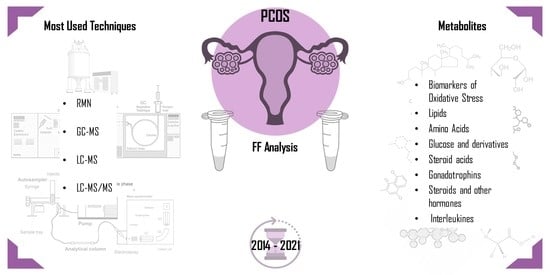
Follicular Fluid: A Powerful Tool for the Understanding and Diagnosis of Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Follicular Fluid (FF)
3. Metabolomics in PCOS
4. Biomarkers in Follicular Fluid
4.1. Biomarkers of Oxidative Stress
4.2. Lipids
4.3. Amino Acids
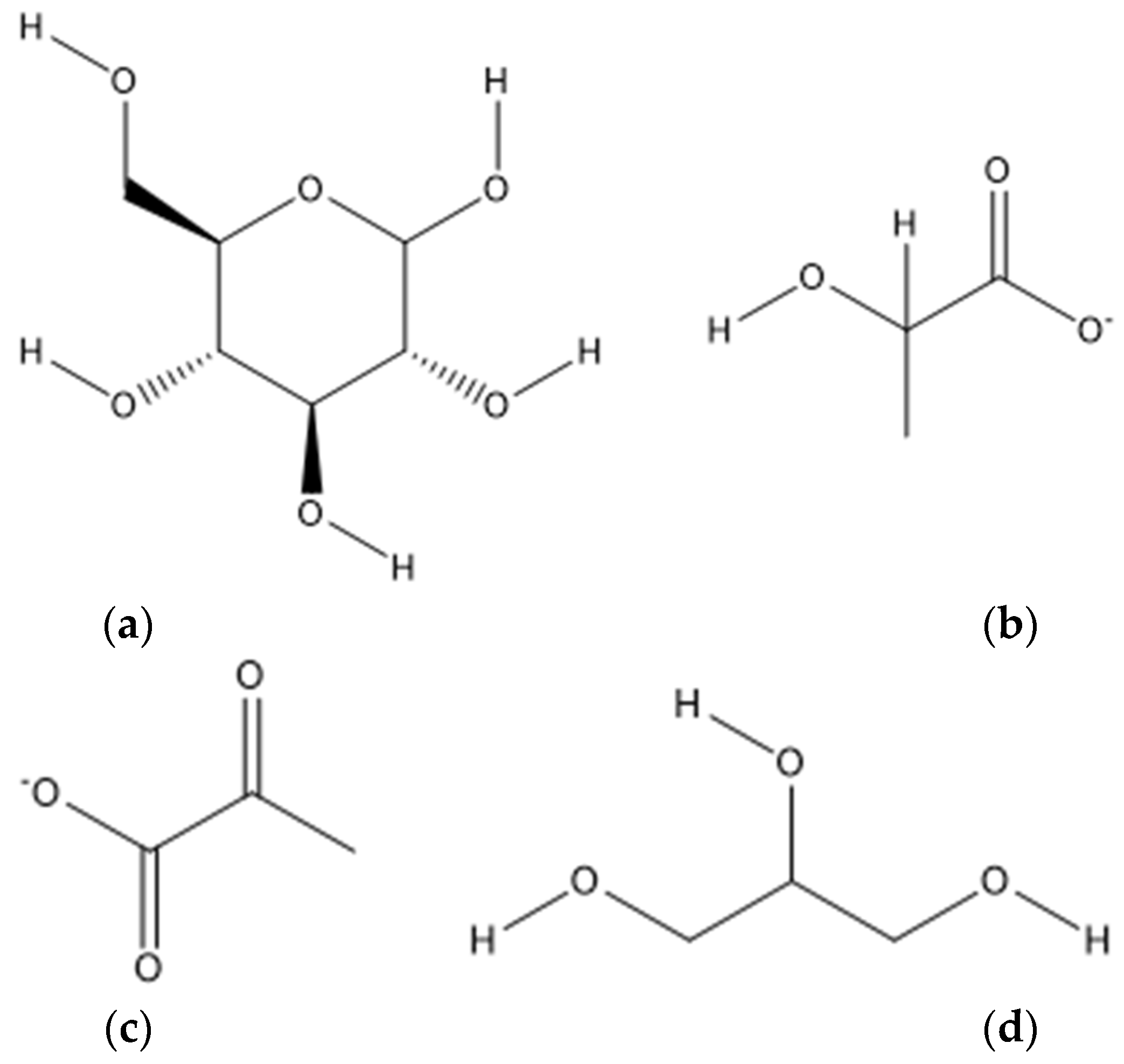
4.4. Glucose and Derivatives
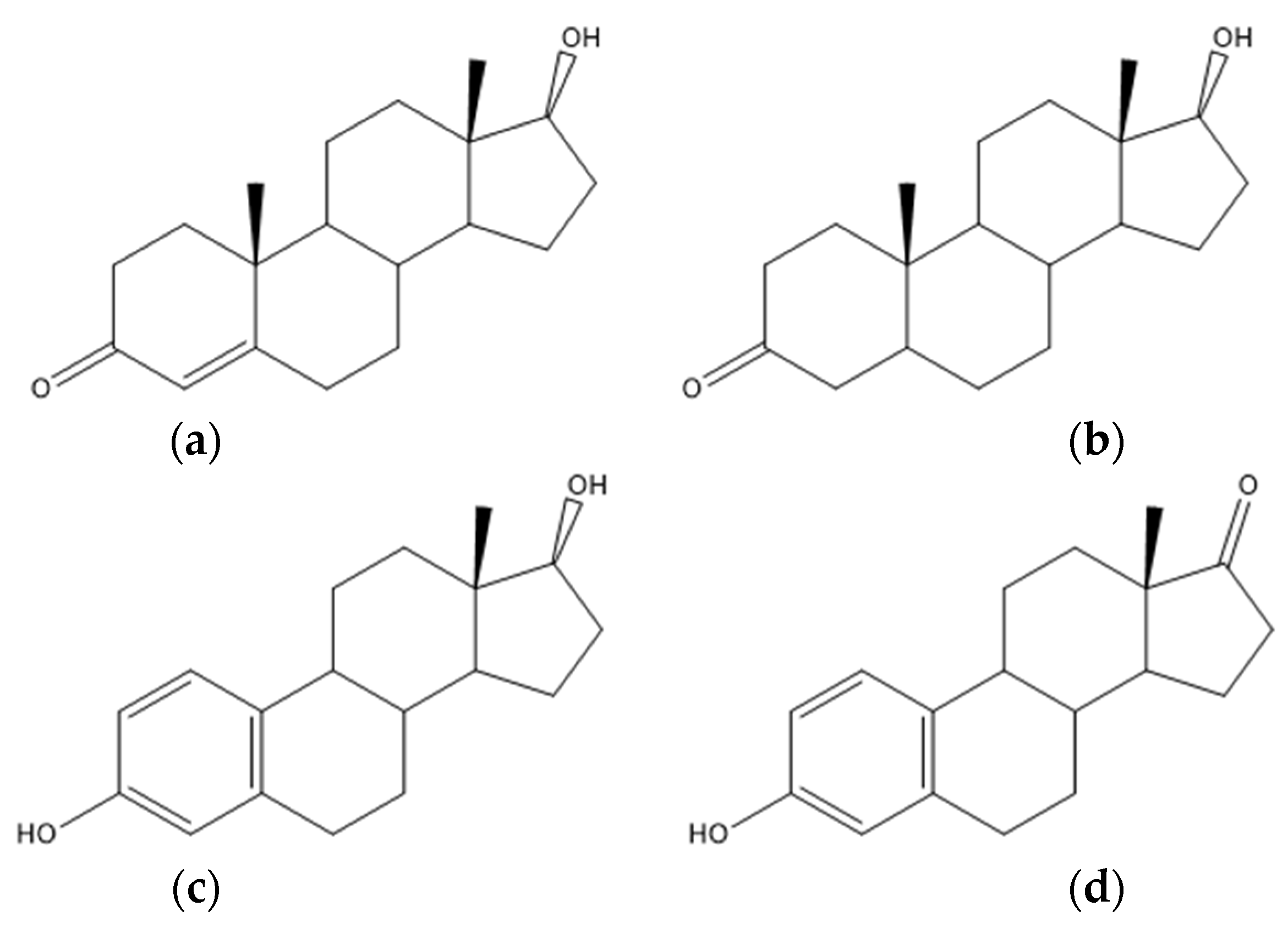
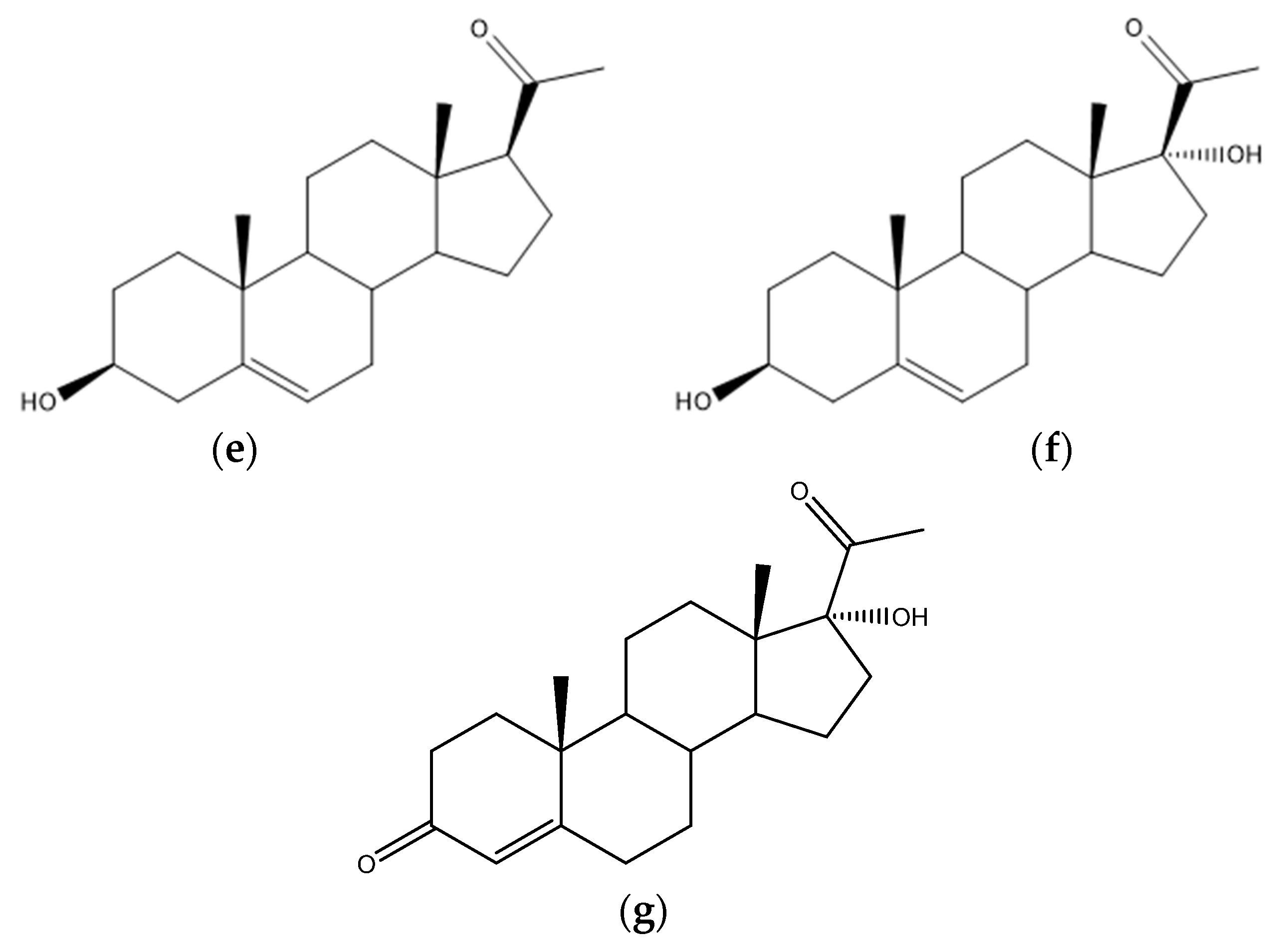
4.5. Steroid Acids
4.6. Gonadotrophines
4.7. Steroids and Other Hormones
4.8. Interleukines
5. Discussion (Perspectives)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Liu, M.; Wang, Z.; Liu, T.; Liu, S.; Wang, B.; Pan, B.; Dong, X.; Guo, W. Correlation between steroid levels in follicular fluid and hormone synthesis related substances in its exosomes and embryo quality in patients with polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2021, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Ran, H.; Chen, Y.; Ma, L. Lipidomics analysis of human follicular fluid form normal-weight patients with polycystic ovary syndrome: A pilot study. J. Ovarian Res. 2021, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pang, Y. Metabolic Syndrome and PCOS: Pathogenesis and the Role of Metabolites. Metabolites 2021, 11, 869. [Google Scholar] [CrossRef]
- Liu, R.; Bai, S.; Zheng, S.; Zhu, X.; Zhang, Y.; Xu, B.; Zhao, W. Identification of the Metabolomics Signature of Human Follicular Fluid from PCOS Women with Insulin Resistance. Dis. Markers 2022, 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, F.J.; Knauff, E.A.H.; Valkenburg, O.; Laven, J.S.; Eijkemans, M.J.; Fauser, B.C.J.M. PCOS according to the Rotterdam consensus criteria: Change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R. PCOS: A diagnostic challenge. Reprod. Biomed. Online 2004, 8, 644–648. [Google Scholar] [CrossRef]
- Azziz, R.; Hincapie, L.A.; Knochenhauer, E.S.; Dewailly, D.; Fox, L.; Boots, L.R. Screening for 21-hydroxylase-deficient nonclassic adrenal hyperplasia among hyperandrogenic women: A prospective study. Fertil. Steril. 1999, 72, 915–925. [Google Scholar] [CrossRef]
- Azziz, R.; Tarlatzis, R.; Dunaif, A.; Ibanez, L.; Pugeat, M.; Taylor, A.; Fauser, C.J.M.; Medicine, R. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Pache, T.D.; Wladimiroff, J.W.; Hop, W.C.J.; Fauser, B.C.J.M. How to discriminate between normal and polycystic ovaries-transvaginal US study. Radiology 1992, 183, 421–423. [Google Scholar] [CrossRef]
- Jonard, S.; Robert, Y.; Cortet-Rudelli, C.; Pigny, P.; Decanter, C.; Dewailly, D. Ultrasound examination of polycystic ovaries: Is it worth counting the follicles? Hum. Reprod. 2003, 18, 598–603. [Google Scholar] [CrossRef]
- Balen, A. Ovulation induction for polycystic ovary syndrome. Hum. Fertil. 2000, 3, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Van Santbrink, E.J.P.; Hop, W.C.; Fauser, B.C.J.M. Classification of normogonadotropic infertility: Polycystic ovaries diagnosed by ultrasound versus endocrine characteristics of polycystic ovary syndrome. Fertil. Steril. 1997, 67, 452–458. [Google Scholar] [CrossRef]
- Christensen, J.T.; Boldsen, J.; Westergaard, J.G. Ovarian volume in gynecologically healthy women using no contraception, or using IUD or oral contraception. Acta Obstet. Gynecol. Scand. 1997, 76, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Dwpolson, D.; Franks, S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br. Med. J. (Clin. Res. Ed.) 1986, 293, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Franks, S. Polycystic Ovary Syndrome: A Changing Perspective. Clin. Endocrinol. 1989, 31, 87–120. [Google Scholar] [CrossRef]
- Carmina, E.; Lobo, R.A. Polycystic ovaries in hirsute women with normal menses. Am. J. Med. 2001, 111, 602–606. [Google Scholar] [CrossRef]
- Cleeman, J.I. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). J. Am. Med. Assoc. 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Mohammad, M.B.; Seghinsara, A.M. Polycystic ovary syndrome (PCOS), diagnostic criteria, and AMH. Asian Pac. J. Cancer Prev. 2017, 18, 17–21. [Google Scholar] [CrossRef]
- de Zegher, F.; López-Bermejo, A.; Ibáñez, L. Central Obesity, Faster Maturation, and ‘PCOS’ in Girls. Trends Endocrinol. Metab. 2018, 29, 815–818. [Google Scholar] [CrossRef]
- Ehrmann, D.A.; Barnes, R.B.; Rosenfield, R.L.; Cavaghan, M.K.; Imperial, J. Prevalence of Impaired Glucose Tolerance and Diabetes in Women Wi t h Polycystic Ovary Syndrome. Diabetes Care 1999, 22, 141–146. [Google Scholar] [CrossRef]
- Dunaif, A.; Legro, R.S. Prevalence and Predictors of Risk for Type 2 Diabetes Mellitus and Impaired Glucose Tolerance in Polycystic Ovary Syndrome-Authors’ Response. J. Clin. Endocrinol. Metab. 1999, 84, 2975–2976. [Google Scholar] [CrossRef]
- Dunaif, A.; Graf, M.; Mandeli, J.; Laumas, V.; Dobrjansky, A. Characterization of Groups of Hyperaiidrogenic Women with Acanthosis Nigricans, Impaired Glucose Tolerance, and/or Hyperinsulinemia. J. Clin. Endocrinol. Metab. 1987, 65, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Kiddy, D.; Gelding, S.V.; Willis, D.; Niththyananthan, R.; Bush, A.; Johnston, D.G.; Franks, S. The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin. Endocrinol. 1993, 39, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, E.; Janson, P.O.; Johansson, S.; Lapidus, L.; Odén, A. Polycystic ovary syndrome and risk for myocardial infarction: Evaluated from a risk factor model based on a prospective population study of women. Acta Obstet. Gynecol. Scand. 1992, 71, 599–604. [Google Scholar] [CrossRef]
- Kiddy, D.S.; Hamilton-Fairley, D.; Bush, A.; Short, F.; Anyaoku, V.; Reed, M.J.; Franks, S. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin. Endocrinol. 1992, 36, 105–111. [Google Scholar] [CrossRef]
- Clark, A.M.; Ledger, W.; Galletly, C.; Tomlinson, L.; Blaney, F.; Wang, X.; Norman, R.J. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum. Reprod. 1995, 10, 2705–2712. [Google Scholar] [CrossRef]
- Huber-Buchholz, M.M.; Carey, D.G.P.; Norman, R.J. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: Role of insulin sensitivity and luteinizing hormone. J. Clin. Endocrinol. Metab. 1999, 84, 1470–1474. [Google Scholar] [CrossRef]
- Morán, C.; Knochenhauer, E.; Boots, L.R.; Azziz, R. Adrenal androgen excess in hyperandrogenism: Relation to age and body mass. Fertil. Steril. 1999, 71, 671–674. [Google Scholar] [CrossRef]
- Dunaif, A.; Segal, K.R.; Futterweit, W.; Dobrjansky, A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989, 38, 1165–1174. [Google Scholar] [CrossRef]
- Niu, Z.; Lin, N.; Gu, R.; Sun, Y.; Feng, Y. Associations between insulin resistance, free fatty acids, and oocyte quality in polycystic ovary syndrome during in vitro fertilization. J. Clin. Endocrinol. Metab. 2014, 99, E2269–E2276. [Google Scholar] [CrossRef]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Crews, L. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: Executive summary. Am. J. Clin. Nutr. 1998, 68, 899–917. [Google Scholar] [CrossRef]
- Gongadashetti, K.; Gupta, P.; Dada, R.; Malhotra, N. Follicular fluid oxidative stress biomarkers and art outcomes in PCOS women undergoing in vitro fertilization: A cross-sectional study. Int. J. Reprod. Biomed. 2021, 19, 449–456. [Google Scholar] [CrossRef]
- Dahlgren, E.; Johansson, S.; Lindstedt, G.; Knutsson, F.; Oden, A.; Janson, P.O.; Mattson, L.A.; Crona, N.; Lundberg, P.A. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: A long-term follow-up focusing on natural history and circulating hormones. Fertil. Steril. 1992, 57, 505–513. [Google Scholar] [CrossRef]
- Wild, S.; Pierpoint, T.; McKeigue, P.; Jacobs, H. Cardiovascular disease in women with polycystic ovary syndrome at long- term follow-up: A retrospective cohort study. Clin. Endocrinol. 2000, 52, 595–600. [Google Scholar] [CrossRef]
- Wild, R.A. Long-term health consequences of PCOS. Hum. Reprod. Update 2002, 8, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Conway, G.S.; Agrawal, R.; Betteridge, D.J.; Jacobs, H.S. Risk factors for coronary artery disease in lean and obese women with the polycystic ovary syndrome. Clin. Endocrinol. 1992, 37, 119–125. [Google Scholar] [CrossRef]
- Robinson, S.; Henderson, A.D.; Gelding, S.V.; Kiddy, D.; Niththyananthan, R.; Bush, A.; Richmond, W.; Johnston, D.G.; Franks, S. Dyslipidaemia is associated with insulin resistance in women with polycystic ovaries. Clin. Endocrinol. 1996, 44, 277–284. [Google Scholar] [CrossRef]
- Talbott, E.; Clerici, A.; Berga, S.L.; Kuller, L.; Guzick, D.; Detre, K.; Daniels, T.; Engberg, R.A. Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: Results of a case-control study. J. Clin. Epidemiol. 1998, 51, 415–422. [Google Scholar] [CrossRef]
- Legro, R.S.; Kunselman, A.R.; Dunaif, A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am. J. Med. 2001, 111, 607–613. [Google Scholar] [CrossRef]
- Paradisi, G.; Steinberg, H.O.; Hempfling, A.; Cronin, J.; Hook, G.; Shepard, M.K.; Baron, A.D. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 2001, 103, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Christian, R.C.; Dumesic, D.A.; Behrenbeck, T.; Oberg, A.L.; Sheedy, P.F.; Fitzpatrick, L.A. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Talbott, E.O.; Guzick, D.S.; Sutton-Tyrrell, K.; McHugh-Pemu, K.P.; Zborowski, J.V.; Remsberg, K.E.; Kuller, L.H. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arter. Thromb. Vasc. Biol. 2000, 20, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, P.; Pillay, O.S.; Atiomo, W. Polycystic ovary syndrome and endometrial carcinoma. Lancet 2003, 361, 1810–1812. [Google Scholar] [CrossRef]
- Naessen, T.; Kushnir, M.M.; Chaika, A.; Nosenko, J.; Mogilevkina, I.; Rockwood, A.L.; Carlstrom, K.; Bergquist, J.; Kirilovas, D. Steroid profiles in ovarian follicular fluid in women with and without polycystic ovary syndrome, analyzed by liquid chromatography-tandem mass spectrometry. Fertil. Steril. 2010, 94, 2228–2233. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Kouli, C.R.; Bergiele, A.T.; Filandra, F.A.; Tsianateli, T.C.; Spina, G.G.; Zapanti, E.D.; Bartzis, M.I. A survey of the polycystic ovary syndrome in the Greek Island of Lesbos: Hormonal and metabolic profile. J. Clin. Endocrinol. Metab. 1999, 84, 4006–4011. [Google Scholar] [CrossRef] [PubMed]
- Slayden, S.M.; Moran, C.; Sams, W.M.; Boots, L.R.; Azziz, R. Hyperandrogenemia in patients presenting with acne. Fertil. Steril. 2001, 75, 889–892. [Google Scholar] [CrossRef]
- Futterweit, W.; Dunaif, A.; Yeh, H.C.; Kingsley, P. The prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. J. Am. Acad. Dermatol. 1988, 19, 831–836. [Google Scholar] [CrossRef]
- Laven, J.S.E.; Imani, B.; Eijkemans, M.J.C.; Fauser, B.C.J.M. New approach to polycystic ovary syndrome and other forms of anovulatory infertility. Obstet. Gynecol. Surv. 2002, 57, 755–767. [Google Scholar] [CrossRef]
- Knochenhauer, E.S.; Key, T.J.; Kahsar-Miller, M.; Waggoner, W.; Boots, L.R.; Azziz, R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the Southeastern United States: A prospective study. J. Clin. Endocrinol. Metab. 1998, 83, 3078–3082. [Google Scholar] [CrossRef]
- Pugeat, M.; Nicolas, M.H.; Craves, J.C.; Alvarado-, C.; Fimbel, S.; Dechaud, H.; Lyon, H.C. De Androgens in Polycystic Ovarian Syndrome. Androg. Polycystic Ovarian Syndr. 1993, 687, 124–135. [Google Scholar]
- Balen, A.H.; Conway, G.S.; Kaltsas, G.; Techatraisak, K.; Manning, P.J.; West, C.; Jacobs, H.S. Andrology: Polycystic ovary syndrome: The spectrum of the disorder in 1741 patients. Hum. Reprod. 1995, 10, 2107–2111. [Google Scholar] [CrossRef] [PubMed]
- Asunción, M.; Calvo, R.M.; San Millá, J.L.; Sancho, J.; Avila, S.; Escobar-Morreale, H.F. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J. Clin. Endocrinol. Metab. 2000, 85, 2434–2438. [Google Scholar] [CrossRef]
- Legro, R.S.; Driscoll, D.; Strauss, J.F.; Fox, J.; Dunaif, A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc. Natl. Acad. Sci. USA 1998, 95, 14956–14960. [Google Scholar] [CrossRef] [PubMed]
- Boots, L.R.; Potter, S.; Potter, H.D.; Azziz, R. Measurement of total serum testosterone levels using commercially available kits: High degree of between-kit variability. Fertil. Steril. 1998, 69, 286–292. [Google Scholar] [CrossRef]
- Rosner, W. Errors in the measurement of plasma free testosterone. J. Clin. Endocrinol. Metab. 1997, 82, 2014–2015. [Google Scholar] [CrossRef][Green Version]
- Vermeulen, A.; Verdonck, L.; Kaufman, J.M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab. 1999, 84, 3666–3672. [Google Scholar] [CrossRef]
- Sorimachi, K. Improved chromatographic methods for the separation of thyroid hormones and their metabolites. Anal. Biochem. 1979, 93, 31–36. [Google Scholar] [CrossRef]
- Stanczyk, F.Z.; Clarke, N.J. Advantages and challenges of mass spectrometry assays for steroid hormones. J. Steroid Biochem. Mol. Biol. 2010, 121, 491–495. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Liquid chromatography methodologies for the determination of steroid hormones in aquatic environmental systems. Trends Environ. Anal. Chem. 2014, 3, 14–27. [Google Scholar] [CrossRef]
- Rittmaster, R.S. Androgen conjugates: Physiology and clinical significance. Endocr. Rev. 1993, 14, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Bili, H.; Laven, J.; Imani, B.; Eijkemans, M.J.C.; Fauser, B.C.J.M. Age-related differences in features associated with polycystic ovary syndrome in normogonadotrophic oligo-amenorrhoeic infertile women of reproductive years. Eur. J. Endocrinol. 2001, 145, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Hill, M.; Starka, L. The best correlation of the new index of hyperandrogenism with the grade of increased body hair. Eur. J. Endocrinol. 2000, 143, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Imani, B.; Eijkemans, M.J.C.; De Jong, F.H.; Payne, N.N.; Bouchard, P.; Giudice, L.C.; Fauser, B.C.J.M. Free androgen index and leptin are the most prominent endocrine predictors of ovarian response during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J. Clin. Endocrinol. Metab. 2000, 85, 676–682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meldrum, D.R.; Abraham, G.E. Peripheral and ovarian venous concentrations of various steroid hormones in virilizing ovarian tumors. Obstet. Gynecol. 1979, 53, 36–43. [Google Scholar] [PubMed]
- Fauser, B.C.J.M.; Pache, T.D.; Lamberts, S.W.J.; Hop, W.C.J.; De Jong, F.H.; Dahl, K.D. Serum bioactive and immunoreactive luteinizing hormone and follicle-stimulating hormone levels in women with cycle abnormalities, with or without polycystic ovarian disease. J. Clin. Endocrinol. Metab. 1991, 73, 811–817. [Google Scholar] [CrossRef]
- Taylor, A.E.; Mccourt, B.; Martin, K.A.; Anderson, E.J.; Adams, J.M.; Schoenfeld, D.; Hall, J.E. Determinants of Abnormal Gonadotropin Secretion in Clinically Defined Women with Polycystic Ovary Syndrome * Prospective evaluation of consensus criteria for Polycystic Ovary Syndrome: Evidence for subgroups char-acterized by inverse defects of LH and insu. J. Clin. Endocrinol. Metab. 1997, 82, 2248–2256. [Google Scholar]
- Waldstreicher, J.; Santoro, N.F.; Hall, J.E.; Filicori, M.; Crowley, W.F. Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: Indirect evidence for partial gonadotroph desensitization. J. Clin. Endocrinol. Metab. 1988, 66, 165–172. [Google Scholar] [CrossRef]
- Balen, A.H.; Tan, S.L.; Macdougall, J.; Jacobs, H.S. Miscarriage rates following in-vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with buserelin. Hum. Reprod. 1993, 8, 959–964. [Google Scholar] [CrossRef]
- Tarlatzis, B.C.; Grimbizis, G.; Pournaropoulos, F.; Bontis, J.; Lagos, S.; Spanos, E.; Mantalenakis, S. The prognostic value of basal luteinizing hormone: Follicle-stimulating hormone ratio in the treatment of patients with polycystic ovarian syndrome by assisted reproduction techniques. Hum. Reprod. 1995, 10, 2545–2549. [Google Scholar] [CrossRef]
- Gordon, U.D.; Harrison, R.F.; Fawzy, M.; Hennelly, B.; Gordon, A.C. A randomized prospective assessor-blind evaluation of luteinizing hormone dosage and in vitro fertilization outcome. Fertil. Steril. 2001, 75, 324–331. [Google Scholar] [CrossRef]
- Mendoza, C.; Ruiz-Requena, E.; Ortega, E.; Cremades, N.; Martinez, F.; Bernabeu, R.; Greco, E.; Tesarik, J. Follicular fluid markers of oocyte developmental potential. Hum. Reprod. 2002, 17, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Homburg, R.; Levy, T.; Berkovitz, D.; Farchi, J.; Feldberg, D.; Ashkenazi, J.; Ben-Rafael, Z. Gonadotropin-releasing hormone agonist reduces the miscarriage rate for pregnancies achieved in women with polycystic ovarian syndrome. Fertil. Steril. 1993, 59, 527–531. [Google Scholar] [CrossRef]
- Clifford, K.; Rai, R.; Watson, H.; Franks, S.; Regan, L. Does suppressing luteinising hormone secretion reduce the miscarriage rate? Results of a randomised controlled trial. BMJ 1996, 312, 1508–1511. [Google Scholar] [CrossRef]
- Younglai, E.V.; Foster, W.G.; Hughes, E.G.; Trim, K.; Jarrell, J.F. Levels of environmental contaminants in human follicular fluid, serum, and seminal plasma of couples undergoing in vitro fertilization. Arch. Environ. Contam. Toxicol. 2002, 43, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, R.; Qi, D.; Fu, L.; Song, T.; Wang, Y.; Bian, Y.; Shi, Y. Profile of Bile Acid Metabolomics in the Follicular Fluid of PCOS Patients. Metabolites 2021, 11, 845. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, A.; Wallace, M.; Cottell, E.; Gibney, M.J.; McAuliffe, F.M.; Wingfield, M.; Brennan, L. Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction 2013, 146, 389–395. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, H.; Lian, F.; Zhang, X.; Pang, C.; Guo, Y.; Song, J.; Wang, A.; Shi, L.; Han, L. Human Follicular Fluid Metabolomics Study of Follicular Development and Oocyte Quality. Chromatographia 2017, 80, 901–909. [Google Scholar] [CrossRef]
- Luti, S.; Fiaschi, T.; Magherini, F.; Modesti, P.A.; Piomboni, P.; Governini, L.; Luddi, A.; Amoresano, A.; Illiano, A.; Pinto, G.; et al. Relationship between the metabolic and lipid profile in follicular fluid of women undergoing in vitro fertilization. Mol. Reprod. Dev. 2020, 87, 986–997. [Google Scholar] [CrossRef]
- Luddi, A.; Governini, L.; Capaldo, A.; Campanella, G.; De Leo, V.; Piomboni, P.; Morgante, G. Characterization of the age-dependent changes in antioxidant defenses and protein’s sulfhydryl/carbonyl stress in human follicular fluid. Antioxidants 2020, 9, 927. [Google Scholar] [CrossRef]
- Kalinina, E.A.; Malushko, A.V.; Zubareva, T.M.; Sitkin, S.I.; Dedul, A.G.; Sheveleva, T.S.; Gamzatova, Z.H.; Bejenar, V.F.; Komlichenko, E.V. Metabolomics: The perspective search of methods to overcome infertility. Gynecol. Endocrinol. 2015, 31, 79–82. [Google Scholar] [CrossRef]
- Gupta, S.; Ghulmiyyah, J.; Sharma, R.; Halabi, J.; Agarwal, A. Power of proteomics in linking oxidative stress and female infertility. BioMed Res. Int. 2014, 2014, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Mirabi, P.; Chaichi, M.J.; Esmaeilzadeh, S.; Jorsaraei, S.G.A.; Bijani, A.; Ehsani, M. Does different BMI influence oocyte and embryo quality by inducing fatty acid in follicular fluid? Taiwan. J. Obstet. Gynecol. 2017, 56, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yin, T.-L.; Chen, Y.; Li, Y.; Yin, L.; Ding, J.; Yang, J.; Feng, H.L. Follicular dynamics of glycerophospholipid and sphingolipid metabolisms in polycystic ovary syndrome patients. J. Steroid Biochem. Mol. Biol. 2018, 185, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Piomboni, P.; Focarelli, R.; Capaldo, A.; Stendardi, A.; Cappelli, V.; Cianci, A.; La Marca, A.; Luddi, A.; De Leo, V. Protein modification as oxidative stress marker in follicular fluid from women with polycystic ovary syndrome: The effect of inositol and metformin. J. Assist. Reprod. Genet. 2014, 31, 1269–1276. [Google Scholar] [CrossRef]
- Tarín, J.J.; Pérez-Albalá, S.; Cano, A. Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Mol. Reprod. Dev. 2002, 61, 385–397. [Google Scholar] [CrossRef]
- Luddi, A.; Capaldo, A.; Focarelli, R.; Gori, M.; Morgante, G.; Piomboni, P.; De Leo, V. Antioxidants reduce oxidative stress in follicular fluid of aged women undergoing IVF. Reprod. Biol. Endocrinol. 2016, 14, 1–7. [Google Scholar] [CrossRef]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.R.; Harrison, D.G.; Bhatnagar, A. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement from the American Heart Association. Circ. Res. 2016, 119, e39–e75. [Google Scholar] [CrossRef]
- Freitas, C.; Neto, A.C.; Matos, L.; Silva, E.; Ribeiro, Â.; Silva-Carvalho, J.L.; Almeida, H. Follicular Fluid redox involvement for ovarian follicle growth. J. Ovarian Res. 2017, 10, 44. [Google Scholar] [CrossRef]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian aging: Mechanisms and clinical consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef]
- Nelson, S.M.; Telfer, E.E.; Anderson, R.A. The ageing ovary and uterus: New biological insights. Hum. Reprod. Update 2013, 19, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Di Simplicio, P.; Franconi, F.; Frosalí, S.; Di Giuseppe, D. Thiolation and nitrosation of cysteines in biological fluids and cells. Amino Acids 2003, 25, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Borowiecka, M.; Wojsiat, J.; Polac, I.; Radwan, M.; Radwan, P.; Zbikowska, H.M. Oxidative stress markers in follicular fluid of women undergoing in vitro fertilization and embryo transfer. Syst. Biol. Reprod. Med. 2012, 58, 301–305. [Google Scholar] [CrossRef]
- Elizur, S.E.; Lebovitz, O.; Orvieto, R.; Dor, J.; Zan-Bar, T. Reactive oxygen species in follicular fluid may serve as biochemical markers to determine ovarian aging and follicular metabolic age. Gynecol. Endocrinol. 2014, 30, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, J.; Kawano, S.; Kumagai, S. Oxidative stress and autoimmune diseases. Oxidative Stress Dis. Cancer 2006, 88, 461–476. [Google Scholar] [CrossRef]
- Cross, C.E.; Halliwell, B.; Borish, E.T.; Pryor, W.A.; Ames, B.N.; Saul, R.L.; McCord, J.M.; Harman, D. Oxygen radicals and human disease. Davis conference. Ann. Intern. Med. 1987, 107, 526–545. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R. Oxidative stress and its implications in female infertility—A clinician’s perspective. Reprod. Biomed. Online 2005, 11, 641–650. [Google Scholar] [CrossRef]
- Weitzel, K.; Chemie, F.; Rev, M.S.; Introduction, I.; Reference, C. Bond-Dissociation Energies of Cations—Pushing the limits to quantum state resolution. WHO Libr. Cat. Data 2011, 30, 221–235. [Google Scholar] [CrossRef]
- Gérard, N.; Loiseau, S.; Duchamp, G.; Seguin, F. Analysis of the variations of follicular fluid composition during follicular growth and maturation in the mare using proton nuclear magnetic resonance (1H NMR). Reproduction 2002, 124, 241–248. [Google Scholar] [CrossRef]
- Bianchi, L.; Gagliardi, A.; Campanella, G.; Landi, C.; Capaldo, A.; Carleo, A.; Armini, A.; De Leo, V.; Piomboni, P.; Focarelli, R.; et al. A methodological and functional proteomic approach of human follicular fluid en route for oocyte quality evaluation. J. Proteom. 2013, 90, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, S.; Ciavardelli, D.; Di Giuseppe, F.; Eleuterio, E.; Sulpizio, M.; Tiboni, G.M.; Giampietro, F.; Palumbo, P.; Di Ilio, C. Proteome analysis of human follicular fluid. Biochim. Biophys. Acta-Proteins Proteom. 2006, 1764, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, T.; Cordeiro, F.B.; Da Costa, L.D.V.T.; Pilau, E.J.; Ferreira, C.R.; Gozzo, F.C.; Eberlin, M.N.; Bertolla, R.P.; Cedenho, A.P.; Lo Turco, E.G. Lipid profiling of follicular fluid from women undergoing IVF: Young poor ovarian responders versus normal responders. Hum. Fertil. 2013, 16, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.X.; Zhu, Y.M.; Luo, Q.; Wu, Y.T.; Gao, H.J.; Zhu, X.M.; Xu, C.M.; Huang, H.F. Specific peptide patterns of follicular fluids at different growth stages analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Biochim. Biophys. Acta-Gen. Subj. 2007, 1770, 29–38. [Google Scholar] [CrossRef]
- Dambala, K.; Paschou, S.A.; Michopoulos, A.; Siasos, G.; Goulis, D.G.; Vavilis, D.; Tarlatzis, B.C. Biomarkers of Endothelial Dysfunction in Women With Polycystic Ovary Syndrome. Angiology 2019, 70, 797–801. [Google Scholar] [CrossRef]
- Rajska, A.; Buszewska-Forajta, M.; Rachoń, D.; Markuszewski, M.J. Metabolomic insight into polycystic ovary syndrome—An overview. Int. J. Mol. Sci. 2020, 21, 4853. [Google Scholar] [CrossRef]
- Bracewell-Milnes, T.; Saso, S.; Abdalla, H.; Nikolau, D.; Norman-Taylor, J.; Johnson, M.; Holmes, E.; Thum, M.Y. Metabolomics as a tool to identify biomarkers to predict and improve outcomes in reproductive medicine: A systematic review. Hum. Reprod. Update 2017, 23, 723–736. [Google Scholar] [CrossRef]
- Revelli, A.; Piane, L.D.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 40. [Google Scholar] [CrossRef]
- Wörheide, M.A.; Krumsiek, J.; Kastenmüller, G.; Arnold, M. Multi-omics integration in biomedical research—A metabolomics-centric review. Anal. Chim. Acta 2021, 1141, 144–162. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Yin, T.L.; Yang, J.; Xiong, C.L. Follicular metabolic changes and effects on oocyte quality in polycystic ovary syndrome patients. Oncotarget 2017, 8, 80472–80480. [Google Scholar] [CrossRef]
- Karaer, A.; Tuncay, G.; Mumcu, A.; Dogan, B. Metabolomics analysis of follicular fluid in women with ovarian endometriosis undergoing in vitro fertilization. Syst. Biol. Reprod. Med. 2019, 65, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Castiglione Morelli, M.A.; Iuliano, A.; Schettini, S.C.A.; Petruzzi, D.; Ferri, A.; Colucci, P.; Viggiani, L.; Cuviello, F.; Ostuni, A. NMR metabolic profiling of follicular fluid for investigating the different causes of female infertility: A pilot study. Metabolomics 2019, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, N.; Amato, J.; Pagano, B.; Pagano, A.; D’Oriano, L.; Pelliccia, S.; Giustiniano, M.; Brancaccio, D.; Merlino, F.; Novellino, E.; et al. 1H NMR-based metabolomics study on follicular fluid from patients with Polycystic Ovary Syndrome Nunzia. Biochim. Clin. 2018, 42, 26–31. [Google Scholar] [CrossRef]
- Hou, E.; Zhao, Y.; Hang, J.; Qiao, J. Metabolomics and correlation network analysis of follicular fluid reveals associations between l-tryptophan, l-tyrosine and polycystic ovary syndrome. Biomed. Chromatogr. 2021, 35, e4993. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Y.; Li, T.; Li, M.; Li, J.; Li, R.; Liu, P.; Yu, Y.; Qiao, J. Metabolism alteration in follicular niche: The nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic. Biol. Med. 2015, 86, 295–307. [Google Scholar] [CrossRef]
- Sun, Z.; Chang, H.M.; Wang, A.; Song, J.; Zhang, X.; Guo, J.; Leung, P.C.K.; Lian, F. Identification of potential metabolic biomarkers of polycystic ovary syndrome in follicular fluid by SWATH mass spectrometry. Reprod. Biol. Endocrinol. 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Li, S.; Qi, J.; Tao, Y.; Zhu, Q.; Huang, R.; Liao, Y.; Yue, J.; Liu, W.; Zhao, H.; Yin, H.; et al. Elevated levels of arachidonic acid metabolites in follicular fluid of PCOS patients. Reproduction 2020, 159, 159–169. [Google Scholar] [CrossRef]
- Jóźwik, M.; Jóźwik, M.; Milewska, A.J.; Battaglia, F.C.; Jóźwik, M. Competitive inhibition of amino acid transport in human preovulatory ovarian follicles. Syst. Biol. Reprod. Med. 2017, 63, 311–317. [Google Scholar] [CrossRef]
- Luti, S.; Fiaschi, T.; Magherini, F.; Modesti, P.A.; Piomboni, P.; Semplici, B.; Morgante, G.; Amoresano, A.; Illiano, A.; Pinto, G.; et al. Follicular microenvironment: Oxidative stress and adiponectin correlated with steroids hormones in women undergoing in vitro fertilization. Mol. Reprod. Dev. 2021, 88, 175–184. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, W.; Zhou, C.; Zhou, Y.; Liu, X.; Ding, G.; Hu, Y.; Pan, J.; Sheng, J.; Jin, L.; et al. Steroid metabolome profiling of follicular fluid in normo- and hyperandrogenic women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2021, 206, 105806. [Google Scholar] [CrossRef]
- Patil, K.; Yelamanchi, S.; Kumar, M.; Hinduja, I.; Prasad, T.S.K.; Gowda, H.; Mukherjee, S. Quantitative mass spectrometric analysis to unravel glycoproteomic signature of follicular fluid in women with polycystic ovary syndrome. PLoS ONE 2019, 14, e0214742. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, T.; Wang, X.; Sun, X.; Zhang, J.; Zhou, K.; Ji, X.; Sun, R.; Wang, X.; Chen, M.; et al. Metabolic alterations associated with polycystic ovary syndrome: A UPLC Q-Exactive based metabolomic study. Clin. Chim. Acta 2020, 502, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, F.B.; Ferreira, C.R.; Sobreira, T.J.P.; Yannell, K.E.; Jarmusch, A.K.; Cedenho, A.P.; Lo Turco, E.G.; Cooks, R.G. Multiple reaction monitoring (MRM)-profiling for biomarker discovery applied to human polycystic ovarian syndrome. Rapid Commun. Mass Spectrom. 2017, 31, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Fabjan, T.; Vrtačnik-Bokal, E.; Virant-Klun, I.; Bedenk, J.; Kumer, K.; Osredkar, J. Antimüllerian hormone and oxidative stress biomarkers as predictors of successful pregnancy in polycystic ovary syndrome, endometriosis and tubal infertility factor. Acta Chim. Slov. 2020, 67, 885–895. [Google Scholar] [CrossRef]
- Garg, D.; Grazi, R.; Lambert-Messerlian, G.M.; Merhi, Z. Correlation between follicular fluid levels of sRAGE and vitamin D in women with PCOS. J. Assist. Reprod. Genet. 2017, 34, 1507–1513. [Google Scholar] [CrossRef]
- Eskandari, Z.; Sadrkhanlou, R.A.; Nejati, V.; Tizro, G. PCOS women show significantly higher homocysteine level, independent to glucose and E2 level. Int. J. Reprod. Biomed. 2016, 14, 495–500. [Google Scholar] [CrossRef]
- Vinaixa, M.; Schymanski, E.L.; Neumann, S.; Navarro, M.; Salek, R.M.; Yanes, O. Mass spectral databases for LC/MS- and GC/MS-based metabolomics: State of the field and future prospects. TrAC Trends Anal. Chem. 2016, 78, 23–35. [Google Scholar] [CrossRef]
- Wishart, D.S. Quantitative metabolomics using NMR. TrAC Trends Anal. Chem. 2008, 27, 228–237. [Google Scholar] [CrossRef]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef]
- Fan, T.W.M.; Lane, A.N. Applications of NMR spectroscopy to systems biochemistry. Prog. Nucl. Magn. Reson. Spectrosc. 2016, 92–93, 18–53. [Google Scholar] [CrossRef]
- Nagana Gowda, G.A.; Raftery, D. Can NMR solve some significant challenges in metabolomics? J. Magn. Reson. 2015, 260, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Nagana Gowda, G.A.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. Nmr spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Silva Elipe, M.V. Advantages and disadvantages of nuclear magnetic resonance spectroscopy as a hyphenated technique. Anal. Chim. Acta 2003, 497, 1–25. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Wishart, D.S. Computational strategies for metabolite identification in metabolomics. Bioanalysis 2009, 1, 1579–1596. [Google Scholar] [CrossRef]
- Wishart, D.S. Advances in metabolite identification. Bioanalysis 2011, 3, 1769–1782. [Google Scholar] [CrossRef]
- Majchrzak, T.; Wojnowski, W.; Lubinska-Szczygeł, M.; Różańska, A.; Namieśnik, J.; Dymerski, T. PTR-MS and GC-MS as complementary techniques for analysis of volatiles: A tutorial review. Anal. Chim. Acta 2018, 1035, 1–13. [Google Scholar] [CrossRef]
- Kranenburg, R.F.; Verduin, J.; Stuyver, L.I.; de Ridder, R.; van Beek, A.; Colmsee, E.; van Asten, A.C. Benefits of derivatization in GC–MS-based identification of new psychoactive substances. Forensic Chem. 2020, 20, 100273. [Google Scholar] [CrossRef]
- Garby, D.M.; Cheryk, L.A. Synthetic opioid analysis by LC-MS/MS. Methods Mol. Biol. 2012, 902, 65–73. [Google Scholar] [CrossRef]
- Gika, H.G.; Wilson, I.D.; Theodoridis, G.A. LC-MS-based holistic metabolic profiling. Problems, limitations, advantages, and future perspectives. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 966, 1–6. [Google Scholar] [CrossRef]
- Lim, C.K.; Lord, G. Current developments in LC-MS for pharmaceutical analysis. Biol. Pharm. Bull. 2002, 25, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Bingol, K.; Bruschweiler-Li, L.; Li, D.; Zhang, B.; Xie, M.; Brüschweiler, R. Emerging new strategies for successful metabolite identification in metabolomics. Bioanalysis 2016, 8, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Tiziani, S.; Kang, Y.; Choi, J.S.; Roberts, W.; Paternostro, G. Metabolomic high-content nuclear magnetic resonance-based drug screening of a kinase inhibitor library. Nat. Commun. 2011, 2, 510–545. [Google Scholar] [CrossRef] [PubMed]
- Lewis, I.A.; Schommer, S.C.; Markley, J.L. rNMR: Open source software for identifying and quantifying metabolites in NMR spectra. Magn. Reson. Chem. 2009, 47, S123–S126. [Google Scholar] [CrossRef]
- Wittmann, C. Fluxome analysis using GC-MS. Microb. Cell Fact. 2007, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Villas-Bôas, S.G.; Mas, S.; Åkesson, M.; Smedsgaard, J.; Nielsen, J. Mass spectrometry in metabolome analysis. Mass Spectrom. Rev. 2005, 24, 613–646. [Google Scholar] [CrossRef]
- Des Rosiers, C.; Di Donato, L.; Comte, B.; Laplante, A.; Marcoux, C.; David, F.; Fernandez, C.A.; Brunengraber, H. Isotopomer analysis of citric acid cycle and gluconeogenesis in rat liver. Reversibility of isocitrate dehydrogenase and involvement of ATP-citrate lyase in gluconeogenesis. J. Biol. Chem. 1995, 270, 10027–10036. [Google Scholar] [CrossRef]
- Hellerstein, M.K.; Neese, R.A.; Linfoot, P.; Christiansen, M.; Turner, S.; Letscher, A. Hepatic gluconeogenic fluxes and glycogen turnover during fasting in humans. A stable isotope study. J. Clin. Investig. 1997, 100, 1305–1319. [Google Scholar] [CrossRef]
- Hellerstein, M.K.; Neese, R.A.; Schwarz, J.M.; Turner, S.; Faix, D.; Wu, K. Altered fluxes responsible for reduced hepatic glucose production and gluconeogenesis by exogenous glucose in rats. Am. J. Physiol. Endocrinol. Metab. 1997, 272, E163–E172. [Google Scholar] [CrossRef]
- Di Donato, L.; Des Rosiers, C.; Montgomery, J.A.; David, F.; Garneau, M.; Brunengraber, H. Rates of gluconeogenesis and citric acid cycle in perfused livers, assessed from the mass spectrometric assay of the 13C labeling pattern of glutamate. J. Biol. Chem. 1993, 268, 4170–4180. [Google Scholar] [CrossRef]
- Maurer, H.H. Multi-analyte procedures for screening for and quantification of drugs in blood, plasma, or serum by liquid chromatography-single stage or tandem mass spectrometry (LC-MS or LC-MS/MS) relevant to clinical and forensic toxicology. Clin. Biochem. 2005, 38, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Maurer, H. Screening Procedures for Simultaneous Detection of Several Drug Classes Used for High Throughput Toxicological Analyses and Doping Control. A Review. Comb. Chem. High Throughput Screen. 2000, 3, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Van Bocxlaer, J.F.; Clauwaert, K.M.; Lambert, W.E.; Deforce, D.L.; Van Den Eeckhout, E.G.; De Leenheer, A.P. Liquid chromatography-mass spectrometry in forensic toxicology. Mass Spectrom. Rev. 2000, 19, 165–214. [Google Scholar] [CrossRef]
- Marquet, P. Progress of liquid chromatography-mass spectrometry in clinical and forensic toxicology. Ther. Drug Monit. 2002, 24, 255–276. [Google Scholar] [CrossRef]
- Maurer, H.H. Position of chromatographic techniques in screening for detection of drugs or poisons in clinical and forensic toxicology and/or doping control. Clin. Chem. Lab. Med. 2004, 42, 1310–1324. [Google Scholar] [CrossRef]
- Maurer, H.H.; Kraemer, T.; Kratzsch, C.; Peters, F.T.; Weber, A.A. Negative ion chemical ionization gas chromatography-mass spectrometry and atmospheric pressure chemical ionization liquid chromatography-mass spectrometry of low-dosed and/or polar drugs in plasma. Ther. Drug Monit. 2002, 24, 117–124. [Google Scholar] [CrossRef]
- Saint-Marcoux, F.; Lachâtre, G.; Marquet, P. Evaluation of an improved general unknown screening procedure using liquid-chromatography-electrospray-mass spectrometry by comparison with gas chromatography and high-performance liquid-chromatography—Diode array detection. J. Am. Soc. Mass Spectrom. 2002, 14, 14–22. [Google Scholar] [CrossRef][Green Version]
- Weinmann, W.; Stoertzel, M.; Vogt, S.; Wendt, J. Tune compounds for electrospray ionisation/in-source collision-induced dissociation with mass spectral library searching. J. Chromatogr. A 2001, 926, 199–209. [Google Scholar] [CrossRef]
- Rivier, L. Criteria for the identification of compounds by liquid chromatography-mass spectrometry and liquid chromatography-multiple mass spectrometry in forensic toxicology and doping analysis. Anal. Chim. Acta 2003, 492, 69–82. [Google Scholar] [CrossRef]
- Lips, A.G.A.M.; Lameijer, W.; Fokkens, R.H.; Nibbering, N.M.M. Methodology for the development of a drug library based upon collision-induced fragmentation for the identification of toxicologically relevant drugs in plasma samples. J. Chromatogr. B Biomed. Sci. Appl. 2001, 759, 191–207. [Google Scholar] [CrossRef]
- Hough, J.M.; Haney, C.A.; Voyksner, R.D.; Bereman, R.D. Evaluation of electrospray transport CID for the generation of searchable libraries. Anal. Chem. 2000, 72, 2265–2270. [Google Scholar] [CrossRef] [PubMed]
- Mallet, C.R.; Lu, Z.; Mazzeo, J.R. A study of ion suppression effects in electrospray ionization from mobile phase additives and solid-phase extracts. Rapid Commun. Mass Spectrom. 2004, 18, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Annesley, T.M. Ion suppression in mass spectrometry. Clin. Chem. 2003, 49, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.R.; Foltz, R.L.; Meng, M.; Bennett, P. Ionization enhancement in atmospheric pressure chemical ionization and suppression in electrospray ionization between target drugs and stable-isotope-labeled internal standards in quantitative liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2815–2821. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Li, H.; Jiang, W.; Liu, H.; Yan, J.; Chen, Z.; Li, W. Leukaemia inhibitory factor in serum and follicular fluid of women with polycystic ovary syndrome and its correlation with IVF outcome. Reprod. Biomed. Online 2018, 36, 483–489. [Google Scholar] [CrossRef]
- Cordeiro, F.B.; Cataldi, T.R.; de Souza, B.Z.; Rochetti, R.C.; Fraietta, R.; Labate, C.A.; Lo Turco, E.G. Hyper response to ovarian stimulation affects the follicular fluid metabolomic profile of women undergoing IVF similarly to polycystic ovary syndrome. Metabolomics 2018, 14, 51. [Google Scholar] [CrossRef]
- Oral, O.; Kutlu, T.; Aksoy, E.; Fıçıcıoğlu, C.; Uslu, H.; Tuğrul, S. The effects of oxidative stress on outcomes of assisted reproductive techniques. J. Assist. Reprod. Genet. 2006, 23, 81–85. [Google Scholar] [CrossRef]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front. Endocrinol. (Lausanne) 2019, 10, 811. [Google Scholar] [CrossRef]
- Igarashi, H.; Takahashi, T.; Nagase, S. Oocyte aging underlies female reproductive aging: Biological mechanisms and therapeutic strategies. Reprod. Med. Biol. 2015, 14, 159–169. [Google Scholar] [CrossRef]
- Appasamy, M.; Jauniaux, E.; Serhal, P.; Al-Qahtani, A.; Groome, N.P.; Muttukrishna, S. Evaluation of the relationship between follicular fluid oxidative stress, ovarian hormones, and response to gonadotropin stimulation. Fertil. Steril. 2008, 89, 912–921. [Google Scholar] [CrossRef]
- Malhotra, N.; Gongadashetti, K.; Dada, R.; Singh, N. Oxidative stress biomarkers in follicular fluid of women with PCOS and tubal factor infertility-is there a correaltion with in-vitro-fertilization outcome? Fertil. Steril. 2014, 102, e86. [Google Scholar] [CrossRef]
- Kuşçu, N.K.; Var, A. Oxidative stress but not endothelial dysfunction exists in non-obese, young group of patients with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 2009, 88, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Atiomo, W.; Khalid, S.; Parameshweran, S.; Houda, M.; Layfield, R. Proteomic biomarkers for the diagnosis and risk stratification of polycystic ovary syndrome: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Sova, H.; Morin-Papunen, L.; Puistola, U.; Karihtala, P. Distinctively low levels of serum 8-hydroxydeoxyguanosine in women with polycystic ovary syndrome. Fertil. Steril. 2010, 94, 2670–2673. [Google Scholar] [CrossRef]
- Chen, Y.H.; Heneidi, S.; Lee, J.M.; Layman, L.C.; Stepp, D.W.; Gamboa, G.M.; Chen, B.S.; Chazenbalk, G.; Azziz, R. Mirna-93 inhibits glut4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 2013, 62, 2278–2286. [Google Scholar] [CrossRef]
- Cordeiro, F.B.; Cataldi, T.R.; do Vale Teixeira da Costa, L.; de Lima, C.B.; Stevanato, J.; Zylbersztejn, D.S.; Ferreira, C.R.; Eberlin, M.N.; Cedenho, A.P.; Turco, E.G. Lo Follicular fluid lipid fingerprinting from women with PCOS and hyper response during IVF treatment. J. Assist. Reprod. Genet. 2015, 32, 45–54. [Google Scholar] [CrossRef]
- Blunsom, N.J.; Cockcroft, S. Phosphatidylinositol synthesis at the endoplasmic reticulum. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158471. [Google Scholar] [CrossRef]
- Montani, D.A.; Cordeiro, F.B.; Regiani, T.; Victorino, A.B.; Pilau, E.J.; Gozzo, F.C.; Ferreira, C.R.; Fraietta, R.; Lo Turco, E.G. The follicular microenviroment as a predictor of pregnancy: MALDI-TOF MS lipid profile in cumulus cells. J. Assist. Reprod. Genet. 2012, 29, 1289–1297. [Google Scholar] [CrossRef]
- Lucki, N.C.; Sewer, M.B. The interplay between bioactive sphingolipids and steroid hormones. Steroids 2010, 75, 390–399. [Google Scholar] [CrossRef]
- Ecker, J.; Liebisch, G. Application of stable isotopes to investigate the metabolism of fatty acids, glycerophospholipid and sphingolipid species. Prog. Lipid Res. 2014, 54, 14–31. [Google Scholar] [CrossRef]
- Dong, F.; Deng, D.; Chen, H.; Cheng, W.; Li, Q.; Luo, R.; Ding, S. Serum metabolomics study of polycystic ovary syndrome based on UPLC-QTOF-MS coupled with a pattern recognition approach. Anal. Bioanal. Chem. 2015, 407, 4683–4695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, F.; Qi, B.; Hao, S.; Li, Y.; Li, Y.; Zou, L.; Lu, C.; Xu, G.; Hou, L. Serum metabolomics study of polycystic ovary syndrome based on liquid chromatography-mass spectrometry. J. Proteome Res. 2014, 13, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Zhang, X.J.; Huang, J.; Zhou, S.J.; Liu, F.; Jiang, L.L.; Chen, M.; Jian-Bo, W.; Yang, D.Z. UHPLC/Q-TOFMS-based plasma metabolomics of polycystic ovary syndrome patients with and without insulin resistance. J. Pharm. Biomed. Anal. 2016, 121, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lauber, K.; Bohn, E.; Kröber, S.M.; Xiao, Y.J.; Blumenthal, S.G.; Lindemann, R.K.; Marini, P.; Wiedig, C.; Zobywalski, A.; Baksh, S.; et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 2003, 113, 717–730. [Google Scholar] [CrossRef]
- Yea, K.; Kim, J.; Yoon, J.H.; Kwon, T.; Kim, J.H.; Lee, B.D.; Lee, H.J.; Lee, S.J.; Kim, J.I.; Lee, T.G.; et al. Lysophosphatidylcholine activates adipocyte glucose uptake and lowers blood glucose levels in murine models of diabetes. J. Biol. Chem. 2009, 284, 33833–33840. [Google Scholar] [CrossRef]
- Brisson, D.; Vohl, M.C.; St-Pierre, J.; Hudson, T.J.; Gaudet, D. Glycerol: A neglected variable in metabolic processes? BioEssays 2001, 23, 534–542. [Google Scholar] [CrossRef]
- Larner, J.M.; Pahuja, S.L.; Shackleton, C.H.; McMurray, W.J.; Giordano, G.; Hochberg, R.B. The isolation and characterization of estradiol-fatty acid esters in human ovarian follicular fluid. Identification of an endogenous long-lived and potent family of estrogens. J. Biol. Chem. 1993, 268, 13893–13899. [Google Scholar] [CrossRef]
- Matorras, R.; Ruiz, J.I.; Mendoza, R.; Ruiz, N.; Sanjurjo, P.; Rodriguez-Escudero, F.J. Fatty acid composition of fertilization-failed human oocytes. Hum. Reprod. 1998, 13, 2227–2230. [Google Scholar] [CrossRef][Green Version]
- Li, H.W.R.; Lee, V.C.Y.; Lau, E.Y.L.; Yeung, W.S.B.; Ho, P.C.; Ng, E.H.Y. Cumulative live-birth rate in women with polycystic ovary syndrome or isolated polycystic ovaries undergoing in-vitro fertilisation treatment. J. Assist. Reprod. Genet. 2014, 31, 205–211. [Google Scholar] [CrossRef]
- Engmann, L.; Maconochie, N.; Sladkevicius, P.; Bekir, J.; Campbell, S.; Tan, S.L. The outcome of in-vitro fertilization treatment in women with sonographic evidence of polycystic ovarian morphology. Hum. Reprod. 1999, 14, 167–171. [Google Scholar] [CrossRef]
- Ray, P.F.; Conaghan, J.; Winston, R.M.L.; Handyside, A.H. Increased number of cells and metabolic activity in male human preimplantation embryos following in vitro fertilization. J. Reprod. Fertil. 1995, 104, 165–171. [Google Scholar] [CrossRef] [PubMed]
- McCommis, K.S.; Finck, B.N. Pdk 1. Biochem. J. 2015, 446, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Wolosker, H.; Sheth, K.N.; Takahashi, M.; Mothet, J.P.; Brady, R.O.; Ferris, C.D.; Snyder, S.H. Purification of serine racemase: Biosynthesis of the neuromodulator D-serine. Proc. Natl. Acad. Sci. USA 1999, 96, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Chen, Z.; Feng, W.-J.; Long, S.-L.; Mo, Z.-C. Sex hormone-binding globulin and polycystic ovary syndrome. Clin. Chim. Acta 2019, 499, 142–148. [Google Scholar] [CrossRef]
- Ciepiela, P.; Bączkowski, T.; Drozd, A.; Kazienko, A.; Stachowska, E.; Kurzawa, R. Arachidonic and linoleic acid derivatives impact oocyte ICSI fertilization—A prospective analysis of follicular fluid and a matched oocyte in a “one follicle—One retrieved oocyte—One resulting embryo” investigational setting. PLoS ONE 2015, 10, e0119087. [Google Scholar] [CrossRef]
- Kim, K.; Ramirez, V.D. Effects of prostaglandin E2, forskolin and cholera toxin on cAMP production and in vitro LH-RH release from the rat hypothalamus. Brain Res. 1986, 386, 258–265. [Google Scholar] [CrossRef]
- Calder, M.D.; Caveney, A.N.; Westhusin, M.E.; Watson, A.J. Cyclooxygenase-2 and prostaglandin E2(PGE2) receptor messenger RNAs are affected by bovine oocyte maturation time and cumulus-oocyte complex quality, and PGE2 induces moderate expansion of the bovine cumulus in vitro. Biol. Reprod. 2001, 65, 135–140. [Google Scholar] [CrossRef][Green Version]
- Marei, W.F.; Wathes, D.C.; Fouladi-Nashta, A.A. Impact of linoleic acid on bovine oocyte maturation and embryo development. Reproduction 2010, 139, 979–988. [Google Scholar] [CrossRef]
- Narumiya, S.; Fukushima, M. Δ12-prostaglandin J2, an ultimate metabolite of prostaglandin D2 exerting cell growth inhibition. Biochem. Biophys. Res. Commun. 1985, 127, 739–745. [Google Scholar] [CrossRef]
- Jiang, C.; Ting, A.T.; Seed, B. PPAR-gama agonists inhibit production ofmonocyte inflammatorycytokines Chengyu. Nature 1998, 391, 82–86. [Google Scholar] [CrossRef]
- Komar, C.M. Peroxisome proliferator-activated receptors (PPARs) and ovarian function—Implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod. Biol. Endocrinol. 2005, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Goto, M.; Harata, T.; Takigawa, S.; Nakahara, T.; Suzuki, K.; Manabe, S.; Kikkawa, F. Insulin attenuates the insulin-like growth factor-I (IGF-I)-akt pathway, not IGF-I-extracellularly regulated kinase pathway, in luteinized granulosa cells with an increase in PTEN. J. Clin. Endocrinol. Metab. 2009, 94, 2184–2191. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Chou, C.H.; Chen, S.U.; Yang, W.S.; Yang, Y.S.; Ho, H.N. The effect of androgens on ovarian follicle maturation: Dihydrotestosterone suppress FSH-stimulated granulosa cell proliferation by upregulating PPARÎ 3-dependent PTEN expression. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Valledor, A.F.; Ricote, M. Nuclear receptor signaling in macrophages. Biochem. Pharmacol. 2004, 67, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.O.; Wittlin, B.M.; Anger, D.B.C.; Martins, D.O.; Sannomiya, P.; Jancar, S. Early phase of allergic airway inflammation in diabetic rats: Role of insulin on the signaling pathways and mediators. Cell. Physiol. Biochem. 2010, 26, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Song, N.Y.; Na, H.K.; Baek, J.H.; Surh, Y.J. Docosahexaenoic acid inhibits insulin-induced activation of sterol regulatory-element binding protein 1 and cyclooxygenase-2 expression through upregulation of SIRT1 in human colon epithelial cells. Biochem. Pharmacol. 2014, 92, 142–148. [Google Scholar] [CrossRef]
- Martins, J.O.; Ferracini, M.; Ravanelli, N.; Landgraf, R.G.; Jancar, S. Insulin suppresses LPS-induced iNOS and COX-2 expression and NF-κB activation in alveolar macrophages. Cell. Physiol. Biochem. 2008, 22, 279–286. [Google Scholar] [CrossRef]
- Xu, J.; Cao, L.; Suo, Y.; Xu, X.; Sun, H.; Xu, S.; Zhu, X.; Yu, H.; Cao, W. Chitosan-microcapsulated insulin alleviates mesenteric microcirculation dysfunction via modulating COX-2 and VCAM-1 expression in rats with diabetes mellitus. Int. J. Nanomed. 2018, 13, 6829–6837. [Google Scholar] [CrossRef]
- Wallace, M.; Cottell, E.; Gibney, M.J.; McAuliffe, F.M.; Wingfield, M.; Brennan, L. An investigation into the relationship between the metabolic profile of follicular fluid, oocyte developmental potential, and implantation outcome. Fertil. Steril. 2012, 97, 1078–1084. [Google Scholar] [CrossRef]
- Pan, J.X.; Zhang, J.Y.; Ke, Z.H.; Wang, F.F.; Barry, J.A.; Hardiman, P.J.; Qu, F. Androgens as double-edged swords: Induction and suppression of follicular development. Hormones 2015, 14, 190–200. [Google Scholar] [CrossRef]
- Murri, M.; Insenser, M.; Escobar-Morreale, H.F. Metabolomics in polycystic ovary syndrome. Clin. Chim. Acta 2014, 429, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Daolio, J.; La Sala, G.B. Oocyte Competence in Women with Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2017, 28, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Collado-Fernandez, E.; Picton, H.M.; Dumollard, R. Metabolism throughout follicle and oocyte development in mammals. Int. J. Dev. Biol. 2012, 56, 799–808. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Mishra, B.P.; Khan, T.; Chattopadhayay, R.; Lodh, I.; Datta Ray, C.; Bose, G.; Sarkar, H.S.; Srivastava, S.; Joshi, M.V.; et al. Serum metabolomics of Indian women with polycystic ovary syndrome using 1H NMR coupled with a pattern recognition approach. Mol. Biosyst. 2016, 12, 3407–3416. [Google Scholar] [CrossRef]
- Toney, M.D. Reaction specificity in pyridoxal phosphate enzymes. Arch. Biochem. Biophys. 2005, 433, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Toney, M.D. Aspartate aminotransferase: An old dog teaches new tricks. Arch. Biochem. Biophys. 2014, 544, 119–127. [Google Scholar] [CrossRef]
- Percudani, R.; Peracchi, A. A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep. 2003, 4, 850–854. [Google Scholar] [CrossRef]
- Richard, J.P.; Amyes, T.L.; Crugeiras, J.; Rios, A. Pyridoxal 5′-phosphate: Electrophilic catalyst extraordinaire. Curr. Opin. Chem. Biol. 2009, 13, 475–483. [Google Scholar] [CrossRef]
- De Miranda, J.; Santoro, A.; Engelender, S.; Wolosker, H. Human serine racemase: Moleular cloning, genomic organization and functional analysis. Gene 2000, 256, 183–188. [Google Scholar] [CrossRef]
- Chen, C.H.; Yeh, E.L.; Chen, C.C.; Huang, S.C.; Huang, Y.C. Vitamin B-6, Independent of Homocysteine, Is a Significant Factor in Relation to Inflammatory Responses for Chronic Kidney Disease and Hemodialysis Patients. BioMed Res. Int. 2017, 2017, 7367831. [Google Scholar] [CrossRef]
- Holton, K. The role of diet in the treatment of fibromyalgia. Pain Manag. 2016, 6, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Fong, M.Y.; McDunn, J.; Kakar, S.S. Identification of metabolites in the normal ovary and their transformation in primary and metastatic ovarian cancer. PLoS ONE 2011, 6, e19963. [Google Scholar] [CrossRef] [PubMed]
- Bonvin, D.; Aschauer, U.J.; Bastiaansen, J.A.M.; Stuber, M.; Hofmann, H.; Ebersold, M.M. Versatility of pyridoxal phosphate as a coating of iron oxide nanoparticles. Nanomaterials 2017, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Dajnowicz, S.; Johnston, R.C.; Parks, J.M.; Blakeley, M.P.; Keen, D.A.; Weiss, K.L.; Gerlits, O.; Kovalevsky, A.; Mueser, T.C. Direct visualization of critical hydrogen atoms in a pyridoxal 5′-phosphate enzyme. Nat. Commun. 2017, 8, 955. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, R.; Saito, N.; Shimada, T.; Tanaka, K. Identification of YbhA as the pyridoxal 5’-phosphate (PLP) phosphatase in escherichia coli: Importance of PLP homeostasis on the bacterial growth. J. Gen. Appl. Microbiol. 2017, 63, 362–368. [Google Scholar] [CrossRef]
- Colinas, M.; Fitzpatrick, T.B. Interaction between vitamin B6metabolism, nitrogen metabolism and autoimmunity. Plant Signal. Behav. 2016, 11, e1161876. [Google Scholar] [CrossRef][Green Version]
- Mills, P.B.; Camuzeaux, S.S.M.; Footitt, E.J.; Mills, K.A.; Gissen, P.; Fisher, L.; Das, K.B.; Varadkar, S.M.; Zuberi, S.; McWilliam, R.; et al. Epilepsy due to PNPO mutations: Genotype, environment and treatment affect presentation and outcome. Brain 2014, 137, 1350–1360. [Google Scholar] [CrossRef]
- Maleedhu, P.; Vijayabhaskar, M.; Sharma, S.S.B.; Kodumuri, P.K.; Vasundhara Devi, D. Status of homocysteine in polycystic ovary syndrome. J. Clin. Diagn. Res. 2014, 8, 31–33. [Google Scholar] [CrossRef]
- Ren, S.G.; Melmed, S. Pyridoxal phosphate inhibits pituitary cell proliferation and hormone secretion. Endocrinology 2006, 147, 3936–3942. [Google Scholar] [CrossRef]
- Christensen, C.E.; Karlsson, M.; Winther, J.R.; Jensen, P.R.; Lerche, M.H. Non-invasive in-cell determination of free cytosolic [NAD+]/[NADH] ratios using hyperpolarized glucose show large variations in metabolic phenotypes. J. Biol. Chem. 2014, 289, 2344–2352. [Google Scholar] [CrossRef]
- Gull, I.; Geva, E.; Lerner-Geva, L.; Lessing, J.B.; Wolman, I.; Amit, A. Anaerobic glycolysisThe metabolism of the preovulatory human oocyte. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 85, 225–228. [Google Scholar] [CrossRef]
- Sugiura, K.; Pendola, F.L.; Eppig, J.J. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: Energy metabolism. Dev. Biol. 2005, 279, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Latham, T.; MacKay, L.; Sproul, D.; Karim, M.; Culley, J.; Harrison, D.J.; Hayward, L.; Langridge-Smith, P.; Gilbert, N.; Ramsahoye, B.H. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res. 2012, 40, 4794–4803. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fu, L.; Li, R.; Wang, L.N.; Yang, Y.; Liu, N.N.; Zhang, C.M.; Wang, Y.; Liu, P.; Tu, B.-B.; et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: Plasma metabolomics analysis. BMC Med. 2012, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hu, W.; Liu, Q.; Hao, Q.; Sun, B.; Zhang, Q.; Mao, S.; Qiao, J.; Yan, X. Metabonomics reveals plasma metabolic changes and inflammatory marker in polycystic ovary syndrome patients. J. Proteome Res. 2012, 11, 2937–2946. [Google Scholar] [CrossRef]
- Piñero-Sagredo, E.; Nunes, S.; De Los Santos, M.J.; Celda, B.; Esteve, V. NMR metabolic profile of human follicular fluid. NMR Biomed. 2010, 23, 485–495. [Google Scholar] [CrossRef]
- Rice, S.; Christoforidis, N.; Gadd, C.; Nikolaou, D.; Seyani, L.; Donaldson, A.; Margara, R.; Hardy, K.; Franks, S. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum. Reprod. 2005, 20, 373–381. [Google Scholar] [CrossRef]
- Smith, L.P.; Nierstenhoefer, M.; Yoo, S.W.; Penzias, A.S.; Tobiasch, E.; Usheva, A. The bile acid synthesis pathway is present and functional in the human ovary. PLoS ONE 2009, 4, e7333. [Google Scholar] [CrossRef]
- Cela, V.; Obino, M.E.R.; Alberga, Y.; Pinelli, S.; Sergiampietri, C.; Casarosa, E.; Simi, G.; Papini, F.; Artini, P.G. Ovarian response to controlled ovarian stimulation in women with different polycystic ovary syndrome phenotypes. Gynecol. Endocrinol. 2017, 34, 518–523. [Google Scholar] [CrossRef]
- Apter, D.; Butsow, T.; Laughlin, G.A.; Yen, S.S.C. Metabolic Features of Polycistic Ovary Syndrome Are Found in Adolescent Girls with Hyperandrogenism. J. Clin. Endocrinol. Metab. 1995, 80, 2966–2973. [Google Scholar]
- Kirilovas, D.; Chaika, A.; Bergstro, M.; Carlstro, K.; Bergstro, E.; Naessen, T. Granulosa cell aromatase enzyme activity: Effects of follicular fluid from patients with polycystic ovary syndrome, using aromatase conversion and [11C] vorozole-binding assays. Gynecol. Endocrinol. 2006, 22, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Wickenheisser, J.K.; Nelson-degrave, V.L.; Mcallister, J.M. Messenger Ribonucleic Acid Stability in Theca Cells Isolated from Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Velasco, J.A.; Moreno, L.; Pacheco, A.; Guillén, A.; Duque, L.; Requena, A.; Pellicer, A. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: A pilot study. Fertil. Steril. 2005, 84, 82–87. [Google Scholar] [CrossRef]
- Pasquali, R.; Patton, L.; Pocognoli, P.; Cognigni, G.E.; Gambineri, A. 17-Hydroxyprogesterone responses to gonadotropin-releasing hormone disclose distinct phenotypes of functional ovarian hyperandrogenism and polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 4208–4217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eden, J.A.; Jones, J.; Carter, G.D.; Alaghband-Zadeh, J. Follicular Fluid Concentrations of Insulin-Like Growth Factor 1, Epidermal Growth Factor, Transforming Growth Factor-Alpha and Sex-Steroids in Volume Matched Normal and Polycystic Human Follicles. Clin. Endocrinol. 1990, 32, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, I.A.J.; Broekmans, F.J.M.; Te Velde, E.R.; Fauser, B.C.J.M.; Bancsi, L.F.J.M.M.; De Jong, F.H.; Themmen, A.P.N. Serum anti-Müllerian hormone levels: A novel measure of ovarian reserve. Hum. Reprod. 2002, 17, 3065–3071. [Google Scholar] [CrossRef]
- Takahashi, C.; Fujito, A.; Kazuka, M.; Sugiyama, R.; Ito, H.; Isaka, K. Anti-Müllerian hormone substance from follicular fluid is positively associated with success in oocyte fertilization during in vitro fertilization. Fertil. Steril. 2008, 89, 586–591. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yang, Y.J.; Tang, C.L.; Wang, K.; Chen, J.J.; Teng, X.M.; Ruan, Y.C.; Yang, J.Z. Elevation of antimüllerian hormone in women with polycystic ovary syndrome undergoing assisted reproduction: Effect of insulin. Fertil. Steril. 2019, 111, 157–167. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chiu, W.C.; Wu, C.H.; Tzeng, C.R.; Hsu, C.S.; Hsu, M.I. Antimüllerian hormone and polycystic ovary syndrome. Fertil. Steril. 2011, 96, 230–235. [Google Scholar] [CrossRef]
- Kadoura, S.; Alhalabi, M.; Nattouf, A.H. Correlations between Follicular Fluid AMH and IVF/ICSI Outcomes among Polycystic Ovary Syndrome Women Using Different Controlled Hyperstimulation Protocols. 2022, pp. 1–21, Research Square. Available online: https://www.researchsquare.com/article/rs-1521860/v1 (accessed on 25 March 2022).
- Aghajanova, L. Update on the role of leukemia inhibitory factor in assisted reproduction. Curr. Opin. Obstet. Gynecol. 2010, 22, 213–219. [Google Scholar] [CrossRef]

| Technique | Advantages | Disadvantages | References |
|---|---|---|---|
| NMR | Highly reproducible; Minimal sample handling and non-destructive; Identification of a wide range of low-molecular-weight compounds; Used for non-selective approaches. | Low sensitivity due to overlapped peaks; Requires expensive deuterated solvents; Suppression of residual protonated solvents; Difficult to find compatibility between the volume of the chromatographic peak and the volume of the flow cell. | [113,127,128,129,130,131,132,133] |
| GC-MS | Presents well-established libraries of both commercial and ‘in house’ metabolite databases available; Highly used for metabolite profiling and quantification. | Requires derivatization; Long analysis time; Does not allow real-time analysis or direct quantitative determinations; Presents a limit of sample capacity. | [134,135,136,137,138] |
| LC-MS | Wide metabolite coverage that presents high sensitivity and specificity; Versatility technology. | Untargeted screening is highly challenging; Lack of reference libraries. | [139,140,141] |
| LC-MS/MS | Higher specificity and selectivity; Gives more structural information. | Lack of reference libraries. | [139,140,141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinca, A.T.; Ramalhinho, A.C.; Sousa, Â.; Oliani, A.H.; Breitenfeld, L.; Passarinha, L.A.; Gallardo, E. Follicular Fluid: A Powerful Tool for the Understanding and Diagnosis of Polycystic Ovary Syndrome. Biomedicines 2022, 10, 1254. https://doi.org/10.3390/biomedicines10061254
Brinca AT, Ramalhinho AC, Sousa Â, Oliani AH, Breitenfeld L, Passarinha LA, Gallardo E. Follicular Fluid: A Powerful Tool for the Understanding and Diagnosis of Polycystic Ovary Syndrome. Biomedicines. 2022; 10(6):1254. https://doi.org/10.3390/biomedicines10061254
Chicago/Turabian StyleBrinca, Ana Teresa, Ana Cristina Ramalhinho, Ângela Sousa, António Hélio Oliani, Luiza Breitenfeld, Luís A. Passarinha, and Eugenia Gallardo. 2022. "Follicular Fluid: A Powerful Tool for the Understanding and Diagnosis of Polycystic Ovary Syndrome" Biomedicines 10, no. 6: 1254. https://doi.org/10.3390/biomedicines10061254
APA StyleBrinca, A. T., Ramalhinho, A. C., Sousa, Â., Oliani, A. H., Breitenfeld, L., Passarinha, L. A., & Gallardo, E. (2022). Follicular Fluid: A Powerful Tool for the Understanding and Diagnosis of Polycystic Ovary Syndrome. Biomedicines, 10(6), 1254. https://doi.org/10.3390/biomedicines10061254