To Explore the Stem Cells Homing to GBM: The Rise to the Occasion
Abstract
1. Introduction
2. General Characteristics of Mesenchymal Stem Cells (MSC)
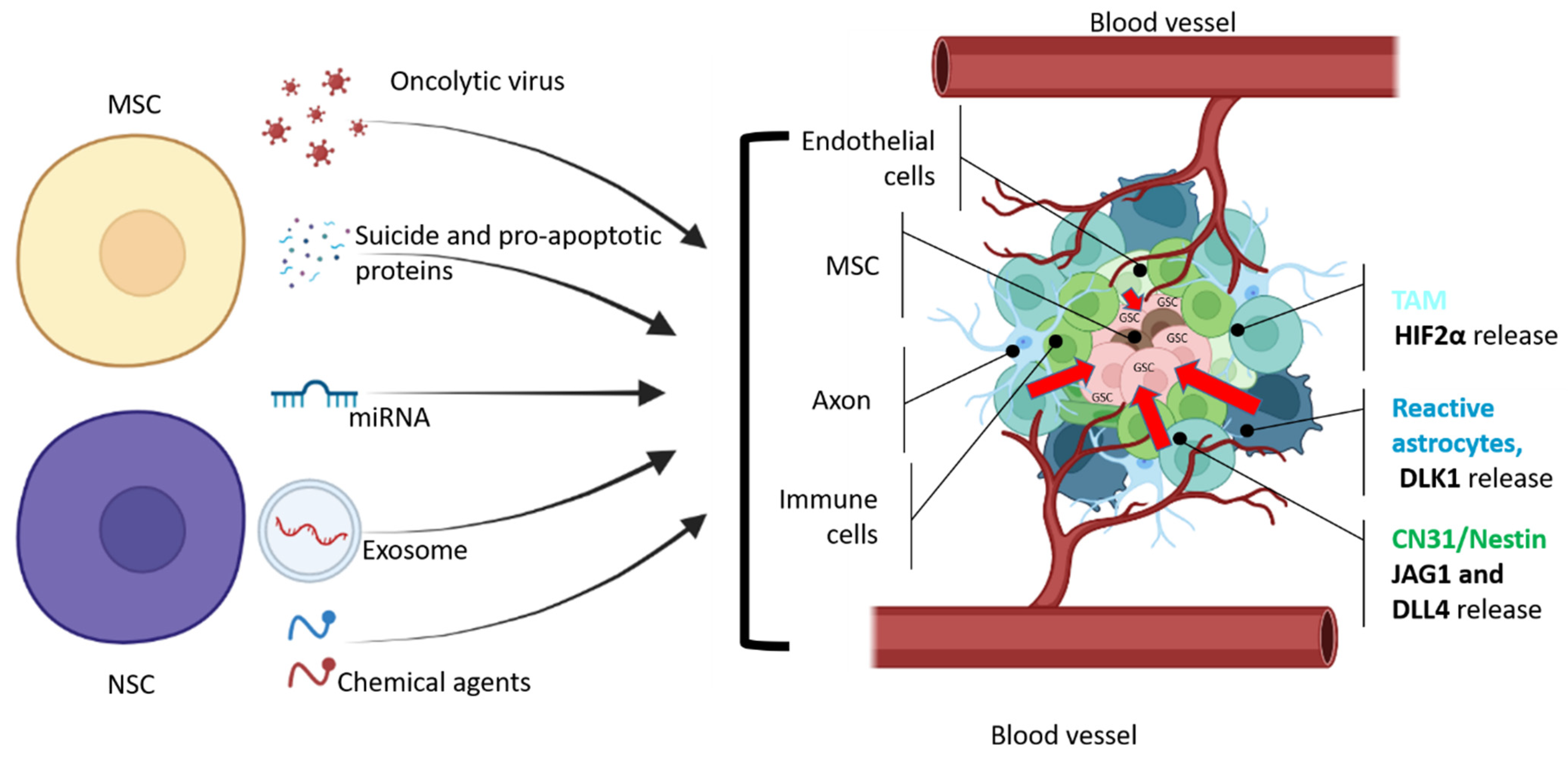
3. MSC—Cell Vectors for Migration and Delivery of Therapeutic Proteins
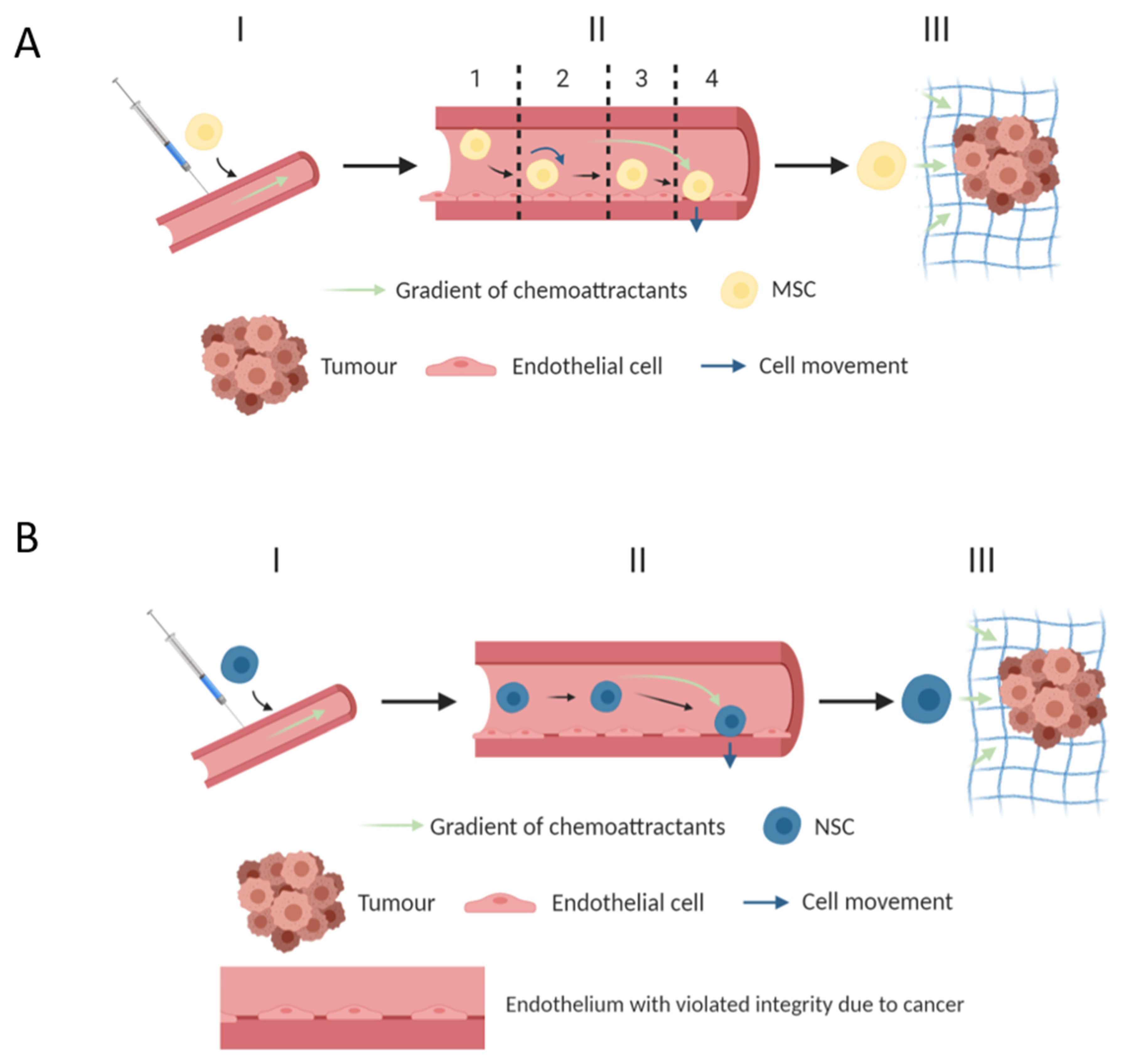
4. Methods and Factors That Increase the Migration Properties of MSCs
5. Modeling MSC Migration In Vitro
6. Induced Hypoxia
7. MSCs Have Been Genetically Modified to Increase Their Migration and Survival in Tumors
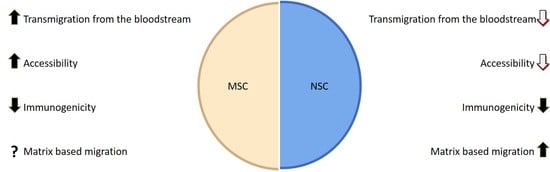
8. General Characteristics of Neural Stem Cells (NSCs)
9. NSC—Cell Vectors for Migration into the Tumor
10. Methods and Factors That Increase the Migration Properties of NSC
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ostrom, Q.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-Oncology 2014, 16, iv1–iv63. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Bredel, M.; Scholtens, D.M.; Harsh, G.R.; Bredel, C.; Chandler, J.P.; Renfrow, J.J.; Yadav, A.K.; Vogel, O.H.; Scheck, A.; Tibshirani, R.; et al. A network model of a cooperative genetic landscape in brain tumors. JAMA 2009, 302, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Renfrow, J.J.; Scholtens, D.M.; Xie, H.; Duran, G.E.; Bredel, C.; Vogel, H.; Chandler, J.P.; Chakravarti, A.; Robe, P.A.; et al. Monosomy of chromosome 10 as-sociated with dysregulation of epidermal growth factor signaling in glioblastomas. JAMA 2009, 302, 276–289. [Google Scholar] [CrossRef]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef]
- Sack, B.K.; Herzog, R.W. Evading the immune response upon in vivo gene therapy with viral vectors. Curr. Opin. Mol. Ther. 2009, 11, 493–503. [Google Scholar]
- Plotkin, S.A. Vaccines: Past, present and future. Nat. Med. 2005, 11, S5–S11. [Google Scholar] [CrossRef]
- Schuster, J.; Lai, R.K.; Recht, L.D.; Reardon, D.A.; Paleologos, N.A.; Groves, M.D.; Mrugala, M.M.; Jensen, R.; Baehring, J.M.; Sloan, A.; et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: The ACT III study. Neuro-Oncology 2015, 17, 854–861. [Google Scholar] [CrossRef]
- Sampson, J.H.; Heimberger, A.B.; Archer, G.E.; Aldape, K.D.; Friedman, A.H.; Friedman, H.S.; Gilbert, M.R.; Herndon, J.E., 2nd; McLendon, R.E.; Mitchell, D.A.; et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010, 28, 4722–4729. [Google Scholar] [CrossRef]
- Thomas, A.A.; Ernstoff, M.S.; Fadul, C.E. Immunotherapy for the treatment of glioblastoma. Cancer J. 2012, 18, 59–68. [Google Scholar] [CrossRef]
- Robbins, P.D.; Ghivizzani, S.C. Viral vectors for gene therapy. Pharmacol. Ther. 1998, 80, 35–47. [Google Scholar] [CrossRef]
- Kaufmann, J.K.; Chiocca, E.A. Glioma virus therapies between bench and bedside. Neuro-Oncology 2014, 16, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Rohle, D.; Popovici-Muller, J.; Palaskas, N.; Turcan, S.; Grommes, C.; Campos, C.; Tsoi, J.; Clark, O.; Oldrini, B.; Komisopoulou, E.; et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 2013, 340, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-X.; Zou, X.-H.; Jiang, S.-Y.; Lu, N.-N.; Han, M.; Zhao, J.-H.; Guo, X.-J.; Zhao, S.-C.; Lu, Z.-Z. Prevalence of serum neutralizing antibodies to adenovirus type 5 (Ad5) and 41 (Ad41) in children is associated with age and sanitary conditions. Vaccine 2016, 34, 5579–5586. [Google Scholar] [CrossRef]
- Yu, B.; Zhou, Y.; Wu, H.; Wang, Z.; Zhan, Y.; Feng, X.; Geng, R.; Wu, Y.; Kong, W.; Yu, X. Seroprevalence of neutralizing antibodies to human adenovirus type 5 in healthy adults in China. J. Med. Virol. 2012, 84, 1408–1414. [Google Scholar] [CrossRef]
- Mok, W.; Stylianopoulos, T.; Boucher, Y.; Jain, R.K. Mathematical Modeling of Herpes Simplex Virus Distribution in Solid Tumors: Implications for Cancer Gene Therapy. Clin. Cancer Res. 2009, 15, 2352–2360. [Google Scholar] [CrossRef]
- Gonzalez-Morales, A.; Zabaleta, A.; Garcia-Moure, M.; Alonso, M.M.; Fernandez-Irigoyen, J.; Santamaria, E. Oncolytic adeno-virus Delta-24-RGD induces a widespread glioma proteotype remodeling during autophagy. J. Proteom. 2019, 194, 168–178. [Google Scholar] [CrossRef]
- Tran, C.; Damaser, M.S. Stem cells as drug delivery methods: Application of stem cell secretome for regeneration. Adv. Drug Deliv. Rev. 2014, 82–83, 1–11. [Google Scholar] [CrossRef]
- Daley, G.Q.; Scadden, D.T. Prospects for stem cell-based therapy. Cell 2008, 132, 544–548. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, J.-H. Stem cells tropism for malignant gliomas. Neurosci. Bull. 2007, 23, 363–369. [Google Scholar] [CrossRef][Green Version]
- Xinaris, C.; Morigi, M.; Benedetti, V.; Imberti, B.; Fabricio, A.; Squarcina, E.; Benigni, A.; Gagliardini, E.; Remuzzi, G. A novel strategy to enhance mesenchymal stem cell migration capacity and promote tissue repair in an injury specific fashion. Cell Transplant. 2013, 22, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Shin, J.Y.; Lee, B.R.; Kim, H.O.; Lee, P.H. Mesenchymal stem cells augment neurogenesis in the subventricular zone and enhance differentiation of neural precursor cells into dopaminergic neurons in the substantia nigra of a parkinsonian model. Cell Transplant. 2012, 21, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Ode, A.; Kopf, J.; Kurtz, A.; Schmidt-Bleek, K.; Schrade, P.; Kolar, P.; Buttgerei, F.; Lehmann, K.; Hutmacher, D.; Duda, G.; et al. CD73 and CD29 concurrently mediate the mechanically induced decrease of migratory capacity of mesenchymal stromal cells. Eur. Cells Mater. 2011, 22, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.M.; Gumin, J.; Camstra, K.M.; Collins, D.E.; Chen, M.M.; Shpall, E.J.; Kerrigan, B.C.P.; Johnson, J.N.; Chen, S.R.; Fueyo, J.; et al. Endovascular Selective Intra-Arterial Infusion of Mesenchymal Stem Cells Loaded with Delta-24 in a Canine Model. Neurosurgery 2020, 88, E102–E113. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.U.; Tyler, M.A.; Thaci, B.; Alexiades, N.G.; Han, Y.; Ulasov, I.V.; Lesniak, M.S. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol. Pharm. 2011, 8, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Yong, R.L.; Shinojima, N.; Fueyo, J.; Gumin, J.; Vecil, G.G.; Marini, F.C.; Bogler, O.; Andreeff, M.; Lang, F.F. Human bone mar-row-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009, 69, 8932–8940. [Google Scholar] [CrossRef]
- Dührsen, L.; Hartfuss, S.; Hirsch, D.; Geiger, S.; Maire, C.L.; Sedlacik, J.; Guenther, C.; Westphal, M.; Lamszus, K.; Hermann, F.G.; et al. Preclinical analysis of human mesenchymal stem cells: Tumor tropism and therapeutic efficiency of local HSV-TK suicide gene therapy in glioblastoma. Oncotarget 2019, 10, 6049–6061. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, M.; Guo, R.; Miao, Y.; Li, B. Bone Marrow–Derived Mesenchymal Stem Cell–Mediated Dual-Gene Therapy for Glioblastoma. Hum. Gene Ther. 2019, 30, 106–117. [Google Scholar] [CrossRef]
- Wei, D.; Hou, J.; Zheng, K.; Jin, X.; Xie, Q.; Cheng, L.; Sun, X. Suicide Gene Therapy Against Malignant Gliomas by the Local Delivery of Genetically Engineered Umbilical Cord Mesenchymal Stem Cells as Cellular Vehicles. Curr. Gene Ther. 2019, 19, 330–341. [Google Scholar] [CrossRef]
- Kurogi, R.; Nakamizo, A.; Suzuki, S.O.; Mizoguchi, M.; Yoshimoto, K.; Amano, T.; Amemiya, T.; Takagishi, S.; Iihara, K. Inhibition of glioblastoma cell invasion by hsa-miR-145-5p and hsa-miR-31-5p co-overexpression in human mesenchymal stem cells. J. Neurosurg. 2018, 130, 44–55. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Didiot, M.-C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Fitch, S.; Wang, C.; Wilson, C.; Li, J.; Grant, G.A.; Yang, F. Nanoparticle engineered TRAIL-overexpressing adi-pose-derived stem cells target and eradicate glioblastoma via intracranial delivery. Proc. Natl. Acad. Sci. USA 2016, 113, 13857–13862. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.-Y.; Jung, J.-H.; Kim, A.A.; Marasini, S.; Lee, Y.J.; Paek, S.H.; Kim, S.-S.; Suh-Kim, H. Combined effects of mesenchymal stem cells carrying cytosine deaminase gene with 5-fluorocytosine and temozolomide in orthotopic glioma model. Am. J. Cancer Res. 2020, 10, 1429–1441. [Google Scholar] [PubMed]
- Yin, J.; Kim, J.K.; Moon, J.H.; Beck, S.; Piao, D.; Jin, X.; Kim, S.H.; Lim, Y.C.; Nam, D.H.; You, S.; et al. hMSC-mediated concurrent delivery of endostatin and carboxylesterase to mouse xenografts suppresses glioma initiation and recurrence. Mol. Ther. 2011, 19, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Tamura, K.; Khajuria, R.K.; Bhere, D.; Nesterenko, I.; Lawler, J.; Shah, K. Antiangiogenic variant of TSP-1 targets tumor cells in glioblastomas. Mol. Ther. 2015, 23, 235–243. [Google Scholar] [CrossRef]
- Pacioni, S.; D’Alessandris, Q.G.; Giannetti, S.; Morgante, L.; De Pascalis, I.; Coccè, V.; Bonomi, A.; Pascucci, L.; Alessandri, G.; Pessina, A.; et al. Mesenchymal stromal cells loaded with paclitaxel induce cytotoxic damage in glioblastoma brain xenografts. Stem Cell Res. Ther. 2015, 6, 194. [Google Scholar] [CrossRef]
- Choi, S.H.; Stuckey, D.W.; Pignatta, S.; Reinshagen, C.; Khalsa, J.K.; Roozendaal, N.; Martinez-Quintanilla, J.; Tamura, K.; Keles, E.; Shah, K. Tumor Resection Recruits Effector T Cells and Boosts Therapeutic Efficacy of Encapsulated Stem Cells Expressing IFNbeta in Glioblastomas. Clin. Cancer Res. 2017, 23, 7047–7058. [Google Scholar] [CrossRef]
- Chavakis, E.; Urbich, C.; Dimmeler, S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J. Mol. Cell. Cardiol. 2008, 45, 514–522. [Google Scholar] [CrossRef]
- Naderi-Meshkin, H.; Matin, M.M.; Heirani-Tabasi, A.; Mirahmadi, M.; Irfan-Maqsood, M.; Edalatmanesh, M.A.; Shahriyari, M.; Ahmadiankia, N.; Moussavi, N.S.; Bidkhori, H.R.; et al. Injectable hydrogel delivery plus preconditioning of mesen-chymal stem cells: Exploitation of SDF-1/CXCR4 axis toward enhancing the efficacy of stem cells’ homing. Cell Biol. Int. 2016, 40, 730–741. [Google Scholar] [CrossRef]
- Huang, W.; Wang, T.; Zhang, D.; Zhao, T.; Dai, B.; Ashraf, A.; Wang, X.; Xu, M.; Millard, R.W.; Fan, G.C.; et al. Mesenchymal stem cells overexpressing CXCR4 attenuate remodeling of postmyocardial infarction by releasing matrix metalloproteinase-9. Stem Cells Dev. 2012, 21, 778–789. [Google Scholar] [CrossRef]
- Liu, H.; Xue, W.; Ge, G.; Luo, X.; Li, Y.; Xiang, H.; Ding, X.; Tian, P.; Tian, X. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1α in MSCs. Biochem. Biophys. Res. Commun. 2010, 401, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Pavon, L.F.; Sibov, T.T.; De Souza, A.V.; da Cruz, E.F.; Malheiros, S.M.F.; Cabral, F.R.; De Souza, J.G.; Boufleur, P.; Oliveira, D.; De Toledo, S.R.C.; et al. Tropism of mesenchymal stem cell toward CD133+ stem cell of glioblastoma in vitro and promote tumor proliferation in vivo. Stem Cell Res. Ther. 2018, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Kuan, I.-I.; Lee, C.-C.; Chen, C.-H.; Lu, J.; Kuo, Y.-S.; Wu, H.-C. The extracellular domain of epithelial cell adhesion molecule (EpCAM) enhances multipotency of mesenchymal stem cells through EGFR–LIN28–LET7 signaling. J. Biol. Chem. 2019, 294, 7769–7786. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Majka, M.; Kucia, M.; Drukala, J.; Pietrzkowski, Z.; Peiper, S.; Janowska-Wieczorek, A. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells 2003, 21, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Okada, M.; Suzuki, S.; Togashi, K.; Sugai, A.; Yamamoto, M.; Kitanaka, C. Targeting Folate Metabolism Is Selectively Cytotoxic to Glioma Stem Cells and Effectively Cooperates with Differentiation Therapy to Eliminate Tumor-Initiating Cells in Glioma Xenografts. Int. J. Mol. Sci. 2021, 22, 11633. [Google Scholar] [CrossRef]
- Lathia, J.D.; Gallagher, J.; Heddleston, J.M.; Wang, J.; Eyler, C.E.; MacSwords, J.; Wu, Q.; Vasanji, A.; McLendon, R.E.; Hjelmeland, A.B.; et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 2010, 6, 421–432. [Google Scholar] [CrossRef]
- Hira, V.V.; Wormer, J.R.; Kakar, H.; Breznik, B.; Van Der Swaan, B.; Hulsbos, R.; Tigchelaar, W.; Tonar, Z.; Khurshed, M.; Molenaar, R.J.; et al. Periarteriolar Glioblastoma Stem Cell Niches Express Bone Marrow Hematopoietic Stem Cell Niche Proteins. J. Histochem. Cytochem. 2018, 66, 155–173. [Google Scholar] [CrossRef]
- Breznik, B.; Stokin, C.L.; Kos, J.; Khurshed, M.; Hira, V.V.V.; Bosnjak, R.; Lah, T.T.; Van Noorden, C.J.F. Cysteine cathepsins B, X and K expression in peri-arteriolar glioblastoma stem cell niches. Histochem. J. 2018, 49, 481–497. [Google Scholar] [CrossRef]
- Hira, V.V.; Breznik, B.; Vittori, M.; de Jong, A.L.; Mlakar, J.; Oostra, R.-J.; Khurshed, M.; Molenaar, R.J.; Lah, T.; Van Noorden, C.J. Similarities Between Stem Cell Niches in Glioblastoma and Bone Marrow: Rays of Hope for Novel Treatment Strategies. J. Histochem. Cytochem. 2019, 68, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.; Fiorelli, R.; Barrientos, E.S.; Melendez, E.L.; Sanai, N.; Mehta, S.; Nikkhah, M. A three-dimensional (3D) organotypic microfluidic model for glioma stem cells—Vascular interactions. Biomaterials 2018, 198, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Warrier, N.M.; Agarwal, P.; Kumar, P. Integrative Analysis to Identify Genes Associated with Stemness and Immune Infiltration in Glioblastoma. Cells 2021, 10, 2765. [Google Scholar] [CrossRef] [PubMed]
- Pillat, M.M.; Oliveira-Giacomelli, A.; Oliveira, M.D.N.; Andrejew, R.; Turrini, N.; Baranova, J.; Turnšek, T.L.; Ulrich, H. Mesenchymal stem cell-glioblastoma interactions mediated via kinin receptors unveiled by cytometry. Cytom. Part A 2021, 99, 152–163. [Google Scholar] [CrossRef]
- Uyar, R. Glioblastoma microenvironment: The stromal interactions. Pathol. Res. Pract. 2022, 232, 153813. [Google Scholar] [CrossRef]
- Vinogradov, S.; Wei, X. Cancer stem cells and drug resistance: The potential of nanomedicine. Nanomedicine 2012, 7, 597–615. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Chen, J.T.; Zheng, M.C.; Yang, L.J.; Wang, J.M.; Liu, Q.L.; Chen, L.F.; Ye, Z.C.; Lin, J.M.; Lin, Z.X. Nestin+/CD31+ cells in the hypoxic perivascular niche regulate glioblastoma chemoresistance by upregulating JAG1 and DLL4. Neuro-Oncology 2021, 23, 905–919. [Google Scholar] [CrossRef]
- Grassi, E.S.; Jeannot, P.; Pantazopoulou, V.; Berg, T.J.; Pietras, A. Niche-derived soluble DLK1 promotes glioma growth. Neoplasia 2020, 22, 689–701. [Google Scholar] [CrossRef]
- Kvisten, M.; Mikkelsen, V.E.; Solheim, O.; Van Der Want, J.; Torp, S.H. Microglia and macrophages in human glioblastomas: A morphological and immunohistochemical study. Mol. Clin. Oncol. 2019, 11, 31–36. [Google Scholar] [CrossRef]
- Wang, Q.; He, Z.; Huang, M.; Liu, T.; Wang, Y.; Xu, H.; Duan, H.; Ma, P.; Zhang, L.; Zamvil, S.S.; et al. Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2α. Nat. Commun. 2018, 9, 559. [Google Scholar] [CrossRef]
- Bordji, K.; Grandval, A.; Cuhna-Alves, L.; Lechapt-Zalcman, E.; Bernaudin, M. Hypoxia-inducible factor-2alpha (HIF-2alpha), but not HIF-1alpha, is essential for hypoxic induction of class III beta-tubulin expression in human glioblastoma cells. FEBS J. 2014, 281, 5220–5236. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zeng, L.; Liu, S.; Dangelmajer, S.; Kahlert, U.D.; Huang, H.; Han, Y.; Chi, X.; Zhu, M.; Lei, T. Transforming Growth Fac-tor-beta Promotes Homing and Therapeutic Efficacy of Human Mesenchymal Stem Cells to Glioblastoma. J. Neuropathol. Exp. Neurol. 2019, 78, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Sadhukha, T.; Panyam, J.; Prabha, S. Nano-Engineered Mesenchymal Stem Cells Increase Therapeutic Efficacy of Anticancer Drug Through True Active Tumor Targeting. Mol. Cancer Ther. 2018, 17, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, L.M.; Jin, X.; Dmitriev, I.P.; Kashentseva, E.A.; Powell, M.A.; Mutch, D.G.; Dietz, A.B.; Curiel, D.T.; Hawkins, W.G.; Spitzer, D. Adenovirus platform enhances transduction efficiency of human mesenchymal stem cells: An opportunity for cel-lular carriers of targeted TRAIL-based TR3 biologics in ovarian cancer. PLoS ONE 2017, 12, e0190125. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Deedigan, L.; Albarenque, S.M.; Mohr, A.; Zwacka, R.M. Delivery of sTRAIL variants by MSCs in combination with cytotoxic drug treatment leads to p53-independent enhanced antitumor effects. Cell Death Dis. 2013, 4, e503. [Google Scholar] [CrossRef]
- Thomas, J.G.; Kerrigan, B.C.P.; Hossain, A.; Gumin, J.; Shinojima, N.; Nwajei, F.; Ezhilarasan, R.; Love, P.; Sulman, E.P.; Lang, F.F. Ionizing radiation augments glioma tropism of mesenchymal stem cells. J. Neurosurg. 2018, 128, 287–295. [Google Scholar] [CrossRef]
- Motaln, H.; Turnsek, T.L. Cytokines play a key role in communication between mesenchymal stem cells and brain cancer cells. Protein Pept. Lett. 2015, 22, 322–331. [Google Scholar] [CrossRef]
- Karp, J.M.; Teo, G.S.L. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell 2009, 4, 206–216. [Google Scholar] [CrossRef]
- Smith, C.L.; Chaichana, K.L.; Lee, Y.M.; Lin, B.; Stanko, K.M.; O’Donnell, T.; Gupta, S.; Shah, S.R.; Wang, J.; Wijesekera, O.; et al. Pre-exposure of human adipose mesenchymal stem cells to soluble factors enhances their homing to brain cancer. Stem Cells Transl. Med. 2015, 4, 239–251. [Google Scholar] [CrossRef]
- Bexell, D.; Gunnarsson, S.; Tormin, A.; Darabi, A.; Gisselsson, D.; Roybon, L.; Scheding, S.; Bengzon, J. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol. Ther. 2009, 17, 183–190. [Google Scholar] [CrossRef]
- Velpula, K.K.; Dasari, V.R.; Rao, J.S. The homing of human cord blood stem cells to sites of inflammation: Unfolding mysteries of a novel therapeutic paradigm for glioblastoma multiforme. Cell Cycle 2012, 11, 2303–2313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qiu, Y.; Marquez-Curtis, L.A.; Janowska-Wieczorek, A. Mesenchymal stromal cells derived from umbilical cord blood migrate in response to complement C1q. Cytotherapy 2012, 14, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, L.; You, Y.; Zou, C.; Zhang, Y.; Liu, Q.; Cheng, F. Erythropoietin combined with granulocyte colony-stimulating factor enhances MMP-2 expression in mesenchymal stem cells and promotes cell migration. Mol. Med. Rep. 2011, 4, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. Biomed. Res. Int. 2013, 2013, 561098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Foudi, A.; Geay, J.; Berthebaud, M.; Buet, D.; Jarrier, P.; Jalil, A.; Vainchenker, W.; Louache, F. Intracellular localization and constitutive endocytosis of CXCR4 in human CD34+ hematopoietic progenitor cells. Stem Cells 2004, 22, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Qian, F.; Tchabo, N.; Mhawech-Fauceglia, P.; Beck, A.; Qian, Z.; Wang, X.; Huss, W.J.; Lele, S.B.; Morrison, C.D.; et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE 2014, 9, e84941. [Google Scholar] [CrossRef]
- Bache, M.; Rot, S.; Kessler, J.; Güttler, A.; Wichmann, H.; Greither, T.; Wach, S.; Taubert, H.; Söling, A.; Bilkenroth, U.; et al. mRNA expression levels of hypoxia-induced and stem cell-associated genes in human glioblastoma. Oncol. Rep. 2015, 33, 3155–3161. [Google Scholar] [CrossRef]
- Annabi, B.; Lee, Y.; Turcotte, S.; Naud, E.; Desrosiers, R.R.; Champagne, M.; Eliopoulos, N.; Galipeau, J.; Béliveau, R. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells 2003, 21, 337–347. [Google Scholar] [CrossRef]
- Valorani, M.G.; Montelatici, E.; Germani, A.; Biddle, A.; D’Alessandro, D.; Strollo, R.; Patrizi, M.P.; Lazzari, L.; Nye, E.; Otto, W.R.; et al. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif. 2012, 45, 225–238. [Google Scholar] [CrossRef]
- Hu, X.; Chen, P.; Wu, Y.; Wang, K.; Xu, Y.; Chen, H.; Zhang, L.; Wu, R.; Webster, K.A.; Yu, H.; et al. MiR-211/STAT5A Signaling Modulates Migration of Mesenchymal Stem Cells to Improve its Therapeutic Efficacy. Stem Cells 2016, 34, 1846–1858. [Google Scholar] [CrossRef]
- Allahverdi, A.; Arefian, E.; Soleimani, M.; Ai, J.; Nahanmoghaddam, N.; Yousefi-Ahmadipour, A.; Ebrahimi-Barough, S. Mi-croRNA-4731-5p delivered by AD-mesenchymal stem cells induces cell cycle arrest and apoptosis in glioblastoma. J. Cell Physiol. 2020, 235, 8167–8175. [Google Scholar] [CrossRef] [PubMed]
- Bobis-Wozowicz, S.; Miekus, K.; Wybieralska, E.; Jarocha, D.; Zawisz, A.; Madeja, Z.; Majka, M. Genetically modified adipose tissue-derived mesenchymal stem cells overexpressing CXCR4 display increased motility, invasiveness, and homing to bone marrow of NOD/SCID mice. Exp. Hematol. 2011, 39, 686–696.e4. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ponnazhagan, S. Bone homing of mesenchymal stem cells by ectopic α4 integrin expression. FASEB J. 2007, 21, 3917–3927. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Peters, C.; Etminan, N.; Börger, V.; Schimanski, A.; Sabel, M.C.; Sorg, R.V. Migration of mesenchymal stem cells towards glioblastoma cells depends on hepatocyte-growth factor and is enhanced by aminolaevulinic acid-mediated photodynamic treatment. Biochem. Biophys. Res. Commun. 2013, 431, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Bennewitz, M.F.; Tang, K.S.; Markakis, E.A.; Shapiro, E.M. Specific chemotaxis of magnetically labeled mesenchymal stem cells: Implications for MRI of glioma. Mol. Imaging Biol. 2012, 14, 676–687. [Google Scholar] [CrossRef]
- Consentius, C.; Reinke, P.; Volk, H.-D. Immunogenicity of allogeneic mesenchymal stromal cells: What has been seen in vitro and in vivo. Regen. Med. 2015, 10, 305–315. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef]
- Deuse, T.; Stubbendorff, M.; Tang-Quan, K.; Phillips, N.; Kay, M.A.; Eiermann, T.; Phan, T.T.; Volk, H.-D.; Reichenspurner, H.; Robbins, R.C.; et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011, 20, 655–667. [Google Scholar] [CrossRef]
- Ming, G.-L.; Song, H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005, 28, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef]
- Pellegrino, G.; Trubert, C.; Terrien, J.; Pifferi, F.; Leroy, D.; Loyens, A.; Migaud, M.; Baroncini, M.; Maurage, C.-A.; Fontaine, C.; et al. A comparative study of the neural stem cell niche in the adult hypothalamus of human, mouse, rat and gray mouse lemur (Microcebus murinus). J. Comp. Neurol. 2017, 526, 1419–1443. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.; Hale, A.T.; Chambless, L.B.; Weaver, K.D.; Thompson, R.C.; Ihrie, R.A. Influence of glioblastoma contact with the lateral ventricle on survival: A meta-analysis. J. Neuro-Oncology 2016, 131, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J.E.; Kahng, J.Y.; Kim, S.H.; Park, J.S.; Yoon, S.J.; Um, J.Y.; Kim, W.K.; Lee, J.K.; Park, J.; et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature 2018, 560, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Filippo, T.R.; Galindo, L.T.; Barnabe, G.F.; Ariza, C.B.; Mello, L.E.; Juliano, M.A.; Juliano, L.; Porcionatto, M.A. CXCL12 N-terminal end is sufficient to induce chemotaxis and proliferation of neural stem/progenitor cells. Stem Cell Res. 2013, 11, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yao, W.-L.; Tan, W.; Zhang, C.-H. SDF-1 and CXCR4 play an important role in adult SVZ lineage cell proliferation and differentiation. Brain Res. 2016, 1657, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Ling, T.Y.; Lin, H.Y.; Liou, J.T.; Liu, F.C.; Chen, I.C.; Lee, S.W.; Hsu, Y.; Lai, D.M.; Liou, H.H. SDF-1/CXCR4 Signaling Maintains Stemness Signature in Mouse Neural Stem/Progenitor Cells. Stem Cells Int. 2017, 2017, 2493752. [Google Scholar] [CrossRef]
- Liao, A.; Shi, R.; Jiang, Y.; Tian, S.; Li, P.; Song, F.; Qu, Y.; Li, J.; Yun, H.; Yang, X. SDF-1/CXCR4 Axis Regulates Cell Cycle Pro-gression and Epithelial-Mesenchymal Transition via Up-regulation of Survivin in Glioblastoma. Mol. Neurobiol. 2016, 53, 210–215. [Google Scholar] [CrossRef]
- Goffart, N.; Lombard, A.; Lallemand, F.; Kroonen, J.; Nassen, J.; Di Valentin, E.; Berendsen, S.; Dedobbeleer, M.; Willems, E.; Robe, P.A.; et al. CXCL12 mediates glioblastoma resistance to radiotherapy in the subventricular zone. Neuro-Oncology 2017, 19, 66–77. [Google Scholar] [CrossRef]
- Jin, C.; Zhao, J.; Zhang, Z.; Wu, M.; Li, J.; Liu, B.; Bin Liao, X.; Liao, Y.; Liu, J. CircRNA EPHB4 modulates stem properties and proliferation of gliomas via sponging miR-637 and up-regulating SOX10. Mol. Oncol. 2020, 15, 596–622. [Google Scholar] [CrossRef]
- Hira, V.V.; Verbovsek, U.; Breznik, B.; Srdic, M.; Novinec, M.; Kakar, H.; Wormer, J.; der Swaan, B.V.; Lenarcic, B.; Juliano, L.; et al. Cathepsin K cleavage of SDF-1alpha inhibits its chemotactic activity towards glioblastoma stem-like cells. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 594–603. [Google Scholar] [CrossRef]
- Marques-Torrejon, M.A.; Gangoso, E.; Pollard, S.M. Modelling glioblastoma tumour-host cell interactions using adult brain organotypic slice co-culture. Dis. Model. Mech. 2017, 11, 031435. [Google Scholar] [CrossRef] [PubMed]
- Aboody, K.S.; Brown, A.; Rainov, N.G.; Bower, K.A.; Liu, S.; Yang, W.; Small, J.E.; Herrlinger, U.; Ourednik, V.; Black, P.M.; et al. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc. Natl. Acad. Sci. USA 2000, 97, 12846–12851. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Ahn, Y.; Kim, S.U.; Wang, K.-C.; Cho, B.-K.; Phi, J.H.; Park, I.H.; Black, P.M.; Carroll, R.S.; Lee, J. Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clin. Cancer Res. 2009, 15, 4925–4934. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Segovia, J.; López-Ornelas, A.; Puerta-Guardo, H.; Ludert, J.E.; Chavez, B.; Meraz-Cruz, N.; González-Mariscal, L. Transmigration of Neural Stem Cells across the Blood Brain Barrier Induced by Glioma Cells. PLoS ONE 2013, 8, e60655. [Google Scholar] [CrossRef] [PubMed]
- Goncharova, V.; Das, S.; Niles, W.; Schraufstatter, I.; Wong, A.K.; Povaly, T.; Wakeman, D.; Miller, L.; Snyder, E.Y.; Khaldoyanidi, S.K. Homing of neural stem cells from the venous compartment into a brain infarct does not involve conventional in-teractions with vascular endothelium. Stem Cells Transl. Med. 2014, 3, 229–240. [Google Scholar] [CrossRef]
- Magge, S.N.; Malik, S.Z.; Royo, N.C.; Chen, H.I.; Yu, L.; Snyder, E.Y.; O’Rourke, D.M.; Watson, D.J. Role of monocyte chemo-attractant protein-1 (MCP-1/CCL2) in migration of neural progenitor cells toward glial tumors. J. Neurosci. Res. 2009, 87, 1547–1555. [Google Scholar] [CrossRef]
- Ziu, M.; Schmidt, N.O.; Cargioli, T.G.; Aboody, K.S.; Black, P.M.; Carroll, R.S. Glioma-produced extracellular matrix influences brain tumor tropism of human neural stem cells. J. Neuro-Oncology 2006, 79, 125–133. [Google Scholar] [CrossRef]
- Friedlander, D.R.; Zagzag, D.; Shiff, B.; Cohen, H.; Allen, J.C.; Kelly, P.J.; Grumet, M. Migration of brain tumor cells on extra-cellular matrix proteins in vitro correlates with tumor type and grade and involves alphaV and beta1 integrins. Cancer Res. 1996, 56, 1939–1947. [Google Scholar]
- Giese, A.; Westphal, M. Glioma invasion in the central nervous system. Neurosurgery 1996, 39, 235–250, discussion 250–252. [Google Scholar] [CrossRef]
- Ferent, J.; Zaidi, D.; Francis, F. Extracellular Control of Radial Glia Proliferation and Scaffolding during Cortical Development and Pathology. Front. Cell Dev. Biol. 2020, 8, 578341. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Lou, J.-Y.; Bai, H.-Y.; Wang, Y.-L.; Li, J.-F.; Yin, H.-L. Protective effect of bone marrow mesenchymal stem cells on PC12 cells apoptosis mediated by TAG1. Int. J. Clin. Exp. Pathol. 2015, 8, 12093–12100. [Google Scholar] [PubMed]
- Suman, S.; Das, T.P.; Damodaran, C. Silencing NOTCH signaling causes growth arrest in both breast cancer stem cells and breast cancer cells. Br. J. Cancer 2013, 109, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Mohamet, L.; Lea, M.L.; Ward, C.M. Abrogation of E-cadherin-mediated cellular aggregation allows proliferation of pluripotent mouse embryonic stem cells in shake flask bioreactors. PLoS ONE 2010, 5, e12921. [Google Scholar] [CrossRef] [PubMed]
- Sadler, N.M.; Harris, B.R.; Metzger, B.A.; Kirshner, J. N-cadherin impedes proliferation of the multiple myeloma cancer stem cells. Am. J. Blood Res. 2013, 3, 271–285. [Google Scholar]
- Cook, P.J.; Thomas, R.; Kingsley, P.J.; Shimizu, F.; Montrose, D.C.; Marnett, L.J.; Tabar, V.S.; Dannenberg, A.J.; Benezra, R. Cox-2-derived PGE2induces Id1-dependent radiation resistance and self-renewal in experimental glioblastoma. Neuro-Oncology 2016, 18, 1379–1389. [Google Scholar] [CrossRef]
- Brocard, E.; Oizel, K.; Lalier, L.; Pecqueur, C.; Paris, F.; Vallette, F.; Oliver, L. Radiation-induced PGE2 sustains human glioma cell growth and survival through EGF signaling. Oncotarget 2015, 6, 6840–6849. [Google Scholar] [CrossRef]
- Ayuso-Sacido, A.; Moliterno, J.A.; Kratovac, S.; Kapoor, G.S.; O’Rourke, D.; Holland, E.C.; García-Verdugo, J.M.; Roy, N.S.; Boockvar, J.A. Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells. J. Neuro-Oncology 2009, 97, 323–337. [Google Scholar] [CrossRef]
- Carey-Ewend, M.A.G.; Hagler, S.B.; Bomba, H.N.; Goetz, M.M.J.; Bago, J.R.; Hingtgen, S.D. Developing Bioinspired Three-Dimensional Models of Brain Cancer to Evaluate Tumor-Homing Neural Stem Cell Therapy. Tissue Eng. Part. A 2021, 27, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Meng, H.; Guan, Y.; Yin, Y.; Zhao, Z.; Sun, G.; Wu, A.; Chen, L.; Yu, X. Neural stem cells promote glioblastoma formation in nude mice. Clin. Transl. Oncol. 2019, 21, 1551–1560. [Google Scholar] [CrossRef]
- Tobias, A.L.; Thaci, B.; Auffinger, B.; Rincón, E.; Balyasnikova, I.V.; Kim, C.K.; Han, Y.; Zhang, L.; Aboody, K.S.; Ahmed, A.U.; et al. The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Transl. Med. 2013, 2, 655–666. [Google Scholar] [CrossRef]
- Chakritbudsabong, W.; Sariya, L.; Jantahiran, P.; Chaisilp, N.; Chaiwattanarungruengpaisan, S.; Rungsiwiwut, R.; Ferreira, J.N.; Rungarunlert, S. Generation of Porcine Induced Neural Stem Cells Using the Sendai Virus. Front. Veter. Sci. 2022, 8, 806785. [Google Scholar] [CrossRef] [PubMed]
- Han, M.J.; Lee, W.J.; Choi, J.; Hong, Y.J.; Uhm, S.J.; Choi, Y.; Do, J.T. Inhibition of neural stem cell aging through the transient induction of reprogramming factors. J. Comp. Neurol. 2020, 529, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Canton, R.; Perez-Villalba, A.; Morante-Redolat, J.M.; Marques-Torrejon, M.A.; Pallas, M.; Perez-Sanchez, F.; Farinas, I. Regulation of the p19(Arf)/p53 pathway by histone acetylation underlies neural stem cell behavior in senescence-prone SAMP8 mice. Aging Cell 2015, 14, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J. Concise Review: MSC Adhesion Cascade—Insights into Homing and Transendothelial Migration. Stem Cells 2017, 35, 1446–1460. [Google Scholar] [CrossRef]
- Gouirand, V.; Guillaumond, F.; Vasseur, S. Influence of the Tumor Microenvironment on Cancer Cells Metabolic Reprogramming. Front. Oncol. 2018, 8, 117. [Google Scholar] [CrossRef]
- Kološa, K.; Motaln, H.; Herold-Mende, C.; Koršič, M.; Lah, T.T. Paracrine effects of mesenchymal stem cells induce senescence and differentiation of glioblastoma stem-like cells. Cell Transplant. 2015, 24, 631–644. [Google Scholar] [CrossRef]
- Dasari, V.R.; Velpula, K.K.; Kaur, K.; Fassett, D.; Klopfenstein, J.D.; Dinh, D.H.; Gujrati, M.; Rao, J.S. Cord blood stem cell-mediated induction of apoptosis in glioma downregulates X-linked inhibitor of apoptosis protein (XIAP). PLoS ONE 2010, 5, e11813. [Google Scholar] [CrossRef]
- Akimoto, K.; Kimura, K.; Nagano, M.; Takano, S.; Salazar, G.; Yamashita, T.; Ohneda, O. Umbilical cord blood-derived mesenchymal stem cells inhibit, but adipose tissue-derived mesenchymal stem cells promote, glioblastoma multiforme proliferation. Stem Cells Dev. 2013, 22, 1370–1386. [Google Scholar] [CrossRef]
- Koutroulis, I.; Zarros, A.; Theocharis, S. The role of matrix metalloproteinases in the pathophysiology and progression of human nervous system malignancies: A chance for the development of targeted therapeutic approaches. Expert Opin. Ther. Targets 2008, 12, 1577–1586. [Google Scholar] [CrossRef]
- Onzi, G.R.; Ledur, P.F.; Hainzenreder, L.D.; Bertoni, A.P.; Silva, A.O.; Lenz, G.; Wink, M.R. Analysis of the safety of mesenchymal stromal cells secretome for glioblastoma treatment. Cytotherapy 2016, 18, 828–837. [Google Scholar] [CrossRef]
- Hossain, A.; Gumin, J.; Gao, F.; Figueroa, J.; Shinojima, N.; Takezaki, T.; Priebe, W.; Villarreal, D.; Kang, S.G.; Joyce, C.; et al. Mesenchymal Stem Cells Isolated from Human Gliomas Increase Proliferation and Maintain Stemness of Glioma Stem Cells Through the IL-6/gp130/STAT3 Pathway. Stem Cells 2015, 33, 2400–2415. [Google Scholar] [CrossRef] [PubMed]
- Bajetto, A.; Pattarozzi, A.; Corsaro, A.; Barbieri, F.; Daga, A.; Bosio, A.; Gatti, M.; Pisaturo, V.; Sirito, R.; Florio, T. Different Effects of Human Umbilical Cord Mesenchymal Stem Cells on Glioblastoma Stem Cells by Direct Cell Interaction or Via Released Soluble Factors. Front. Cell. Neurosci. 2017, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.P.; Carle, G.; Faneca, H.; de Lima, M.P.; Pierrefite-Carle, V. Suicide gene therapy in cancer: Where do we stand now. Cancer Lett. 2012, 324, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharboosh, R.; ReFaey, K.; Lara-Velazquez, M.; Grewal, S.S.; Imitola, J.; Quiñones-Hinojosa, A. Inflammatory Mediators in Glioma Microenvironment Play a Dual Role in Gliomagenesis and Mesenchymal Stem Cell Homing: Implication for Cellular Therapy. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 443–459. [Google Scholar] [CrossRef]
- Richardson, P.J. CXCR4 and Glioblastoma. Anticancer Agents Med. Chem. 2016, 16, 59–74. [Google Scholar] [CrossRef]
- Zhao, D.; Najbauer, J.; Garcia, E.; Metz, M.Z.; Gutova, M.; Glackin, C.A.; Kim, S.U.; Aboody, K.S. Neural stem cell tropism to glioma: Critical role of tumor hypoxia. Mol. Cancer Res. 2008, 6, 1819–1829. [Google Scholar] [CrossRef]
- Bagó, J.R.; Okolie, O.; Dumitru, R.; Ewend, M.G.; Parker, J.S.; Werff, R.V.; Underhill, T.M.; Schmid, R.S.; Miller, C.R.; Hingtgen, S.D. Tumor-homing cytotoxic human induced neural stem cells for cancer therapy. Sci. Transl. Med. 2017, 9, 375. [Google Scholar] [CrossRef]
- Deng, L.; Stafford, J.H.; Liu, S.C.; Chernikova, S.B.; Merchant, M.; Recht, L.; Brown, J.M. SDF-1 Blockade Enhances Anti-VEGF Therapy of Glioblastoma and Can Be Monitored by MRI. Neoplasia 2017, 19, 1–7. [Google Scholar] [CrossRef]
- Chiorean, R.; Braicu, C.; Florian, I.S.; Leucuta, D.; Montefrancesco, C.; Crisan, D.; Berindan-Neagoe, I.; Cernea, V. Quantitative expression of serum biomarkers involved in angiogenesis and inflammation, in patients with glioblastoma multiforme: Correlations with clinical data. Cancer Biomark. 2014, 14, 185–194. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, R.; Zhao, T.; Yang, X.; Han, L.; Ye, F.; Lei, T.; Wan, F. Neural stem cells preferentially migrate to glioma stem cells and reduce their stemness phenotypes. Int. J. Oncol. 2014, 45, 1989–1996. [Google Scholar] [CrossRef]
- Schichor, C.; Birnbaum, T.; Etminan, N.; Schnell, O.; Grau, S.; Miebach, S.; Aboody, K.; Padovan, C.; Straube, A.; Tonn, J.-C.; et al. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC). Exp. Neurol. 2006, 199, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Rath, P.; Lal, B.; Ajala, O.; Li, Y.; Xia, S.; Kim, J.; Laterra, J. In Vivo c-Met Pathway Inhibition Depletes Human Glioma Xenografts of Tumor-Propagating Stem-Like Cells. Transl. Oncol. 2013, 6, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Keunen, O.; Johansson, M.; Oudin, A.; Sanzey, M.; Rahim, S.A.A.; Fack, F.; Thorsen, F.; Taxt, T.; Bartos, M.; Jirik, R.; et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc. Natl. Acad. Sci. USA 2011, 108, 3749–3754. [Google Scholar] [CrossRef] [PubMed]
- Sproull, M.; Mathen, P.; Miller, C.A.; Mackey, M.; Cooley, T.; Smart, D.; Shankavaram, U.; Camphausen, K. A Serum Proteomic Signature Predicting Survival in Patients with Glioblastoma. J. Biochem. Anal. Stud. 2020, 4, 117. [Google Scholar]
- Gondi, C.S.; Lakka, S.S.; Yanamandra, N.; Siddique, K.; Dinh, D.H.; Olivero, W.C.; Gujrati, M.; Rao, J.S. Expression of antisense uPAR and antisense uPA from a bicistronic adenoviral construct inhibits glioma cell invasion, tumor growth, and angiogenesis. Oncogene 2003, 22, 5967–5975. [Google Scholar] [CrossRef]
- Bindal, A.K.; Hammoud, M.; Shi, W.M.; Wu, S.Z.; Sawaya, R.; Rao, J.S. Prognostic significance of proteolytic enzymes in human brain tumors. J. Neuro-Oncology 1994, 22, 101–110. [Google Scholar] [CrossRef]
- López Ponte, A.; Marais, E.; Gallay, N.; Langonné, A.; Delorme, B.; Hérault, O.; Charbord, P.; Domenech, J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef]
- Heese, O.; Disko, A.; Zirkel, D.; Westphal, M.; Lamszus, K. Neural stem cell migration toward gliomas in vitro. Neuro-Oncology 2005, 7, 476–484. [Google Scholar] [CrossRef]
- Liu, T.; Ma, W.; Xu, H.; Huang, M.; Zhang, D.; He, Z.; Zhang, L.; Brem, S.; O’Rourke, D.M.; Gong, Y.; et al. PDGF-mediated mesenchymal transformation renders endothelial resistance to anti-VEGF treatment in glioblastoma. Nat. Commun. 2018, 9, 3439. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Kao, C.Y.; Chung, Y.F.; Lee, D.C.; Liu, J.W.; Chiu, I.M. Activation of Aurora A kinase through the FGF1/FGFR signaling axis sustains the stem cell characteristics of glioblastoma cells. Exp. Cell Res. 2016, 344, 153–166. [Google Scholar] [CrossRef]
- Sato, H.; Kuwashima, N.; Sakaida, T.; Hatano, M.; Dusak, J.E.; Fellows-Mayle, W.K.; Papworth, G.D.; Watkins, S.; Gambotto, A.; Pollack, I.F.; et al. Epidermal growth factor receptor-transfected bone marrow stromal cells exhibit enhanced migratory response and therapeutic potential against murine brain tumors. Cancer Gene Ther. 2005, 12, 757–768. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, Y.; Bollen, A.W.; Aldape, K.D.; Gupta, N. Nuclear FABP7 immunoreactivity is preferentially expressed in infiltrative glioma and is associated with poor prognosis in EGFR-overexpressing glioblastoma. BMC Cancer 2006, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Hou, S.-C.; Hung, C.-M.; Lin, J.-N.; Chen, W.-C.; Ho, C.-T.; Kuo, S.-C.; Way, T.-D. Inhibition of the insulin-like growth factor 1 receptor by CHM-1 blocks proliferation of glioblastoma multiforme cells. Chem. Interact. 2015, 231, 119–126. [Google Scholar] [CrossRef]
- Maule, F.; Bresolin, S.; Rampazzo, E.; Boso, D.; Della Puppa, A.; Esposito, G.; Porcù, E.; Mitola, S.; Lombardi, G.; Accordi, B.; et al. Annexin 2A sustains glioblastoma cell dissemination and proliferation. Oncotarget 2016, 7, 54632–54649. [Google Scholar] [CrossRef][Green Version]
- Guo, G.; Gong, K.; Puliyappadamba, V.T.; Panchani, N.; Pan, E.; Mukherjee, B.; Damanwalla, Z.; Bharia, S.; Hatanpaa, K.J.; Gerber, D.E.; et al. Efficacy of EGFR plus TNF inhibition in a preclinical model of temozolomide-resistant glioblastoma. Neuro-Oncology 2019, 21, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Nie, E.; Jin, X.; Miao, F.; Yu, T.; Zhi, T.; Shi, Z.; Wang, Y.; Zhang, J.; Xie, M.; You, Y. TGF-beta1 modulates temozolomide resistance in glioblastoma via altered microRNA processing and elevated MGMT. Neuro-Oncology 2021, 23, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-B.; Zhang, C.-H.; Wang, S.-Q.; Ai, P.-H.; Chen, K.; Zhu, L.; Sun, Z.-L.; Feng, D.-F. Transforming growth factor beta induced (TGFBI) is a potential signature gene for mesenchymal subtype high-grade glioma. J. Neuro-Oncol. 2018, 137, 395–407. [Google Scholar] [CrossRef]

| NSC | ||||
|---|---|---|---|---|
| Reference | Type of Study | Cargo | Treatment | Results |
| Chen et al., 2013 | Clinical trial | - | Radiation therapy for SVZ | Radiation increases patient’s PFS and OS PFS:15.1 vs. 10.3 OS:17.5 vs. 15.6 (months) |
| Lee et al., 2013 | Clinical trial | - | Radiation therapy for SVZ | Radiation improves PFS PFS:12.6 vs. 9.9 |
| Portnow et al., 2017 | Clinical trial | CD | HB1.F3.CD.C21; CD-NSCs + oral administration of 5-FC | Migration of NSC to the tumor and locally produces chemotherapy is confirmed. |
| Aboody et al., 2013 | Preclinical study | CD | HB1.F3.CD.C21; CD-NSCs + intraperitoneal injection of 5-FC | Inhibition of GBM progression and prolongation of mice with GBM xenografts survival |
| Bago et al., 2016 | Preclinical study | TRAIL | iNSC modified with TRAIL | Inhibition of GBM progression and prolongation of mice with GBM xenografts survival |
| Dey et al., 2016 | Preclinical study | Oncolytic Adenovirus CRAd-S-pK7 | Overexpressed CXCR4 in NSCs and loaded with CRAd-S-pk7 | Increased mice survival with GBM xenografts |
| MSC | ||||
| NCT03896568 | Clinical trial | Oncolytic Adenovirus DNX-2401 | Mesenchymal stem cells loaded with a tumor selective oncolytic adenovirus, DNX-2401 | Recruiting |
| NCT04657315 | Clinical trial | CD | Mesenchymal stem cells into which cytosine deaminase the suicide gene was injected | Recruiting |
| Oraee-Yazdani et al., 2021 | Clinical trial | HSV-TK | Autologous mesenchymal stem cells as HSV-TK gene vehicle | Biosafety of MSC PFS:23.7 OS: 32.2 (months) |
| Mohme et al., 2020 | Preclinical study | IL-12, IL-7 | Intra-tumoral of genetically modified MSCs that co-express high levels of IL-12 and IL-7 | Intra-tumoral administration of MSC IL7/12 induced significant tumor growth inhibition and remission of established intracranial tumors |
| Novak et al., 2020 | Preclinical study | TRAIL | TRAIL-secreting MSC/nanomedicine spheroid system | The hybrid spheroid inhibited the tumor growth efficiently |
| Shimizu et al., 2021 | Preclinical study | Oncolytic Adenovirus Delta-24-RGD | Bone marrow-derived human mesenchymal stem cells loaded with Delta-24-RGD | Intravascular administration of PD-BM-MSC-D24 increased the survival of mice harboring U87MG gliomas |
| Factors | Receptor Presence at the Stem Cells | Clinical Significance for the Patients with GBM | ||
|---|---|---|---|---|
| Tumor-Derived Factors | MSC | NSC | Impact for Therapy/References | Correlation with Survival |
| SDF-1 | + [134] | + [135,136,137] | Inhibition of SDF-1α enhances anti-VEGF therapy [138] | |
| IL6 | + [134] | Inhibition Il6 negatively affects GBM viability | No correlation [139] | |
| IL1β | + [134] | |||
| HGF | + [84] | + [137,140,141] | Inhibits tumor stem-like cells [142] | |
| VEGF | + [141] | + [136,140] | Anti-VEGF treatment inhibits angiogenesis and strongly increases cell invasion and tumor hypoxia [143] | [144] |
| uPA | + [136] | Inhibition uPA/uPAR attenuates invasion, angiogenesis in glioblastoma cells [145] | [146] | |
| PDGFAA/BB | + [66,147] | + [148] | Ablation of PDGF signaling sensitizes anti-VEGF/VEGFR treatment in GBM [149] | [139] |
| FGF ligands | + [148] | FGF1/FGFR signaling axis sustains the stem cell characteristics of GBM cells [150] | ||
| EGF | + [151] | + [140,148] | Inhibition EGF suppresses GBM migration [152] | |
| IGF | + [147] | + [148] | Inhibition IGF reduces GBM proliferation [153] | [139] |
| Annexin A2 | + | Annexin A2 acts at multiple levels in determining the disseminating and aggressive behavior of GBM cells [154] | [154] | |
| TNF-a | + [134] | + | EGFR plus TNF inhibition is effective in TMZ-resistant recurrent GBM [155] | No correlation [139] |
| TGF b | + [134] | + [148] | TGF-β1 modulates temozolomide resistance in glioblastoma [156] | [157] No correlation [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsibulnikov, S.; Drefs, N.M.; Timashev, P.S.; Ulasov, I.V. To Explore the Stem Cells Homing to GBM: The Rise to the Occasion. Biomedicines 2022, 10, 986. https://doi.org/10.3390/biomedicines10050986
Tsibulnikov S, Drefs NM, Timashev PS, Ulasov IV. To Explore the Stem Cells Homing to GBM: The Rise to the Occasion. Biomedicines. 2022; 10(5):986. https://doi.org/10.3390/biomedicines10050986
Chicago/Turabian StyleTsibulnikov, Sergey, Natalya M. Drefs, Peter S. Timashev, and Ilya V. Ulasov. 2022. "To Explore the Stem Cells Homing to GBM: The Rise to the Occasion" Biomedicines 10, no. 5: 986. https://doi.org/10.3390/biomedicines10050986
APA StyleTsibulnikov, S., Drefs, N. M., Timashev, P. S., & Ulasov, I. V. (2022). To Explore the Stem Cells Homing to GBM: The Rise to the Occasion. Biomedicines, 10(5), 986. https://doi.org/10.3390/biomedicines10050986