Macrophage-Derived Extracellular Vesicles: A Promising Tool for Personalized Cancer Therapy
Abstract
:1. Introduction
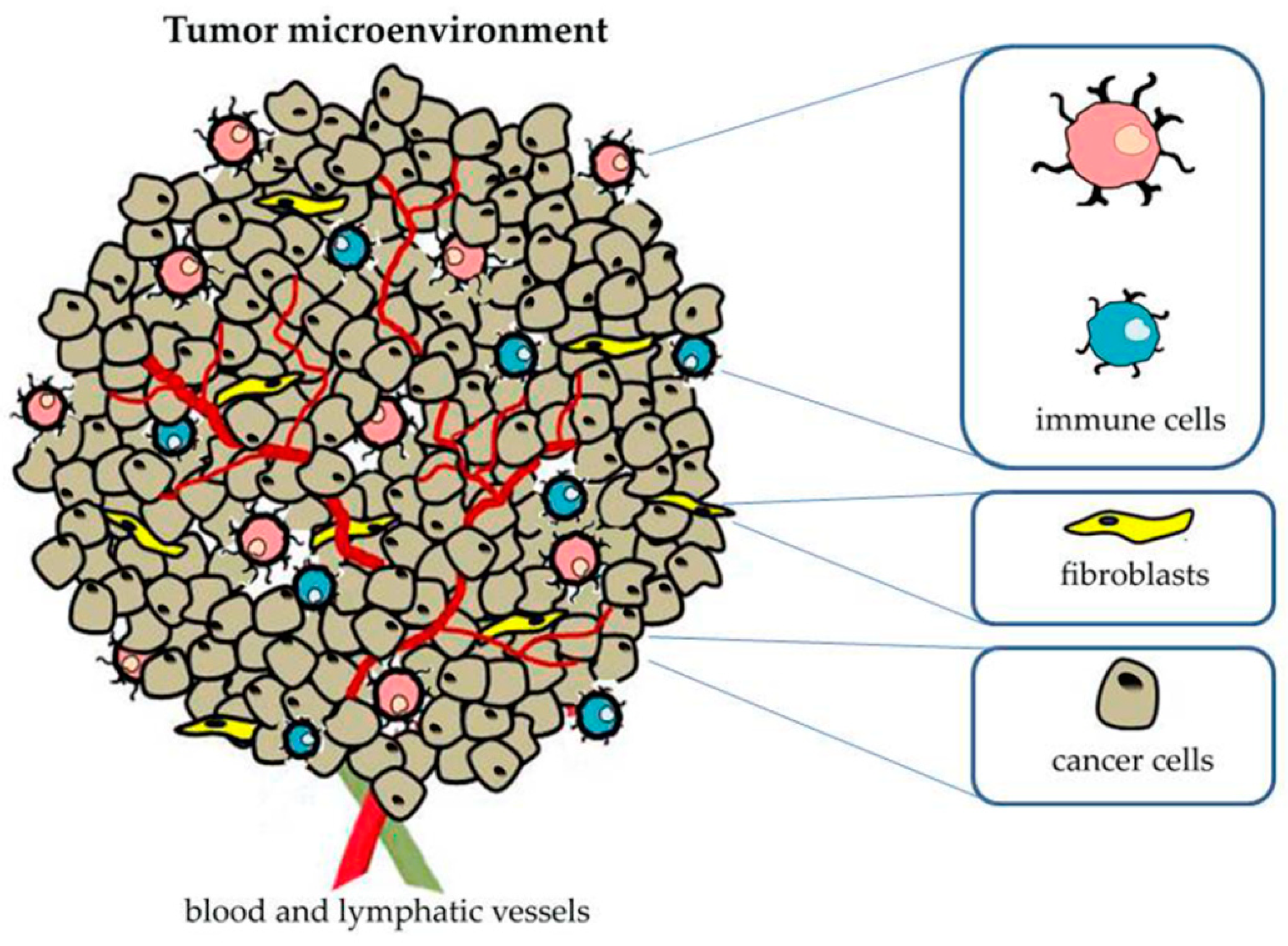
2. Role of Tumor Microenvironment in Cancer Progression
Tumor-Associated Macrophages
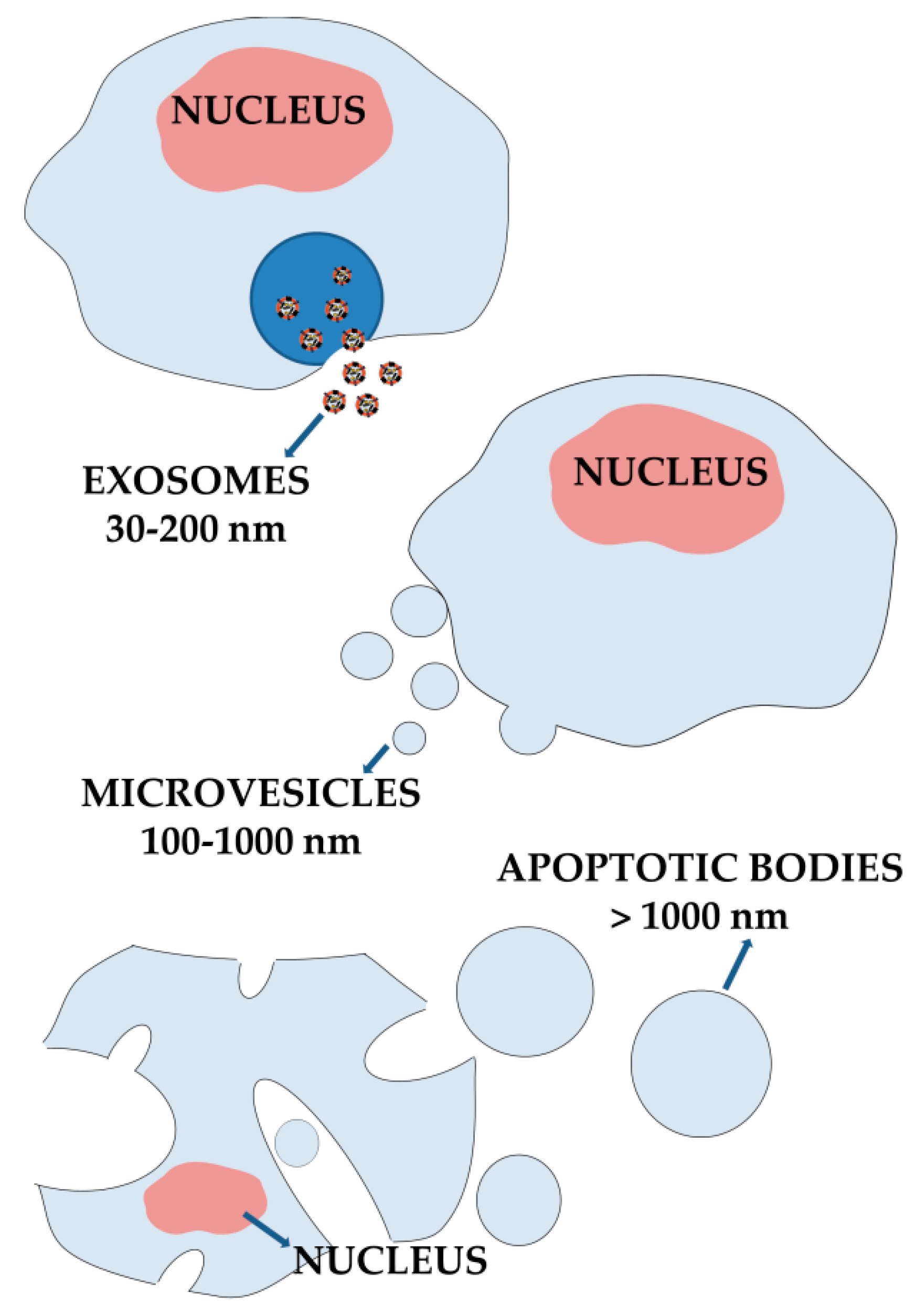
3. Extracellular Vesicles from Cellular Communication Mediators to Promising Nanomedicine
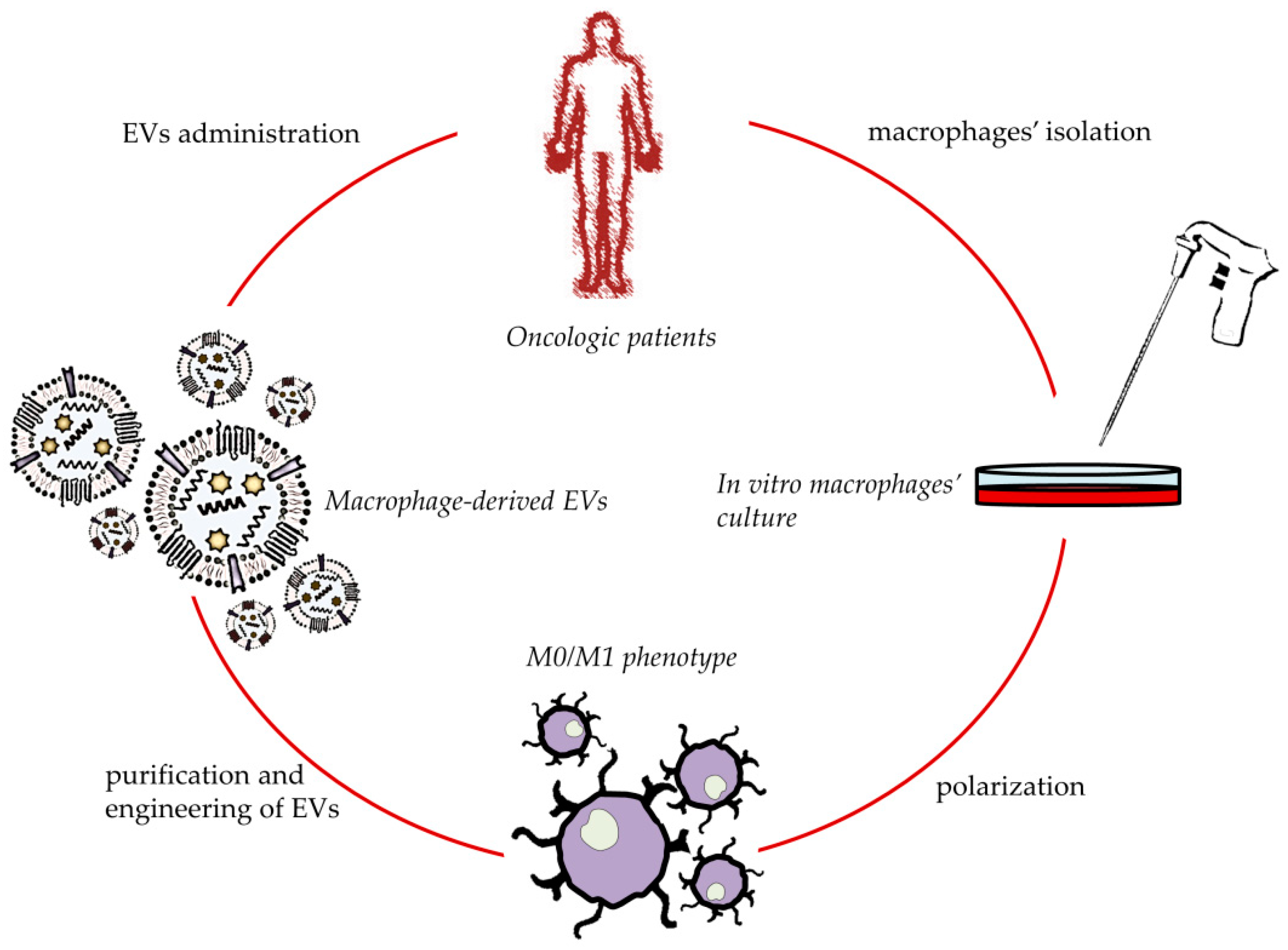
4. Macrophage-Derived EVs for Cancer Treatment
5. Macrophage-Derived EVs for In Situ TAMs Phenotype’s Shifting
6. Engineered Macrophage-Derived EVs for Anticancer Application
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Giddings, E.L.; Champagne, D.P.; Wu, M.-H.; Laffin, J.M.; Thornton, T.M.; Valenca-Pereira, F.; Culp-Hill, R.; Fortner, K.A.; Romero, N.; East, J. Mitochondrial ATP fuels ABC transporter-mediated drug efflux in cancer chemoresistance. Nat. Commun. 2021, 12, 2804. [Google Scholar] [CrossRef] [PubMed]
- Robey, I.F.; Lien, A.D.; Welsh, S.J.; Baggett, B.K.; Gillies, R.J. Hypoxia-inducible factor-1α and the glycolytic phenotype in tumors. Neoplasia 2005, 7, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Assaraf, Y.G.; Brozovic, A.; Gonçalves, A.C.; Jurkovicova, D.; Linē, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.; Vasconcelos, M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updat. 2019, 46, 100645. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-M.; Zhang, J.-G.; Zhang, X.; Li, Q. Targeting cancer stem cells for reversing therapy resistance: Mechanism, signaling, and prospective agents. Signal Transduct Target Ther. 2021, 6, 1–17. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, W.; Cai, X.; Wang, X.; Dang, W.; Tang, H.; Cao, H.; Wang, L.; Chen, T. Hydrazinocurcumin Encapsuled nanoparticles “re-educate” tumor-associated macrophages and exhibit anti-tumor effects on breast cancer following STAT3 suppression. PLoS ONE 2013, 8, e65896. [Google Scholar] [CrossRef]
- Dai, X.; Lu, L.; Deng, S.; Meng, J.; Wan, C.; Huang, J.; Sun, Y.; Hu, Y.; Wu, B.; Wu, G. USP7 targeting modulates anti-tumor immune response by reprogramming Tumor-associated Macrophages in Lung Cancer. Theranostics 2020, 10, 9332. [Google Scholar] [CrossRef]
- Zhang, Y.; Bush, X.; Yan, B.; Chen, J.A. Gemcitabine nanoparticles promote antitumor immunity against melanoma. Biomaterials 2019, 189, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Cang, H.; Guo, B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med. 2019, 8, 4709–4721. [Google Scholar] [CrossRef] [PubMed]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: Technicalities and challenges in routine clinical practice. Front. Oncol. 2020, 9, 1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sica, A. Role of tumour-associated macrophages in cancer-related inflammation. Exp. Oncol. 2010, 32, 153–158. [Google Scholar] [PubMed]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-associated macrophages: Recent insights and therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Genard, G.; Lucas, S.; Michiels, C. Reprogramming of tumor-associated macrophages with anticancer therapies: Radiotherapy versus chemo-and immunotherapies. Front. Immunol. 2017, 8, 828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Somiya, M.; Kuroda, S.i. Enhancing antibody-dependent cellular phagocytosis by Re-education of tumor-associated macrophages with resiquimod-encapsulated liposomes. Biomaterials 2021, 268, 120601. [Google Scholar] [CrossRef]
- Ye, J.; Yang, Y.; Dong, W.; Gao, Y.; Meng, Y.; Wang, H.; Li, L.; Jin, J.; Ji, M.; Xia, X. Drug-free mannosylated liposomes inhibit tumor growth by promoting the polarization of tumor-associated macrophages. Int. J. Nanomed. 2019, 14, 3203. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Li, L.; Yu, B.; Xu, E.; Sun, N.; Li, X.; Xing, Z.; Han, X.; Cui, Y.; Wang, X. Extracellular vesicles mediated proinflammatory macrophage phenotype induced by radiotherapy in cervical cancer. BMC Cancer 2022, 22, 1–12. [Google Scholar] [CrossRef]
- Maisano, D.; Mimmi, S.; Dattilo, V.; Marino, F.; Gentile, M.; Vecchio, E.; Fiume, G.; Nisticò, N.; Aloisio, A.; De Santo, M.P. A novel phage display based platform for exosome diversity characterization. Nanoscale 2022. [Google Scholar] [CrossRef]
- Möller, A.; Lobb, R.J. The evolving translational potential of small extracellular vesicles in cancer. Nat. Rev. Cancer. 2020, 20, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Veerman, R.E.; Akpinar, G.G.; Eldh, M.; Gabrielsson, S. Immune cell-derived extracellular vesicles–functions and therapeutic applications. Trends Mol. Med. 2019, 25, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Seeger, R.C.; Fabbri, M.; Wang, L.; Wayne, A.S.; Jong, A.Y. Biological roles and potential applications of immune cell-derived extracellular vesicles. J. Extracell Vesicles 2017, 6, 1400370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Chen, Z.; Li, Z.; Li, S.; Wen, Z.; Cao, L.; Chen, Y.; Xue, P.; Li, H.; Zhang, D. Tumor-associated macrophages promote cholangiocarcinoma progression via exosomal Circ_0020256. Cell Death Dis. 2022, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Peng, R.; Fang, F.; Mao, L.; Chen, Z.; Yang, S.; Dai, C.; Wu, H.; Wang, C.; Feng, N. Tumor-associated macrophages promote prostate cancer progression via exosome-mediated miR-95 transfer. J. Cell. Physiol. 2020, 235, 9729–9742. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.; Kuttan, G. Role of macrophages in tumour progression. Immunol. Lett. 2009, 123, 97–102. [Google Scholar] [CrossRef]
- Galli, F.; Aguilera, J.V.; Palermo, B.; Markovic, S.N.; Nisticò, P.; Signore, A. Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. J. Exp. Clin. Cancer Res. 2020, 39, 1–21. [Google Scholar] [CrossRef]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef]
- Bellei, B.; Caputo, S.; Migliano, E.; Lopez, G.; Marcaccini, V.; Cota, C.; Picardo, M. Simultaneous Targeting Tumor Cells and Cancer-Associated Fibroblasts with a Paclitaxel–Hyaluronan Bioconjugate: In Vitro Evaluation in Non-Melanoma Skin Cancer. Biomedicines 2021, 9, 597. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Song, Y.; Si, M.; Sun, Y.; Liu, X.; Cui, S.; Qu, X.; Yu, X. Exosomal miR-146a-5p and miR-155–5p promote CXCL12/CXCR7-induced metastasis of colorectal cancer by crosstalk with cancer-associated fibroblasts. Cell Death Dis. 2022, 13, 380. [Google Scholar] [CrossRef]
- Chiu, K.-J.; Chiou, H.-Y.C.; Huang, C.-H.; Lu, P.-C.; Kuo, H.-R.; Wang, J.-W.; Lin, M.-H. Natural Compounds Targeting Cancer-Associated Fibroblasts against Digestive System Tumor Progression: Therapeutic Insights. Biomedicines 2022, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Barone, A.; Cristiano, M.C.; Cianflone, E.; Fresta, M.; Paolino, D. Cardiac Stem Cell-Loaded Delivery Systems: A New Challenge for Myocardial Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 7701. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of extracellular matrix in development and cancer progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. Int. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yuan, Y.; Li, C.; Mao, H.; Liu, B.; Jiang, X. Modulating Tumor Extracellular Matrix by Simultaneous Inhibition of Two Cancer Cell Receptors. Adv. Mater. 2022, 34, 2109376. [Google Scholar] [CrossRef]
- Chiang, Y.-T.; Lo, C.-L. pH-responsive polymer-liposomes for intracellular drug delivery and tumor extracellular matrix switched-on targeted cancer therapy. Biomaterials 2014, 35, 5414–5424. [Google Scholar] [CrossRef]
- Koh, J.; Kim, H.Y.; Lee, Y.; Park, I.K.; Kang, C.H.; Kim, Y.T.; Kim, J.-E.; Choi, M.; Lee, W.-W.; Jeon, Y.K. IL23-producing human lung cancer cells promote tumor growth via conversion of innate lymphoid cell 1 (ILC1) into ILC3. Clin. Cancer Res. 2019, 25, 4026–4037. [Google Scholar] [CrossRef] [Green Version]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H. The clinical role of the TME in solid cancer. Br. J. Cancer. 2019, 120, 45–53. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Liu, L.; Chen, M.; Wang, X.; Yang, J.; Gong, Y.; Ding, B.-S.; Wei, Y.; Wei, X. Tumor cells induce LAMP2a expression in tumor-associated macrophage for cancer progression. EBioMedicine 2019, 40, 118–134. [Google Scholar] [CrossRef] [Green Version]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [Green Version]
- Twomey, J.D.; Zhang, B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 2021, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Takacova, M.; Kajanova, I.; Kolarcikova, M.; Lapinova, J.; Zatovicova, M.; Pastorekova, S. Understanding metabolic alterations and heterogeneity in cancer progression through validated immunodetection of key molecular components: A case of carbonic anhydrase IX. Cancer Metastasis Rev. 2022, 40, 1035–1053. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sinha, S.; Shrivastava, N. Therapeutic Targeting Hypoxia-Inducible Factor (HIF-1) in Cancer: Cutting Gordian Knot of Cancer Cell Metabolism. Front. Genet. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Kobayashi, M.; Koyasu, S.; Chow, C.C.; Harada, H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int. J. Mol. Sci. 2019, 20, 238. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; He, F.; Hou, Y.; Tu, G.; Li, Q.; Jin, T.; Zeng, H.; Qin, Y.; Wan, X.; Qiao, Y. A novel hypoxic long noncoding RNA KB-1980E6. 3 maintains breast cancer stem cell stemness via interacting with IGF2BP1 to facilitate c-Myc mRNA stability. Oncogene 2021, 40, 1609–1627. [Google Scholar] [CrossRef]
- Cui, X.-g.; Han, Z.-t.; He, S.-h.; Wu, X.-d.; Chen, T.-r.; Shao, C.-h.; Chen, D.-l.; Su, N.; Chen, Y.-m.; Wang, T. HIF1/2α mediates hypoxia-induced LDHA expression in human pancreatic cancer cells. Oncotarget 2017, 8, 24840. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Li, P.; Bai, Y.; Pang, J.; Fan, L.; Tian, W. Ruthenium (II)-Coordinated Supramolecular Metallodrug Complex Realizing Oxygen Self-Supply In Situ for Overcoming Hypoxic Tumors. Adv. Funct. Mater. 2021, 31, 2105837. [Google Scholar] [CrossRef]
- Singh, S.V.; Chaube, B.; Mayengbam, S.S.; Singh, A.; Malvi, P.; Mohammad, N.; Deb, A.; Bhat, M.K. Metformin induced lactic acidosis impaired response of cancer cells towards paclitaxel and doxorubicin: Role of monocarboxylate transporter. Biochim. Biophys. Acta Mol. Basis. Dis. 2021, 1867, 166011. [Google Scholar] [CrossRef]
- Errea, A.; Cayet, D.; Marchetti, P.; Tang, C.; Kluza, J.; Offermanns, S.; Sirard, J.-C.; Rumbo, M. Lactate inhibits the pro-inflammatory response and metabolic reprogramming in murine macrophages in a GPR81-independent manner. PLoS ONE 2016, 11, e0163694. [Google Scholar] [CrossRef] [Green Version]
- Peng, M.; Yin, N.; Chhangawala, S.; Xu, K.; Leslie, C.S.; Li, M.O. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 2016, 354, 481–484. [Google Scholar] [CrossRef]
- Zhang, J.; Muri, J.; Fitzgerald, G.; Gorski, T.; Gianni-Barrera, R.; Masschelein, E.; D’Hulst, G.; Gilardoni, P.; Turiel, G.; Fan, Z.; et al. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab. 2020, 31, 1136–1153. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Khan, S.; Zaki, A.; Tabassum, G.; Mohsin, M.; Bhutto, H.N.; Ahmad, T.; Fatma, T.; Syed, M.A. Integration of chemokine signaling with non-coding RNAs in tumor microenvironment and heterogeneity in different cancers. Semin. Cancer Biol. 2022; in press. [Google Scholar] [CrossRef]
- Wu, H.-J.; Hao, M.; Yeo, S.K.; Guan, J.-L. FAK signaling in cancer-associated fibroblasts promotes breast cancer cell migration and metastasis by exosomal miRNAs-mediated intercellular communication. Oncogene 2020, 39, 2539–2549. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, J.; Zhou, H.; Zeng, X.; Ruan, Z.; Pu, Z.; Jiang, X.; Matsui, A.; Zhu, L.; Amoozgar, Z. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 2022, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dey, D.; Kim, K.; Kim, S.; Kim, E.; Kang, S.; Bajpai, V.; Huh, Y. Hypoxia-responsive nanomedicine to overcome tumor microenvironment-mediated resistance to chemo-photodynamic therapy. Mater. Today Adv. 2022, 14, 100218. [Google Scholar] [CrossRef]
- Lahmar, Q.; Keirsse, J.; Laoui, D.; Movahedi, K.; Van Overmeire, E.; Van Ginderachter, J.A. Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochim. Biophys. Acta Rev. Cancer 2016, 1865, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Luo, Y. Targeting macrophages in cancer immunotherapy. Signal Transduct. Target Ther. 2021, 6, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-F.; Wang, C.; Zhang, L.; Li, Q. Reversing chemokine/chemokine receptor mismatch to enhance the antitumor efficacy of CAR-T cells. Immunotherapy 2022, 14, 459–473. [Google Scholar] [CrossRef]
- Günes Günsel, G.; Conlon, T.M.; Jeridi, A.; Kim, R.; Ertüz, Z.; Lang, N.J.; Ansari, M.; Novikova, M.; Jiang, D.; Strunz, M. The arginine methyltransferase PRMT7 promotes extravasation of monocytes resulting in tissue injury in COPD. Nat. Commun. 2022, 13, 1303. [Google Scholar] [CrossRef]
- Nobs, S.P.; Kopf, M. Tissue-resident macrophages: Guardians of organ homeostasis. Trends Immunol. 2021, 42, 495–507. [Google Scholar] [CrossRef]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.-S.; Wen, Z.-J.; Wang, J.-H.; Zhu, F.-F.; Guo, F.; Zhou, J.-L.; Xu, J.-J.; Zhong, H.-J. Legumain protease-sheddable PEGylated, tuftsin-modified nanoparticles for selective targeting to tumour-associated macrophages. J. Drug Target 2022, 30, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-associated macrophages in human breast, colorectal, lung, ovarian and prostate cancers. Front. Oncol. 2020, 2232. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Tanaka, T.; Yamamoto, Y.; Akasaki, Y.; Sasaki, H. Dual role of macrophage in tumor immunity. Immunotherapy 2018, 10, 899–909. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, N.; Zhou, Y.; Chen, J.; Wei, Q.; Han, M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm. Sin. B 2020, 10, 2156–2170. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.-S.; Lin, J.; Xing, Y.-M.; Gao, W.-L.; Jiang, Y.-X.; Chen, L.-X.; Zhang, X.-P.; Dai, Z.-L. OVOL2 inhibits macrophage M2 polarization by regulating IL-10 transcription, and thus inhibits the tumor metastasis by modulating the tumor microenvironment. Immunol. Lett. 2022, 242, 17–26. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Z.; Chen, W.; Wang, X.; Cao, M.; Han, X.; Zhang, K.; Teng, B.; Cao, J.; Wu, W. M2 macrophage-derived exosomes promote angiogenesis and growth of pancreatic ductal adenocarcinoma by targeting E2F2. Mol. Ther. 2021, 29, 1226–1238. [Google Scholar] [CrossRef]
- Peterson, T.; Kirkpatrick, N.; Huang, Y.; Farrar, C.; Marjit, K.; Kloepper, J.; Datta, M.; Amoozgar, Z.; Seano, G.; Jung, K. Tmic-07. Dual Inhibition of Ang-2 and Vegf Receptors Normalizes Tumor Vasculature and Prolongs Survival in Glioblastoma By Altering Macrophages. Proc. Natl. Acad. Sci. USA 2016, 113, 4470–4475. [Google Scholar] [CrossRef] [Green Version]
- Rigo, A.; Gottardi, M.; Zamò, A.; Mauri, P.; Bonifacio, M.; Krampera, M.; Damiani, E.; Pizzolo, G.; Vinante, F. Macrophages may promote cancer growth via a GM-CSF/HB-EGF paracrine loop that is enhanced by CXCL12. Mol. Cancer 2010, 9, 273. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.-Q.; Du, W.-L.; Cai, M.-H.; Yao, J.-Y.; Zhao, Y.-Y.; Mou, X.-Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lepland, A.; Scodeller, P.; Fontana, F.; Torrieri, G.; Tiboni, M.; Shahbazi, M.A.; Casettari, L.; Kostiainen, M.A.; Hirvonen, J. Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy. Acta Biomater. 2021, 133, 231–243. [Google Scholar] [CrossRef]
- Hou, L.; Gong, X.; Yang, J.; Zhang, H.; Yang, W.; Chen, X. Hybrid-Membrane-Decorated Prussian Blue for Effective Cancer Immunotherapy via Tumor-Associated Macrophages Polarization and Hypoxia Relief. Adv. Mater. Technol. 2022, 34, 2200389. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, J.-H.; Guo, F.; Zhu, F.-F.; Wen, Z.-J.; Zhong, H.-J.; Liang, D.-S. Legumain protease-activated tuftsin-functionalized nanoparticles for dual-targeting TAMs and cancer chemotherapy. Colloids Surf. B 2021, 197, 111442. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Ma, Z.; Yang, F.; Zhao, X.; Jiang, W.; Pan, C.; Li, Z.; Pan, X.; He, Z.; Xu, J. M2 macrophage-derived exosomes promote lung adenocarcinoma progression by delivering miR-942. Cancer Lett. 2022, 526, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, X.; Guo, Q.; Zhu, S.; Li, P.; Zhang, S.; Min, L. M2 Macrophage Derived Extracellular Vesicle-Mediated Transfer of MiR-186–5p Promotes Colon Cancer Progression by Targeting DLC1. Int. J. Biol. Sci. 2022, 18, 1663. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, Y.; Wei, R.; Zhang, X.; Wang, C.; Feng, M. Proinflammatory macrophage-derived microvesicles exhibit tumor tropism dependent on CCL2/CCR2 signaling axis and promote drug delivery via SNARE-mediated membrane fusion. Theranostics 2020, 10, 6581. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Harding, C.; Stahl, P. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem. Biophys. Res. Commun. 1983, 113, 650–658. [Google Scholar] [CrossRef]
- Pan, B.-T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Manna, I.; De Benedittis, S.; Quattrone, A.; Maisano, D.; Iaccino, E.; Quattrone, A. Exosomal miRNAs as potential diagnostic biomarkers in Alzheimer’s disease. Pharmaceuticals 2020, 13, 243. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Iglesias-Aguirre, C.E.; Cortés-Martín, A.; Vallejo, F.; Cattivelli, A.; del Pozo-Acebo, L.; Del Saz, A.; López de las Hazas, M.C.; Dávalos, A.; Espín, J.C. Milk-Derived Exosomes as Nanocarriers to Deliver Curcumin and Resveratrol in Breast Tissue and Enhance Their Anticancer Activity. Int. J. Mol. Sci. 2022, 23, 2860. [Google Scholar] [CrossRef]
- Matuk, R.; Pereira, M.; Baird, J.; Dooner, M.; Cheng, Y.; Wen, S.; Rao, S.; Quesenberry, P.; Raukar, N.P. The role of salivary vesicles as a potential inflammatory biomarker to detect traumatic brain injury in mixed martial artists. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Krušić Alić, V.; Malenica, M.; Biberić, M.; Zrna, S.; Valenčić, L.; Šuput, A.; Kalagac Fabris, L.; Wechtersbach, K.; Kojc, N.; Kurtjak, M. Extracellular Vesicles from Human Cerebrospinal Fluid Are Effectively Separated by Sepharose CL-6B—Comparison of Four Gravity-Flow Size Exclusion Chromatography Methods. Biomedicines 2022, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.N.T.; Kim, M.G.; Koo, B.; Liu, H.; Jang, Y.O.; Lee, H.J.; Kim, Y.; Park, Y.Y.; Kim, H.S.; Kim, C.S. Chimeric nanocomposites for the rapid and simple isolation of urinary extracellular vesicles. J. Extracell. Vesicles 2022, 11, e12195. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef]
- Gutiérrez García, G.; Galicia García, G.; Zalapa Soto, J.; Izquierdo Medina, A.; Rotzinger-Rodríguez, M.; Casas Aguilar, G.A.; López Pacheco, C.P.; Aguayo, Á.; Aguilar-Hernandez, M.M. Analysis of RNA yield in extracellular vesicles isolated by membrane affinity column and differential ultracentrifugation. PLoS ONE 2020, 15, e0238545. [Google Scholar] [CrossRef]
- Niu, Z.; Pang, R.T.; Liu, W.; Li, Q.; Cheng, R.; Yeung, W.S. Polymer-based precipitation preserves biological activities of extracellular vesicles from an endometrial cell line. PLoS ONE 2017, 12, e0186534. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.; Shin, D.W.; Kim, S.; Lee, Y.-S.; Mankhong, S.; Yang, S.W.; Lee, P.H.; Park, D.-H.; Kwak, H.-B.; Lee, J.-S. Enrichment of exosome-like extracellular vesicles from plasma suitable for clinical vesicular miRNA biomarker research. J. Clin. Med. 2019, 8, 1995. [Google Scholar] [CrossRef] [Green Version]
- Foers, A.D.; Chatfield, S.; Dagley, L.F.; Scicluna, B.J.; Webb, A.I.; Cheng, L.; Hill, A.F.; Wicks, I.P.; Pang, K.C. Enrichment of extracellular vesicles from human synovial fluid using size exclusion chromatography. J. Extracell. Vesicles 2018, 7, 1490145. [Google Scholar] [CrossRef]
- Davis, C.N.; Phillips, H.; Tomes, J.J.; Swain, M.T.; Wilkinson, T.J.; Brophy, P.M.; Morphew, R.M. The importance of extracellular vesicle purification for downstream analysis: A comparison of differential centrifugation and size exclusion chromatography for helminth pathogens. PLoS Negl. Trop. Dis. 2019, 13, e0007191. [Google Scholar] [CrossRef]
- Zeng, X.; Yi, X.; Chen, L.; Zhang, H.; Zhou, R.; Wu, J.; Chen, Y.; Huang, W.; Zhang, L.; Zheng, J. Characterization and bioassays of extracellular vesicles extracted by tangential flow filtration. Regen. Med. 2022. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Mantel, P.-Y.; Halleck, A.E.; Trachtenberg, A.J.; Soria, C.E.; Oquin, S.; Bonebreak, C.M.; Saracoglu, E. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013, 394, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, J.; Park, J. Methods to isolate extracellular vesicles for diagnosis. Micro Nano Syst. Lett. 2017, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gholizadeh, S.; Draz, M.S.; Zarghooni, M.; Sanati-Nezhad, A.; Ghavami, S.; Shafiee, H.; Akbari, M. Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: Current status and future directions. Biosens. Bioelectron. 2017, 91, 588–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Huang, L.-F.; Zhao, L.; Zeng, Z.; Wang, X.; Cao, D.; Yang, L.; Ye, Z.; Chen, X.; Liu, B. Microvesicles (MIVs) secreted from adipose-derived stem cells (ADSCs) contain multiple microRNAs and promote the migration and invasion of endothelial cells. Genes Dis. 2020, 7, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ma, Z.; Tayebi, M.; Ai, Y. Submicron particle focusing and exosome sorting by wavy microchannel structures within viscoelastic fluids. Anal. Chem. 2019, 91, 4577–4584. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lässer, C.; Szabó, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzás, E.I.; Lötvall, J. Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J. Extracell Vesicles 2013, 2, 20677. [Google Scholar] [CrossRef]
- Yokoi, A.; Ochiya, T. Exosomes and extracellular vesicles: Rethinking the essential values in cancer biology. Semin. Cancer Biol. 2021, 74, 79–91. [Google Scholar] [CrossRef]
- Kakarla, R.; Hur, J.; Kim, Y.J.; Kim, J.; Chwae, Y.-J. Apoptotic cell-derived exosomes: Messages from dying cells. Exp. Mol. Med. 2020, 52, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Arandjelovic, S.; Ravichandran, K.S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 2015, 16, 907–917. [Google Scholar] [CrossRef] [Green Version]
- Clancy, J.W.; Schmidtmann, M.; D’Souza-Schorey, C. The ins and outs of microvesicles. FASEB BioAdv. 2021, 3, 399–406. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.; Bebawy, M. Proteins regulating microvesicle biogenesis and multidrug resistance in cancer. Proteomics 2019, 19, 1800165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 1–18. [Google Scholar] [CrossRef]
- Kenific, C.M.; Zhang, H.; Lyden, D. An exosome pathway without an ESCRT. Cell Res. 2021, 31, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umeda, R.; Satouh, Y.; Takemoto, M.; Nakada-Nakura, Y.; Liu, K.; Yokoyama, T.; Shirouzu, M.; Iwata, S.; Nomura, N.; Sato, K. Structural insights into tetraspanin CD9 function. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tkach, M.; Thalmensi, J.; Timperi, E.; Gueguen, P.; Névo, N.; Grisard, E.; Sirven, P.; Cocozza, F.; Gouronnec, A.; Martin-Jaular, L. Extracellular vesicles from triple negative breast cancer promote pro-inflammatory macrophages associated with better clinical outcome. Proc. Natl. Acad. Sci. USA. 2022, 119, e2107394119. [Google Scholar] [CrossRef]
- Pan, J.; Sheng, S.; Ye, L.; Xu, X.; Ma, Y.; Feng, X.; Qiu, L.; Fan, Z.; Wang, Y.; Xia, X. Extracellular vesicles derived from glioblastoma promote proliferation and migration of neural progenitor cells via PI3K-Akt pathway. Cell Commun. Signal. 2022, 20, 1–16. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Belhadj, Z.; He, B.; Deng, H.; Song, S.; Zhang, H.; Wang, X.; Dai, W.; Zhang, Q. A combined “eat me/don’t eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J. Extracell Vesicles 2020, 9, 1806444. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, G.; Xia, Y.; Li, H.; Yuan, J.; Zhang, J.; Chen, Y.; Guo, H.; Yang, Y.; Wang, Y. Eliminating the original cargos of glioblastoma cell-derived small extracellular vesicles for efficient drug delivery to glioblastoma with improved biosafety. Bioact. Mater. 2022, 16, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prada, I.; Meldolesi, J. Binding and fusion of extracellular vesicles to the plasma membrane of their cell targets. Int. J. Mol. Sci. 2016, 17, 1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdera, H.C.; Gitz-Francois, J.J.; Schiffelers, R.M.; Vader, P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J. Control. Release 2017, 266, 100–108. [Google Scholar] [CrossRef]
- Pedrioli, G.; Paganetti, P. Hijacking Endocytosis and Autophagy in Extracellular Vesicle Communication: Where the Inside Meets the Outside. Front. Cell Dev. Biol. 2021, 8, 595515. [Google Scholar] [CrossRef]
- Vlaeminck-Guillem, V. Extracellular vesicles in prostate cancer carcinogenesis, diagnosis, and management. Front. Oncol. 2018, 8, 222. [Google Scholar] [CrossRef]
- Corbeil, D.; Santos, M.F.; Karbanová, J.; Kurth, T.; Rappa, G.; Lorico, A. Uptake and fate of extracellular membrane vesicles: Nucleoplasmic reticulum-associated late endosomes as a new gate to intercellular communication. Cells 2020, 9, 1931. [Google Scholar] [CrossRef]
- Aubertin, K.; Silva, A.K.; Luciani, N.; Espinosa, A.; Djemat, A.; Charue, D.; Gallet, F.; Blanc-Brude, O.; Wilhelm, C. Massive release of extracellular vesicles from cancer cells after photodynamic treatment or chemotherapy. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019, 9, 8001. [Google Scholar] [CrossRef]
- Li, Y.-J.; Wu, J.-Y.; Wang, J.-M.; Hu, X.-B.; Cai, J.-X.; Xiang, D.-X. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020, 101, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Piffoux, M.; Volatron, J.; Cherukula, K.; Aubertin, K.; Wilhelm, C.; Silva, A.K.; Gazeau, F. Engineering and loading therapeutic extracellular vesicles for clinical translation: A data reporting frame for comparability. Adv. Drug Deliv. Rev. 2021, 178, 113972. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Han, Y.; Sun, Q.; Li, Z. Harnessing anti-tumor and tumor-tropism functions of macrophages via nanotechnology for tumor immunotherapy. Exploration. 2022, 20210166. [Google Scholar] [CrossRef]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, R.; Martinez, J.O.; Zinger, A.; De Vita, A.; Storci, G.; Arrighetti, N.; De Rosa, E.; Hartman, K.A.; Basu, N.; Taghipour, N. Leukocyte-mimicking nanovesicles for effective doxorubicin delivery to treat breast cancer and melanoma. Biomater. Sci. 2020, 8, 333–341. [Google Scholar] [CrossRef]
- Molinaro, R.; Corbo, C.; Martinez, J.O.; Taraballi, F.; Evangelopoulos, M.; Minardi, S.; Yazdi, I.K.; Zhao, P.; De Rosa, E.; Sherman, M. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 2016, 15, 1037–1046. [Google Scholar] [CrossRef]
- Liu, J.; Wu, F.; Zhou, H. Macrophage-derived exosomes in cancers: Biogenesis, functions and therapeutic applications. Immunol. Lett. 2020, 227, 102–108. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Haney, M.J.; Zhao, Y.; Jin, Y.S.; Li, S.M.; Bago, J.R.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC) therapy. J. Neuroimmune. Pharm. 2020, 15, 487–500. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Huang, Q.; Peng, C.; Yao, L.; Chen, H.; Qiu, Z.; Wu, Y.; Wang, L.; Chen, W. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics 2019, 9, 1714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, Y.; Zhu, Y.; Zhang, Y.; Zhu, H.; Liu, T. M1 Macrophage-Derived Exosomes Loaded with Gemcitabine and Deferasirox against Chemoresistant Pancreatic Cancer. Pharmaceutics 2021, 13, 1493. [Google Scholar] [CrossRef] [PubMed]
- Jorquera-Cordero, C.; Lara, P.; Cruz, L.J.; Schomann, T.; van Hofslot, A.; de Carvalho, T.G.; Guedes, P.M.D.M.; Creemers, L.; Koning, R.I.; Chan, A.B. Extracellular Vesicles from M1-Polarized Macrophages Combined with Hyaluronic Acid and a β-Blocker Potentiate Doxorubicin’s Antitumor Activity by Downregulating Tumor-Associated Macrophages in Breast Cancer. Pharmaceutics 2022, 14, 1068. [Google Scholar] [CrossRef]
- Choo, Y.W.; Kang, M.; Kim, H.Y.; Han, J.; Kang, S.; Lee, J.-R.; Jeong, G.-J.; Kwon, S.P.; Song, S.Y.; Go, S. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano 2018, 12, 8977–8993. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, H.; Li, Z.; Peng, Y.; Tan, H.; Wang, C.; Huang, G.; Li, W.; Ma, G.; Wei, W. Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects. Sig. Transduct. Target Ther. 2022, 7, 1–16. [Google Scholar] [CrossRef]
- Armstrong, J.P.; Holme, M.N.; Stevens, M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano 2017, 11, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Evers, M.J.; van de Wakker, S.I.; de Groot, E.M.; de Jong, O.G.; Gitz-François, J.J.; Seinen, C.S.; Sluijter, J.P.; Schiffelers, R.M.; Vader, P. Functional siRNA Delivery by Extracellular Vesicle–Liposome Hybrid Nanoparticles. Adv. Healthc. Mater. 2022, 11, 2101202. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, X.; Tang, J.; Lv, Q.; Liu, J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials 2021, 275, 120964. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, T.; He, W.; Jin, H.; Liu, C.; Yang, Z.; Ren, J. Methotrexate-loaded extracellular vesicles functionalized with therapeutic and targeted peptides for the treatment of glioblastoma multiforme. ACS Appl. Mater. Interfaces 2018, 10, 12341–12350. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 195–204. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Ding, F.; Yang, J.; Li, J.; Gao, X.; Zhang, C.; Feng, J. Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale 2020, 12, 10854–10862. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Tian, J.; Wang, Z.; Gao, Y.; Wu, X.; Ding, X.; Qiang, L.; Li, G.; Han, Z.; Yuan, Y. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J. Nanobiotechnology 2019, 17, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danhier, F.; Le Breton, A.; Préat, V.r. RGD-based strategies to target alpha (v) beta (3) integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef]
- Li, R.; Gong, X.; Hong, C.; Wang, H.; Chen, Y.; Tan, K.; Liu, X.; Wang, F. An efficient photochemotherapy nanoplatform based on the endogenous biosynthesis of photosensitizer in macrophage-derived extracellular vesicles. Biomaterials 2021, 279, 121234. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Cheng, L.; Lu, Y.; Zhang, X.; Wang, Y.; Deng, J.; Zhou, J.; Liu, B.; Liu, J. Thermosensitive Exosome–Liposome Hybrid Nanoparticle-Mediated Chemoimmunotherapy for Improved Treatment of Metastatic Peritoneal Cancer. Adv. Sci. 2020, 7, 2000515. [Google Scholar] [CrossRef]
- Rayamajhi, S.; Nguyen, T.D.T.; Marasini, R.; Aryal, S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019, 94, 482–494. [Google Scholar] [CrossRef]
| Method | Functional Principle | Main Advantages/Disadvantages | References |
|---|---|---|---|
| Differential centrifugation | Multi step precipitation | Cheap method/low EVs recovery rate and unspecific method | [85,86] |
| Polymer-based precipitation | Salting out method | Fast method/unspecific method and low purity | [87,88] |
| Size-exclusion chromatography | Hydrodynamic radius-based separation | High purity of EVs recovered/ low yield | [89,90] |
| Tangential flow filtration (TFF) | Cross-flow filtration | Large scale isolation/unspecific method | [91]/NA |
| Magnetic beads affinity | Immunocapture | Efficient multi-step method/expensive and unsuitable for large sample volumes | [92,93] |
| Microfluidic platforms | Immunocapture | Single-step method/Shear stress can damage EVs | [93,94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, A.; d’Avanzo, N.; Cristiano, M.C.; Paolino, D.; Fresta, M. Macrophage-Derived Extracellular Vesicles: A Promising Tool for Personalized Cancer Therapy. Biomedicines 2022, 10, 1252. https://doi.org/10.3390/biomedicines10061252
Barone A, d’Avanzo N, Cristiano MC, Paolino D, Fresta M. Macrophage-Derived Extracellular Vesicles: A Promising Tool for Personalized Cancer Therapy. Biomedicines. 2022; 10(6):1252. https://doi.org/10.3390/biomedicines10061252
Chicago/Turabian StyleBarone, Antonella, Nicola d’Avanzo, Maria Chiara Cristiano, Donatella Paolino, and Massimo Fresta. 2022. "Macrophage-Derived Extracellular Vesicles: A Promising Tool for Personalized Cancer Therapy" Biomedicines 10, no. 6: 1252. https://doi.org/10.3390/biomedicines10061252
APA StyleBarone, A., d’Avanzo, N., Cristiano, M. C., Paolino, D., & Fresta, M. (2022). Macrophage-Derived Extracellular Vesicles: A Promising Tool for Personalized Cancer Therapy. Biomedicines, 10(6), 1252. https://doi.org/10.3390/biomedicines10061252









