In Vitro Anticancer Activity of Two Ferrocene-Containing Camphor Sulfonamides as Promising Agents against Lung Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Cell Culture
2.3. In Vitro Cytotoxicity Test
2.4. Flow Cytometry
2.5. Immunofluorescence Microscopy
2.6. Reactive Oxygen Species (ROS) Analysis
2.7. JC-1 Mitochondrial Membrane Potential Assay
2.8. Statistical Analysis
3. Results
3.1. Chemistry
3.2. Solubility
3.3. In Vitro Evaluation of DK-164 and CC-78 Cytotoxicity
3.4. Study of Apoptosis in Lung Cells Treated with DK-164 and CC-78
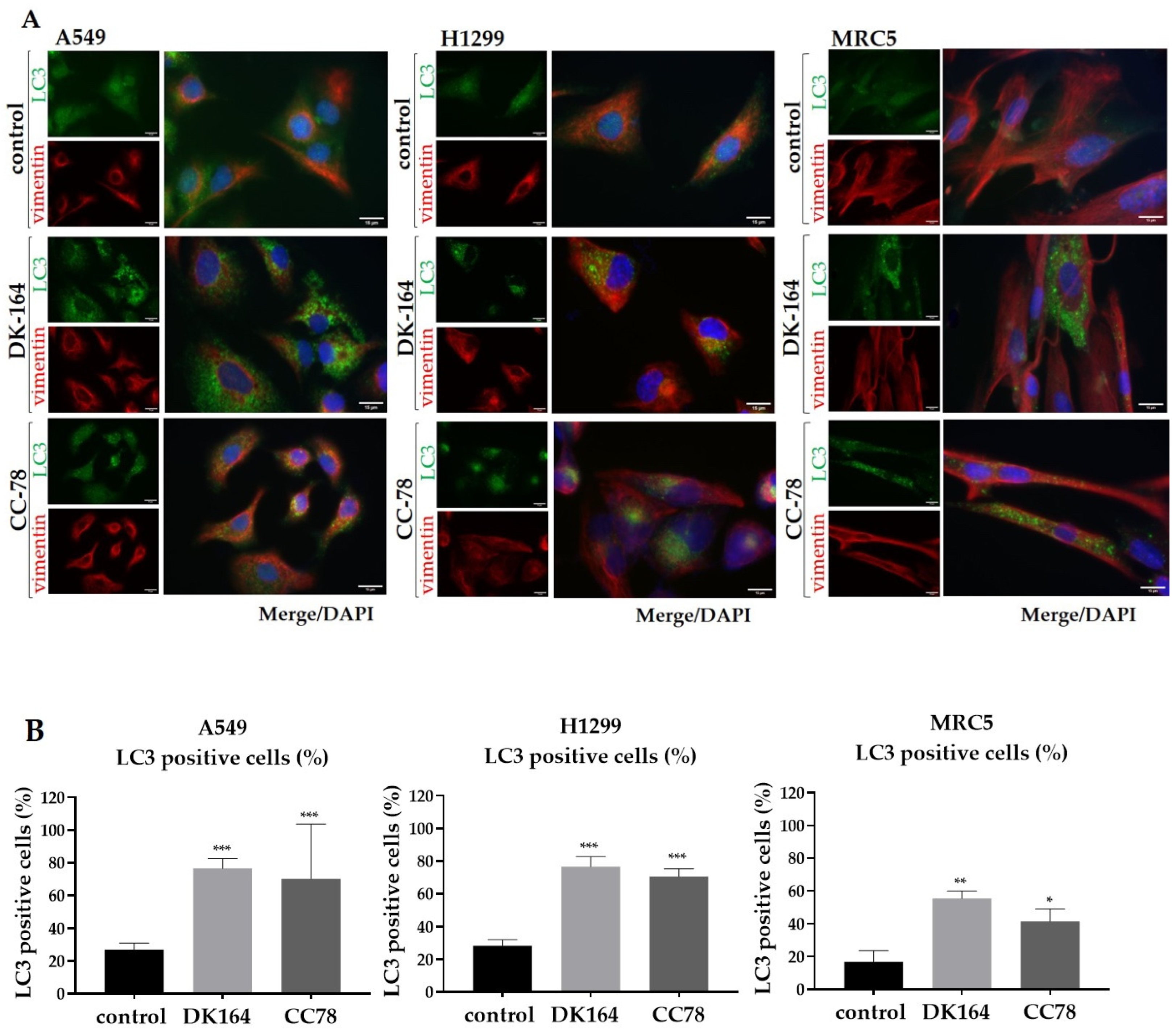
3.5. Autophagy in Lung Cells Induced by Treatment with DK-164 and CC-78
3.6. The Effect of DK-164 and CC-78 on the Cellular Localisation of the Regulatory Proteins NF-kB and p53 in Lung Cells
3.7. Activation of Reactive Oxygen Species (ROS) in Lung Cancer Cells by DK-164, CC-78
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verband Forschender Arzneimittelherstellere e.V. Neue Chancen für Krebspatienten. In Perspektive 2023. Neue Medikamente in Entwicklung; Verband Forschender Arzneimittelhersteller e.V: Berlin, Germany, 2020; pp. 8–10. [Google Scholar]
- Müller-Schiffmann, A.; März-Berberich, J.; Andreyeva, A.; Rönicke, R.; Bartnik, D.; Brener, O.; Kutzsche, J.; Horn, A.H.C.; Hellmert, M.; Polkowska, J.; et al. Combining Independent Drug Classes into Superior, Synergistically Acting Hybrid Molecules. Angew. Chem. Int. Ed. 2010, 49, 8743–8746. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Riddell, I.A. Cisplatin and Oxaliplatin: Our Current Understanding of Their Actions. Metal. Ions Life Sci. 2018, 18, 1–42. [Google Scholar] [CrossRef]
- Kvardova, V.; Hrstka, R.; Walerych, D.; Muller, P.; Matoulkova, E.; Hruskova, V.; Stelclova, D.; Sova, P.; Vojtesek, B. The new platinum(IV) derivative LA-12 shows stronger inhibitory effect on Hsp90 function compared to cisplatin. Mol. Cancer 2010, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Dyson, P.J. Bioorganometallic chemistry—from teaching paradigms to medicinal applications. Chem. Soc. Rev. 2009, 38, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Metzler-Nolte, N. The potential of organometallic complexes in medicinal chemistry. Curr. Opin. Chem. Biol. 2012, 16, 84–91. [Google Scholar] [CrossRef]
- Harding, M.; Mokdsi, G. Antitumour Metallocenes: Structure-Activity Studies and Interactions with Biomolecules. Curr. Med. Chem. 2001, 7, 1289–1303. [Google Scholar] [CrossRef]
- Kealy, T.J.; Pauson, P.L. A New Type of Organo-Iron Compound. Nature 1951, 168, 1039–1040. [Google Scholar] [CrossRef]
- Cooper, W.A.; Lam, D.C.; O′Toole, S.A.; Minna, J.D. Molecular biology of lung cancer. J. Thorac. Dis. 2013, 5 (Suppl. S5), S479–S490. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Reviews. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Schröder, M.; Yusein-Myashkova, S.; Petrova, M.; Dobrikov, G.; Kamenova-Nacheva, M.; Todorova, J.; Pasheva, E.; Ugrinova, I. The Effect of a Ferrocene Containing Camphor Sulfonamide DK-164 on Breast Cancer Cell Lines. Anti Cancer Agents Med. Chem. 2019, 19, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Kamenova-Nacheva, M.; Schröder, M.; Pasheva, E.; Slavchev, I.; Dimitrov, V.; Momekov, G.; Nikolova, R.; Shivachev, B.; Ugrinova, I.; Dobrikov, G.M. Synthesis of ferrocenylmethylidene and arylidene substituted camphane based compounds as potential anticancer agents. New J. Chem. 2017, 41, 9103–9112. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Saha, S.; Panigrahi, D.P.; Patil, S.; Bhutia, S.K. Autophagy in health and disease: A comprehensive review. Biomed. Pharmacother. 2018, 104, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Jeon, J.; Go, G.; Lee, J.H.; Lee, S.H. The Dual Role of Autophagy in Cancer Development and a Therapeutic Strategy for Cancer by Targeting Autophagy. Int. J. Mol. Sci. 2020, 22, 179. [Google Scholar] [CrossRef]
- Eskelinen, E.-L. The dual role of autophagy in cancer. Curr. Opin. Pharmacol. 2011, 11, 294–300. [Google Scholar] [CrossRef]
- Perera, R.M.; Stoykova, S.; Nicolay, B.N.; Ross, K.N.; Fitamant, J.; Boukhali, M.; Lengrand, J.; Deshpande, V.; Selig, M.K.; Ferrone, C.R.; et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature 2015, 524, 361–365. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Chaturvedi, M.M.; Sung, B.; Yadav, V.R.; Kannappan, R.; Aggarwal, B.B. NF-κB addiction and its role in cancer: ‘one size does not fit all’. Oncogene 2011, 30, 1615–1630. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio Protoc. 2019, 9, e3128. [Google Scholar] [CrossRef] [PubMed]
- Karalı, N.; Akdemir, A.; Göktaş, F.; Eraslan Elma, P.; Angeli, A.; Kızılırmak, M.; Supuran, C.T. Novel sulfonamide-containing 2-indolinones that selectively inhibit tumor-associated alpha carbonic anhydrases. Bioorganic Med. Chem. 2017, 25, 3714–3718. [Google Scholar] [CrossRef]
- Pastorekova, S.; Gillies, R.J. The role of carbonic anhydrase IX in cancer development: Links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev. 2019, 38, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Michard, Q.; Jaouen, G.; Vessieres, A.; Bernard, B.A. Evaluation of cytotoxic properties of organometallic ferrocifens on melanocytes, primary and metastatic melanoma cell lines. J. Inorg. Biochem. 2008, 102, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Heldt, J.M.; Guille-Collignon, M.; Lemaître, F.; Jaouen, G.; Vessières, A.; Amatore, C. Quantitative analyses of ROS and RNS production in breast cancer cell lines incubated with ferrocifens. ChemMedChem 2014, 9, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, G.; Vessières, A.; Top, S. Ferrocifen type anti cancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef] [PubMed]
- Vessières, A.; Corbet, C.; Heldt, J.M.; Lories, N.; Jouy, N.; Laïos, I.; Leclercq, G.; Jaouen, G.; Toillon, R.A. A ferrocenyl derivative of hydroxytamoxifen elicits an estrogen receptor-independent mechanism of action in breast cancer cell lines. J. Inorg. Biochem. 2010, 104, 503–511. [Google Scholar] [CrossRef]
- Bruyère, C.; Mathieu, V.; Vessières, A.; Pigeon, P.; Top, S.; Jaouen, G.; Kiss, R. Ferrocifen derivatives that induce senescence in cancer cells: Selected examples. J. Inorg. Biochem. 2014, 141, 144–151. [Google Scholar] [CrossRef]
- de Oliveira, A.C.; da Silva, E.G.; Rocha, D.D.; Hillard, E.A.; Pigeon, P.; Jaouen, G.; Rodrigues, F.A.; de Abreu, F.C.; da Rocha Ferreira, F.; Goulart, M.O.; et al. Molecular mechanism of action of 2-ferrocenyl-1,1-diphenylbut-1-ene on HL-60 leukemia cells. ChemMedChem 2014, 9, 2580–2586. [Google Scholar] [CrossRef]
- Shen, S.; Kepp, O.; Michaud, M.; Martins, I.; Minoux, H.; Métivier, D.; Maiuri, M.C.; Kroemer, R.T.; Kroemer, G. Association and dissociation of autophagy, apoptosis and necrosis by systematic chemical study. Oncogene 2011, 30, 4544–4556. [Google Scholar] [CrossRef]
- Kocaturk, N.M.; Akkoc, Y.; Kig, C.; Bayraktar, O.; Gozuacik, D.; Kutlu, O. Autophagy as a molecular target for cancer treatment. Eur. J. Pharm. Sci. 2019, 134, 116–137. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Vats, S.; Chia, A.Y.; Tan, T.Z.; Deng, S.; Ong, M.S.; Arfuso, F.; Yap, C.T.; Goh, B.C.; Sethi, G.; et al. Dual role of autophagy in hallmarks of cancer. Oncogene 2018, 37, 1142–1158. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Alnemri, E.S.; Altucci, L.; Andrews, D.; Annicchiarico-Petruzzelli, M.; et al. Essential versus accessory aspects of cell death: Recommendations of the NCCD 2015. Cell Death Differ. 2015, 22, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Kanzawa, T.; Germano, I.M.; Komata, T.; Ito, H.; Kondo, Y.; Kondo, S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004, 11, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Knizhnik, A.V.; Roos, W.P.; Nikolova, T.; Quiros, S.; Tomaszowski, K.H.; Christmann, M.; Kaina, B. Survival and death strategies in glioma cells: Autophagy, senescence and apoptosis triggered by a single type of temozolomide-induced DNA damage. PLoS ONE 2013, 8, e55665. [Google Scholar] [CrossRef]
- Prieto-Domínguez, N.; Ordóñez, R.; Fernández, A.; García-Palomo, A.; Muntané, J.; González-Gallego, J.; Mauriz, J.L. Modulation of Autophagy by Sorafenib: Effects on Treatment Response. Front. Pharmacol. 2016, 7, 151. [Google Scholar] [CrossRef]
- Würstle, S.; Schneider, F.; Ringel, F.; Gempt, J.; Lämmer, F.; Delbridge, C.; Wu, W.; Schlegel, J. Temozolomide induces autophagy in primary and established glioblastoma cells in an EGFR independent manner. Oncol. Lett. 2017, 14, 322–328. [Google Scholar] [CrossRef]
- Burgos, M.H.; Ito, S.; Segal, S.J.; Tran, P.T. Effect of Gossypol on the Ultrastructure of Spisula Sperm. Biol. Bull. 1997, 193, 228–229. [Google Scholar] [CrossRef]
- Voss, V.; Senft, C.; Lang, V.; Ronellenfitsch, M.W.; Steinbach, J.P.; Seifert, V.; Kögel, D. The pan-Bcl-2 inhibitor (-)-gossypol triggers autophagic cell death in malignant glioma. Mol. Cancer Res. 2010, 8, 1002–1016. [Google Scholar] [CrossRef]
- Lian, J.; Wu, X.; He, F.; Karnak, D.; Tang, W.; Meng, Y.; Xiang, D.; Ji, M.; Lawrence, T.S.; Xu, L. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell Death Differ. 2011, 18, 60–71. [Google Scholar] [CrossRef]
- Wolter, K.G.; Wang, S.J.; Henson, B.S.; Wang, S.; Griffith, K.A.; Kumar, B.; Chen, J.; Carey, T.E.; Bradford, C.R.; D′Silva, N.J. (-)-gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia 2006, 8, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Tang, W.; Dai, Y.; Wu, X.; Liu, M.; Ji, Q.; Ji, M.; Pienta, K.; Lawrence, T.; Xu, L. Natural BH3 mimetic (-)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol. Cancer Ther. 2008, 7, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Vara, D.; Salazar, M.; Olea-Herrero, N.; Guzmán, M.; Velasco, G.; Díaz-Laviada, I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: Role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011, 18, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.; Carracedo, A.; Salanueva, I.J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S.; et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef]
- Nakanishi, C.; Toi, M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer 2005, 5, 297–309. [Google Scholar] [CrossRef]
- Liu, F.; Bardhan, K.; Yang, D.; Thangaraju, M.; Ganapathy, V.; Waller, J.L.; Liles, G.B.; Lee, J.R.; Liu, K. NF-κB directly regulates Fas transcription to modulate Fas-mediated apoptosis and tumor suppression. J. Biol. Chem. 2012, 287, 25530–25540. [Google Scholar] [CrossRef]
- Ashikawa, K.; Shishodia, S.; Fokt, I.; Priebe, W.; Aggarwal, B.B. Evidence that activation of nuclear factor-kappaB is essential for the cytotoxic effects of doxorubicin and its analogues. Biochem. Pharmacol. 2004, 67, 353–364. [Google Scholar] [CrossRef]
- Rai, A.; Kapoor, S.; Singh, S.; Chatterji, B.P.; Panda, D. Transcription factor NF-κB associates with microtubules and stimulates apoptosis in response to suppression of microtubule dynamics in MCF-7 cells. Biochem. Pharmacol. 2015, 93, 277–289. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Kaltschmidt, C.; Hofmann, T.G.; Hehner, S.P.; Dröge, W.; Schmitz, M.L. The pro- or anti-apoptotic function of NF-kappaB is determined by the nature of the apoptotic stimulus. Eur. J. Biochem. 2000, 267, 3828–3835. [Google Scholar] [CrossRef]
- Pérez, W.I.; Soto, Y.; Ortíz, C.; Matta, J.; Meléndez, E. Ferrocenes as potential chemotherapeutic drugs: Synthesis, cytotoxic activity, reactive oxygen species production and micronucleus assay. Bioorganic Med. Chem. 2015, 23, 471–479. [Google Scholar] [CrossRef][Green Version]
- Pelicano, H.; Feng, L.; Zhou, Y.; Carew, J.S.; Hileman, E.O.; Plunkett, W.; Keating, M.J.; Huang, P. Inhibition of mitochondrial respiration: A novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J. Biol. Chem. 2003, 278, 37832–37839. [Google Scholar] [CrossRef] [PubMed]
- Kirshner, J.R.; He, S.; Balasubramanyam, V.; Kepros, J.; Yang, C.Y.; Zhang, M.; Du, Z.; Barsoum, J.; Bertin, J. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol. Cancer Ther. 2008, 7, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F. Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 2012, 143, 383–396. [Google Scholar] [CrossRef] [PubMed]








| H1299 | A549 | MRC5 | |
|---|---|---|---|
| DK-164 | 22 ± 5.2 | 18.7 ± 2.3 | 130.7 ± 8.6 |
| CC-78 | 19 ± 3.7 | 12.4 ± 2.8 | 10 ± 0.8 |
| cisplatin | 28.6 ± 4.6 | 19.7 ± 3.5 | 15.4 ± 1.9 |
| tamoxifen | 35.5 ± 5.8 | 17.8 ± 2.1 | 26.9 ± 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schröder, M.; Petrova, M.; Vlahova, Z.; Dobrikov, G.M.; Slavchev, I.; Pasheva, E.; Ugrinova, I. In Vitro Anticancer Activity of Two Ferrocene-Containing Camphor Sulfonamides as Promising Agents against Lung Cancer Cells. Biomedicines 2022, 10, 1353. https://doi.org/10.3390/biomedicines10061353
Schröder M, Petrova M, Vlahova Z, Dobrikov GM, Slavchev I, Pasheva E, Ugrinova I. In Vitro Anticancer Activity of Two Ferrocene-Containing Camphor Sulfonamides as Promising Agents against Lung Cancer Cells. Biomedicines. 2022; 10(6):1353. https://doi.org/10.3390/biomedicines10061353
Chicago/Turabian StyleSchröder, Maria, Maria Petrova, Zlatina Vlahova, Georgi M. Dobrikov, Ivaylo Slavchev, Evdokia Pasheva, and Iva Ugrinova. 2022. "In Vitro Anticancer Activity of Two Ferrocene-Containing Camphor Sulfonamides as Promising Agents against Lung Cancer Cells" Biomedicines 10, no. 6: 1353. https://doi.org/10.3390/biomedicines10061353
APA StyleSchröder, M., Petrova, M., Vlahova, Z., Dobrikov, G. M., Slavchev, I., Pasheva, E., & Ugrinova, I. (2022). In Vitro Anticancer Activity of Two Ferrocene-Containing Camphor Sulfonamides as Promising Agents against Lung Cancer Cells. Biomedicines, 10(6), 1353. https://doi.org/10.3390/biomedicines10061353





