueda, T.; Matsuoka, H.; Yamaguchi, M.; Jinnai, M.; Muro, S.; Hirai, T.; Ito, Y.; et al. High sensitivity C-reactive protein in asthma. Eur. Respir. J. 2006, 27, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.D.; Knoebel, E.; Fenta, Y.; Gabriel, S.E.; Leibson, C.L.; Loftus, E.V.; Roger, V.; Yawn, B.P.; Li, B.; Juhn, Y.J. Asthma and Proinflammatory Conditions: A Population-Based Retrospective Matched Cohort Study. Mayo Clin. Proc. 2012, 87, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Nakachi, K.; Hayashi, T.; Imai, K.; Kusunoki, Y. Perspectives on cancer immuno-epidemiology. Cancer Sci. 2004, 95, 921–929. [Google Scholar] [CrossRef]
- Woodall, M.J.; Neumann, S.; Campbell, K.; Pattison, S.T.; Young, S.L. The Effects of Obesity on Anti-Cancer Immunity and Cancer Immunotherapy. Cancers 2020, 12, 1230. [Google Scholar] [CrossRef]
- Connelly, M.A.; Gruppen, E.G.; Otvos, J.D.; Dullaart, R.P. Inflammatory glycoproteins in cardiometabolic disorders, autoimmune diseases and cancer. Clin. Chim. Acta 2016, 459, 177–186. [Google Scholar] [CrossRef]
- Divella, R.; Mazzocca, A.; Daniele, A.; Sabbà, C.; Paradiso, A. Obesity, Nonalcoholic Fatty Liver Disease and Adipocytokines Network in Promotion of Cancer. Int. J. Biol. Sci. 2019, 15, 610–616. [Google Scholar] [CrossRef]
- Wong, S.Y.-S.; Woo, J.; Lynn, H.S.H.; Leung, J.; Tang, Y.N.; Leung, P.C. Risk of depression in patients with chronic respiratory diseases: Results from two large cohort studies in Chinese elderly from Hong Kong. Int. J. Geriatr. Psychiatry 2006, 21, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Ogino, K.; Obase, Y.; Ito, T.; Fujimura, M.; Eguchi, E.; Kubo, M.; Nagaoka, K.; Nakamura, H. Relationship between serum arginase I and l-arginine or exhaled nitric oxide in asthma. Free Radic. Res. 2016, 50, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lu, X. Inhaled Glucocorticoid with or without Tiotropium Bromide for Asthma-Chronic Obstructive Pulmonary Disease Overlap Syndrome. J. Coll. Physicians Surg. Pak. 2019, 29, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Juvonen, R.; Bloigu, A.; Peitso, A.; Silvennoinen-Kassinen, S.; Saikku, P.; Leinonen, M.; Hassi, J.; Harju, T. Training Improves Physical Fitness and Decreases CRP Also in Asthmatic Conscripts. J. Asthma 2008, 45, 237–242. [Google Scholar] [CrossRef]
- Montarello, N.J.; Nguyen, M.T.; Wong, D.T.; Nicholls, S.J.; Psaltis, P.J. Inflammation in Coronary Atherosclerosis and Its Therapeutic Implications. Cardiovasc. Drugs Ther. 2020, 36, 347–362. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Wu, P.-H.; Sheu, C.-C.; Hsu, Y.-L.; Chang, W.A.; Hung, J.-Y.; Yang, C.-J.; Yang, Y.-H.; Kuo, P.-L.; Huang, M.-S. Cysteinyl Leukotriene Receptor Antagonists Decrease Cancer Risk in Asthma Patients. Sci. Rep. 2016, 6, 23979. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFayden, J.G.; Thurner, T.; Everett, B.M.; Libby, P.; Glynn, R.J.; CANTOS Trial Group. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: Exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1833–1842. [Google Scholar] [CrossRef]
- El-Zein, M.; Parent, M.-E.; Kâ, K.; Siemiatycki, J.; St-Pierre, Y.; Rousseau, M.-C. History of asthma or eczema and cancer risk among men: A population-based case-control study in Montreal, Quebec, Canada. Ann. Allergy Asthma Immunol. 2010, 104, 378–384. [Google Scholar] [CrossRef]
- Turner, M.C.; Chen, Y.; Krewski, D.; Ghadirian, P.; Thun, M.J.; Calle, E.E. Cancer Mortality among US Men and Women with Asthma and Hay Fever. Am. J. Epidemiol. 2005, 162, 212–221. [Google Scholar] [CrossRef]
- Kantor, E.D.; Hsu, M.; Du, M.; Signorello, L.B. Allergies and Asthma in Relation to Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1395–1403. [Google Scholar] [CrossRef]
- Hwang, C.-Y.; Chen, Y.-J.; Lin, M.-W.; Chen, T.-J.; Chu, S.-Y.; Chen, C.-C.; Lee, D.-D.; Chang, Y.-T.; Wang, W.-J.; Liu, H.-N. Cancer risk in patients with allergic rhinitis, asthma and atopic dermatitis: A nationwide cohort study in Taiwan. Int. J. Cancer 2011, 130, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Shu, X.; Li, X.; Sundquist, K.; Hemminki, K.; Sundquist, J. Cancer risk in hospitalised asthma patients. Br. J. Cancer 2009, 100, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.; Lee, S.W.; Koh, H.Y.; Kim, M.A.; Han, M.Y.; Yon, D.K. Incidence of cancer after asthma development: 2 independent population-based cohort studies. J. Allergy Clin. Immunol. 2021, 147, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sandelin, M.; Mindus, S.; Thuresson, M.; Lisspers, K.; Ställberg, B.; Johansson, G.; Larsson, K.; Janson, C. Factors associated with lung cancer in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1833–1839. [Google Scholar] [CrossRef]
- Senthilselvan, A.; Beach, J. Characteristics of asthma and COPD overlap syndrome (ACOS) in the Canadian population. J. Asthma 2018, 56, 1129–1137. [Google Scholar] [CrossRef]
- Lange, P.; Tøttenborg, S.S.; Sorknæs, A.D.; Andersen, J.S.; Søgaard, M.; Nielsen, H.; Thomsen, R.; Nielsen, K.A. Danish Register of chronic obstructive pulmonary disease. Clin. Epidemiol. 2016, 8, 673–678. [Google Scholar] [CrossRef]
- Schmidt, M.; Pedersen, L.; Sørensen, H.T. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 2014, 29, 541–549. [Google Scholar] [CrossRef]
- Schmidt, M.; Schmidt, S.A.J.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sørensen, H.T. The Danish National Patient Registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef]
- Ehrenstein, V.; Antonsen, S.; Pedersen, L. Existing data sources for clinical epidemiology: Aarhus University Prescription Database. Clin. Epidemiol. 2010, 2, 273–279. [Google Scholar] [CrossRef][Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Department of Quantitative Health Sciences Mayo Clinic. Available online: http://bioinformaticstools.mayo.edu/research/gmatch/.2022 (accessed on 21 January 2022).
- McCaffrey, D.F.; Griffin, B.A.; Almirall, D.; Slaughter, M.E.; Ramchand, R.; Burgette, L. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat. Med. 2013, 32, 3388–3414. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J. NewSurv Macro. 2021. Available online: https://communities.sas.com/t5/SAS-Communities-Library/Kaplan-Meier-Survival-Plotting-Macro-NEWSURV/ta-p/4797471 (accessed on 21 January 2021).
- Tourangeau, R.; Yan, T.; Sun, H.; Hyland, A.; Stanton, C.A. Population Assessment of Tobacco and Health (PATH) reliability and validity study: Selected reliability and validity estimates. Tob. Control 2018, 28, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, K.; Takaku, Y.; Ohta, C.; Takayanagi, N.; Yanagisawa, T.; Kanauchi, T.; Takahashi, O. Smoking history and emphysema in asthma–COPD overlap. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 3523–3532. [Google Scholar] [CrossRef]
| Characteristic | No Previous Cancer * | Previous Cancer * | ||
|---|---|---|---|---|
| Patients with Asthma-COPD Overlap (N = 8793) | Patients with COPD without Asthma (N = 17,210) | Patients with Asthma-COPD Overlap (N = 1114) | Patients with COPD without Asthma (N = 2217) | |
| Age, years | 67.7 (58.7–76.0) | 68.0 (59.9–75.6) | 71.9 (65.4–77.9) | 71.9 (65.5–78.3) |
| Gender, female | 5074 (57.7) | 9746 (56.6) | 650 (58.3) | 1287 (58.1) |
| Tobacco exposure: | ||||
| Passive smoking | 1 (0.0) | 0 (0.0) | 1 (0.1) | 0 (0.0) |
| Previous smoker | 5157 (58.6) | 9745 (56.6) | 678 (60.9) | 1301 (58.9) |
| Active smoker | 2881 (32.8) | 6186 (35.9) | 308 (27.6) | 682 (30.8) |
| Unknown tobacco exposure | 754 (8.6) | 1279 (7.4) | 127 (11.4) | 234 (10.6) |
| MRC | 3 (2–4) | 3 (2–4) | 3 (3–4) | 3 (3–4) |
| BMI | 25 (22–29) | 25 (22–29) | 25 (22–28) | 25 (22–28) |
| FEV1 in percentage of expected | 49 (36–61) | 49 (36–61) | 49 (36–59) | 49 (36–59) |
| Malignancy within five years: | 1114 (100.0) | 2217 (100.0) | ||
| Oropharyngeal cancer | 82 (7.4) | 135 (6.1) | ||
| Intestinal cancer | 139 (12.5) | 256 (11.5) | ||
| Pancreas cancer | 9 (0.8) | 9 (0.4) | ||
| Other digestive organ cancer | 41 (3.7) | 53 (2.4) | ||
| Lung cancer | 221 (19.8) | 537 (24.2) | ||
| Other respiratory and intrathoracic cancers | 50 (4.5) | 82 (3.7) | ||
| Malignant melanomas | 29 (2.6) | 49 (2.2) | ||
| Mesothelial, soft tissue, bone, cartilage, nervous system, eye and brain cancers | 29 (2.6) | 42 (1.9) | ||
| Malignant neoplasms of the breast | 190 (17.1) | 422 (19.0) | ||
| Female genital organ cancers | 54 (4.8) | 81 (3.7) | ||
| Male genital organ cancers | 148 (13.3) | 298 (13.4) | ||
| Kidney and urinary tract cancers | 84 (7.5) | 169 (7.6) | ||
| Endo- and neuroendocrine cancers | 7 (0.6) | 18 (0.8) | ||
| Other cancers (non-defined) | 122 (11.0) | 279 (12.6) | ||
| Malignant lymphomas | 45 (4.0) | 123 (5.5) | ||
| Malignant leukemia | 63 (5.7) | 130 (5.9) | ||
| Concomitant cancers | 199 (17.9) | 447 (20.2) | ||
| Comorbidities: | ||||
| Hypertension | 2747 (31.2) | 5105 (29.7) | 452 (40.6) | 838 (37.8) |
| Hypercholesterolemia | 1075 (12.2) | 2152 (12.5) | 201 (18.0) | 335 (15.1) |
| Atrial fibrillation | 1155 (13.1) | 2294 (13.3) | 210 (18.9) | 415 (18.7) |
| Diabetes | 1135 (12.9) | 1990 (11.6) | 165 (14.8) | 287 (12.9) |
| Osteoporosis or osteopenia | 1956 (22.2) | 3104 (18.0) | 341 (30.6) | 568 (25.6) |
| Renal insufficiency | 3086 (35.1) | 5710 (33.2) | 500 (44.9) | 908 (41.0) |
| Liver insufficiency | 264 (3.0) | 528 (3.1) | 41 (3.7) | 85 (3.8) |
| Atopy or allergy | 1338 (15.2) | 552 (3.2) | 156 (14.0) | 81 (3.7) |
| Depression | 512 (5.8) | 760 (4.4) | 78 (7.0) | 114 (5.1) |
| Exacerbations requiring admission within the last year prior to inclusion | 3017 (34.3) | 5005 (29.1) | 428 (38.4) | 689 (31.1) |
| Medical treatment for respiratory disease within the last year prior to inclusion: | ||||
| Oral corticosteroid | 4932 (56.1) | 6958 (40.4) | 670 (60.1) | 970 (43.8) |
| Inhaled corticosteroid (ICS) | 7744 (88.1) | 11,647 (67.8) | 994 (89.2) | 1508 (68.0) |
| Long acting β2-agonist (LABA) | 7679 (87.3) | 12,743 (74.0) | 1005 (90.2) | 1690 (76.2) |
| Long acting muscarinic receptor antagonist (LAMA) | 6246 (71.0) | 11,954 (69.5) | 861 (77.3) | 1595 (71.9) |
| Short acting β2-agonist (SABA) | 6774 (7.0) | 10,886 (63.3) | 892 (80.1) | 1389 (62.7) |
| Short acting muscarinic receptor antagonist (SAMA) | 371 (4.2) | 515 (3.0) | 68 (6.1) | 100 (4.5) |
| Endpoint | Patients without a Diagnosis of Malignancy within 5 Years Prior to Inclusion | Patients with a Diagnosis of Malignancy within 5 Years Prior to Inclusion | ||
|---|---|---|---|---|
| Patients with Asthma-COPD Overlap (N = 8793) | Patients with COPD without Asthma (N = 17,210) | Patients with Asthma-COPD Overlap (N = 1114) | Patients with COPD without Asthma (N = 2217) | |
| Cancer events | ||||
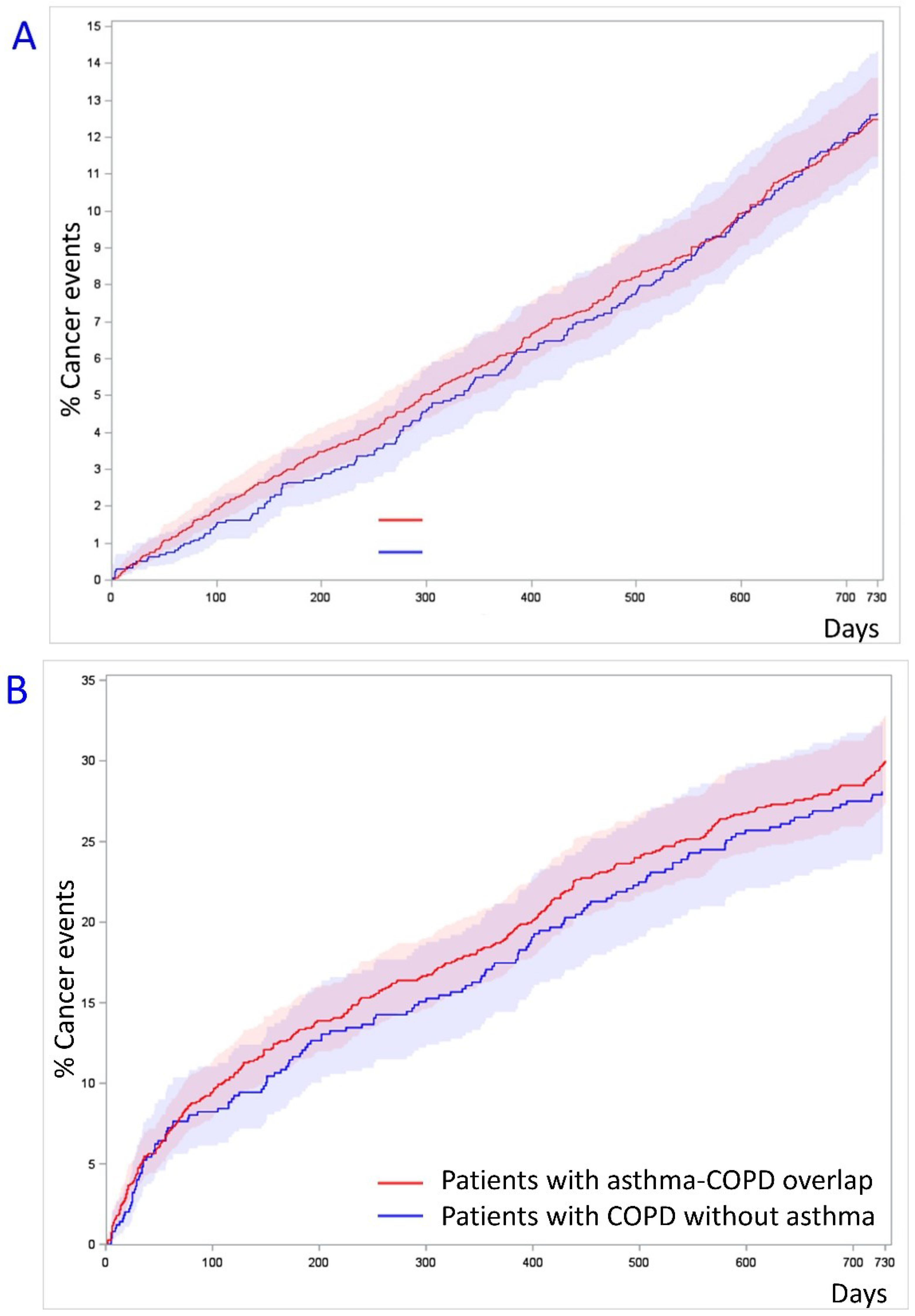
| N (%) | 219 (2.5) | 457 (2.7) | 140 (12.6) | 335 (15.1) |
| # HR | 0.92 (0.78–1.08) | Reference | 1.04 (0.85–1.26) | Reference |
| Lung cancer events | ||||
| N (%) | 52 (0.6) | 129 (0.7) | 21 (1.9) | 77 (3.5) |
| # HR | 0.77 (0.56–1.07) | Reference | 0.68 (0.42–1.10) | Reference |
| Cancer events: | ||||
| Oropharyngeal cancer | 4 (0.0) | 13 (0.1) | 1 (0.1) | 5 (0.2) |
| Intestinal cancer | 35 (0.4) | 63 (0.4) | 10 (0.9) | 19 (0.9) |
| Pancreas cancer | 3 (0.0) | 2 (0.0) | 0 (0.0) | 2 (0.1) |
| Other digestive organ cancers | 9 (0.1) | 29 (0.2) | 2 (0.2) | 9 (0.4) |
| Other respiratory and intrathoracic cancers | 2 (0.0) | 12 (0.1) | 1 (0.1) | 2 (0.1) |
| Mesothelial, soft tissue, bone, cartilage, nervous system, eye, and brain cancers | 1 (0.0) | 7 (0.0) | 3 (0.3) | 4 (0.2) |
| Malignant melanomas | 6 (0.1) | 5 (0.0) | 1 (0.1) | 5 (0.2) |
| Malignant neoplasms of the breast | 40 (0.5) | 72 (0.4) | 28 (2.5) | 47 (2.1) |
| Female genital organ cancers | 4 (0.0) | 6 (0.0) | 2 (0.2) | 2 (0.1) |
| Male genital organ cancers | 21 (0.2) | 52 (0.3) | 26 (2.3) | 59 (2.7) |
| Urinary tract cancers | 21 (0.2) | 24 (0.1) | 12 (1.1) | 21 (0.9) |
| Endo- and neuroendocrine cancers | 3 (0.0) | 3 (0.0) | 2 (0.2) | 2 (0.1) |
| Other cancers (non-defined) | 6 (0.1) | 5 (0.0) | 11 (1.0) | 24 (1.1) |
| Malignant lymphomas | 6 (0.1) | 17 (0.1) | 6 (0.5) | 28 (1.3) |
| Malignant leukemia | 6 (0.1) | 18 (0.1) | 14 (1.3) | 29 (1.3) |
| Non-malignancy related mortality | ||||
| N (%) | 1347 (15.3) | 2786 (16.2) | 162 (14.5) | 339 (15.3) |
| # HR | 1.04 (0.97–1.10) | Reference | 0.89 (0.73–1.07) | Reference |
| Characteristic | No Inhaled Corticosteroid (N = 11,665) | Low-Dose Inhaled Corticosteroid (N = 9309) | Medium-Dose Inhaled Corticosteroid (N = 6761) | High-Dose Inhaled Corticosteroid (N = 8235) | p-Value for Difference between ICS Treatment Groups |
|---|---|---|---|---|---|
| Age, years | 69.5 (61.2–77.2) | 71.0 (62.9–78.4) | 72.5 (65.4–79.1) | 72.7 (65.8–79.2) | <0.0001 * <0.0001 ** <0.0001 *** |
| Gender, female | 5379 (46.1) | 4593 (49.3) | 3522 (52.1) | 4731 (57.4) | <0.0001 |
| Tobacco exposure: | <0.0001 | ||||
| Passive smoking | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Previous smoker | 5463 (46.1) | 5171 (55.5) | 4300 (63.6) | 5250 (63.8) | |
| Active smoker | 4628 (39.7) | 3384 (36.4) | 2057 (30.4) | 2552 (31.0) | |
| Unknown tobacco exposure | 1574 (13.5) | 754 (8.1) | 404 (6.0) | 433 (5.3) | |
| MRC | 3 (2–3) | 3 (2–3) | 3 (3–4) | 3 (3–4) | <0.0001 * <0.0001 ** <0.0001 *** |
| BMI | 25 (22–28) | 25 (22–29) | 25 (21–28) | 25 (21–27) | =0.41 * <0.0001 ** <0.0001 *** |
| FEV1 in percentage of expected | 54 (47–69) | 49 (40–62) | 44 (33–55) | 39 (29–49) | <0.0001 * <0.0001 ** <0.0001 *** |
| Comorbidities: | |||||
| Hypertension | 3824 (32.8) | 3046 (32.7) | 2146 (31.7) | 2517 (30.6) | 0.003 |
| Hypercholesterolemia | 1837 (15.7) | 1352 (14.5) | 789 (11.7) | 830 (10.1) | <0.0001 |
| Atrial fibrillation | 1821 (15.6) | 1563 (16.8) | 1040 (15.4) | 1149 (14.0) | <0.0001 |
| Diabetes | 1473 (12.6) | 1142 (12.3) | 702 (10.4) | 831 (10.1) | <0.0001 |
| Osteoporosis or osteopenia | 1662 (14.2) | 1476 (15.9) | 1393 (20.6) | 2094 (25.4) | <0.0001 |
| Renal insufficiency | 4227 (36.2) | 3347 (36.0) | 2310 (34.2) | 2748 (33.4) | <0.0001 |
| Liver insufficiency | 385 (3.3) | 276 (3.0) | 167 (3.3) | 175 (2.1) | <0.0001 |
| Atopy or allergy | 354 (3.0) | 289 (3.1) | 184 (2.7) | 194 (2.4) | 0.01 |
| Depression | 496 (4.3) | 442 (4.7) | 313 (4.6) | 371 (4.5) | 0.35 |
| Exacerbations requiring admission within the last year prior to inclusion | 1939 (16.6) | 2932 (31.5) | 2473 (36.6) | 3480 (42.3) | <0.0001 |
| Medical treatment for respiratory disease within the last year prior to inclusion: | |||||
| Oral corticosteroid | 2444 (21.0) | 3697 (39.7) | 3546 (52.4) | 5107 (62.0) | <0.0001 |
| Long acting β2-agonist (LABA) | 3317 (28.4) | 8686 (93.3) | 6580 (07.3) | 8079 (98.1) | <0.0001 |
| Long acting muscarinic receptor antagonist (LAMA) | 5635 (48.3) | 6531 (70.2) | 5711 (84.5) | 7316 (88.8) | <0.0001 |
| Short acting β2-agonist (SABA) | 4304 (36.9) | 6118 (65.7) | 5163 (76.4) | 6910 (83.9) | <0.0001 |
| Short acting muscarinic receptor antagonist (SAMA) | 215 (1.8) | 260 (2.8) | 289 (4.3) | 339 (4.1) | <0.0001 |
| Endpoint | No Inhaled Corticosteroid (N = 11,665) | Low-Dose Inhaled Corticosteroid (N = 9309) | Medium-Dose Inhaled Corticosteroid (N = 6761) | High-Dose Inhaled Corticosteroid (N = 8235) |
|---|---|---|---|---|
| Cancer events | Reference | |||
| # HR | 1.01 (0.92–1.11) | 0.97 (0.92–1.01) | 0.96 (0.93–0.99) * | |
| p-value | 0.88 | 0.12 | 0.01 | |
| Lung cancer events | Reference | |||
| # HR | 1.09 (0.90–1.31) | 0.96 (0.89–1.05) | 1.00 (0.94–1.05) | |
| p-value | 0.37 | 0.41 | 0.86 | |
| Non-malignancy related mortality | Reference | |||
| # HR | 1.04 (1.01–1.09) * | 1.01 (1.00–1.03) | 1.03 (1.02–1.04) | |
| p-value | 0.03 | 0.15 | * p < 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnesen, B.; Sivapalan, P.; Jordan, A.; Pedersen, J.W.; Bergsøe, C.M.; Eklöf, J.; Toennesen, L.L.; Jensen, S.G.; Naqibullah, M.; Saghir, Z.; et al. Risk of Malignancy in Patients with Asthma-COPD Overlap Compared to Patients with COPD without Asthma. Biomedicines 2022, 10, 1463. https://doi.org/10.3390/biomedicines10071463
Bonnesen B, Sivapalan P, Jordan A, Pedersen JW, Bergsøe CM, Eklöf J, Toennesen LL, Jensen SG, Naqibullah M, Saghir Z, et al. Risk of Malignancy in Patients with Asthma-COPD Overlap Compared to Patients with COPD without Asthma. Biomedicines. 2022; 10(7):1463. https://doi.org/10.3390/biomedicines10071463
Chicago/Turabian StyleBonnesen, Barbara, Pradeesh Sivapalan, Alexander Jordan, Johannes Wirenfeldt Pedersen, Christina Marisa Bergsøe, Josefin Eklöf, Louise Lindhardt Toennesen, Sidse Graff Jensen, Matiullah Naqibullah, Zaigham Saghir, and et al. 2022. "Risk of Malignancy in Patients with Asthma-COPD Overlap Compared to Patients with COPD without Asthma" Biomedicines 10, no. 7: 1463. https://doi.org/10.3390/biomedicines10071463
APA StyleBonnesen, B., Sivapalan, P., Jordan, A., Pedersen, J. W., Bergsøe, C. M., Eklöf, J., Toennesen, L. L., Jensen, S. G., Naqibullah, M., Saghir, Z., & Jensen, J.-U. S. (2022). Risk of Malignancy in Patients with Asthma-COPD Overlap Compared to Patients with COPD without Asthma. Biomedicines, 10(7), 1463. https://doi.org/10.3390/biomedicines10071463








