Theophylline Induces Remyelination and Functional Recovery in a Mouse Model of Peripheral Neuropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Theophylline Treatment
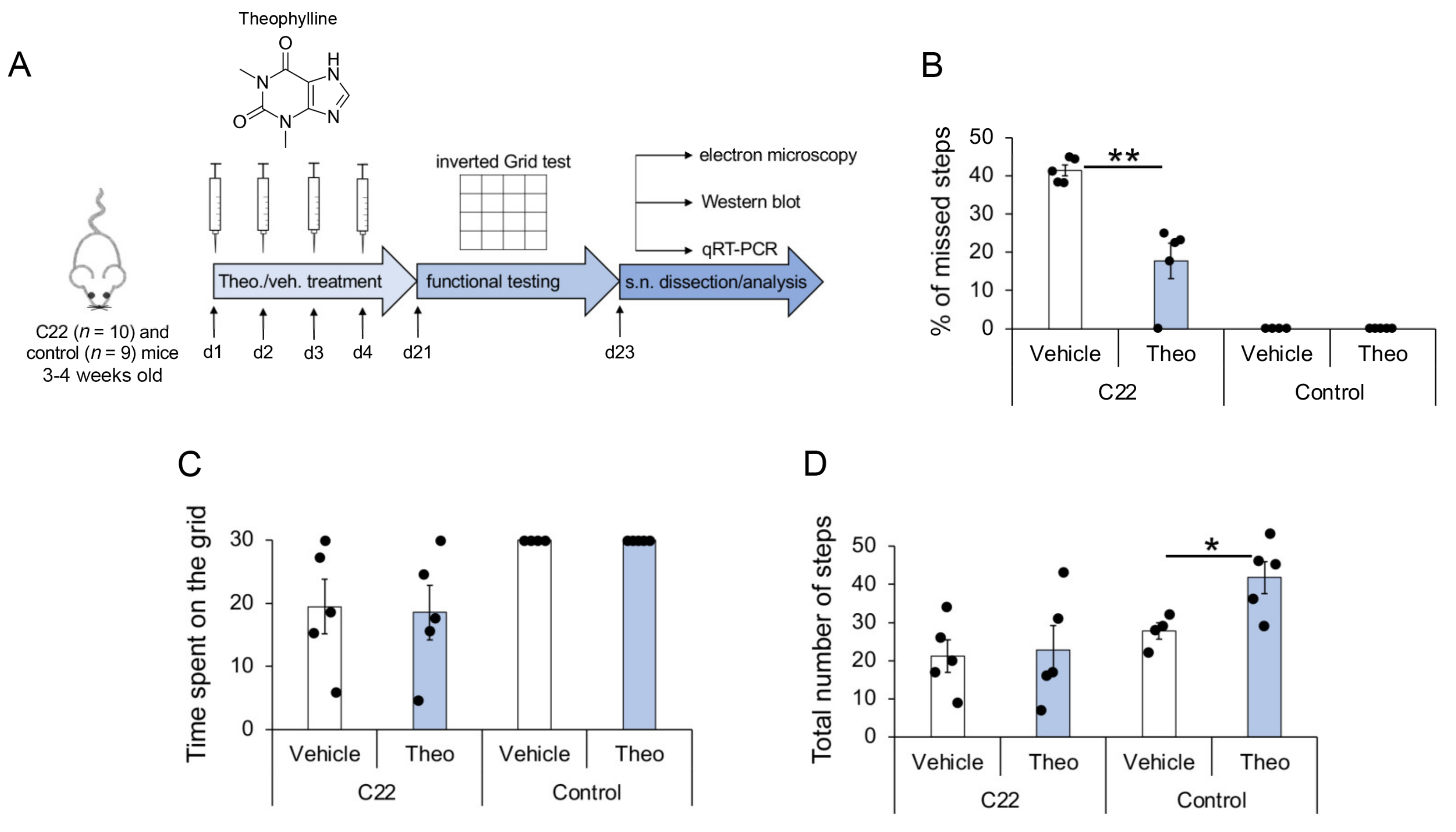
2.2. Inverted Grid Test
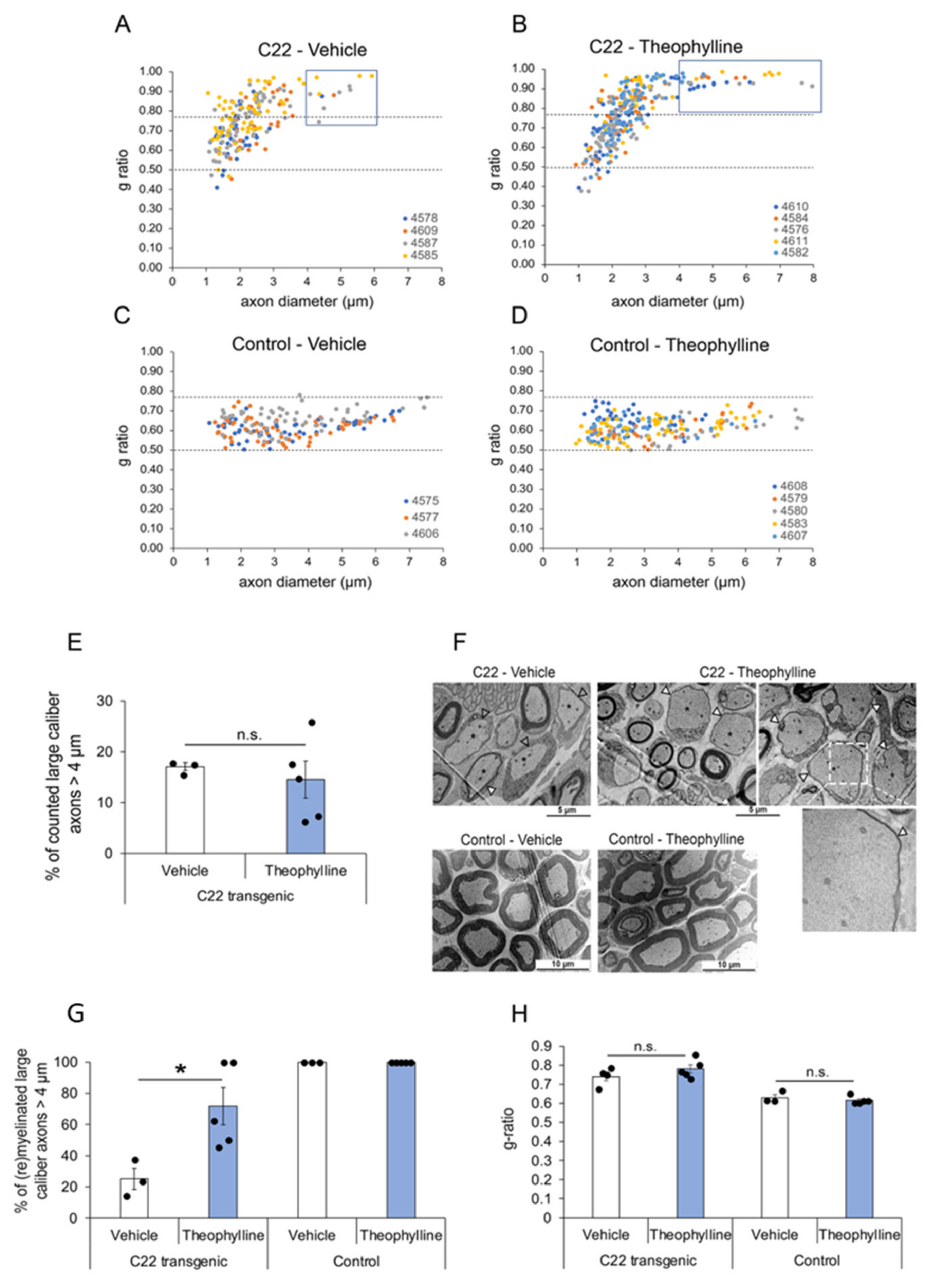
2.3. Transmission Electron Microscopy
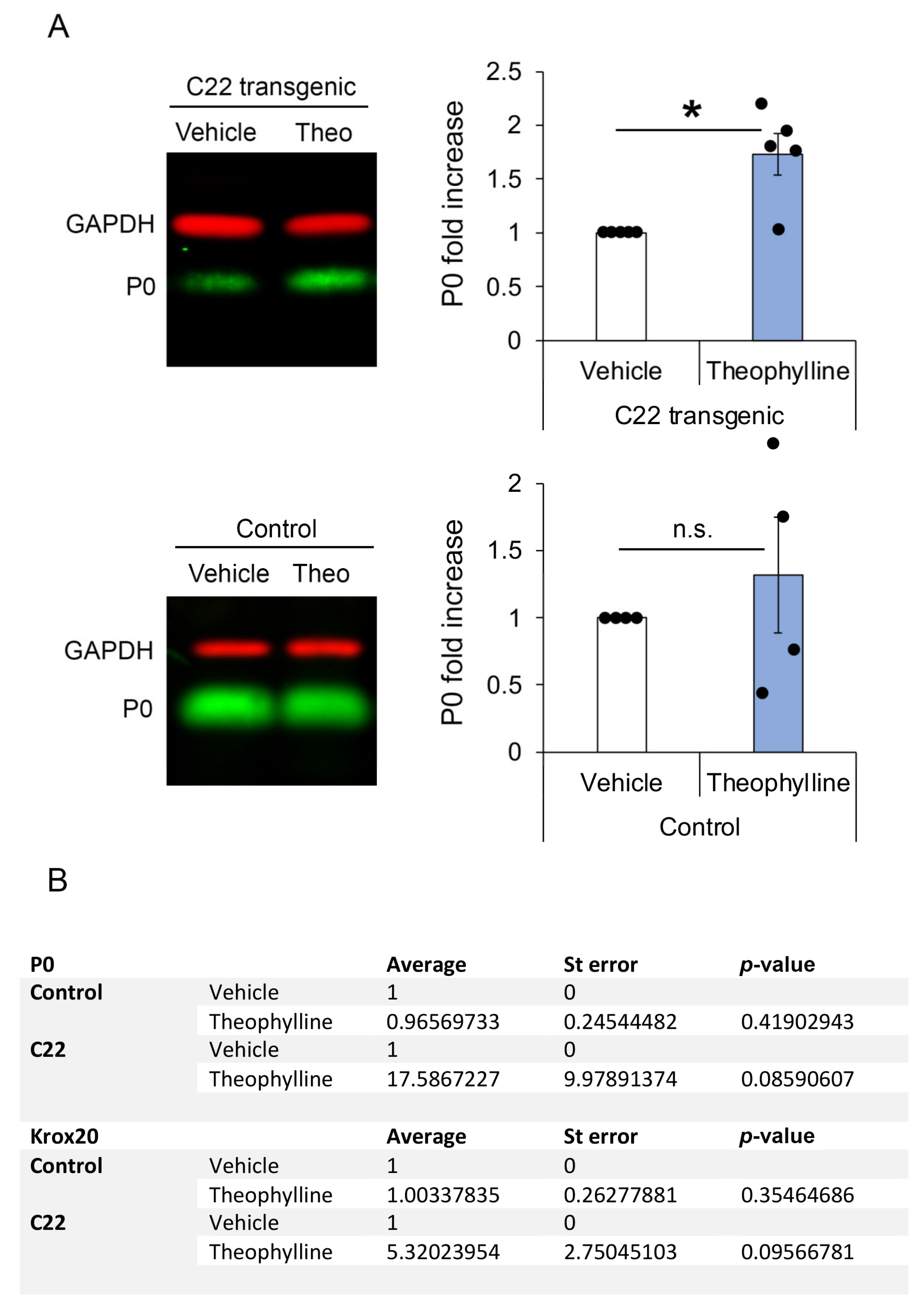
2.4. Protein Expression
2.5. mRNA Expression
2.6. Statistical Analysis
3. Results
3.1. Theophylline Treatment Leads to Partial Functional Recovery in C22 Transgenic Mice
3.2. Theophylline Treatment Induces (Re)Myelination in C22 Transgenic Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schenone, A.; Mancardi, G.L. Molecular basis of inherited neuropathies. Curr. Opin. Neurol. 1999, 12, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Morena, J.; Gupta, A.; Hoyle, J.C. Charcot-Marie-Tooth: From molecules to therapy. Int. J. Mol. Sci. 2019, 20, 3419. [Google Scholar] [CrossRef] [PubMed]
- Wrabetz, L.; Feltri, M.L.; Kleopa, K.A.; Scherer, S.S. Myelin Biology and Disorders; Lazzarini, R.A., Ed.; Elsevier Academic Press: San Diego, CA, USA, 2008; pp. 905–951. [Google Scholar]
- Gemelli, C.; Geroldi, A.; Massucco, S.; Trevisan, L.; Callegari, I.; Marinelli, L.; Ursino, G.; Hamedani, M.; Mennella, G.; Stara, S.; et al. Genetic Workup for Charcot-Marie-Tooth Neuropathy: A Retrospective Single-Site Experience Covering 15 Years. Life 2022, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Parker, B.; Martyn, C.; Natarajan, C.; Guo, J. The PMP22 gene and its related diseases. Mol. Neurobiol. 2013, 47, 673–698. [Google Scholar] [CrossRef] [PubMed]
- van Paassen, B.W.; van der Kooi, A.J.; van Spaendonck-Zwarts, K.Y.; Verhamme, C.; Baas, F.; de Visser, M. PMP22 related neuropathies: Charcot-Marie-Tooth disease type 1A and Hereditary Neuropathy with liability to Pressure Palsies. Orphanet J. Rare Dis. 2014, 9, 38. [Google Scholar] [CrossRef]
- Hanemann, C.O.; Müller, H.W. Pathogenesis of Charcot-Marie-Tooth 1A (CMT1A) neuropathy. Trends Neurosci. 1998, 21, 282–286. [Google Scholar] [CrossRef]
- Pareyson, D.; Marchesi, C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol. 2009, 8, 654–667. [Google Scholar] [CrossRef]
- Juneja, M.; Burns, J.; Saporta, M.A.; Timmerman, V. Challenges in modelling the Charcot-Marie-Tooth neuropathies for therapy development. J. Neurol. Neurosurg. Psychiatry 2019, 90, 58–67. [Google Scholar] [CrossRef]
- Boutary, S.; Echaniz-Laguna, A.; Adams, D.; Loisel-Duwattez, J.; Schumacher, M.; Massaad, C.; Massaad-Massade, L. Treating PMP22 gene duplication-related Charcot-Marie-Tooth disease: The past, the present and the future. Transl. Res. 2021, 227, 100–111. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Belfiore, L.; Chu, T.H.; Fleming, T.; Midha, R.; Biernaskie, J.; Schuurmans, C. Insights into the role and potential of schwann cells for peripheral nerve repair from studies of development and injury. Front. Mol. Neurosci. 2021, 13, 608442. [Google Scholar] [CrossRef] [PubMed]
- Duman, M.; Vaquie, A.; Nocera, G.; Heller, M.; Stumpe, M.; Siva Sankar, D.; Dengjel, J.; Meijer, D.; Yamaguchi, T.; Matthias, P.; et al. EEF1A1 deacetylation enables transcriptional activation of remyelination. Nat. Commun. 2020, 11, 3420. [Google Scholar] [CrossRef]
- Jacob, C.; Christen, C.N.; Pereira, J.A.; Somandin, C.; Baggiolini, A.; Lötscher, P.; Özçelik, M.; Tricaud, N.; Meijer, D.; Yamaguchi, T.; et al. HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nat. Neurosci. 2011, 14, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Brügger, V.; Duman, M.; Bochud, M.; Münger, E.; Heller, M.; Ruff, S.; Jacob, C. Delaying histone deacetylase early response to injury accelerates conversion into repair Schwann cells and nerve regeneration. Nat. Commun. 2017, 8, 14272. [Google Scholar] [CrossRef] [PubMed]
- Duman, M.; Martinez-Moreno, M.; Jacob, C.; Tapinos, N. Functions of histone modifications and histone modifiers in Schwann cells. Glia 2020, 68, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C. Chromatin-remodeling enzymes in control of Schwann cell development, maintenance and plasticity. Curr. Opin. Neurobiol. 2017, 47, 24–30. [Google Scholar] [CrossRef]
- Brügger, V.; Engler, S.; Pereira, J.A.; Ruff, S.; Horn, M.; Welzl, H.; Münger, E.; Vaquié, A.; Sidiropoulos, P.N.M.; Egger, B.; et al. HDAC1/2-dependent P0 expression maintains paranodal and nodal integrity independently of myelin stability through interactions with neurofascins. PLoS Biol. 2015, 13, e1002258. [Google Scholar] [CrossRef]
- Jacob, C.; Lötscher, P.; Engler, S.; Baggiolini, A.; Varum Tavares, S.; Brügger, V.; John, N.; Büchmann-Møller, S.; Snider, P.L.; Conway, S.J.; et al. HDAC1 and HDAC2 control the specification of neural crest cells into peripheral glia. J. Neurosci. 2014, 34, 6112–6122. [Google Scholar] [CrossRef]
- Britsch, S.; Goerich, D.E.; Riethmacher, D.; Peirano, R.I.; Rossner, M.; Nave, K.A.; Birchmeier, C.; Wegner, M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001, 15, 66–78. [Google Scholar] [CrossRef]
- Weider, M.; Reiprich, S.; Wegner, M. Sox appeal—Sox10 attracts epigenetic and transcriptional regulators in myelinating glia. Biol. Chem. 2013, 394, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Fröb, F.; Bremer, M.; Finzsch, M.; Kichko, T.; Reeh, P.; Tamm, E.R.; Charnay, P.; Wegner, M. Establishment of myelinating Schwann cells and barrier integrity between central and peripheral nervous systems depend on Sox10. Glia 2012, 60, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Mirsky, R.; Parkinson, D.B.; Dong, Z.; Meier, C.; Calle, E.; Brennan, A.; Topilko, P.; Harris, B.S.; Stewart, H.J.; Jessen, K.R. Regulation of genes involved in Schwann cell development and differentiation. Prog. Brain Res. 2001, 132, 3–11. [Google Scholar] [CrossRef]
- Stolt, C.C.; Wegner, M. Schwann cells and their transcriptional network: Evolution of key regulators of peripheral myelination. Brain Res. 2016, 1641, 101–110. [Google Scholar] [CrossRef]
- Jagalur, N.B.; Ghazvini, M.; Mandemakers, W.; Driegen, S.; Maas, A.; Jones, E.A.; Jaegle, M.; Grosveld, F.; Svaren, J.; Meijer, D. Functional dissection of the Oct6 Schwann cell enhancer reveals an essential role for dimeric Sox10 binding. J. Neurosci. 2011, 31, 8585–8594. [Google Scholar] [CrossRef]
- Ghislain, J.; Charnay, P. Control of myelination in Schwann cells: A Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep. 2006, 7, 52–58. [Google Scholar] [CrossRef]
- Topilko, P.; Levi, G.; Merlo, G.; Mantero, S.; Desmarquet, C.; Mancardi, G.; Charnay, P. Differential regulation of the zinc finger genes Krox-20 and Krox-24 (Egr-1) suggests antagonistic roles in Schwann cells. J. Neurosci. Res. 1997, 50, 702–712. [Google Scholar] [CrossRef]
- Reiprich, S.; Kriesch, J.; Schreiner, S.; Wegner, M. Activation of Krox20 gene expression by Sox10 in myelinating Schwann cells. J. Neurochem. 2010, 112, 744–754. [Google Scholar] [CrossRef]
- Svaren, J.; Meijer, D. The molecular machinery of gene transcription in Schwann cells. Glia 2008, 56, 1541–1551. [Google Scholar] [CrossRef]
- Peirano, R.I.; Goerich, D.E.; Riethmacher, D.; Wegner, M. Protein zero gene expression is regulated by the glial transcription factor Sox10. Mol. Cell. Biol. 2000, 20, 3198–3209. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.E.; Ward, R.M.; Svaren, J. Neuropathy-associated Egr2 mutants disrupt cooperative activation of myelin protein zero by Egr2 and Sox10. Mol. Cell. Biol. 2007, 27, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W.; Svaren, J. Induction of myelin protein zero by early growth response 2 through upstream and intragenic elements. J. Biol. Chem. 2009, 284, 20111–20120. [Google Scholar] [CrossRef]
- LeBlanc, S.E.; Jang, S.W.; Ward, R.M.; Wrabetz, L.; Svaren, J. Direct regulation of myelin protein zero expression by the Egr2 transactivator. J. Biol. Chem. 2006, 281, 5453–5460. [Google Scholar] [CrossRef] [PubMed]
- Raasakka, A.; Kursula, P. How Does Protein Zero Assemble Compact Myelin? Cells 2020, 9, 1832. [Google Scholar] [CrossRef]
- Siems, S.B.; Jahn, O.; Eichel, M.A.; Kannaiyan, N.; Wu, L.M.N.; Sherman, D.L.; Kusch, K.; Hesse, D.; Jung, R.B.; Fledrich, R.; et al. Proteome profile of peripheral myelin in healthy mice and in a neuropathy model. Elife 2020, 9, e51406. [Google Scholar] [CrossRef]
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef]
- Huxley, C.; Passage, E.; Manson, A.; Putzu, G.; Figarella-Branger, D.; Pellissier, J.F.; Fontes, M. Construction of a mouse model of Charcot-Marie-Tooth disease type 1A by pronuclear injection of human YAC DNA. Hum. Mol. Genet. 1996, 5, 563–569. [Google Scholar] [CrossRef]
- Kinter, J.; Lazzati, T.; Schmid, D.; Zeis, T.; Erne, B.; Lutzelschwab, R.; Steck, A.J.; Pareyson, D.; Peles, E.; Schaeren-Wiemers, N. An essential role of MAG in mediating axon-myelin attachment in Charcot-Marie-Tooth 1A disease. Neurobiol. Dis. 2013, 49, 221–231. [Google Scholar] [CrossRef][Green Version]
- Robertson, A.M.; Perea, J.; McGuigan, A.; King, R.H.; Muddle, J.R.; Gabreels-Festen, A.A.; Thomas, P.K.; Huxley, C. Comparison of a new Pmp22 transgenic mouse line with other mouse models and human patients with CMT1A. J. Anat. 2002, 200, 377–390. [Google Scholar] [CrossRef]
- Tillerson, J.L.; Cohen, A.D.; Philhower, J.; Miller, G.W.; Zigmond, M.J.; Schallert, T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J. Neurosci. 2001, 21, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Tillerson, J.L.; Miller, G.W. Grid performance test to measure behavioral impairment in the MPTP-treated-mouse model of parkinsonism. J. Neurosci. Methods 2003, 123, 189–200. [Google Scholar] [CrossRef]
- Pereira, J.A.; Benninger, Y.; Baumann, R.; Gonçalves, A.F.; Özçelik, M.; Thurnherr, T.; Tricaud, N.; Meijer, D.; Fässler, R.; Suter, U.; et al. Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J. Cell Biol. 2009, 185, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, A.; Yancopoulos, G.D.; Alt, F.W. Methods for Cloning and Analysis of Eukaryotic Genes; Jones and Bartlett Publishers: Boston, MA, USA, 1990. [Google Scholar]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Robaglia-Schlupp, A.; Pizant, J.; Norreel, J.C.; Passage, E.; Saberan-Djoneidi, D.; Ansaldi, J.L.; Vinay, L.; Figarella-Branger, D.; Levy, N.; Clarac, F.; et al. PMP22 overexpression causes dysmyelination in mice. Brain 2002, 125, 2213–2221. [Google Scholar] [CrossRef]
- Sancho, S.; Magyar, J.P.; Aguzzi, A.; Suter, U. Distal axonopathy in peripheral nerves of PMP22-mutant mice. Brain 1999, 122, 1563–1577. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; Du, J.; Qing, L.; Mee, T.; Xu, X.; Wang, Z.; Xu, C.; Jia, A. Identification of sensory and motor nerve fascicles by immunofluorescence staining after peripheral nerve injury. J. Transl. Med. 2021, 19, 207. [Google Scholar] [CrossRef]
- Prior, R.; Verschoren, S.; Vints, K.; Jaspers, T.; Rossaert, E.; Klingl, Y.E.; Silva, A.; Hersmus, N.; Van Damme, P.; Van Den Bosch, L. HDAC3 Inhibition Stimulates Myelination in a CMT1A Mouse Model. Mol. Neurobiol. 2022, 59, 3414–3430. [Google Scholar] [CrossRef]
- Kaliyaperumal, S.; Wilson, K.; Aeffner, F.; Dean, C. Animal Models of Peripheral Pain: Biology Review and Application for Drug Discovery. Toxicol. Pathol. 2020, 48, 202–219. [Google Scholar] [CrossRef]
- Lamoine, S.; Cumenal, M.; Barriere, D.A.; Pereira, V.; Fereyrolles, M.; Prival, L.; Barbier, J.; Boudieu, L.; Brasset, E.; Bertin, B.; et al. The class I HDAC inhibitor, MS-275, prevents oxaliplatin-induced chronic neuropathy and potentiates its antiproliferative activity in mice. Int. J. Mol. Sci. 2021, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Denk, F.; Huang, W.; Sidders, B.; Bithell, A.; Crow, M.; Grist, J.; Sharma, S.; Ziemek, D.; Rice, A.S.C.; Buckley, N.J.; et al. HDAC inhibitors attenuate the development of hypersensitivity in models of neuropathic pain. Pain® 2013, 154, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Janitschke, D.; Lauer, A.A.; Bachmann, C.M.; Grimm, H.S.; Hartmann, T.; Grimm, M.O.W. Methylxanthines and neurodegenerative diseases: An update. Nutrients 2021, 13, 803. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duman, M.; Jaggi, S.; Enz, L.S.; Jacob, C.; Schaeren-Wiemers, N. Theophylline Induces Remyelination and Functional Recovery in a Mouse Model of Peripheral Neuropathy. Biomedicines 2022, 10, 1418. https://doi.org/10.3390/biomedicines10061418
Duman M, Jaggi S, Enz LS, Jacob C, Schaeren-Wiemers N. Theophylline Induces Remyelination and Functional Recovery in a Mouse Model of Peripheral Neuropathy. Biomedicines. 2022; 10(6):1418. https://doi.org/10.3390/biomedicines10061418
Chicago/Turabian StyleDuman, Mert, Stephanie Jaggi, Lukas Simon Enz, Claire Jacob, and Nicole Schaeren-Wiemers. 2022. "Theophylline Induces Remyelination and Functional Recovery in a Mouse Model of Peripheral Neuropathy" Biomedicines 10, no. 6: 1418. https://doi.org/10.3390/biomedicines10061418
APA StyleDuman, M., Jaggi, S., Enz, L. S., Jacob, C., & Schaeren-Wiemers, N. (2022). Theophylline Induces Remyelination and Functional Recovery in a Mouse Model of Peripheral Neuropathy. Biomedicines, 10(6), 1418. https://doi.org/10.3390/biomedicines10061418





