Phase II Trial of Sipuleucel-T and Stereotactic Ablative Body Radiation for Patients with Metastatic Castrate-Resistant Prostate Cancer
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Treatment
2.3. Target Lesion Criteria
2.4. Adverse Events (AEs) and Follow-Up
2.5. Cellular Response Measurement by (Interferon) IFNg ELISpot
2.6. Humoral Response Measurement by Luminex Multiplex Assay
2.7. Investigation of Myeloid-Derived Suppressor Cells (MDSCs) by Fluorescence-Activated Cell Sorting (FACS)
2.8. Statistical Analysis
3. Results
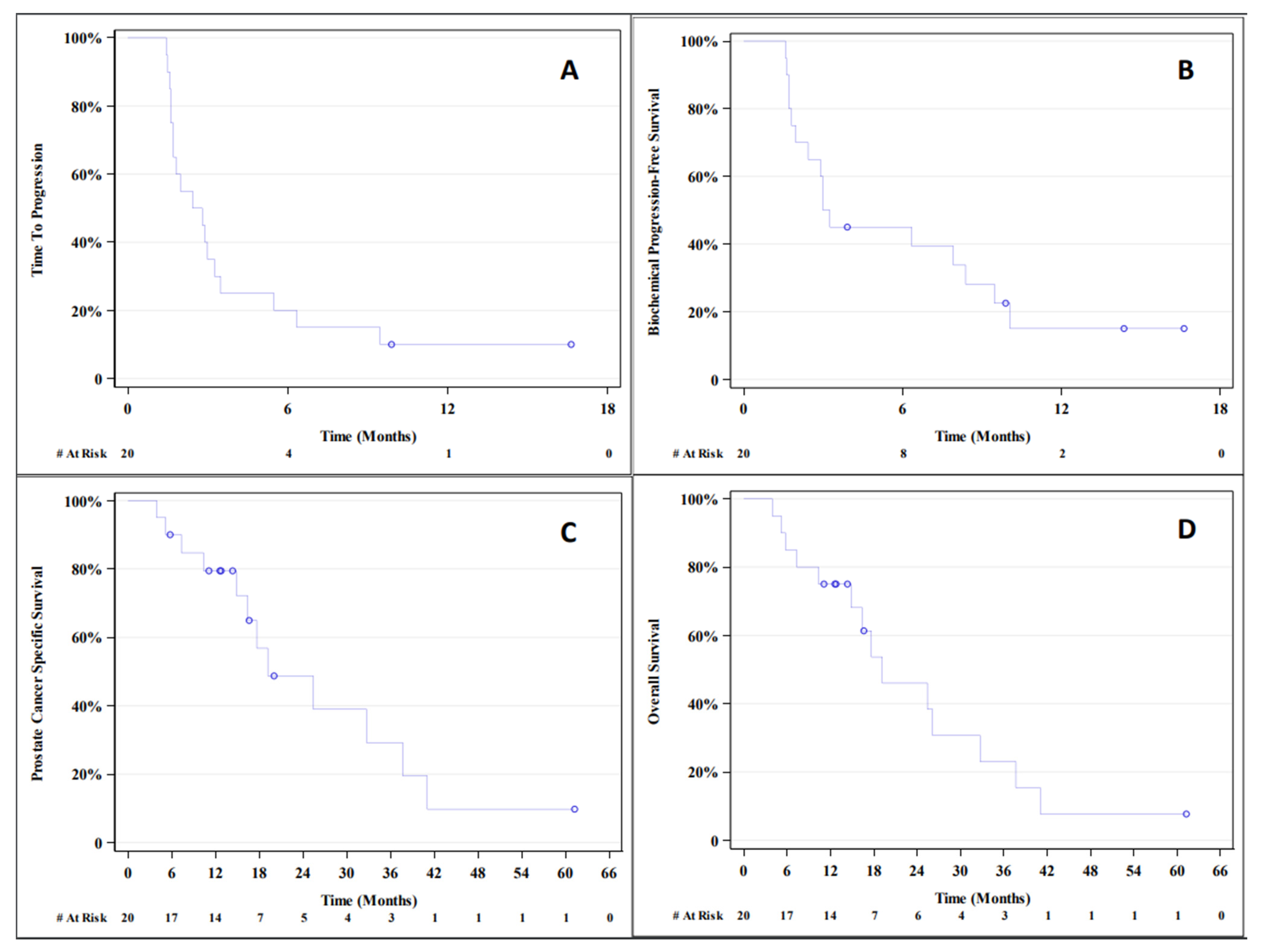
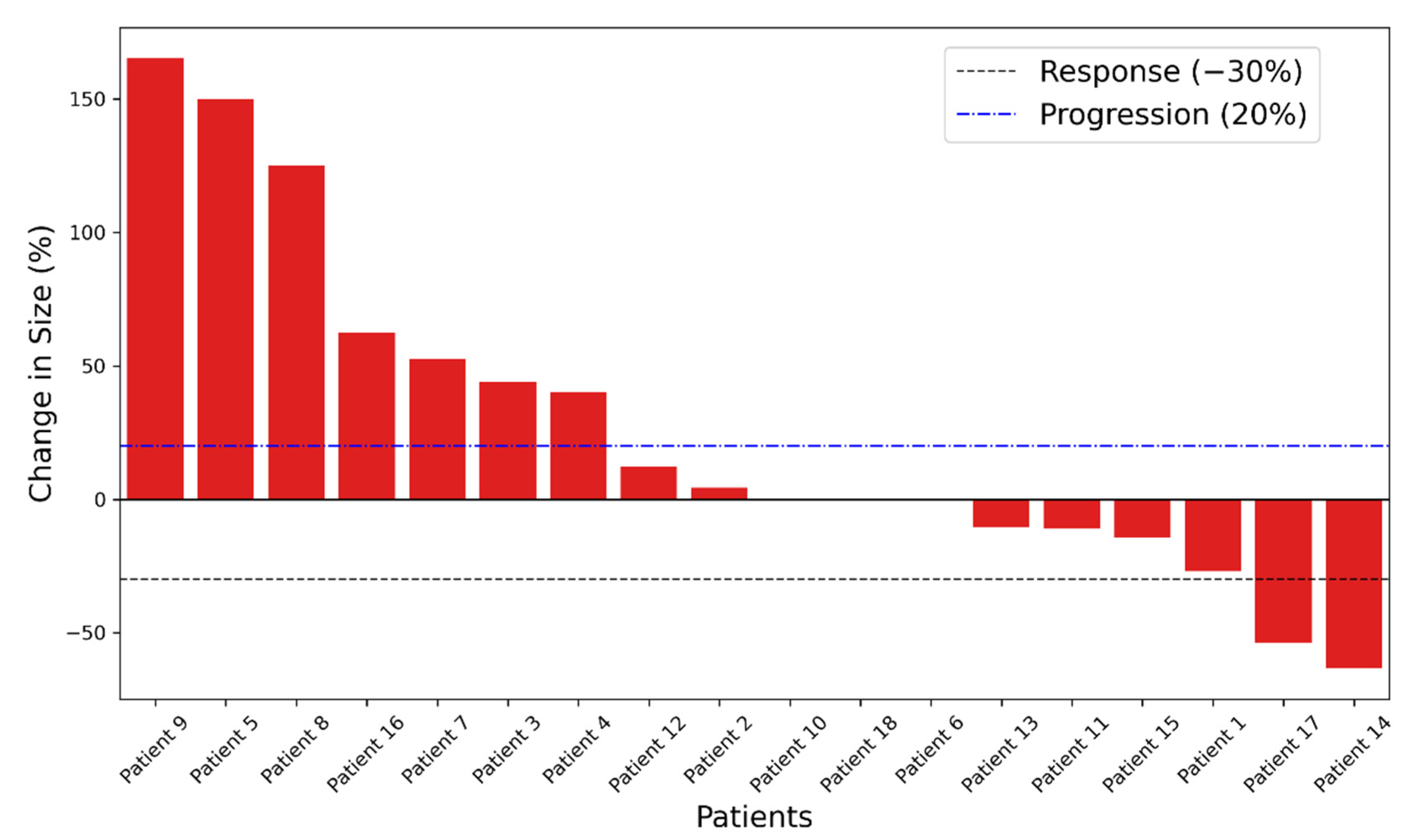
3.1. Outcome
3.2. Toxicity
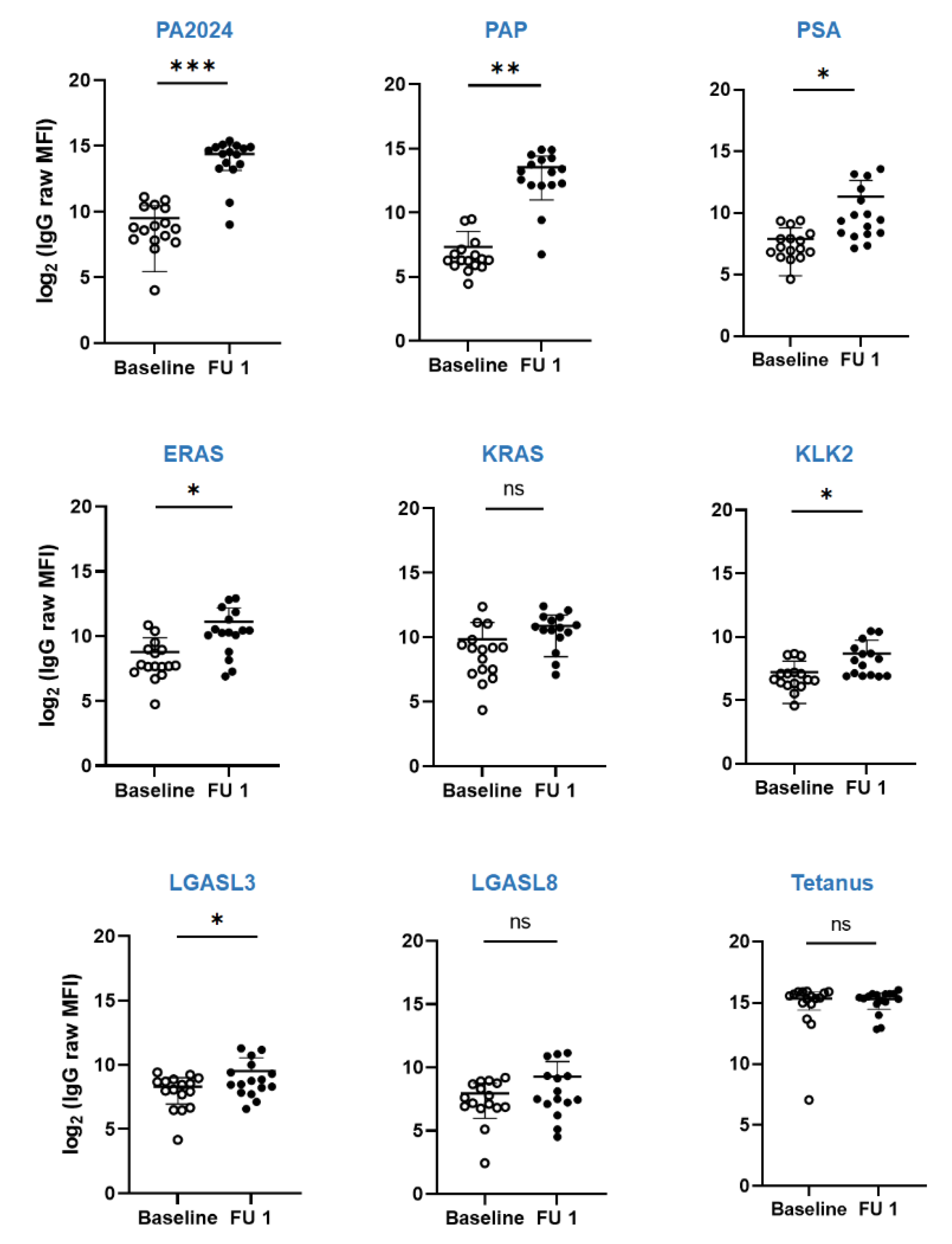
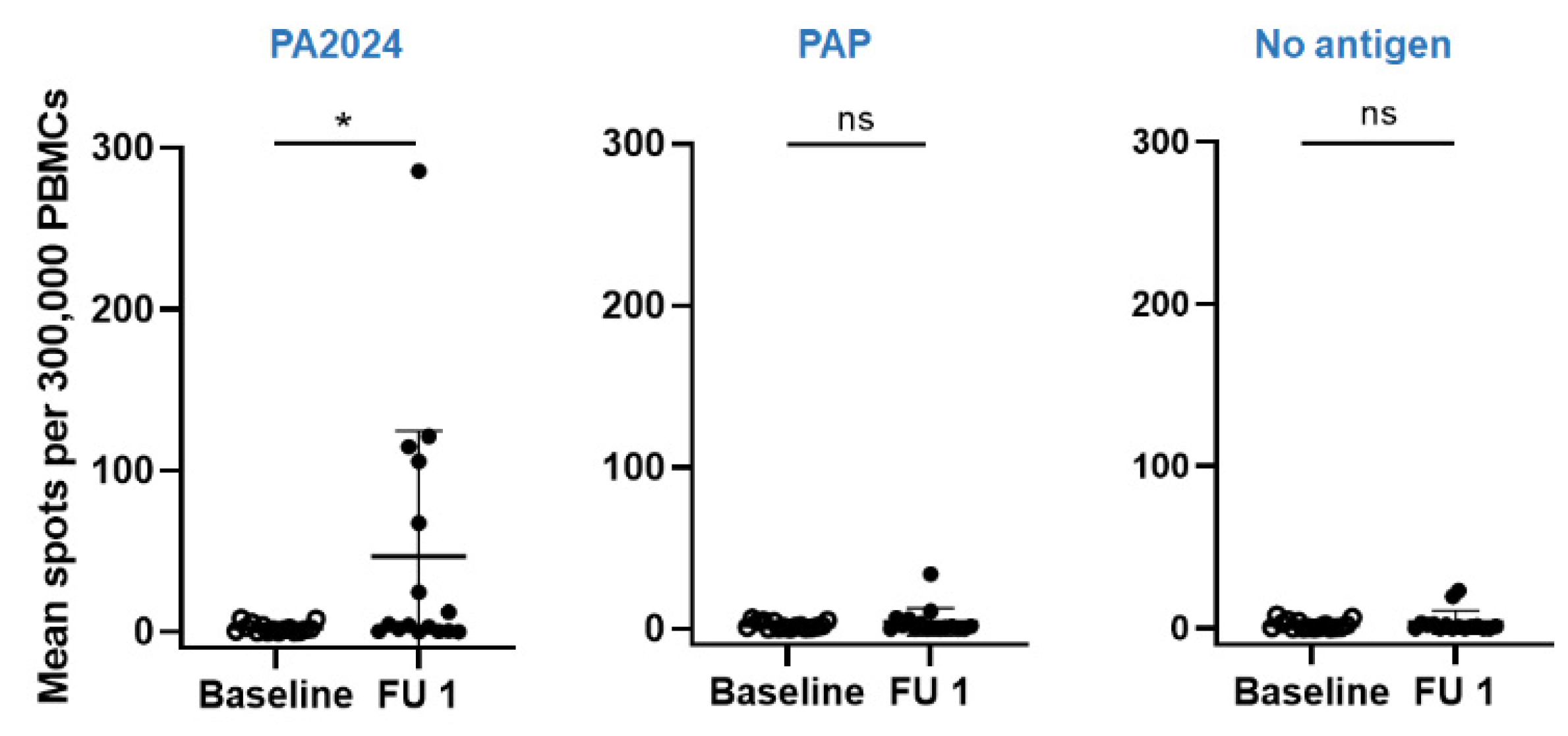
3.3. Immunologic Endpoint
3.4. Responders vs. Non-Responders Characteristics
3.5. Dynamics of Circulating MDSCs in Responders vs. Non-Responders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prostate Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 24 August 2020).
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; De Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.T.; Schellenberg, D.; Shen, J.; Kim, J.; Goodman, K.A.; Fisher, G.A.; Ford, J.M.; Desser, T.; Quon, A.; Koong, A.C. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009, 115, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef] [Green Version]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Chen, B. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002, 94, 1458–1468. [Google Scholar] [CrossRef]
- Stereotactic Radiotherapy for Oligo-Progressive Metastatic Cancer (The STOP Trial)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02756793 (accessed on 20 September 2020).
- Litwin, M.S.; Tan, H.J. The diagnosis and treatment of prostate cancer: A review. J. Am. Med. Assoc. 2017, 317, 2532–2542. [Google Scholar] [CrossRef]
- de Bono, J.S.; Oudard, S.; Özgüroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Small, E.J.; Schellhammer, P.F.; Higano, C.S.; Redfern, C.H.; Nemunaitis, J.J.; Valone, F.H.; Verjee, S.S.; Jones, L.A.; Hershberg, R.M. Placebo-controlled phase III trial of immunologic therapy with Sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 2006, 24, 3089–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boettcher, N.; Usman, A.; Morgans, A.; VanderWeele, D.J.; Sosman, J.; Wu, J.D. Past, Current, and Future of Immunotherapies for Prostate Cancer. Front. Oncol. 2019, 9, 884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corso, D.; Ali, A.N.; Diaz, R. Radiation-induced tumor neoantigens: Imaging and therapeutic implications. Am. J. Cancer Res. 2011, 1, 390–412. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21969260 (accessed on 24 August 2020). [PubMed]
- Deng, L.; Liang, H.; Fu, S.; Weichselbaum, R.R.; Fu, Y.X. From DNA damage to nucleic acid sensing: A strategy to enhance radiation therapy. Clin. Cancer Res. 2016, 22, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnette, B.C.; Liang, H.; Lee, Y.; Chlewicki, L.; Khodarev, N.N.; Weichselbaum, R.R.; Fu, Y.-X.; Auh, S.L. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011, 71, 2488–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golden, E.B.; Chhabra, A.; Chachoua, A.; Adams, S.; Donach, M.; Fenton-Kerimian, M.; Friedman, K.; Ponzo, F.; Babb, J.S.; Goldberg, J.; et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: A proof-of-principle trial. Lancet Oncol. 2015, 16, 795–803. [Google Scholar] [CrossRef]
- Formenti, S.C.; Rudqvist, N.-P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.C.; Postow, M.A.; Orlowski, R.J.; Mick, R.; Bengsch, B.; Manne, S.; Xu, W.; Harmon, S.; Giles, J.R.; Wenz, B.; et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017, 545, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Cushman, T.R.; Gomez, D.; Kumar, R.; Likacheva, A.; Chang, J.Y.; Cadena, A.P.; Paris, S.; Welsh, J.W. Combining radiation plus immunotherapy to improve systemic immune response. J. Thorac. Dis. 2018, 10 (Suppl. S3), S468–S479. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.N.; Medin, P.M.; Timmerman, R.D. Emphasis on Repair, Not Just Avoidance of Injury, Facilitates Prudent Stereotactic Ablative Radiotherapy. Semin. Radiat. Oncol. 2017, 27, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, N.A.; Petrylak, D.; Kantoff, P.W.; dela Rosa, C.; Stewart, F.P.; Kuan, L.-Y.; Whitmore, J.B.; Trager, J.B.; Poehlein, C.H.; Frohlich, M.W.; et al. Sipuleucel-T immune parameters correlate with survival: An analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol. Immunother. 2013, 62, 137–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, A.; Harasymczuk, M.; Schilling, B.; Georgoulias, V.; Argiris, A.; Whiteside, T.L. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J. Immunol. Methods 2012, 381, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.J.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef]
- Twardowski, P.; Wong, J.Y.; Pal, S.K.; Maughan, B.L.; Frankel, P.H.; Franklin, K.; Junqueira, M.; Prajapati, M.R.; Nachaegari, G.; Harwood, D.; et al. Randomized phase II trial of sipuleucel-T immunotherapy preceded by sensitizing radiation therapy and sipuleucel-T alone in patients with metastatic castrate resistant prostate cancer. Cancer Treat. Res. Commun. 2019, 19, 100116. [Google Scholar] [CrossRef]
- Tian, S.; Lei, Z.; Gong, Z.; Sun, Z.; Xu, D.; Piao, M. Clinical implication of prognostic and predictive biomarkers for castration-resistant prostate cancer: A systematic review. Cancer Cell Int. 2020, 20, 26. [Google Scholar] [CrossRef]
- Liu, D.; Kuai, Y.; Zhu, R.; Zhou, C.; Tao, Y.; Han, W.; Chen, Q. Prognosis of prostate cancer and bone metastasis pattern of patients: A SEER-based study and a local hospital based study from China. Sci. Rep. 2020, 10, 9140. [Google Scholar] [CrossRef]
- Higano, C.S.; Schellhammer, P.F.; Small, E.J.; Burch, P.A.; Nemunaitis, J.; Yuh, L.; Provost, N.; Frohlich, M.W. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 2009, 115, 3670–3679. [Google Scholar] [CrossRef]
- Marshall, C.H.; Park, J.C.; Deweese, T.L.; King, S.; Afful, M.; Hurrelbrink, J.; Manogue, C.; Cotogno, P.; Moldawer, N.P.; Barata, P.C.; et al. Randomized phase II study of sipuleucel-T (SipT) with or without radium-223 (Ra223) in men with asymptomatic bone-metastatic castrate-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 130. [Google Scholar] [CrossRef]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local consolidative therapy vs. Maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Hannan, R.; Mohamad, O.; de Leon, A.D.; Manna, S.; Pop, L.M.; Zhang, Z.; Mannala, S.; Christie, A.; Christley, S.; Monson, N.; et al. Outcome and Immune Correlates of a Phase II Trial of High-Dose Interleukin-2 and Stereotactic Ablative Radiotherapy for Metastatic Renal Cell Carcinoma. Clin. Cancer Res. 2021, 27, 6716–6725. [Google Scholar] [CrossRef] [PubMed]
- GuhaThakurta, D.; Sheikh, N.A.; Fan, L.-Q.; Kandadi, H.; Meagher, T.C.; Hall, S.J.; Kantoff, P.W.; Higano, C.S.; Small, E.J.; Gardner, T.A.; et al. Humoral Immune Response against Nontargeted Tumor Antigens after Treatment with Sipuleucel-T and Its Association with Improved Clinical Outcome. Clin. Cancer Res. 2015, 21, 3619–3630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, R.L.; Yu, E.Y. Refining Immuno-Oncology Approaches in Metastatic Prostate Cancer: Transcending Current Limitations. Curr. Treat. Options Oncol. 2021, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, R.; Lilljebjörn, L.; Johansson, M.; Leandersson, K.; Bjartell, A. The STAT3 inhibitor galiellalactone inhibits the generation of MDSC-like monocytes by prostate cancer cells and decreases immunosuppressive and tumorigenic factors. Prostate 2019, 79, 1611–1621. [Google Scholar] [CrossRef] [Green Version]
- Idorn, M.; Køllgaard, T.; Kongsted, P.; Sengeløv, L.; Straten, P.t. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol. Immunother. 2014, 63, 1177–1187. [Google Scholar] [CrossRef]
- Khan, A.N.H.; Emmons, T.R.; Wong, J.T.; Alqassim, E.; Singel, K.L.; Mark, J.; Smith, B.E.; Tario, J.D.; Eng, K.H.; Moysich, K.B.; et al. Quantification of Early-Stage Myeloid-Derived Suppressor Cells in Cancer Requires Excluding Basophils. Cancer Immunol. Res. 2020, 8, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; He, X.; Liu, Y.; Ding, X.; Jiang, H.; Tan, Y.; Lu, H. Prognostic Significance of Serum Uric Acid and Gamma-Glutamyltransferase in Patients with Advanced Gastric Cancer. Dis. Markers 2019, 2019, 1415421. [Google Scholar] [CrossRef]
- Baey, C.; Yang, J.; Ronchese, F.; Harper, J.L. Hyperuricaemic UrahPlt2/Plt2 mice show altered T cell proliferation and defective tumor immunity after local immunotherapy with Poly I:C. PLoS ONE 2018, 13, e0206827. [Google Scholar] [CrossRef]
- Mi, S.; Gong, L.; Sui, Z. Friend or Foe? An Unrecognized Role of Uric Acid in Cancer Development and the Potential Anticancer Effects of Uric Acid-lowering Drugs. J. Cancer 2020, 11, 5236–5244. [Google Scholar] [CrossRef]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients with Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, A.; Mollica, V.; Cimadamore, A.; Santoni, M.; Scarpelli, M.; Giunchi, F.; Cheng, L.; Lopez-Beltran, A.; Fiorentino, M.; Montironi, R.; et al. Is There a Role for Immunotherapy in Prostate Cancer? Cells 2020, 9, 2051. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; Van den Eertwegh, A.J.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef] [Green Version]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef] [Green Version]
- Fizazi, K.; Drake, C.G.; Beer, T.M.; Kwon, E.D.; Scher, H.I.; Gerritsen, W.R.; Bossi, A.; Eertwegh, A.J.V.D.; Krainer, M.; Houede, N.; et al. Final Analysis of the Ipilimumab Versus Placebo Following Radiotherapy Phase III Trial in Postdocetaxel Metastatic Castration-resistant Prostate Cancer Identifies an Excess of Long-term Survivors. Eur. Urol. 2020, 78, 822–830. [Google Scholar] [CrossRef]
| Variables | Mean ± STD |
| Age at Diagnosis (years) | 63 ± 9 |
| Age at Enrollment (years) | 69 ± 8 |
| Prior systemic therapy | 4 ± 2 |
| PSA value at Enrollment (ng/dL) | 93 ± 270 |
| PSA < 2 | N = 1 |
| PSA ≥ 2–10 | N = 10 |
| PSA ≥ 10 | N = 9 |
| Testosterone level (ng/dL) | 7 ± 5 |
| LDH (U/L) | 196 ± 44 |
| CRP (mg/L) | 7.9 ± 8.4 |
| Beta2 (mcg/mL) | 2.5 ± 0.8 |
| Uric Acid (mg/dL) | 5.3 ± 1.7 |
| WBC (×109/L) | 6.0 ± 1.5 |
| Neutrophils (×109/L) | 3.9 ± 1.4 |
| Lymphocytes (×109/L) | 1.3 ± 0.5 |
| Monocytes (×109/L) | 0.5 ± 0.2 |
| Eosinophils (×109/L) | 0.1 ± 0.1 |
| Variables | # (%) |
| Race | |
| White, not Hispanic | 14 (70%) |
| Black, not Hispanic | 4 (20%) |
| Hispanic | 1 (5%) |
| Asian | 1 (5%) |
| ECOG | |
| 0 | 12 (60%) |
| 1 | 8 (40%) |
| Grade Group | |
| 2–3 | 1 (5%) |
| 4 | 4 (22%) |
| 5 | 12 (67%) |
| Original Primary Gleason Score | |
| 5 | 7 (35%) |
| 4 | 10 (50%) |
| 2–3 | 1 (5%) |
| Original Secondary Gleason Score | |
| 5 | 8 (40%) |
| 4 | 7 (35%) |
| 3 | 2 (10%) |
| Stage at Diagnosis | |
| IIC | 1 (5%) |
| IIIB | 4 (21%) |
| IIIC | 5 (26%) |
| IVB | 9 (47%) |
| High Burden Metastatic Disease | |
| Yes | 11 (55 %) |
| No | 9 (45%) |
| Histology | |
| Adenocarcinoma | 20 (100%) |
| Variables | Number |
| SAbR Sites | |
| Vertebral body | 10 |
| Bony pelvis | 3 |
| Non-pelvic/non-vertebral bony metastases | 2 |
| Pelvic lymph nodes | 3 |
| Para-aortic lymph nodes | 1 |
| Supraclavicular lymph nodes | 1 |
| Prostate | 4 |
| Treatment Sites per Patient | |
| 1 | 11 |
| 2 | 5 |
| 3 | 1 |
| Dose/Fraction | |
| 20–21 Gy in 1 fraction | 10 |
| 24, 27, 30 Gy in 3 fractions | 14 |
| Systemic Therapy after sipuleucel-T | |
| Radium | 3 |
| Olaparib | 2 |
| Mitoxantrone | 1 |
| Lupron | 13 |
| Enzalutamide | 5 |
| Docetaxel | 11 |
| Degarelix | 1 |
| Cyclophosphamide | 6 |
| Cabazitaxel | 6 |
| Abiraterone | 6 |
| SL-801 | 1 |
| 177Lu-PSMA-617 | 1 |
| Rucaparib | 1 |
| Prior Systemic Therapy | |
| Abiraterone | 9 |
| Samarium-153 | 1 |
| Bicalutamide | 16 |
| Cabazitaxel | 3 |
| Cyclophosphamide | 1 |
| Degarelix | 1 |
| Docetaxel | 7 |
| Enzalutamide | 13 |
| Flutamide | 1 |
| Itraconazole | 1 |
| Lupron | 20 |
| Nilutamide | 5 |
| Radium | 1 |
| Administeration of sipuleucel-T | |
| 1st line (prior ADT only) | 11 |
| 2nd line | 6 |
| ≥3rd line | 3 |
| (A) | |||
| Parameter | Patients (n = 9) | Healthy Donors (n = 3) | p Value |
| M-MDSC baseline | 9.73 ± 6.68 | 0.67 ± 0.31 | 0.004 |
| e-MDSC baseline | 0.58 ± 0.71 | 0.17 ± 0.26 | 0.362 |
| (B) | |||
| Parameter | Responders (n = 5) | Non-Responders (n = 4) | p Value |
| M-MDSC baseline | 12.06 ± 8.16 | 6.83 ± 3.14 | 0.269 |
| M-MDSC FU | 9.56 ± 4.30 | 12.05 ± 6.59 | 0.514 |
| ∆M-MDSC | −2.50 ± 7.06 | 5.23 ± 3.49 | 0.088 |
| e-MDSC baseline | 0.49 ± 0.39 | 0.69 ± 1.06 | 0.711 |
| e-MDSC FU | 0.45 ± 0.36 | 0.10 ± 0.09 | 0.065 |
| ∆e-MDSC | −0.04 ± 0.54 | −0.59 ± 1.00 | 0.321 |
| (C) | |||
| Parameter | Responders (n = 4) | Non-Responders (n = 5) | p Value |
| M-MDSC baseline | 14.25 ± 7.54 | 6.12 ± 3.14 | 0.063 |
| M-MDSC FU | 10.33 ± 4.56 | 10.94 ± 6.23 | 0.874 |
| ∆M-MDSC | −3.93 ± 7.28 | 4.82 ± 3.16 | 0.044 |
| e-MDSC baseline | 0.55 ± 0.43 | 0.60 ± 0.94 | 0.917 |
| e-MDSC FU | 0.40 ± 0.39 | 0.21 ± 0.27 | 0.420 |
| ∆e-MDSC | −0.26 ± 0.53 | −0.39 ± 0.97 | 0.793 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannan, R.; Dohopolski, M.J.; Pop, L.M.; Mannala, S.; Watumull, L.; Mathews, D.; Gao, A.; Garant, A.; Arriaga, Y.E.; Bowman, I.; et al. Phase II Trial of Sipuleucel-T and Stereotactic Ablative Body Radiation for Patients with Metastatic Castrate-Resistant Prostate Cancer. Biomedicines 2022, 10, 1419. https://doi.org/10.3390/biomedicines10061419
Hannan R, Dohopolski MJ, Pop LM, Mannala S, Watumull L, Mathews D, Gao A, Garant A, Arriaga YE, Bowman I, et al. Phase II Trial of Sipuleucel-T and Stereotactic Ablative Body Radiation for Patients with Metastatic Castrate-Resistant Prostate Cancer. Biomedicines. 2022; 10(6):1419. https://doi.org/10.3390/biomedicines10061419
Chicago/Turabian StyleHannan, Raquibul, Michael J. Dohopolski, Laurentiu M. Pop, Samantha Mannala, Lori Watumull, Dana Mathews, Ang Gao, Aurelie Garant, Yull E. Arriaga, Isaac Bowman, and et al. 2022. "Phase II Trial of Sipuleucel-T and Stereotactic Ablative Body Radiation for Patients with Metastatic Castrate-Resistant Prostate Cancer" Biomedicines 10, no. 6: 1419. https://doi.org/10.3390/biomedicines10061419
APA StyleHannan, R., Dohopolski, M. J., Pop, L. M., Mannala, S., Watumull, L., Mathews, D., Gao, A., Garant, A., Arriaga, Y. E., Bowman, I., Chung, J.-S., Wang, J., Ariizumi, K., Ahn, C., Timmerman, R., & Courtney, K. (2022). Phase II Trial of Sipuleucel-T and Stereotactic Ablative Body Radiation for Patients with Metastatic Castrate-Resistant Prostate Cancer. Biomedicines, 10(6), 1419. https://doi.org/10.3390/biomedicines10061419








