1. Introduction
Cutaneous wound healing is an important physiological process to restore the skin barrier after trauma. This process includes complex and dynamic pathways that are classified into three main consecutive but overlapping stages: the inflammatory, proliferative, and maturation/remodeling phases [
1]. This involves the simultaneous actuation of soluble mediators, blood cells, the extracellular matrix, and epithelial and parenchymal cells [
2].
The inflammatory phase occurs immediately after tissue damage; the hemorrhage triggers the coagulation cascade to restore hemostasis and prevent further blood loss. Hemostasis begins with the formation of a platelet plug, followed by a provisional fibrin matrix that will favor the migration of inflammatory cells. In addition to forming the clot, the thrombocytes secrete various mediators, growth factors and cytokines, which, together with the factors produced by the coagulation cascade itself, recruit inflammatory cells and activate tissue repair mechanisms [
3,
4].
Wounds that fail to progress satisfactorily through the normal phases of healing become chronic wounds, which represent a major problem to healthcare systems [
5,
6]. This may be due to a whole series of potential stimuli such as local tissue ischemia, necrotic tissue, bioburden, or even repeated trauma, which happens when wounds remain in the inflammatory phase, contributing to their chronicity [
7]. However, this problem more frequently occurs in patients with underlying disorders such as peripheral artery disease, diabetes, venous insufficiency, and nutritional deficiencies, as well as other disease states [
8,
9].
These lesions show an altered and sustained inflammatory response, altered protease levels, and a deficient extracellular matrix, as well as impaired granulation tissue formation and maturation; in addition, the precise balance between the production and degradation of collagen is lost, and degradation dominates [
10,
11].
At both clinical and experimental levels, multiple therapies have been designed in attempts to improve healing. Among them, the use of collagen represents a good option for treating cutaneous wounds.
Collagen is a critical component of a healing wound, and the most abundant protein of the extracellular matrix of the dermal tissue. In addition to its important role as a scaffold in skin, this protein is of the utmost importance as a signaling molecule in the regulation of all stages of the reparative process. Regarding the inflammatory stage, it has been demonstrated that collagen breakdown products are chemotactic for a variety of cell types, including macrophages and fibroblasts, required for the formation of granulation tissue, enhancing phagocytosis and immune responses. During the proliferative phase, it promotes the growth of fibroblasts, which contribute to collagen deposition and granulation tissue formation, and keratinocytes in the wound. In the last phase, which is characterized by maturation and tissue remodeling, immature type III collagen is replaced by mature type I collagen, which provides tensile strength to the newly formed tissue [
7].
Collagen is of great interest in regenerative medicine due to its low immunogenicity, remarkable biocompatibility, ease of application, and good biodegradability. Additionally, collagen-based treatments have the ability to absorb wound exudates and maintain a moist environment, which is essential for an optimal healing process. As a biodegradable component, they do not require removal from the wound bed before re-application, preventing loss of fluid and protecting the wound from bacterial infection and other agents [
12]. These properties make collagen an ideal wound therapy agent to improve and accelerate the healing process. Currently, the main sources of collagen are typically bovine, equine, avian, or porcine, although alternative natural (marine) or engineered (recombinant from plant or bacterial material) sources have been considered [
13]. Numerous studies have assessed the collagen formats in wound healing; some of them use collagen as scaffolds/matrices [
14], sponges [
15], hydrogels [
16], or powders [
17].
Collagen-based wound products can be classified into two categories: native collagens, decellularized but maintaining ECM structure, and denatured collagens that are highly modified and have lost their three-dimensional structure [
7,
18]. Collagen powder is well known for promoting cellular recruitment and inflammation phase activation and exerting its activity immediately upon application, as compared to 3D scaffolds. A pilot study demonstrated that treatment of a full-thickness wound with collagen powder enhanced the maturity and strength of wound healing [
19].
A variety of commercial collagen powder products are available on the market, varying in source, purity, manufacturing, and particle size. In some cases, native collagen can be hydrolyzed to produce low-molecular-weight peptides. Denaturation of native collagen produces three α chains in their random coiled form. Once the chains are separated, hydrolysis is carried out by the action of proteolytic enzymes (alcalase, papain, pepsin, and others). The resulting product is commonly called hydrolyzed collagen. It is composed of small peptides with low molecular weight 3–6 KDa [
20]. The advantages of hydrolyzed collagen are that it avoids breakdown by endogenous enzymes, and is highly soluble and easily absorbed by the human body, allowing for immediate signaling. A study carried out in dogs [
21] demonstrated that hydrolyzed collagen powder enhanced the percentage of epithelialization after seven days of treatment compared to the control.
Other commercial collagen-based products, derived from bovine cartilage in the form of powder, have been shown to be effective in the treatment of wounds by secondary intent such as pressure ulcers, venous stasis ulcers, and diabetic ulcers, as well as second-degree burns, post-radiation dermatitis, and wounds unresponsive to conventional treatments [
22].
Researchers and companies have developed and marketed a wide variety of products presenting different compositions, but all focused on promoting wound healing.
Taking into account all these factors, the present preclinical study was designed to compare the effects on wound repair of two collagen-based powder products, a new hydrolyzed bovine dermal collagen powder (not yet on the market) and a non-hydrolyzed commercial collagen derived from bovine cartilage, in a murine model of cutaneous healing. The reparative process was assessed with respect to the evolution over time of the defect and inflammatory response, and the formation and maturation of new tissue.
2. Materials and Methods
2.1. Experimental Animals and Ethics
Female Wistar rats (n = 36) weighing around 250 g were used. The care of the animals used in this study and the experimental procedures were in accordance with current protocols on the use of animals in experimentation (European Directive 2010/63/EU, European Convention of the Council of Europe ETS123 and Spanish Royal Decree 53/2013), and the study was approved by the Animal Experimentation Ethics Committee of Universidad de Alcalá, Spain. In this study, a rat model of wound healing was developed to evaluate the effect of different collagen treatments on the tissue reparative process.
Animals were individually housed under controlled temperature and illumination conditions, with a complete diet (Harlan Laboratories, Houston, TX, USA) and water ad libitum.
2.2. Study Groups
The animals were randomly distributed into three study groups (n = 12), according to the treatment administrated:
- –
Control (n = 12): Rats without treatment
Powder treatment:
- –
T1 (n = 12): rats treated with Catrix® (Lescarden Inc., New York, USA).
Catrix® is a bovine cartilage collagen powder. The particles are composed of natural macromolecules (particle size 35 μm) of collagen arranged in the form of a three-dimensional network.
- –
T2 (n = 12): rats treated with collagen developed by Viscofan, S.A (Tajonar, Navarra, Spain).
This is a hydrolyzed bovine dermal collagen powder, mainly containing low-molecular-weight (3 KDa) type I peptides. These collagen peptides contain a unique composition and a high number of essential amino acids, such as proline, hydroxyproline, and glycine.
2.3. Surgical Technique and Sample Collection
Excisional wounds on the dorsal surface in rats is one of the most commonly used and standardized wound healing models [
23,
24]. These wounds are generated by the surgical removal of all skin layers (epidermis, dermis, and subcutaneous tissue) from the animal. This model allows the investigation of the inflammation, granulation tissue formation, reepithelialization, angiogenesis, and remodeling process.
The animals were anesthetized in an inhalation chamber with a mixture of isoflurane (Forane
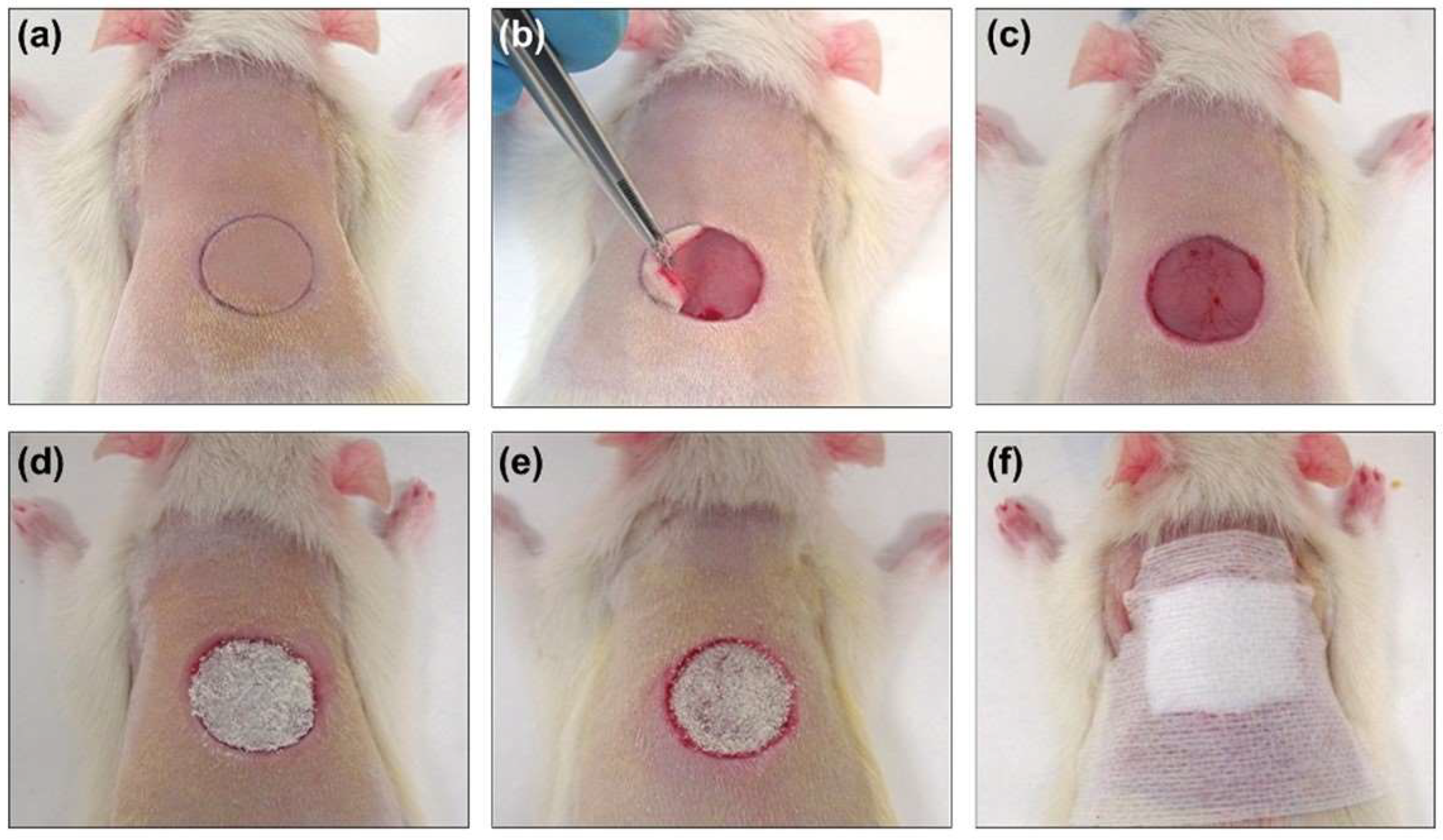
®; AbbVieS.L.U., Madrid, Spain) at 4–5% and oxygen at a flow rate of 0.6–0.7 L/min. During the surgery, anesthesia was maintained by a face mask connected to a calibrated vaporizer, providing an inhalation dose of isoflurane of 2.5–3%. The fur on the backs of the rats was shaved with an electric razor, and the skin was disinfected with iodopovidone. After anesthesia, a circular (1.5 cm diameter) full-thickness defect, previously marked by a calibrated metallic punch and centered approximately 0.5 cm caudal to the scapulae, was created (
Figure 1). The skin was removed with a scalpel and surgical scissors, following the previously marked line. The animals did not require postoperative analgesia. To minimize stress caused by the surgical procedure and individual housing, the animals were in visual, auditory, and olfactory contact with other rats, and environmental enrichment was provided every week.
Once the wound was created, the corresponding treatment was administered to all groups except the control group. Treatments were applied at 0, 3, 5, 7 and 9 days, covering the area with wound-protective devices (
Figure 1). The scab covering the wound was removed before each treatment application to ensure its accessibility in the open area, and no debridement of the wound was performed in any case. The same procedure was carried out in the control group.
At the end of the established study times, the animals were euthanized in a CO2 inhalation chamber. The scar tissue was photographed for morphometric evaluation and subsequently excised. The tissue samples were sectioned into two halves transverse to the body axis for histological processing.
2.4. Morphometric Studies of Wound Evolution
Following surgery, animals were regularly weighed and monitored daily to evaluate the evolution of skin scarring. Immediately following surgery, during the study and at the end of the established study times, measurements of the defect were performed.
To evaluate the wound closure after the defect was performed and at the time of euthanasia, cenital photographs, from a plane just above the experimental animals, of the defects and scar tissue were taken with a ruler for calibration. For this assessment, the initial area of the defect caused at the time of surgery (diameter: 1.5 cm), the area of the defect that remained unclosed after 7 and 18 days, and the contraction area were measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA) (
https://imagej.nih.gov/ij). These measurements were used to calculate the relative values of the processes of wound closure, epithelialization, and contraction (
Figure 2).
2.5. Morphological Studies
For light microscopy analyses, the tissue samples obtained were fixed with solution F13 (60% ethanol, 20% methanol, 7% polyethylene glycol, and 13% distilled water) and paraffin-embedded. Tissue blocks were cut with a Microm HM-325 microtome (Microm International GmbH, Walldorf, Germany) into 5-μm-thick sections and placed onto slides coated with 0.01% polylysine (Sigma-Aldrich, St. Louis, MO, USA). Finally, the sections were dewaxed, rehydrated, and stained with hematoxylin–eosin and Masson’s trichrome (Goldner–Gabe variant), and Sirius red. Samples were examined under a Zeiss Axiophot light microscope (Carl Zeiss, Oberkochen, Germany). For each staining, six sections from the central zone of wound and three sections of the marginal edge were analyzed.
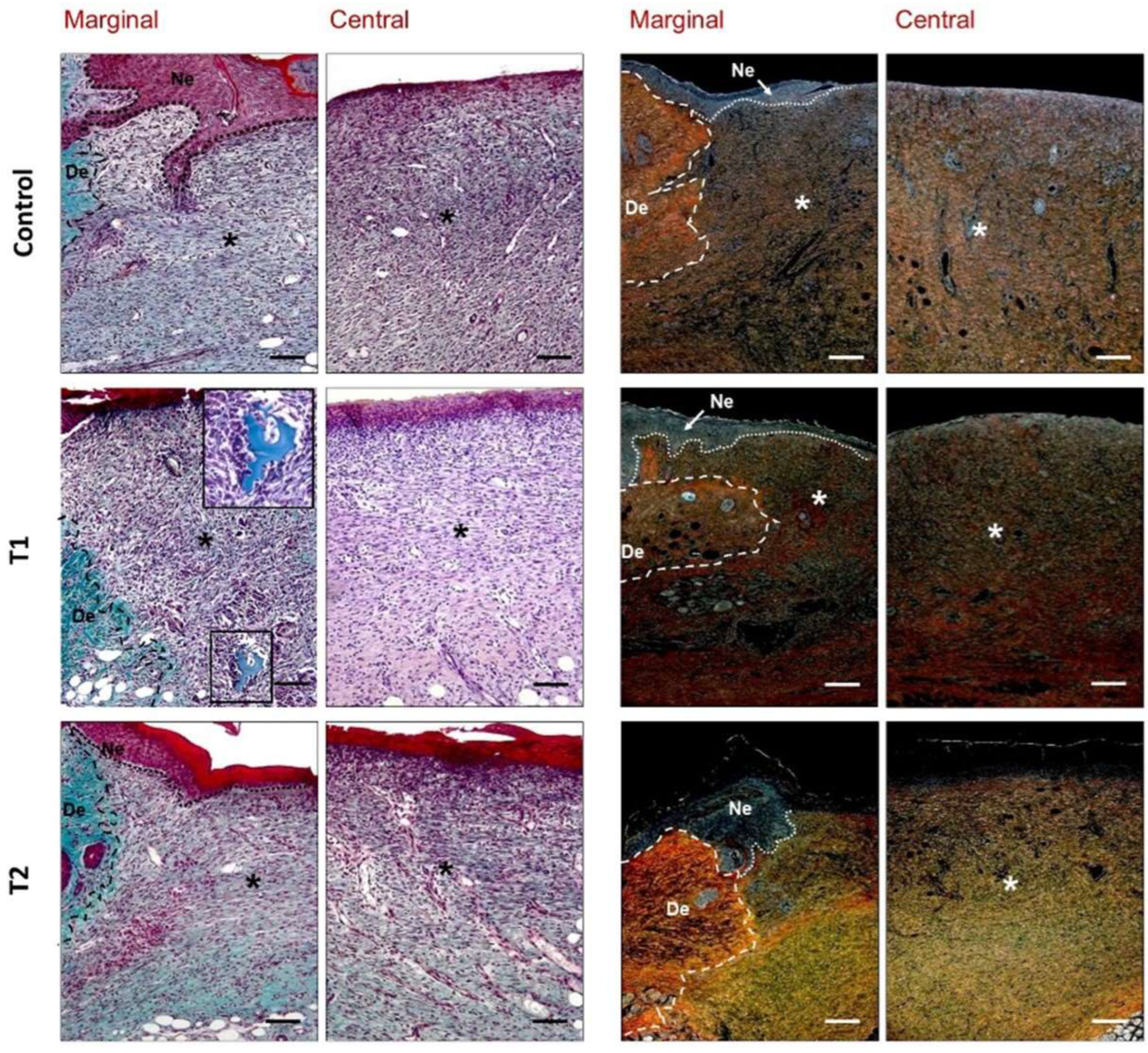
Both hematoxylin–eosin and Masson’s trichrome staining allowed for the general observation of the repairing tissue, granulation tissue, distribution of collagen inflammatory cells, and neoformed vessels. Sirius red staining was utilized to evaluate the organization and maturation of collagen fibers in the repairing tissue. Despite the lack of complete specificity, type I collagen (mature) appears as a reddish-orange stain, while type III collagen (immature) takes on a yellowish-green stain when observed under the polarized light microscope [
25]. Morphological examination was performed by two independent histologists in a blinded fashion.
2.6. Statistical Analysis
The data were expressed as the mean ± standard deviation. To compare different study groups, the Mann–Whitney U test was used. All statistical tests were performed using the software package GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA). Significance was set at p < 0.05.
4. Discussion
The main objective of the wide range of devices or treatments developed to promote wound healing is that this physiological process promotes wound closure as soon as possible and results in a functionally and aesthetically satisfactory scar. For this purpose, the organism must be able to reduce cutaneous discontinuity by generating new granulation tissue in the wounded area. An epithelial barrier must be able to develop on this tissue, reaching continuity and avoiding re-openings through the formation of a resistant extracellular matrix. This results from cell proliferation, synthesis, and maturation of the extracellular matrix [
3].
In general, the wound repair process occurs in almost all tissues after exposure to a destructive stimulus. It must be taken into consideration that, in this study, the physiological healing process was not compromised in any way. All the animals used were healthy animals without pathologies that might have affected healing; therefore, the model showed a standard healing pattern, with characteristics of the three phases of the skin repair process (inflammation, proliferation, and remodeling/maturation) appearing in a progressive and orderly manner until the achievement of newly formed connective tissue with reduced cellularity and a dense and organized extracellular matrix. With these requirements in mind, the treatments’ ability to improve wound closure were evaluated.
Although the study and knowledge of wound healing in humans have advanced considerably, the difficulties and limitations make the use of experimental models necessary for research in this field. For decades, the development of multiple animal models has been used, including dorsal wounds in small rodents, rabbit ear defects or porcine models [
26,
27]. However, the most used models for the study of skin repair are based on rats and mice due to their easy handling and maintenance. It must be taken into account that the use of these animals has some limitations due to differences in healing mechanisms compared to humans. One of them is the greater proportional contribution of the contraction process over epithelialization that occurs in rodents [
27]. Excisional wounds are one of the most commonly used wound healing models because they allow the investigation of inflammation, granulation tissue formation, re-epithelialization, angiogenesis and the remodeling process [
28].
Based on our group’s previous experience [
29], we developed a murine model to evaluate both the initial phases of healing (7 days) and tissue remodeling (18 days) by using an excisional model. In the present experimental study, evidence for collagen powder as an adjunctive therapy in wound healing has been provided. We used one collagen-based product available on the market and obtained from the bovine tracheal cartilage. Catrix
® was approved by the FDA in 1998, and is frequently used in wound care. Several studies have indicated that this collagen powder could be an optimal agent to stimulate the wound healing process [
22]. Clinical evidence from a prospective multicenter study treating resistant pressure ulcers showed a statistically significant improvement in complete healing using this product compared to untreated controls [
30].
For these reasons, in this study we chose Catrix® as a reference product to assess the performance of a new collagen-based product in a rat wound healing model.
In the classic stages of wound repair, inflammatory phase occurs immediately after tissue damage with the development of a platelet plug and a provisional fibrin scaffold that promotes migration of inflammatory cells [
3,
4]. New tissue formation occurs 2–10 days after injury and is characterized by cellular proliferation and the migration of different cell types [
3]. The first event is the migration of keratinocytes to the injured dermis, as we observed in our study groups after seven days. Tissue edges adjacent to the wound showed an active and thickened epithelium, with signs of cell proliferation and migration; neoformed granulation tissue showed signs of inflammatory and proliferative phases of the reparative process and important angiogenesis. The most important positive regulators of angiogenesis are vascular endothelial growth factor A (VEGFA) and fibroblast growth factor 2 (FGF2; also known as bFGF) [
4].
The results of our study revealed, after seven days, some differences between the groups. In our T1 and T2 groups, reduced granulation tissue formation with homogeneous thickness, fewer inflammatory cells, and the induction of vasculature in the neoformed tissue were observed with respect to untreated animals, which exhibited more inflammation and less organized repair tissue. Some other findings indicate that the application of pure undiluted bio-collagen extracted from bovine skin dramatically improved wound healing in rats after seven days in terms of collagen production, wound filling, and the migration and differentiation of keratinocytes, being three times more effective than the commercial Catrix
® [
31].
In the later part of this stage, fibroblasts, which are attracted from the edge of the wound, are stimulated by macrophages, and some differentiate into myofibroblasts [
32], contractile cells that, over time, bring the edges of a wound together and are responsible for the process of wound contraction. Some authors, trying to minimize the important process of wound contraction that occurs in rodents to replicate human physiology, have described models of wound healing utilizing wound splinting, verifying a greater deposition of granulation tissue and not being affected by the rate of re-epithelialization [
33]. In our study, the evolution of the wound closure process revealed a greater proportional contribution of contraction over epithelialization. The highest contraction values in our study were observed in the control group compared to the rest of the groups, although no significant differences were observed between them.
Fibroblasts and myofibroblasts interact and produce an extracellular matrix, mainly in the form of collagen, which ultimately forms the bulk of the mature scar [
34]. Evident immature collagen type III deposition was observed in the repair tissue in our model as the main component of the fibrillar matrix.
The third stage of wound repair—remodeling—begins 2–3 weeks after an injury and lasts for a year or more. Eighteen days after surgery, samples exhibited a significant approximation of the wound edges due to tissue contraction. At the end of the study, all groups showed similar macroscopic closures, except animals treated with the new collagen (T2 group), which showed accelerated wound closure compared to the untreated group. Despite making a limited contribution to total closure in the present model, epithelialization showed an improvement in this group of animals.
The effects of bovine collagen-derived powder treatments, similar to those applied in our T2 group, were evaluated on the healing of open wounds in healthy dogs [
21] and, according to our results, improved wound epithelialization.
During this last stage of healing, all the processes that had been activated after injury progressively decrease their activity until they are finished. Most of the macrophages and myofibroblasts undergo apoptosis or exit from the wound, leaving a mass that contains few cells [
35].
The morphological observations allowed us to verify that repaired tissue appeared more organized than after 7 days, showing higher density of mature collagen type I. Histological assessment of the group of animals treated with hydrolyzed collagen (T2 group) showed a newly stratified epidermis, with dense and organized connective tissue presenting a great number of fibroblasts and few inflammatory cells.
Other preclinical trials using modified collagen gel treatments versus untreated wounds have shown acute inflammatory cell and fibroblast recruitment, collagen I deposition, increased endothelial cells, upregulated vascular endothelial growth factor, and improved blood flow [
36,
37]. In accordance with our results, clinical studies [
19] report that collagen-treated wounds displayed increased neoangiogenesis, less inflammatory granulation tissue, and more organized and well-formed collagen bundles compared to the primary closure of punch biopsies with nonabsorbable sutures.