Vascular Endothelial Growth Factor as a Potential Biomarker of Neuroinflammation and Frontal Cognitive Impairment in Patients with Alcohol Use Disorder
Abstract
1. Introduction
2. Materials and Methods
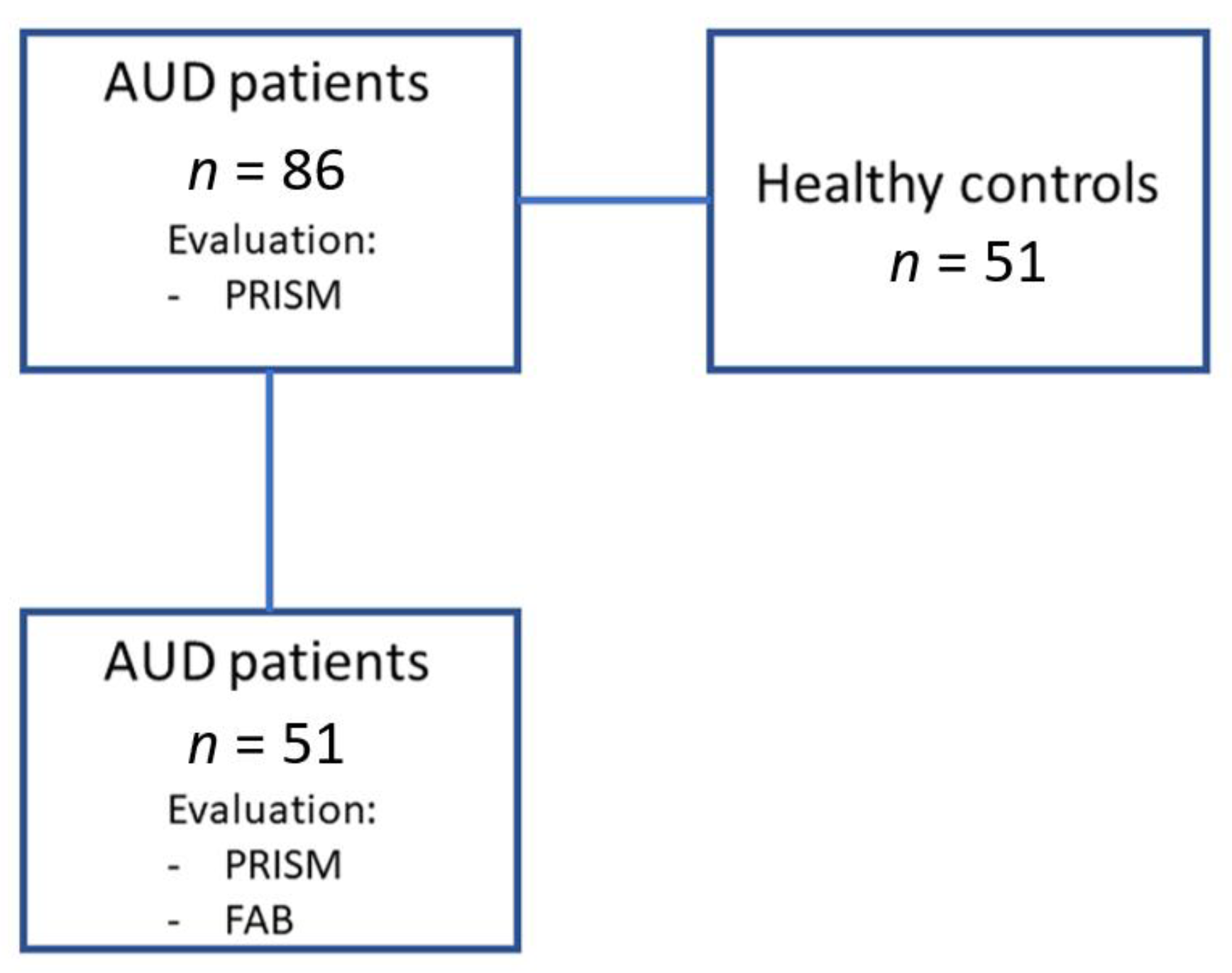
2.1. Recruitment and Screening of Participants
AUD Patients and Control Volunteers
2.2. Ethical Statement
2.3. Psychiatric and Neuropsychological Evaluation
2.4. Obtaining Plasma Samples
2.5. Multiplexed Bead Immunoassay
2.6. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics
3.2. Alcohol-Related Variables in AUD Group
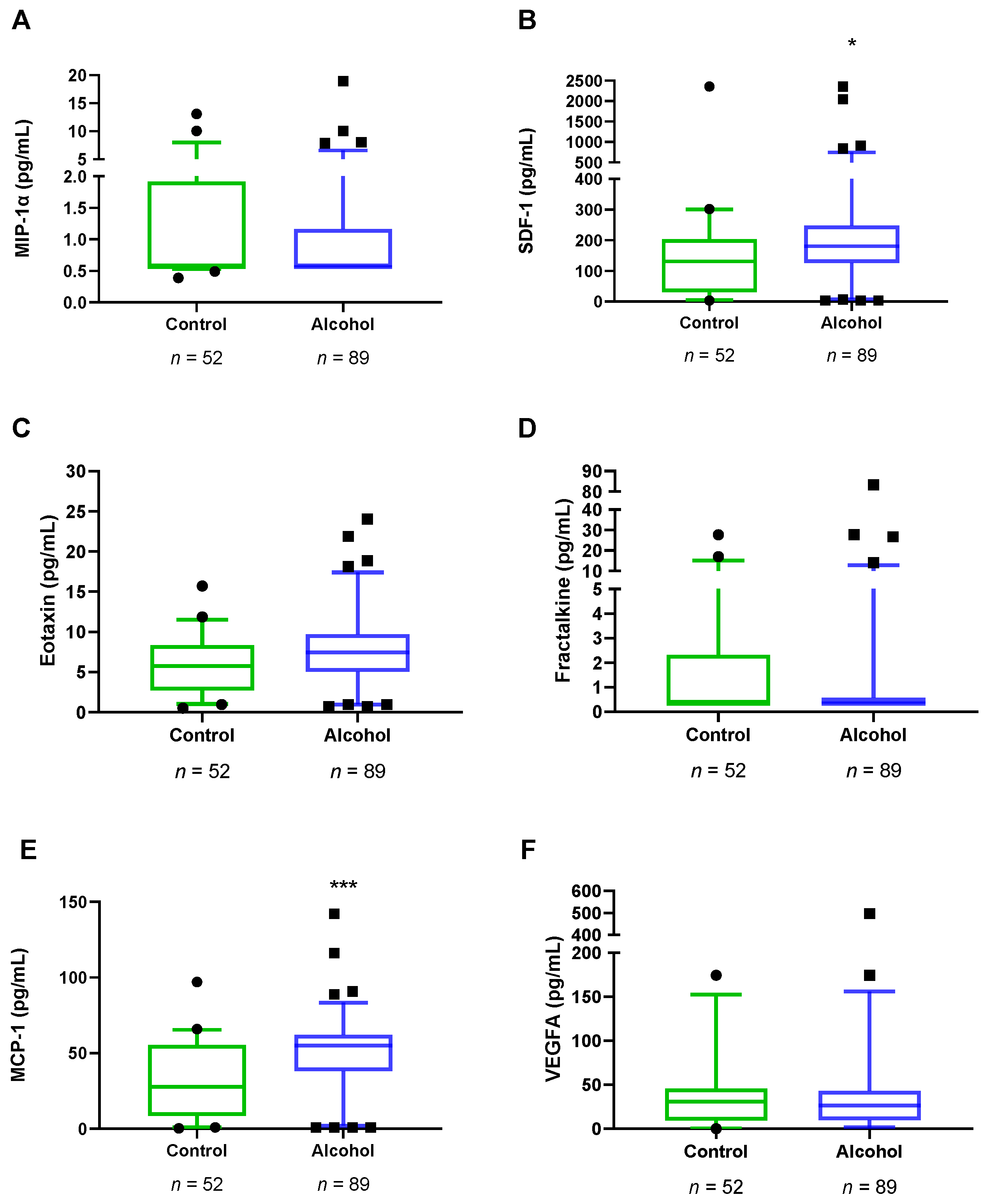
3.3. Plasma Concentrations of VEGFA and Chemokines in Abstinent AUD Patients
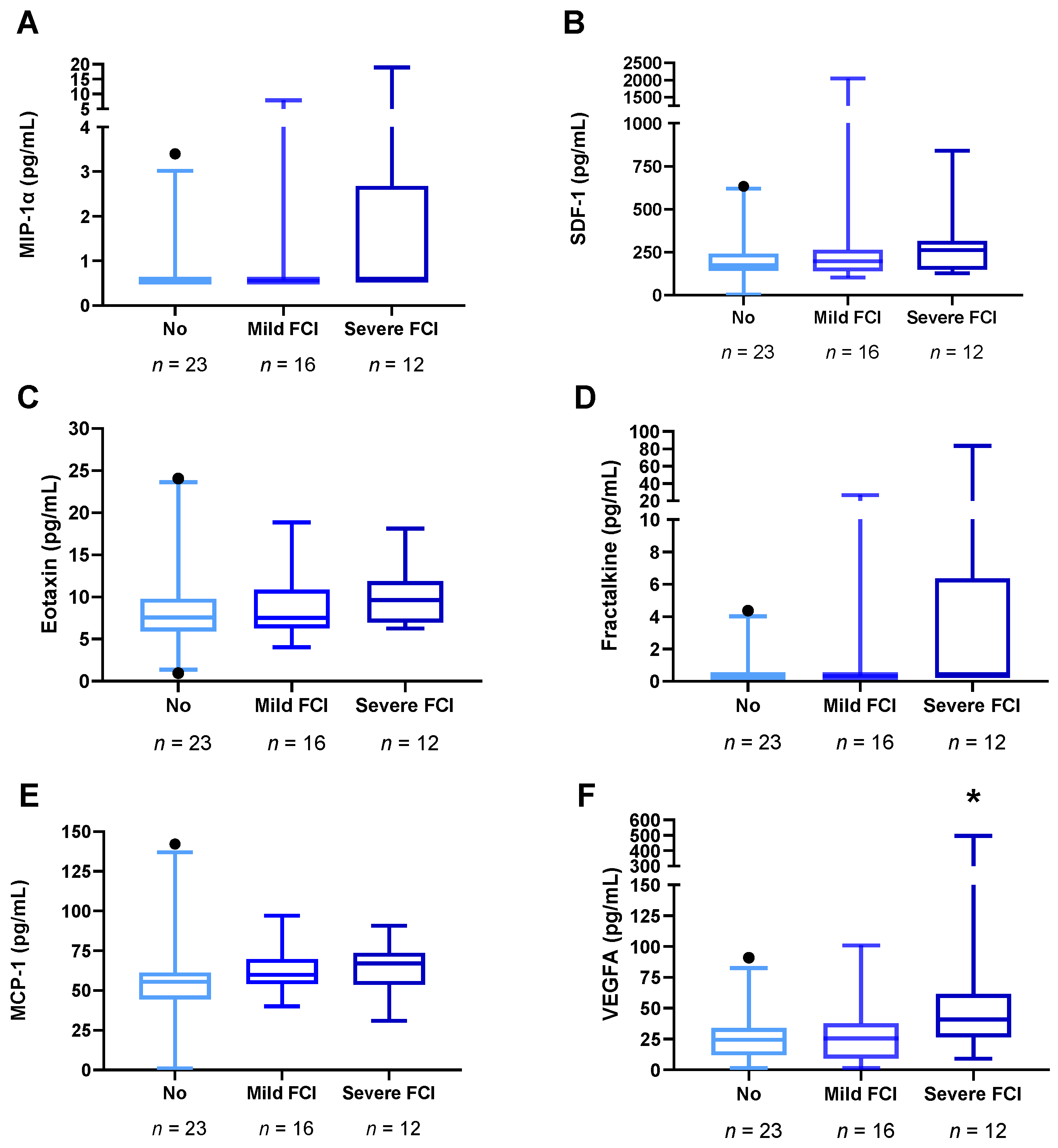
3.4. Plasma Concentrations of VEGFA and Chemokines in Abstinent AUD Patients with Frontal Cognitive Impairment
3.5. Correlation Analyses between Frontal Cognition and Plasma Concentrations of VEGFA and Chemokines in AUD Patients
3.6. Correlation Analyses between Plasma Concentrations of VEGFA and Chemokines in AUD Patients with and without Mild Cognitive Impairment
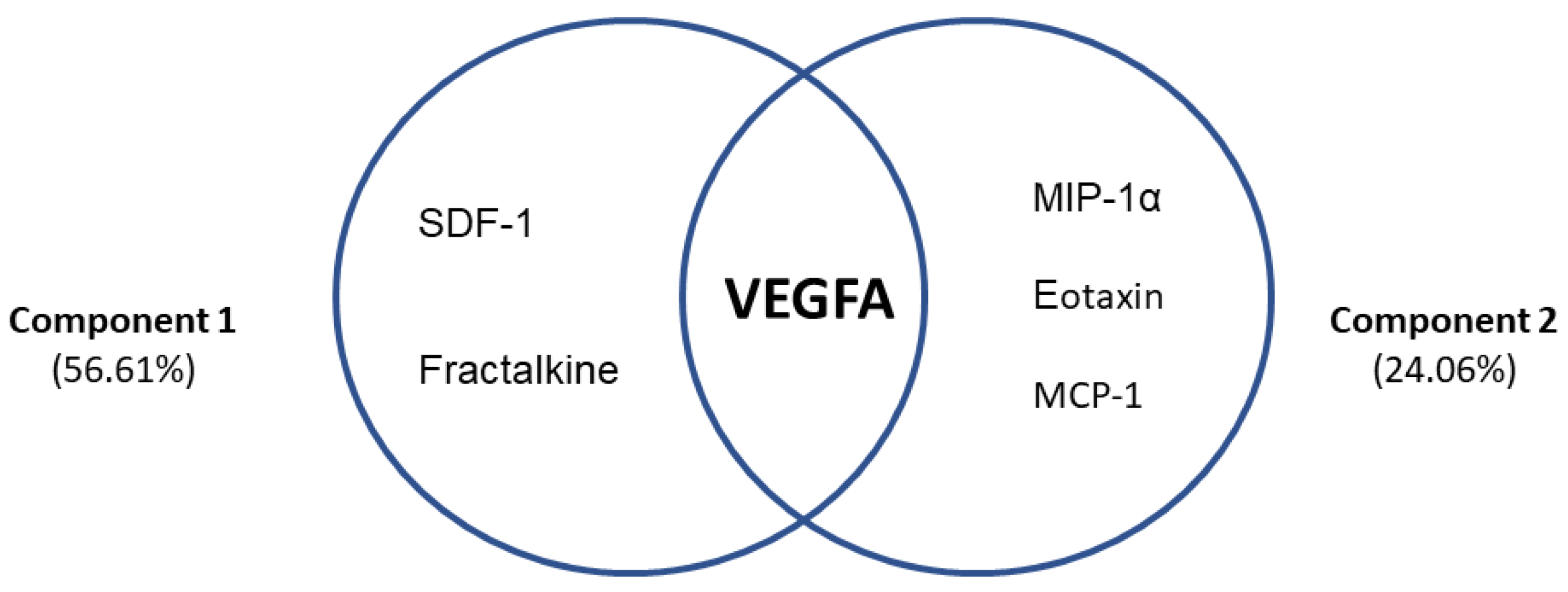
3.7. Differential Profiles Associated with VEGFA and Chemokines in AUD Patients with Frontal Cognitive Impairment
3.8. Plasma Concentrations of VEGFA and Chemokines in Comorbid Medical, Psychiatric Medication and Substance Use Problems in AUD Patients
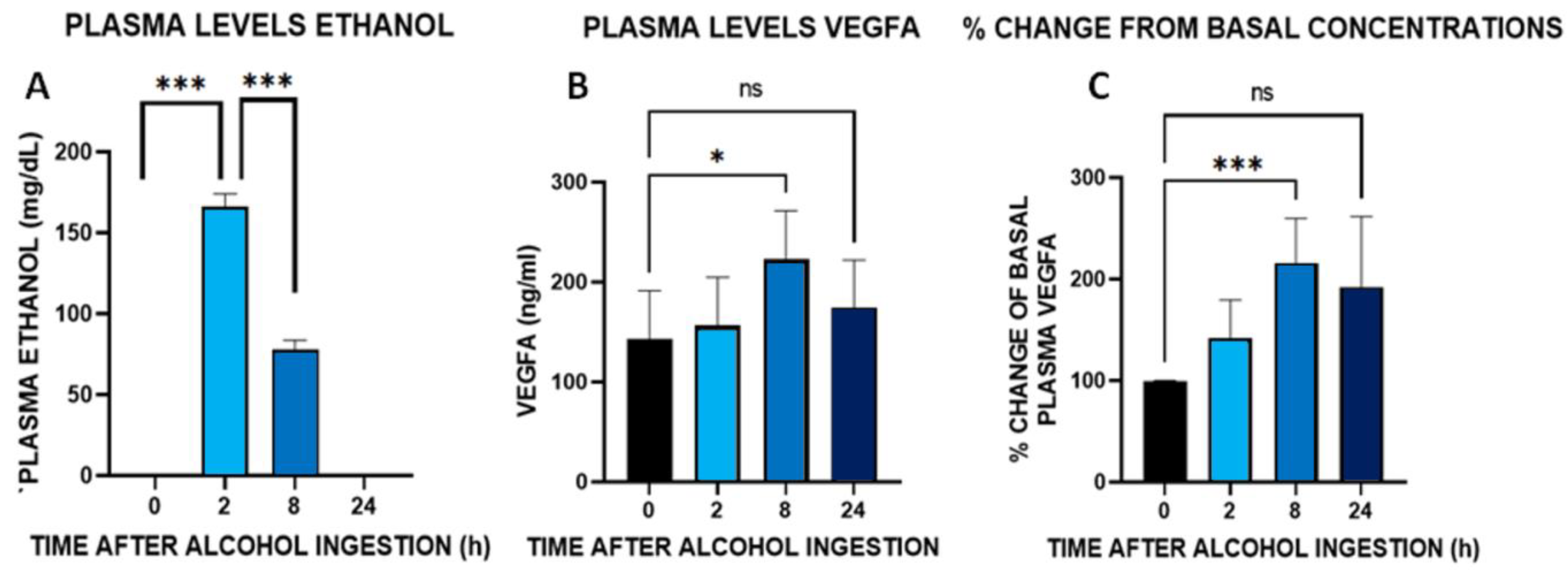
3.9. Time Course of Plasma Concentrations of VEGFA after an Acute Administration of Alcohol (100 g) in Healthy Male Volunteers
4. Discussion
5. Conclusions, Limitations, and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- OEDA. Observatorio Español de las Drogas y las Adicciones (OEDA) [2017]. Informe Anual 2017. Informe EDADES 2017. Plan Nacional Sobre Drogas. 2019. Available online: https://pnsd.sanidad.gob.es/profesionales/sistemasInformacion/home.htm (accessed on 23 February 2022).
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, V.W.; Monroe, K.; Lugea, A.; Yadav, D.; Pandol, S. Uniting Epidemiology and Experimental Disease Models for Alcohol-Related Pancreatic Disease. Alcohol Res. Curr. Rev. 2017, 38, 173–182. [Google Scholar]
- Marchena, N.G.; Araos, P.; Pavón, F.J.; Ponce, G.; Pedraz, M.; Serrano, A.; Arias, F.; Romero-Sanchiz, P.; Suárez, J.; Pastor, A.; et al. Psychiatric comorbidity and plasma levels of 2-acyl-glycerols in outpatient treatment alcohol users. Analysis of gender differences. Adicciones 2016, 29, 83. [Google Scholar] [CrossRef]
- Boden, J.; Fergusson, D.M. Alcohol and depression. Addiction 2011, 106, 906–914. [Google Scholar] [CrossRef]
- Samet, S.; Fenton, M.C.; Nunes, E.; Greenstein, E.; Aharonovich, E.; Hasin, D. Effects of independent and substance-induced major depressive disorder on remission and relapse of alcohol, cocaine and heroin dependence. Addiction 2013, 108, 115–123. [Google Scholar] [CrossRef]
- Schwarzinger, M.; Pollock, B.G.; Hasan, O.S.M.; Dufouil, C.; Rehm, J.; Baillot, S.; Guibert, Q.; Planchet, F.; Luchini, S. Contribution of alcohol use disorders to the burden of dementia in France 2008-13: A nationwide retrospective cohort study. Lancet Public Health 2018, 3, e124–e132. [Google Scholar] [CrossRef]
- Withall, A.; Draper, B.; Seeher, K.; Brodaty, H. The prevalence and causes of younger onset dementia in Eastern Sydney, Australia. Int. Psychogeriatr. 2014, 26, 1955–1965. [Google Scholar] [CrossRef]
- Cheng, C.; Huang, C.-L.; Tsai, C.-J.; Chou, P.-H.; Lin, C.-C.; Chang, C.-K. Alcohol-Related Dementia: A Systemic Review of Epidemiological Studies. J. Psychosom. Res. 2017, 58, 331–342. [Google Scholar] [CrossRef]
- Woods, A.J.; Porges, E.C.; Bryant, V.E.; Seider, T.; Gongvatana, A.; Kahler, C.W.; De La Monte, S.; Monti, P.M.; Cohen, R.A. Current Heavy Alcohol Consumption is Associated with Greater Cognitive Impairment in Older Adults. Alcohol. Clin. Exp. Res. 2016, 40, 2435–2444. [Google Scholar] [CrossRef]
- Hayes, V.; Demirkol, A.; Ridley, N.; Withall, A.; Draper, B. Alcohol-related cognitive impairment: Current trends and future perspectives. Neurodegener. Dis. Manag. 2016, 6, 509–523. [Google Scholar] [CrossRef]
- Ros-Cucurull, E.; Palma-Álvarez, R.F.; Cardona-Rubira, C.; García-Raboso, E.; Jacas, C.; Grau-López, L.; Abad, A.C.; Rodríguez-Cintas, L.; Ros-Montalbán, S.; Casas, M.; et al. Alcohol use disorder and cognitive impairment in old age patients: A 6 months follow-up study in an outpatient unit in Barcelona. Psychiatry Res. 2018, 261, 361–366. [Google Scholar] [CrossRef]
- Horton, L.; Duffy, T.; Martin, C. Neurocognitive, psychosocial and functional status of individuals with alco-hol-related brain damage (ARBD) on admission to specialist residential care. Drugs Educ. Prev. Policy 2015, 22, 416–427. [Google Scholar] [CrossRef]
- Montesinos, J.; Pascual, M.; Pla, A.; Maldonado, C.; Rodríguez-Arias, M.; Miñarro, J.; Guerri, C. TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain Behav. Immun. 2015, 45, 233–244. [Google Scholar] [CrossRef]
- Dwivedi, D.K.; Kumar, D.; Kwatra, M.; Pandey, S.N.; Choubey, P.; Lahkar, M.; Jangra, A. Voluntary alcohol consumption exacerbated high fat diet-induced cognitive deficits by NF-κB-calpain dependent apoptotic cell death in rat hippocampus: Ameliorative effect of melatonin. Biomed. Pharmacother. 2018, 108, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Xue, X.; Tian, H.; Wang, X.-X.; Dong, Y.-X.; Wang, F.; Zhao, Y.-N.; Yao, X.-C.; Cui, W.; Wu, C.-F. Role of microglia in ethanol-induced neurodegenerative disease: Pathological and behavioral dysfunction at different developmental stages. Pharmacol. Ther. 2014, 144, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. Peptides and the blood-brain barrier. Peptides 2015, 72, 16–19. [Google Scholar] [CrossRef]
- Pascual, M.; Baliño, P.; Aragón, C.M.; Guerri, C. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: Role of TLR4 and TLR2. Neuropharmacology 2015, 89, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-H.; Ho, P.-S.; Yeh, Y.-W.; Liang, C.-S.; Kuo, S.-C.; Huang, C.-C.; Chen, C.-Y.; Shih, M.-C.; Ma, K.-H.; Sung, Y.-F.; et al. Differential cytokine levels between early withdrawal and remission states in patients with alcohol dependence. Psychoneuroendocrinology 2017, 76, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Rostène, W.; Kitabgi, P.; Parsadaniantz, S.M. Chemokines: A new class of neuromodulator? Nat. Rev. Neurosci. 2007, 8, 895–903. [Google Scholar] [CrossRef]
- Watson, A.E.S.; Goodkey, K.; Footz, T.; Voronova, A. Regulation of CNS precursor function by neuronal chemokines. Neurosci. Lett. 2020, 715, 134533. [Google Scholar] [CrossRef]
- Comerford, I.; McColl, S.R. Mini-review series: Focus on chemokines. Immunol. Cell Biol. 2011, 89, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Palomino, D.C.T.; Marti, L.C. Chemokines and immunity. Einstein (São Paulo) 2015, 13, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Shurtleff, D.; Harris, R.A. Neuroimmune Mechanisms of Alcohol and Drug Addiction. Int. Rev. Neurobiol. 2014, 118, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lacagnina, M.; Rivera, P.D.; Bilbo, S.D. Glial and Neuroimmune Mechanisms as Critical Modulators of Drug Use and Abuse. Neuropsychopharmacology 2017, 42, 156–177. [Google Scholar] [CrossRef] [PubMed]
- Ivanovska, M.; Abdi, Z.; Murdjeva, M.; Macedo, D.; Maes, A.; Maes, M. CCL-11 or Eotaxin-1: An Immune Marker for Ageing and Accelerated Ageing in Neuro-Psychiatric Disorders. Pharmaceuticals 2020, 13, 230. [Google Scholar] [CrossRef]
- Wang, R.; Li, H. A focus on CXCR4 in Alzheimer’s disease. Brain Circ. 2017, 3, 199–203. [Google Scholar] [CrossRef]
- Stuart, M.; Baune, B. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: A systematic review of biomarker studies. Neurosci. Biobehav. Rev. 2014, 42, 93–115. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Gama, C.S.; Rocha, N.P.; Teixeira, M.M. Revisiting the role of eotaxin-1/CCL11 in psychiatric disorders. Front. Psychiatry 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, L.; Zhang, J.; Cheng, Y. Aberrations in peripheral inflammatory cytokine levels in substance use disorders: A meta-analysis of 74 studies. Addiction 2020, 115, 2257–2267. [Google Scholar] [CrossRef]
- Achur, R.N.; Freeman, W.M.; Vrana, K.E. Circulating Cytokines as Biomarkers of Alcohol Abuse and Alcoholism. J. Neuroimmune Pharmacol. 2010, 5, 83–91. [Google Scholar] [CrossRef]
- Qin, L.; He, J.; Hanes, R.N.; Pluzarev, O.; Hong, J.-S.; Crews, F.T. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflammation 2008, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Xu, M. Chemokines and alcoholic hepatitis: Are chemokines good therapeutic targets? Gut 2014, 63, 1683–1684. [Google Scholar] [CrossRef] [PubMed]
- McClain, C.J.; Shedlofsky, S.; Barve, S.; Hill, D.B. Cytokines and alcoholic liver disease. Alcohol Health Res. World 1997, 21, 317–320. [Google Scholar] [PubMed]
- Lange, C.; Storkebaum, E.; de Almodovar, C.R.; Dewerchin, M.; Carmeliet, P. Vascular endothelial growth factor: A neurovascular target in neurological diseases. Nat. Rev. Neurol. 2016, 12, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Klintsova, A.Y.; Hamilton, G.F.; Boschen, K.E. Long-Term Consequences of Developmental Alcohol Exposure on Brain Structure and Function: Therapeutic Benefits of Physical Activity. Brain Sci. 2012, 3, 1–38. [Google Scholar] [CrossRef]
- Bates, D.O. Vascular endothelial growth factors and vascular permeability. Cardiovasc. Res. 2010, 87, 262–271. [Google Scholar] [CrossRef]
- Tammela, T.; Enholm, B.; Alitalo, K.; Paavonen, K. The biology of vascular endothelial growth factors. Cardiovasc. Res. 2005, 65, 550–563. [Google Scholar] [CrossRef]
- Heberlein, A.; Muschler, M.; Lenz, B.; Frieling, H.; Büchl, C.; Gröschl, M.; Riera, R.; Kornhuber, J.; Bleich, S.; Hillemacher, T. Serum levels of vascular endothelial growth factor a increase during alcohol withdrawal. Addict. Biol. 2010, 15, 362–364. [Google Scholar] [CrossRef]
- Kasztelan-Szczerbinska, B.; Surdacka, A.; Slomka, M.; Rolinski, J.M.; Celinski, K.; Cichoz-Lach, H.; Madro, A.; Szczerbinski, M. Angiogenesis-Related Biomarkers in Patients with Alcoholic Liver Disease: Their Association with Liver Disease Complications and Outcome. Mediat. Inflamm. 2014, 2014, 673032. [Google Scholar] [CrossRef]
- Torrens, M.; Serrano, D.; Astals, M.; Pérez-Domínguez, G.; Martín-Santos, R. Diagnosing Comorbid Psychiatric Disorders in Substance Abusers: Validity of the Spanish Versions of the Psychiatric Research Interview for Substance and Mental Disorders and the Structured Clinical Interview for DSM-IV. Am. J. Psychiatry 2004, 161, 1231–1237. [Google Scholar] [CrossRef]
- Hasin, D.S.; O’Brien, C.P.; Auriacombe, M.; Borges, G.; Bucholz, K.; Budney, A.; Compton, W.M.; Crowley, T.; Ling, W.; Petry, N.M.; et al. DSM-5 Criteria for Substance Use Disorders: Recommendations and Rationale. Am. J. Psychiatry 2013, 170, 834–851. [Google Scholar] [CrossRef] [PubMed]
- Del Alamo, A.R.; Alonso, M.J.C.; Marín, L.C. FAB: A preliminar Spanish application of the frontal assessment battery to 11 groups of patients. Revista de Neurología 2003, 36, 605–608. [Google Scholar]
- Ahearn, O.C.; Watson, M.N.; Rawls, S.M. Chemokines, cytokines and substance use disorders. Drug Alcohol Depend. 2021, 220, 108511. [Google Scholar] [CrossRef] [PubMed]
- Blednov, Y.; Benavidez, J.; Geil, C.; Perra, S.; Morikawa, H.; Harris, R. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav. Immun. 2011, 25, S92–S105. [Google Scholar] [CrossRef] [PubMed]
- Blednov, Y.A.; Ponomarev, I.; Geil, C.; Bergeson, S.; Koob, G.F.; Harris, R.A. Neuroimmune regulation of alcohol consumption: Behavioral validation of genes obtained from genomic studies. Addict. Biol. 2012, 17, 108–120. [Google Scholar] [CrossRef]
- Blednov, Y.A.; Bergeson, S.E.; Walker, D.; Ferreira, V.M.; Kuziel, W.A.; Harris, R.A. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav. Brain Res. 2005, 165, 110–125. [Google Scholar] [CrossRef]
- Bjørkhaug, S.T.; Neupane, S.P.; Bramness, J.G.; Aanes, H.; Skar, V.; Medhus, A.W.; Valeur, J. Plasma cytokine levels in patients with chronic alcohol overconsumption: Relations to gut microbiota markers and clinical correlates. Alcohol 2020, 85, 35–40. [Google Scholar] [CrossRef]
- He, J.; Crews, F.T. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008, 210, 349–358. [Google Scholar] [CrossRef]
- Kim, J.; Connelly, K.L.; Unterwald, E.M.; Rawls, S.M. Chemokines and cocaine: CXCR4 receptor antagonist AMD3100 attenuates cocaine place preference and locomotor stimulation in rats. Brain, Behav. Immun. 2017, 62, 30–34. [Google Scholar] [CrossRef]
- Trecki, J.; Unterwald, E. Modulation of cocaine-induced activity by intracerebral administration of CXCL12. Neuroscience 2009, 161, 13–22. [Google Scholar] [CrossRef][Green Version]
- Trocello, J.M.; Rostène, W.; Melik-Parsadaniantz, S.; Godefroy, D.; Roze, E.; Kitabgi, P.; Kuziel, W.A.; Chalon, S.; Caboche, J.; Apartis, E. Implication of CCR2 Chemokine Receptor in Cocaine-Induced Sensitization. J. Mol. Neurosci. 2011, 44, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Araos, P.; Pedraz, M.; Serrano, A.; Lucena, M.; Barrios, V.; García-Marchena, N.; Campos-Cloute, R.; Ruiz, J.J.; Romero, P.; Suárez, J.; et al. Plasma profile of pro-inflammatory cytokines and chemokines in cocaine users under outpatient treatment: Influence of cocaine symptom severity and psychiatric co-morbidity. Addict. Biol. 2014, 20, 756–772. [Google Scholar] [CrossRef] [PubMed]
- García-Marchena, N.; Barrera, M.; Mestre-Pintó, J.I.; Araos, P.; Serrano, A.; Pérez-Mañá, C.; Papaseit, E.; Fonseca, F.; Ruiz, J.J.; De Fonseca, F.R.; et al. Inflammatory mediators and dual depression: Potential biomarkers in plasma of primary and substance-induced major depression in cocaine and alcohol use disorders. PLoS ONE 2019, 14, e0213791. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, J.; Castilla-Ortega, E.; Sánchez-Marín, L.; Montagud-Romero, S.; Araos, P.; Pedraz, M.; Porras-Perales, Ó.; García-Marchena, N.; Serrano, A.; Suárez, J.; et al. Cocaine-induced changes in CX3CL1 and inflammatory signaling pathways in the hippocampus: Association with IL1β. Neuropharmacology 2020, 162, 107840. [Google Scholar] [CrossRef]
- Romero-Martínez, Á.; Vitoria-Estruch, S.; Moya-Albiol, L. Perfil cognitivo de los alcohólicos abstinentes durante un periodo de tiempo prolongado en comparación con un grupo de hombres que no consumen alcohol. Adicciones 2018, 32, 19–31. [Google Scholar] [CrossRef]
- Joos, L.; Schmaal, L.; Goudriaan, A.E.; Fransen, E.; Brink, W.V.D.; Sabbe, B.G.C.; Dom, G. Age of Onset and Neuropsychological Functioning in Alcohol Dependent Inpatients. Alcohol. Clin. Exp. Res. 2013, 37, 407–416. [Google Scholar] [CrossRef]
- Freeman, C.R.; Wiers, C.E.; Sloan, M.E.; Zehra, A.; Ramirez, V.; Wang, G.-J.; Volkow, N.D. Emotion Recognition Biases in Alcohol Use Disorder. Alcohol. Clin. Exp. Res. 2018, 42, 1541–1547. [Google Scholar] [CrossRef]
- Kopera, M.; Wojnar, M.; Brower, K.; Glass, J.; Nowosad, I.; Gmaj, B.; Szelenberger, W. Cognitive functions in abstinent alcohol-dependent patients. Alcohol 2012, 46, 665–671. [Google Scholar] [CrossRef]
- Sachdeva, A.; Chandra, M.; Choudhary, M.; Dayal, P.; Anand, K.S. Alcohol-Related Dementia and Neurocognitive Impairment: A Review Study. Int. J. High Risk Behav. Addict. 2016, 5, e27976. [Google Scholar] [CrossRef]
- Le Berre, A.-P.; Fama, R.; Sullivan, E.V. Executive Functions, Memory, and Social Cognitive Deficits and Recovery in Chronic Alcoholism: A Critical Review to Inform Future Research. Alcohol. Clin. Exp. Res. 2017, 41, 1432–1443. [Google Scholar] [CrossRef]
- Lechner, W.V.; Day, A.M.; Metrik, J.; Leventhal, A.M.; Kahler, C.W. Effects of alcohol-induced working memory decline on alcohol consumption and adverse consequences of use. Psychopharmacology 2015, 233, 83–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khemiri, L.; Brynte, C.; Stunkel, A.; Klingberg, T.; Jayaram-Lindstrom, N. Working Memory Training in Alcohol Use Disorder: A Randomized Controlled Trial. Alcohol. Clin. Exp. Res. 2019, 43, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Fernández, A.; Marín-Navarrete, R.; Villalobos-Gallegos, L.; Salvador-Cruz, J.; Benjet, C.; Roncero, C. Testing whether cognitive reserve as measured by self-rating of stimulating activities moderates the association of polysubstance use and neurocognitive disorder. Cogn. Neuropsychiatry 2019, 24, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Fernández, A.; Villalobos-Gallegos, L.; Salvador-Cruz, J.; Benjet, C.; Roncero, C.; Marín-Navarrete, R. Differential Effects of Cognitive Reserve on the Neurocognitive Functioning of Polysubstance Users: An Exploratory Analysis Using Mixture Regression. Int. J. Ment. Health Addict. 2019, 18, 500–514. [Google Scholar] [CrossRef]
- Cutuli, D.; De Guevara-Miranda, D.L.; Castilla-Ortega, E.; Santín, L.; Sampedro-Piquero, P. Highlighting the Role of Cognitive and Brain Reserve in the Substance use Disorder Field. Curr. Neuropharmacol. 2019, 17, 1056–1070. [Google Scholar] [CrossRef]
- Sabia, S.; Guéguen, A.; Berr, C.; Berkman, L.; Ankri, J.; Goldberg, M.; Zins, M.; Singh-Manoux, A. High alcohol consumption in middle-aged adults is associated with poorer cognitive performance only in the low socio-economic group. Results from the GAZEL cohort study. Addiction 2011, 106, 93–101. [Google Scholar] [CrossRef]
- Sabia, S.; Fayosse, A.; Dumurgier, J.; Dugravot, A.; Akbaraly, T.; Britton, A.; Kivimaki, M.; Singh-Manoux, A. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ 2018, 362, k2927. [Google Scholar] [CrossRef]
- Requena-Ocaña, N.; Flores-Lopez, M.; Martín, A.S.; García-Marchena, N.; Pedraz, M.; Ruiz, J.J.; Serrano, A.; Suarez, J.; Pavón, F.J.; de Fonseca, F.R.; et al. Influence of gender and education on cocaine users in an outpatient cohort in Spain. Sci. Rep. 2021, 11, 20928. [Google Scholar] [CrossRef]
- Requena-Ocaña, N.; Araos, P.; Flores, M.; García-Marchena, N.; Silva-Peña, D.; Aranda, J.; Rivera, P.; Ruiz, J.J.; Serrano, A.; Pavón, F.J.; et al. Evaluation of neurotrophic factors and education level as predictors of cognitive decline in alcohol use disorder. Sci. Rep. 2021, 11, 15583. [Google Scholar] [CrossRef]
- Tarkowski, E.; Issa, R.; Sjögren, M.; Wallin, A.; Blennow, K.; Tarkowski, A.; Kumar, P. Increased intrathecal levels of the angiogenic factors VEGF and TGF-β in Alzheimer’s disease and vascular dementia. Neurobiol. Aging 2002, 23, 237–243. [Google Scholar] [CrossRef]
- Chiappelli, M.; Borroni, B.; Archetti, S.; Calabrese, E.; Corsi, M.M.; Franceschi, M.; Padovani, A.; Licastro, F. VEGF Gene and Phenotype Relation with Alzheimer’s Disease and Mild Cognitive Impairment. Rejuvenation Res. 2006, 9, 485–493. [Google Scholar] [CrossRef]
- Zhang, N.; Xing, M.; Wang, Y.; Liang, H.; Yang, Z.; Shi, F.; Cheng, Y. Hydroxysafflor yellow A improves learning and memory in a rat model of vascular dementia by increasing VEGF and NR1 in the hippocampus. Neurosci. Bull. 2013, 30, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xing, M.; Wang, Y.; Tao, H.; Cheng, Y. Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF-NMDAR pathways in a rat model of vascular dementia. Neuroscience 2015, 311, 284–291. [Google Scholar] [CrossRef] [PubMed]
- de Almodovar, C.R.; Lambrechts, D.; Mazzone, M.; Carmeliet, P. Role and Therapeutic Potential of VEGF in the Nervous System. Physiol. Rev. 2009, 89, 607–648. [Google Scholar] [CrossRef] [PubMed]
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Chi, O.Z.; Hunter, C.; Liu, X.; Tan, T.; Weiss, H.R. Effects of VEGF on the Blood-Brain Barrier Disruption Caused by Hyperosmolarity. Pharmacology 2008, 82, 187–192. [Google Scholar] [CrossRef]
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.J.; Seto, J.; Kramer, E.G.; et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Investig. 2012, 122, 2454–2468. [Google Scholar] [CrossRef]
- Muneer, P.M.A.; Alikunju, S.; Szlachetka, A.M.; Haorah, J. The Mechanisms of Cerebral Vascular Dysfunction and Neuroinflammation by MMP-Mediated Degradation of VEGFR-2 in Alcohol Ingestion. Arter. Thromb. Vasc. Biol. 2012, 32, 1167–1177. [Google Scholar] [CrossRef]
- Louboutin, J.-P.; Marusich, E.; Gao, E.; Agrawal, L.; Koch, W.J.; Strayer, D.S. Ethanol protects from injury due to ischemia and reperfusion by increasing vascularity via vascular endothelial growth factor. Alcohol 2012, 46, 441–454. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Welsh, M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013, 273, 114–127. [Google Scholar] [CrossRef]
- Rampino, A.; Annese, T.; Torretta, S.; Tamma, R.; Falcone, R.M.; Ribatti, D. Involvement of Vascular Endothelial Growth Factor in schizophrenia. Neurosci. Lett. 2021, 760, 136093. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Luan, D.; Song, R.; Zhang, Z. Value of peripheral neurotrophin levels for the diagnosis of depression and response to treatment: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2020, 41, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ren, Q.; Zhang, J.-C.; Chen, Q.-X.; Hashimoto, K. Altered expression of BDNF, BDNF pro-peptide and their precursor proBDNF in brain and liver tissues from psychiatric disorders: Rethinking the brain-liver axis. Transl. Psychiatry 2017, 7, e1128. [Google Scholar] [CrossRef] [PubMed]
- Sokolowski, J.D.; Chabanon-Hicks, C.N.; Han, C.Z.; Heffron, D.S.; Mandell, J.W. Fractalkine is a “find-me” signal released by neurons undergoing ethanol-induced apoptosis. Front. Cell. Neurosci. 2014, 8, 360. [Google Scholar] [CrossRef]
- Hatori, K.; Nagai, A.; Heisel, R.; Ryu, J.K.; Kim, S.U. Fractalkine and fractalkine receptors in human neurons and glial cells. J. Neurosci. Res. 2002, 69, 418–426. [Google Scholar] [CrossRef]
- Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.; Kidd, G.; Dombrowski, S.; Dutta, R.; et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006, 9, 917–924. [Google Scholar] [CrossRef]
- Perea, J.R.; Lleó, A.; Alcolea, D.; Fortea, J.; Avila, J.; Bolós, M. Decreased CX3CL1 Levels in the Cerebrospinal Fluid of Patients with Alzheimer’s Disease. Front. Neurosci. 2018, 12, 609. [Google Scholar] [CrossRef]
- Strobel, S.; Grünblatt, E.; Riederer, P.; Heinsen, H.; Arzberger, T.; Al-Sarraj, S.; Troakes, C.; Ferrer, I.; Monoranu, C.M. Changes in the expression of genes related to neuroinflammation over the course of sporadic Alzheimer’s disease progression: CX3CL1, TREM2, and PPARγ. J. Neural Transm. 2015, 122, 1069–1076. [Google Scholar] [CrossRef]
- Suresh, P.; Phasuk, S.; Liu, I. Modulation of microglia activation and Alzheimer’s disease: CX3 chemokine ligand 1/CX3CR and P2X7R signaling. Tzu-Chi Med. J. 2021, 33, 1–6. [Google Scholar]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Battaglia, S. Neurobiological advances of learned fear in humans. Adv. Clin. Exp. Med. 2022, 31, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Rodríguez, L.; Echeverry-Alzate, V.; Rincón-Pérez, I.; Calleja-Conde, J.; Bühler, K.M.; Giné, E.; Albert, J.; Hinojosa, J.A.; Huertas, E.; Gómez-Gallego, F.; et al. Gut microbiota and voluntary alcohol consumption. Transl. Psychiatry 2022, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]

| Total Sample (n = 141) | |||||
|---|---|---|---|---|---|
| Variables | Control Group (n = 52) | Alcohol Group (n = 89) | Statistic | p-Value | |
| Age (Mean ± SD) | Years | 47.14 ± 5.29 | 44.16 ± 11.88 | 1867.50 1 | 0.056 |
| Body Mass Index (Mean ± SD) | Kg/m2 | 27.15 ± 3.59 | 26.36 ± 4.84 | 1907.50 1 | 0.117 |
| Sex [n (%)] | Women Men | 17 (32.70) 35 (67.30) | 17 (19.10) 72 (80.90) | 3.313 2 | 0.069 |
| Education Degree [n (%)] | Elementary Secondary University | 13 (25) 20 (38.50) 19 (36.50) | 36 (40.40) 38 (42.70) 15 (16.90) | 7.672 2 | 0.022 |
| Occupation [n (%)] | Employed Unemployed Retired Other | 45 (86.50) 0 3 (5.80) 4 (7.70) | 19 (21.30) 39 (43.80) 13 (14.60) 18 (20.20) | 59.081 2 | <0.001 |
| Variables | AUD Group | |||||
|---|---|---|---|---|---|---|
| Total AUD (n = 89) | AUD with FCI (n = 28) | AUD without FCI (n = 23) | Statistic | p-Value | ||
| Age at first alcohol use (Mean ± SD) | Years | 14.69 ± 4.027 | 14.42 ± 3.62 | 15.62 ± 3.71 | 271 1 | 0.330 |
| Age at onset of AUD (Mean ± SD) | Years | 25.99 ± 9.591 | 28.38 ± 12.31 | 26.20 ± 9.58 | 266.50 1 | 0.417 |
| Length of AUD diagnosis (Mean ± SD) | Years | 15.06 ± 11.314 | 11.46 ± 8.96 | 15.19 ± 11.40 | 228 1 | 0.334 |
| Severity criteria (Mean ± SD) | Criteria [1,2,3,4,5,6,7,8,9,10,11] | 8.09 ± 2.114 | 7.96 ± 2.20 | 8.52 ± 2.32 | 299.50 1 | 0.666 |
| Length of abstinence (Mean ± SD) | Days | 322.12 ± 908.545 | 63.46 ± 60.69 | 432.95 ± 1069.93 | 305.50 1 | 0.961 |
| Comorbid substance use disorders [n (%)] | Tobacco Cocaine Cannabis Sedatives | 69 (77.50) 43 (48.30) 19 (21.30) 7 (7.90) | 21 (75) 12 (42.90) 4 (14.30) - | 21 (91.30) 11 (47.80) 4 (17.40) 4 (17.40) | 2.264 2 0.126 0.090 5.180 | 0.132 0.723 0.764 0.023 |
| Comorbid psychiatric disorders [n (%)] | Mood Anxiety ADHD Personality Psychotic | 44 (49.4) 24 (27) 19 (21.30) 14 (15.70) 8 (9) | 14 (50) 6 (21.40) 3 (10.70) 5 (17.90) 3 (10.70) | 8 (34.80) 5 (21.70) 2 (8.70) 4 (17.40) 1 (4.30) | 1.192 2 0.001 0.057 0.002 0.694 | 0.275 0.979 0.811 0.966 0.405 |
| Psychiatric medication [n (%)] | Antidepressants Anxiolytics Antipsychotics Disulfiram Anticraving | 46 (51.70) 56 (62.90) 10 (11.20) 35 (39.30) 9 (10.10) | 17 (60.70) 15 (53.60) 2 (7.10) 14 (50) 5 (17.90) | 12 (52.20) 18 (78.30) 1 (4.30) 10 (43.50) 1 (4.30) | 0.375 2 3.370 0.175 0.216 2.117 | 0.540 0.066 0.676 0.642 0.204 |
| Variables | FAB (Score) | |
|---|---|---|
| Rho | p-Value | |
| SDF-1 (pg/mL) | −0.228 | 0.111 |
| Eotaxin (pg/mL) | −0.147 | 0.303 |
| MIP-1α (pg/mL) | −0.154 | 0.280 |
| MCP-1 (pg/mL) | −0.203 | 0.152 |
| Fractalkine (pg/mL) | −0.336 | 0.016 |
| VEGFA (pg/mL) | −0.290 | 0.039 |
| AUD Group (n = 89) | ||||
|---|---|---|---|---|
| Variables | Comorbid Psychiatric Disorder (n = 60) | No Comorbid Psychiatric Disorder (n = 29) | Statistics | |
| U-Value | p-Value | |||
| Mean [95% CI] | Mean [95% CI] | |||
| SDF-1 (pg/mL) | 271.9046 [165.9141–377.8952] | 189.3975 [143.9530–234.8421] | 831 | 0.828 |
| Eotaxin (pg/mL) | 7.77278 [6.57250–8.97306] | 7.47531 [5.57394–9.37668] | 806 | 0.575 |
| MIP-1α (pg/mL) | 1.8189 [1.0330–2.6048] | 0.6189 [0.5586–0.6793] | 567 | 0.002 |
| MCP-1 (pg/mL) | 48.9765 [42.4613–55.4917] | 48.4279 [36.5812–60.2747] | 813 | 0.618 |
| Fractalkine (pg/mL) | 2.0546 [0.6521–3.4570] | 0.7399 [0.1778–1.3020] | 741 | 0.160 |
| VEGFA (pg/mL) | 36.4530 [28.3181–44.5879] | 20.1710 [14.3319–26.0100] | 552 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Requena-Ocaña, N.; Flores-Lopez, M.; Papaseit, E.; García-Marchena, N.; Ruiz, J.J.; Ortega-Pinazo, J.; Serrano, A.; Pavón-Morón, F.J.; Farré, M.; Suarez, J.; et al. Vascular Endothelial Growth Factor as a Potential Biomarker of Neuroinflammation and Frontal Cognitive Impairment in Patients with Alcohol Use Disorder. Biomedicines 2022, 10, 947. https://doi.org/10.3390/biomedicines10050947
Requena-Ocaña N, Flores-Lopez M, Papaseit E, García-Marchena N, Ruiz JJ, Ortega-Pinazo J, Serrano A, Pavón-Morón FJ, Farré M, Suarez J, et al. Vascular Endothelial Growth Factor as a Potential Biomarker of Neuroinflammation and Frontal Cognitive Impairment in Patients with Alcohol Use Disorder. Biomedicines. 2022; 10(5):947. https://doi.org/10.3390/biomedicines10050947
Chicago/Turabian StyleRequena-Ocaña, Nerea, María Flores-Lopez, Esther Papaseit, Nuria García-Marchena, Juan Jesús Ruiz, Jesús Ortega-Pinazo, Antonia Serrano, Francisco Javier Pavón-Morón, Magí Farré, Juan Suarez, and et al. 2022. "Vascular Endothelial Growth Factor as a Potential Biomarker of Neuroinflammation and Frontal Cognitive Impairment in Patients with Alcohol Use Disorder" Biomedicines 10, no. 5: 947. https://doi.org/10.3390/biomedicines10050947
APA StyleRequena-Ocaña, N., Flores-Lopez, M., Papaseit, E., García-Marchena, N., Ruiz, J. J., Ortega-Pinazo, J., Serrano, A., Pavón-Morón, F. J., Farré, M., Suarez, J., Rodríguez de Fonseca, F., & Araos, P. (2022). Vascular Endothelial Growth Factor as a Potential Biomarker of Neuroinflammation and Frontal Cognitive Impairment in Patients with Alcohol Use Disorder. Biomedicines, 10(5), 947. https://doi.org/10.3390/biomedicines10050947








