Orchestrating the Dermal/Epidermal Tissue Ratio during Wound Healing by Controlling the Moisture Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wound Dressings
2.2. In Vitro Dehydration Experiments
2.3. Porcine In Vivo Experiments
2.4. Microscopic Analysis of Wounds and Re-Epithelialization Assessment
2.5. In Vivo Dehydration Experiments
2.6. Immunofluorescence Staining
2.7. Assessment of Blood Vessel Density through Imaging Software
2.8. Statistical Analysis
3. Results
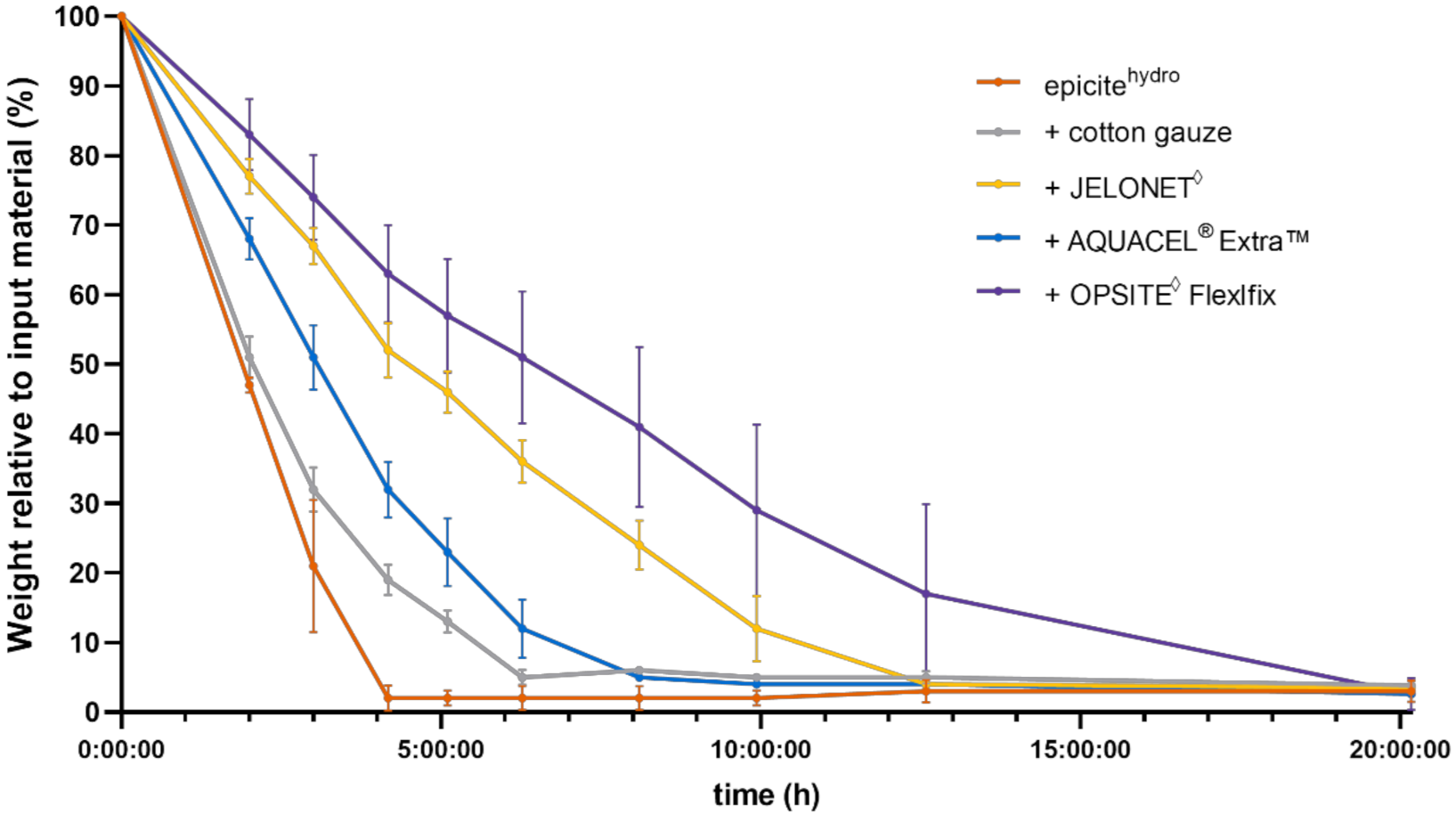
3.1. In Vitro Dehydration Experiments
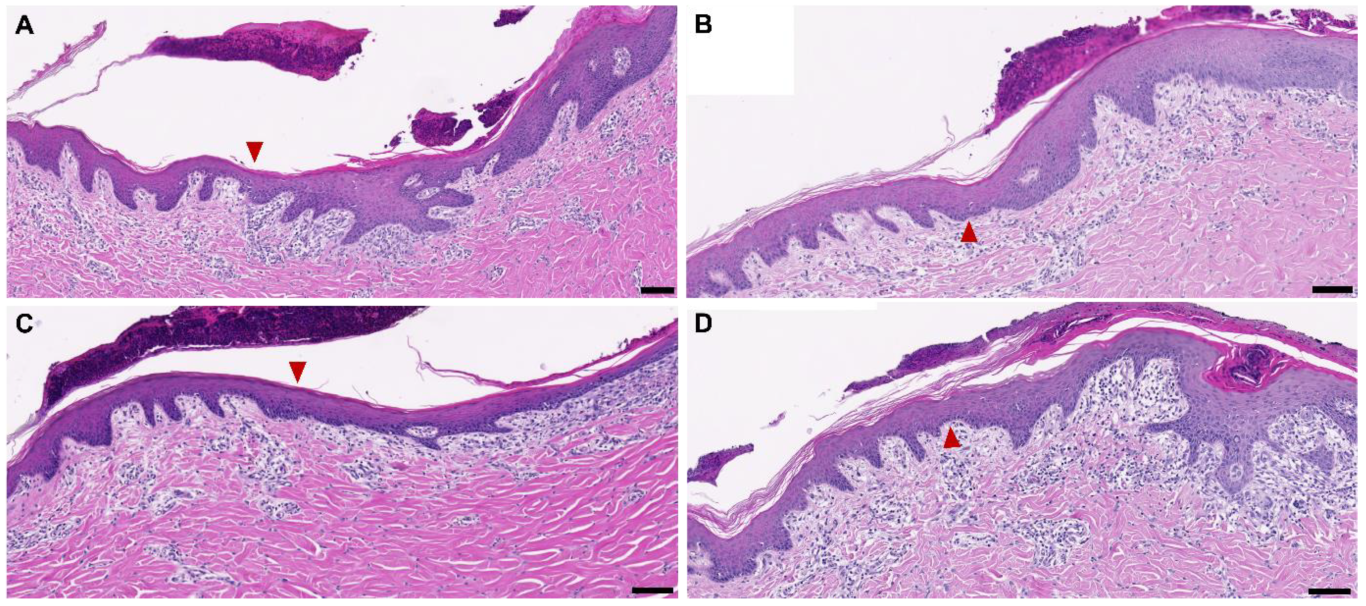
3.2. Dehydration on Wounds In Vivo and New Tissue Formation after Five Days
3.3. Influence of Nonocclusive and Occlusive Dressing after Seven Days of Treatment
4. Discussion
4.1. Comparison of In Vitro and In Vivo Water Evaporation Results
4.2. Moisture Balance on Occlusive Secondary Wound Dressing
4.3. Wound Healing Effect after Different Treatment Times
4.4. Characteristics of the Newly Formed Dermal Tissue
5. Conclusions, Limitations and Clinical Aspects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vowden, K.; Vowden, P. Understanding exudate management and the role of exudate in the healing process. Br. J. Community Nurs. 2003, 8, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.d.O.; Costa, T.F.; Andrade, Z.d.A.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowett, C.; Ayello, E. TIME principles of chronic wound bed preparation and treatment. Br. J. Nurs. 2004, 13, S16–S23. [Google Scholar] [CrossRef]
- Attinger, C.E.; Janis, J.E.; Steinberg, J.; Schwartz, J.; Al-Attar, A.; Couch, K. Clinical approach to wounds: Débridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast. Reconstr. Surg. 2006, 117, 72S–109S. [Google Scholar] [CrossRef]
- Mosti, G. Wound care in venous ulcers. Phlebology 2013, 28 (Suppl. 1), 79–85. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Mustoe, T.A. Occlusion regulates epidermal cytokine production and inhibits scar formation. Wound Repair Regen. 2010, 18, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Mustoe, T.A.; Gurjala, A. The role of the epidermis and the mechanism of action of occlusive dressings in scarring. Wound Repair Regen. 2011, 19, s16–s21. [Google Scholar] [CrossRef]
- Reish, R.G.; Zuhaili, B.; Bergmann, J.; Aflaki, P.; Koyama, T.; Hackl, F.; Waisbren, E.; Canseco, J.A.; Verma, K.D.; Eriksson, E.; et al. Modulation of scarring in a liquid environment in the Yorkshire pig. Wound Repair Regen. 2009, 17, 806–816. [Google Scholar] [CrossRef]
- Ousey, K.; Cutting, K.F.; Rogers, A.A.; Rippon, M.G. The importance of hydration in wound healing: Reinvigorating the clinical perspective. J. Wound Care 2016, 25, 122–130. [Google Scholar] [CrossRef]
- Colwell, J.C.; Ratliff, C.R.; Goldberg, M.; Baharestani, M.M.; Bliss, D.Z.; Gray, M.; Kennedy-Evans, K.L.; Logan, S.; Black, J.M. MASD Part 3. J. Wound Ostomy Cont. Nurs. 2011, 38, 541–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, M.; Weir, D. Prevention and Treatment of Moisture-Associated Skin Damage (Maceration) in the Periwound Skin. J. Wound Ostomy Cont. Nurs. 2007, 34, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Björklund, S.; Engblom, J.; Thuresson, K.; Sparr, E. A water gradient can be used to regulate drug transport across skin. J. Control Release 2010, 143, 191–200. [Google Scholar] [CrossRef]
- Björklund, S.; Ruzgas, T.; Nowacka, A.; Dahi, I.; Topgaard, D.; Sparr, E.; Engblom, J. Skin Membrane Electrical Impedance Properties under the Influence of a Varying Water Gradient. Biophys. J. 2013, 104, 2639–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, G.; Xu, P.; Lawson, L.B.; He, J.; Freytag, L.C.; Clements, J.D.; John, V.T. Hydration Effects on Skin Microstructure as Probed by High-Resolution Cryo-Scanning Electron Microscopy and Mechanistic Implications to Enhanced Transcutaneous Delivery of Biomacromolecules. J. Pharm. Sci. 2010, 99, 730–740. [Google Scholar] [CrossRef] [Green Version]
- Moritz, S.; Wiegand, C.; Wesarg, F.; Hessler, N.; Müller, F.A.; Kralisch, D.; Hipler, U.-C.; Fisher, D. Active wound dressings based on bacterial nanocellulose as drug delivery system for octenidine. Int. J. Pharm. 2014, 471, 45–55. [Google Scholar] [CrossRef]
- Wiegand, C.; Hipler, U.-C. Polymer-based Biomaterials as Dressings for Chronic Stagnating Wounds. Macromol. Symp. 2010, 294, 1–13. [Google Scholar] [CrossRef]
- Lachenbruch, C.; VanGilder, C. Estimates of evaporation rates from wounds for various dressing/support surface combinations. Adv. Skin Wound Care 2012, 25, 29–36. [Google Scholar] [CrossRef]
- Kim, N.T.; Elie, N.; Plancoulaine, B.; Herlin, P.; Coster, M. An Original Approach for Quantification of Blood Vessels on the Whole Tumour Section. Anal. Cell Pathol. 2003, 25, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Rust, R.; Kirabali, T.; Grönnert, L.; Dogancay, B.; Limasale, Y.D.P.; Meinhardt, A.; Werner, C.; Laviña, B.; Kulic, L.; Nitsch, R.M.; et al. A Practical Guide to the Automated Analysis of Vascular Growth, Maturation and Injury in the Brain. Front. Neurosci. 2020, 14, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Xia, H.; He, W.; Li, Z.; Zhao, J.; Liu, B.; Wang, Y.; Lei, Q.; Kong, Y.; Bai, Y.; et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci. Rep. 2016, 6, 24596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattelaens, J.; Turco, L.; Berclaz, L.M.; Huelsse, B.; Hitzl, W.; Vollkommer, T.; Bodenschatz, K.J. The Impact of a Nanocellulose-Based Wound Dressing in the Management of Thermal Injuries in Children: Results of a Retrospective Evaluation. Life 2020, 10, 212. [Google Scholar] [CrossRef]
- Bryan, J. Moist wound healing: A concept that changed our practice. J. Wound Care 2004, 13, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Haith, L.R.; Patton, M.L.; Goldman, W.T. Cultured Epidermal Autograft and the Treatment of the Massive Burn Injury. J. Burn Care Rehabil. 1992, 13, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Okan, D.; Woo, K.; Ayello, E.A.; Sibbald, G. The Role of Moisture Balance in Wound Healing. Adv. Skin Wound Care 2007, 20, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Rietschel, R.L.; Allen, A.M. Effects of prolonged continuous exposure of human skin to water: A reassessment. J. Investig. Dermatol. 1977, 68, 79–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippon, M.G.; Ousey, K.; Cutting, K.F. Wound healing and hyper-hydration: A counterintuitive model. J. Wound Care 2016, 25, 68, 70–75. [Google Scholar] [CrossRef]
- Egawa, M.; Hirao, T.; Takahashi, M. In vivo estimation of stratum corneum thickness from water concentration profiles obtained with Raman spectroscopy. Acta Derm. Venereol. 2007, 87, 4–8. [Google Scholar] [CrossRef] [Green Version]
- de Mattos, I.B.; Holzer, J.C.J.; Tuca, A.-C.; Groeber-Becker, F.; Funk, M.; Popp, D.; Mautner, S.; Birngruber, T.; Kamolz, P. Uptake of PHMB in a bacterial nanocellulose-based wound dressing: A feasible clinical procedure. Burns 2019, 45, 898–904. [Google Scholar] [CrossRef]
- de Mattos, I.B.; Nischwitz, S.P.; Tuca, A.-C.; Groeber-Becker, F.; Funk, M.; Birngruber, T.; Mautner, S.I.; Kamolz, L.-P.; Holzer, J.C.J. Delivery of antiseptic solutions by a bacterial cellulose wound dressing: Uptake, release and antibacterial efficacy of octenidine and povidone-iodine. Burns 2020, 46, 918–927. [Google Scholar] [CrossRef]
- Parnell, L.K.S.; Volk, S.W. The Evolution of Animal Models in Wound Healing Research: 1993–2017. Adv. Wound Care 2019, 8, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The pig as a model for human wound healing. Wound Repair Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef]
- Seaton, M.; Hocking, A.; Gibran, N.S. Porcine Models of Cutaneous Wound Healing. ILAR J. 2015, 56, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.D.; Scales, J.T. Effect of Air Drying and Dressings on the Surface of a Wound. Nature 1963, 197, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Hinman, C.D.; Maibach, H. Effect of Air Exposure and Occlusion on Experimental Human Skin Wounds. Nature 1963, 200, 377–378. [Google Scholar] [CrossRef]
- Ogawa, R. Ideal wound closure methods for minimizing scarring after surgery. In Textbook on Scar Management; Springer International Publishing: Cham, Switzerland, 2020; pp. 185–191. [Google Scholar]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiraldi, M.; Raucci, A.; Muñoz, L.M.; Livoti, E.; Celona, B.; Venereau, E.; Apuzzo, T.; De Marchis, F.; Pedotti, M.; Bachi, A.; et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J. Exp. Med. 2012, 209, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yu, J.; Xu, Y.; Chen, L.; Zhou, F.; Zhai, Q.; Wu, J.; Shu, B.; Qi, S. Epidermal HMGB1 Activates Dermal Fibroblasts and Causes Hypertrophic Scar Formation in Reduced Hydration. J. Investig. Dermatol. 2018, 138, 2322–2332. [Google Scholar] [CrossRef] [Green Version]
- Ploeger, D.T.; Hosper, N.A.; Schipper, M.; Koerts, J.A.; de Rond, S.; Bank, R.A. Cell plasticity in wound healing: Paracrine factors of M1/ M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun. Signal. 2013, 11, 29. [Google Scholar] [CrossRef] [Green Version]




| Score Description | |
|---|---|
| 0 | None in tissue |
| 1 | Very low density in the new dermal tissue at around mid-depth; a few cells could be found when roaming through the tissue |
| 2 | Low density of these cells in the new tissue; 1 to 5 cells may have been found at high magnifications of 200× or 100% of the scan at mid-depth |
| 3 | Significant intermediate density, or around many vessels; 6 to 20 cells noted in about every high-power field (200× or 100% magnification) |
| 4 | High density; at least 21–100 cells in about every high-power field; may focally partially obliterate (erase) the new dermal tissue |
| 5 | Coalescing to diffuse (e.g., a lymph node for small mononuclear cells or a granuloma for epithelioid macrophages/multinucleate giant cells), almost completely erasing the new “granulation tissue” |
| Five Day Treatment | Seven Day Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Residual Water In Vivo | BNC Thickness In Vivo | % Re-Epithelial | New Granulation Tissue | Residual Water In Vivo | % Re-Epithelial | New Granulation Tissue | |
| Evaporation rates in vitro | −0.87 * | −0.79 * | 0.76 * | −0.71 * | −0.84 * | 0.93 * | −0.93 * |
| −0.96 to −0.0 | −0.94 to −0.40 | 0.32 to 0.93 | −0.91 to −0.22 | −0.97 to −0.34 | 0.66 to 0.99 | −0.99 to −0.64 | |
| Residual water in vivo | 0.90* | −0.63 * | 0.70 * | −0.86 * | 0.85 * | ||
| 0.70 to 0.97 | −0.89 to −0.09 | 0.20 to 0.91 | −0.97 to −0.39 | 0.36 to 0.97 | |||
| BNC thickness in vivo | −0.70 * | 0.54 | |||||
| −0.91 to −0.20 | −0.048 to 0.85 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuca, A.-C.; Bernardelli de Mattos, I.; Funk, M.; Winter, R.; Palackic, A.; Groeber-Becker, F.; Kruse, D.; Kukla, F.; Lemarchand, T.; Kamolz, L.-P. Orchestrating the Dermal/Epidermal Tissue Ratio during Wound Healing by Controlling the Moisture Content. Biomedicines 2022, 10, 1286. https://doi.org/10.3390/biomedicines10061286
Tuca A-C, Bernardelli de Mattos I, Funk M, Winter R, Palackic A, Groeber-Becker F, Kruse D, Kukla F, Lemarchand T, Kamolz L-P. Orchestrating the Dermal/Epidermal Tissue Ratio during Wound Healing by Controlling the Moisture Content. Biomedicines. 2022; 10(6):1286. https://doi.org/10.3390/biomedicines10061286
Chicago/Turabian StyleTuca, Alexandru-Cristian, Ives Bernardelli de Mattos, Martin Funk, Raimund Winter, Alen Palackic, Florian Groeber-Becker, Daniel Kruse, Fabian Kukla, Thomas Lemarchand, and Lars-Peter Kamolz. 2022. "Orchestrating the Dermal/Epidermal Tissue Ratio during Wound Healing by Controlling the Moisture Content" Biomedicines 10, no. 6: 1286. https://doi.org/10.3390/biomedicines10061286
APA StyleTuca, A.-C., Bernardelli de Mattos, I., Funk, M., Winter, R., Palackic, A., Groeber-Becker, F., Kruse, D., Kukla, F., Lemarchand, T., & Kamolz, L.-P. (2022). Orchestrating the Dermal/Epidermal Tissue Ratio during Wound Healing by Controlling the Moisture Content. Biomedicines, 10(6), 1286. https://doi.org/10.3390/biomedicines10061286








