Experimental Models for Testing the Efficacy of Pharmacological Treatments for Neonatal Hypoxic-Ischemic Encephalopathy
Abstract
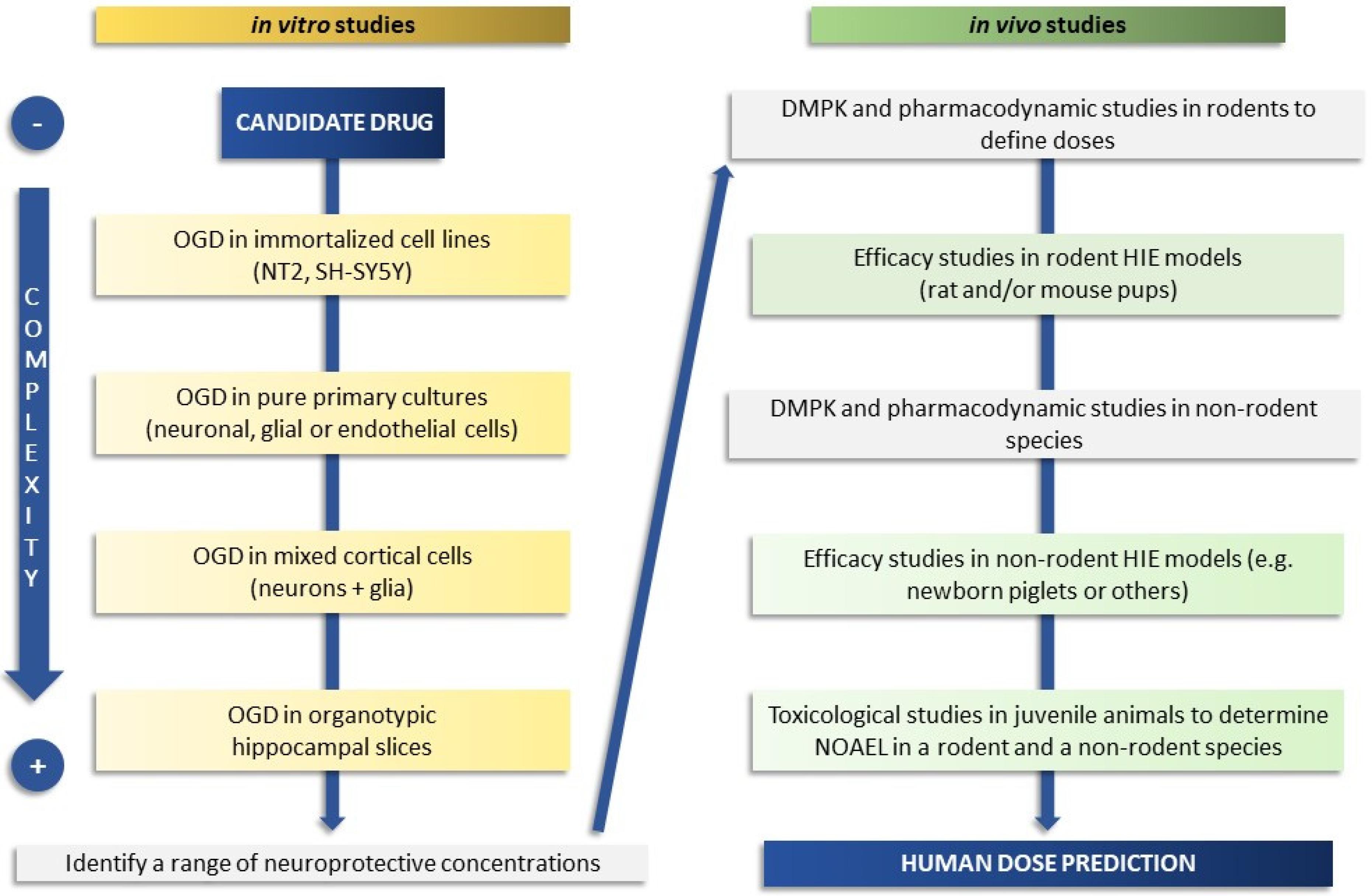
:1. Introduction
2. In Vitro Models
2.1. Dissociated Neuronal and Mixed Glial/Neuronal Cultures
2.2. D Brain Organoids
2.3. Organotypic Hippocampal Slices
3. At Term and Juvenile In Vivo Animal Models
3.1. The Rice–Vannucci Model
3.2. Piglet Model of Neonatal Encephalopathy
4. Near-Term Animal Models
5. The Ideal Timeline and Examples of Practical Approaches
5.1. Erythropoietin
5.2. Memantine and Topiramate
5.3. Cell-Based Treatments
6. State of the Art in Italy
6.1. In Vitro Models of HIE in Italy
6.2. In Vivo Models of HIE in Italy
6.3. HIE Clinical Trials in Italy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nair, J.; Kumar, V. Current and Emerging Therapies in the Management of Hypoxic Ischemic Encephalopathy in Neonates. Children 2018, 5, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, C.M.; Ozen, M.; Burd, I. Perinatal Brain Injury: Mechanisms, Prevention, and Outcomes. Clin. Perinatol. 2018, 45, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.; Singh, N.; Datta, A. In Vitro Oxygen Glucose Deprivation Model of Ischemic Stroke: A Proteomics-Driven Systems Biological Perspective. Mol. Neurobiol. 2022, 59, 2363–2377. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.O.; Wassink, G.; van den Heuij, L.G.; Bennet, L.; Gunn, A.J. Therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy—Where to from here? Front. Neurol. 2015, 6, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yıldız, E.P.; Ekici, B.; Tatlı, B. Neonatal hypoxic ischemic encephalopathy: An update on disease pathogenesis and treatment. Expert Rev. Neurother. 2017, 17, 449–459. [Google Scholar] [CrossRef]
- Arteaga, O.; Álvarez, A.; Revuelta, M.; Santaolalla, F.; Urtasun, A.; Hilario, E. Role of Antioxidants in Neonatal Hypoxic-Ischemic Brain Injury: New Therapeutic Approaches. Int. J. Mol. Sci. 2017, 18, 265. [Google Scholar] [CrossRef] [Green Version]
- Jatana, M.; Singh, I.; Singh, A.K.; Jenkins, D. Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr. Res. 2006, 59, 684–689. [Google Scholar] [CrossRef] [Green Version]
- Silachev, D.N.; Plotnikov, E.Y.; Pevzner, I.B.; Zorova, L.D.; Balakireva, A.V.; Gulyaev, M.V.; Pirogov, Y.A.; Skulachev, V.P.; Zorov, D.B. Neuroprotective Effects of Mitochondria-Targeted Plastoquinone in a Rat Model of Neonatal Hypoxic-Ischemic Brain Injury. Molecules 2018, 23, 1871. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.D.; Pierce, L.; Ciardiello, A.J.; Vannucci, S.J. Neonatal encephalopathy: Pre-clinical studies in neuroprotection. Biochem. Soc. Trans. 2014, 42, 564–568. [Google Scholar] [CrossRef]
- Mitra, S.; Kendall, G.S.; Bainbridge, A.; Sokolska, M.; Dinan, M.; Uria-Avellanal, C.; Price, D.; Mckinnon, K.; Gunny, R.; Huertas-Ceballos, A.; et al. Proton magnetic resonance spectroscopy lactate/N-acetylaspartate within 2 weeks of birth accurately predicts 2-year motor, cognitive and language outcomes in neonatal encephalopathy after therapeutic hypothermia. Arch. Dis. Child.-Fetal Neonatal Ed. 2018, 104, F424–F432. [Google Scholar] [CrossRef]
- Lally, P.J.; Montaldo, P.; Oliveira, V.; Soe, A.; Swamy, R.; Bassett, P.; Mendoza, J.; Atreja, G.; Kariholu, U.; Pattnayak, S.; et al. Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: A prospective multicentre cohort study. Lancet Neurol. 2019, 18, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Hagberg, H.; Mallard, C.; Ferriero, D.M.; Vannucci, S.J.; Levison, S.W.; Vexler, Z.S.; Gressens, P. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 2015, 11, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.J.; Thoresen, M. Animal studies of neonatal hypothermic neuroprotection have translated well in to practice. Resuscitation 2015, 97, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Sabir, H.; Walløe, L.; Dingley, J.; Smit, E.; Liu, X.; Thoresen, M. Combined treatment of Xenon and hypothermia in newborn rats—Additive or synergistic effect? PLoS ONE 2014, 9, e109845. [Google Scholar] [CrossRef]
- Robertson, N.J.; Martinello, K.; Lingam, I.; Avdic-Belltheus, A.; Meehan, C.; Alonso-Alconada, D.; Ragab, S.; Bainbridge, A.; Sokolska, M.; Tachrount, M.; et al. Melatonin as an adjunct to therapeutic hypothermia in a piglet model of neonatal encephalopathy: A translational study. Neurobiol. Dis. 2019, 121, 240–251. [Google Scholar] [CrossRef]
- Ryou, M.G.; Mallet, R.T. An In Vitro Oxygen-Glucose Deprivation Model for Studying Ischemia-Reperfusion Injury of Neuronal Cells. Methods Mol. Biol. 2018, 1717, 229–235. [Google Scholar]
- Guo, H.; Fan, Z.; Wang, S.; Ma, L.; Wang, J.; Yu, D.; Zhang, Z.; Wu, L.; Peng, Z.; Liu, W.; et al. Astrocytic A1/A2 paradigm participates in glycogen mobilization mediated neuroprotection on reperfusion injury after ischemic stroke. J. Neuroinflamm. 2021, 18, 230. [Google Scholar] [CrossRef]
- Liu, J.; Ma, W.; Zang, C.-H.; Wang, G.-D.; Zhang, S.-J.; Wu, H.-J.; Zhu, K.-W.; Xiang, X.-L.; Li, C.-Y.; Liu, K.-P.; et al. Salidroside inhibits NLRP3 inflammasome activation and apoptosis in microglia induced by cerebral ischemia/reperfusion injury by inhibiting the TLR4/NF-κB signaling pathway. Ann. Transl. Med. 2021, 9, 1694. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, J.; Li, X.; Wang, B.; Zhou, X. lncRNA HOTAIR mediates OGD/R-induced cell injury and angiogenesis in a EZH2-dependent manner. Exp. Ther. Med. 2022, 23, 99. [Google Scholar] [CrossRef]
- Goldberg, M.P.; Choi, D.W. Combined oxygen and glucose deprivation in cortical cell culture: Calcium-dependent and calcium-independent mechanisms of neuronal injury. J. Neurosci. 1993, 13, 3510–3524. [Google Scholar] [CrossRef]
- Sloan, S.A.; Andersen, J.; Pașca, A.M.; Birey, F.; Pașca, S.P. Generation and assembly of human brain region-specific three-dimensional cultures. Nat. Protoc. 2018, 13, 2062–2085. [Google Scholar] [CrossRef] [PubMed]
- Pașca, A.M.; Park, J.Y.; Shin, H.W.; Qi, Q.; Revah, O.; Krasnoff, R.; O’Hara, R.; Willsey, A.J.; Palmer, T.D.; Pașca, S.P. Human 3D cellular model of hypoxic brain injury of prematurity. Nat. Med. 2019, 25, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Humpel, C. Organotypic brain slice cultures: A review. Neuroscience 2015, 305, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Laurino, A.; Landucci, E.; Resta, F.; De Siena, G.; Pellegrini-Giampietro, D.E.; Masi, A.; Mannaioni, G.; Raimondi, L. Anticonvulsant and Neuroprotective Effects of the Thyroid Hormone Metabolite 3-Iodothyroacetic Acid. Thyroid 2018, 28, 1387–1397. [Google Scholar] [CrossRef]
- Gerace, E.; Landucci, E.; Totti, A.; Bani, D.; Guasti, D.; Baronti, R.; Moroni, F.; Mannaioni, G.; Pellegrini-Giampietro, D.E. Ethanol Toxicity during Brain Development: Alterations of Excitatory Synaptic Transmission in Immature Organotypic Hippocampal Slice Cultures. Alcohol. Clin. Exp. Res. 2016, 40, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, X.; Wang, J. Organotypic Hippocampal Slices as Models for Stroke and Traumatic Brain Injury. Mol. Neurobiol. 2016, 53, 4226–4237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerace, E.; Landucci, E.; Scartabelli, T.; Moroni, F.; Pellegrini-Giampietro, D.E. Rat hippocampal slice culture models for the evaluation of neuroprotective agents. Methods Mol. Biol. 2012, 846, 343–354. [Google Scholar] [PubMed]
- Carloni, S.; Facchinetti, F.; Pelizzi, N.; Buonocore, G.; Balduini, W. Melatonin Acts in Synergy with Hypothermia to Reduce Oxygen-Glucose Deprivation-Induced Cell Death in Rat Hippocampus Organotypic Slice Cultures. Neonatology 2018, 114, 364–371. [Google Scholar] [CrossRef]
- Landucci, E.; Filippi, L.; Gerace, E.; Catarzi, S.; Guerrini, R.; Pellegrini-Giampietro, D.E. Neuroprotective effects of topiramate and memantine in combination with hypothermia in hypoxic-ischemic brain injury in vitro and in vivo. Neurosci. Lett. 2018, 668, 103–107. [Google Scholar] [CrossRef]
- Taniguchi, H.; Andreasson, K. The Hypoxic Ischemic Encephalopathy Model of Perinatal Ischemia. J. Vis. Exp. 2008, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Klöfers, M.; Kohaut, J.; Bendix, I.; Herz, J.; Boos, V.; Felderhoff-Müser, U.; Dzietko, M. Effects of Poly(ADP-Ribose) Polymerase-1 Inhibition in a Neonatal Rodent Model of Hypoxic-Ischemic Injury. Biomed Res. Int. 2017, 2017, 2924848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klahr, A.C.; Nadeau, C.A.; Colbourne, F. Temperature Control in Rodent Neuroprotection Studies: Methods and Challenges. Ther. Hypothermia Temp. Manag. 2017, 7, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Riddle, A.; Dean, J.; Hohimer, A.R. The Instrumented Fetal Sheep as a Model of Cerebral White Matter Injury in the Premature Infant. Neurotherapeutics 2012, 9, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Sabir, H.; Scull-Brown, E.; Liu, X.; Thoresen, M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats. Stroke 2012, 43, 3364–3370. [Google Scholar] [CrossRef] [PubMed]
- Rumajogee, P.; Bregman, T.; Miller, S.P.; Yager, J.Y.; Fehlings, M.G. Rodent Hypoxia–Ischemia Models for Cerebral Palsy Research: A Systematic Review. Front. Neurol. 2016, 7, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAdams, R.M.; Fleiss, B.; Traudt, C.; Schwendimann, L.; Snyder, J.M.; Haynes, R.L.; Natarajan, N.; Gressens, P.; Juul, S.E. Long-Term Neuropathological Changes Associated with Cerebral Palsy in a Nonhuman Primate Model of Hypoxic-Ischemic Encephalopathy. Dev. Neurosci. 2017, 39, 124–140. [Google Scholar] [CrossRef] [Green Version]
- Traudt, C.M.; McPherson, R.J.; Bauer, L.A.; Richards, T.L.; Burbacher, T.M.; McAdams, R.M.; Juul, S.E. Concurrent erythropoietin and hypothermia treatment improve outcomes in a term nonhuman primate model of perinatal asphyxia. Dev. Neurosci. 2013, 35, 491–503. [Google Scholar] [CrossRef] [Green Version]
- Drury, P.P.; Davidson, J.O.; van den Heuij, L.G.; Tan, S.; Silverman, R.B.; Ji, H.; Blood, A.B.; Fraser, M.; Bennet, L.; Gunn, A.J. Partial neuroprotection by nNOS inhibition during profound asphyxia in preterm fetal sheep. Exp. Neurol. 2013, 250, 282–292. [Google Scholar] [CrossRef] [Green Version]
- Davidson, J.O.; Yuill, C.A.; Zhang, F.G.; Wassink, G.; Bennet, L.; Gunn, A.J. Extending the duration of hypothermia does not further improve white matter protection after ischemia in term-equivalent fetal sheep. Sci. Rep. 2016, 6, 25178. [Google Scholar] [CrossRef] [Green Version]
- Derrick, M.; Drobyshevsky, A.; Ji, X.; Chen, L.; Yang, Y.; Ji, H.; Silverman, R.B.; Tan, S. Hypoxia-ischemia causes persistent movement deficits in a perinatal rabbit model of cerebral palsy: Assessed by a new swim test. Int. J. Dev. Neurosci. 2009, 27, 549–557. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Yu, L.; Drobyshevsky, A.; Ji, H.; Derrick, M.; Silverman, R.B.; Chen, L.; Yang, Y.; Tan, S.; Rao, S.; et al. Involvement of Neuronal Nitric Oxide Synthase in Ongoing Fetal Brain Injury following Near-Term Rabbit Hypoxia-Ischemia. Dev. Neurosci. 2011, 33, 288–298. [Google Scholar]
- Shi, Z.; Luo, K.; Jani, S.; February, M.; Fernandes, N.; Venkatesh, N.; Sharif, N.; Tan, S. Mimicking partial to total placental insufficiency in a rabbit model of cerebral palsy. J. Neurosci. Res. 2021, 24901. [Google Scholar] [CrossRef] [PubMed]
- Montero, M.; Poulsen, F.R.; Noraberg, J.; Kirkeby, A.; van Beek, J.; Leist, M.; Zimmer, J. Comparison of neuroprotective effects of erythropoietin (EPO) and carbamylerythropoietin (CEPO) against ischemia-like oxygen-glucose deprivation (OGD) and NMDA excitotoxicity in mouse hippocampal slice cultures. Exp. Neurol. 2007, 204, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Meloni, B.P.; Tilbrook, P.A.; Boulos, S.; Arthur, P.G.; Knuckey, N.W. Erythropoietin preconditioning in neuronal cultures: Signaling, protection from in vitro ischemia, and proteomic analysis. J. Neurosci. Res. 2006, 83, 584–593. [Google Scholar] [CrossRef]
- Iwai, M.; Cao, G.; Yin, W.; Stetler, R.A.; Liu, J.; Chen, J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke 2007, 38, 2795–2803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larpthaveesarp, A.; Georgevits, M.; Ferriero, D.M.; Gonzalez, F.F. Delayed erythropoietin therapy improves histological and behavioral outcomes after transient neonatal stroke. Neurobiol. Dis. 2016, 93, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Wood, T.R.; Vu, P.T.; Comstock, B.A.; Law, J.B.; Mayock, D.E.; Heagerty, P.J.; Burbacher, T.; Bammler, T.K.; Juul, S.E. Cytokine and chemokine responses to injury and treatment in a nonhuman primate model of hypoxic-ischemic encephalopathy treated with hypothermia and erythropoietin. J. Cereb. Blood Flow Metab. 2021, 41, 2054–2066. [Google Scholar] [CrossRef]
- Pang, R.; Avdic-Belltheus, A.; Meehan, C.; Martinello, K.; Mutshiya, T.; Yang, Q.; Sokolska, M.; Torrealdea, F.; Hristova, M.; Bainbridge, A.; et al. Melatonin and/or erythropoietin combined with hypothermia in a piglet model of perinatal asphyxia. Brain Commun. 2021, 3, fcaa211. [Google Scholar] [CrossRef]
- Wu, Y.W.; Mathur, A.M.; Chang, T.; McKinstry, R.C.; Mulkey, S.B.; Mayock, D.E.; Van Meurs, K.P.; Rogers, E.E.; Gonzalez, F.F.; Comstock, B.A.; et al. High-Dose Erythropoietin and Hypothermia for Hypoxic-Ischemic Encephalopathy: A Phase II Trial. Pediatrics 2016, 137, e20160191. [Google Scholar] [CrossRef] [Green Version]
- Juul, S.E.; Comstock, B.A.; Heagerty, P.J.; Mayock, D.E.; Goodman, A.M.; Hauge, S.; Gonzalez, F.; Wu, Y.W. High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL): A Randomized Controlled Trial-Background, Aims, and Study Protocol. Neonatology 2018, 113, 331–338. [Google Scholar] [CrossRef]
- Yang, G.; Xue, Z.; Zhao, Y. Efficacy of erythropoietin alone in treatment of neonates with hypoxic-ischemic encephalopathy: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e26365. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, N.; Wu, B.; Qiu, Y. Neuroprotective effect of memantine combined with topiramate in hypoxic-ischemic brain injury. Brain Res. 2009, 1282, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Fiorini, P.; Catarzi, S.; Berti, E.; Padrini, L.; Landucci, E.; Donzelli, G.; Bartalena, L.; Fiorentini, E.; Boldrini, A.; et al. Safety and efficacy of topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia (NeoNATI): A feasibility study. J. Matern. Fetal Neonatal Med. 2018, 31, 973–980. [Google Scholar] [CrossRef]
- Nuñez-Ramiro, A.; Benavente-Fernández, I.; Valverde, E.; Cordeiro, M.; Blanco, D.; Boix, H.; Cabañas, F.; Chaffanel, M.; Fernández-Colomer, B.; Fernández-Lorenzo, J.R.; et al. Topiramate plus Cooling for Hypoxic-Ischemic Encephalopathy: A Randomized, Controlled, Multicenter, Double-Blinded Trial. Neonatology 2019, 116, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Serrenho, I.; Rosado, M.; Dinis, A.; Cardoso, C.M.; Grãos, M.; Manadas, B.; Baltazar, G. Stem Cell Therapy for Neonatal Hypoxic-Ischemic Encephalopathy: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 3142. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.S.; Jurek, B.; Karimi-Haghighi, S.; Nezhad, N.J.; Mousavi, S.M.; Hooshmandi, E.; Safari, A.; Dianatpour, M.; Haerteis, S.; Miyan, J.A.; et al. Intranasal application of stem cells and their derivatives as a new hope in the treatment of cerebral hypoxia/ischemia: A review. Rev. Neurosci. 2022. [Google Scholar] [CrossRef]
- Park, Y.; Borlongan, C.; Dezawa, M. Cell-based treatment for perinatal hypoxic-ischemic encephalopathy. Brain Circ. 2021, 7, 13. [Google Scholar]
- Sato, Y.; Tsuji, M. Diverse actions of cord blood cell therapy for hypoxic-ischemic encephalopathy. Pediatr. Int. 2021, 63, 497–503. [Google Scholar] [CrossRef]
- Porrini, V.; Sarnico, I.; Benarese, M.; Branca, C.; Mota, M.; Lanzillotta, A.; Bellucci, A.; Parrella, E.; Faggi, L.; Spano, P.; et al. Neuroprotective and Anti-Apoptotic Effects of CSP-1103 in Primary Cortical Neurons Exposed to Oxygen and Glucose Deprivation. Int. J. Mol. Sci. 2017, 18, 184. [Google Scholar] [CrossRef] [Green Version]
- Faggi, L.; Pignataro, G.; Parrella, E.; Porrini, V.; Vinciguerra, A.; Cepparulo, P.; Cuomo, O.; Lanzillotta, A.; Mota, M.; Benarese, M.; et al. Synergistic Association of Valproate and Resveratrol Reduces Brain Injury in Ischemic Stroke. Int. J. Mol. Sci. 2018, 19, 172. [Google Scholar] [CrossRef] [Green Version]
- Secondo, A.; Petrozziello, T.; Tedeschi, V.; Boscia, F.; Vinciguerra, A.; Ciccone, R.; Pannaccione, A.; Molinaro, P.; Pignataro, G.; Annunziato, L. ORAI1/STIM1 Interaction Intervenes in Stroke and in Neuroprotection Induced by Ischemic Preconditioning through Store-Operated Calcium Entry. Stroke 2019, 50, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Baldassarro, V.A.; Marchesini, A.; Giardino, L.; Calza, L. Vulnerability of primary neurons derived from Tg2576 Alzheimer mice to oxygen and glucose deprivation: Role of intraneuronal amyloid-β accumulation and astrocytes. Dis. Model. Mech. 2017, 10, 671–678. [Google Scholar] [CrossRef] [Green Version]
- DE, P.-G.; A, C.; F, P.; P, L.; E, M.; R, P.; F, M. 1-Aminoindan-1,5-dicarboxylic acid and (S)-(+)-2-(3′-carboxybicyclo[1.1.1] pentyl)-glycine, two mGlu1 receptor-preferring antagonists, reduce neuronal death in in vitro and in vivo models of cerebral ischaemia. Eur. J. Neurosci. 1999, 11, 3637–3647. [Google Scholar]
- Meli, E.; Pangallo, M.; Picca, R.; Baronti, R.; Moroni, F.; Pellegrini-Giampietro, D.E. Differential role of poly(ADP-ribose) polymerase-1in apoptotic and necrotic neuronal death induced by mild or intense NMDA exposure in vitro. Mol. Cell. Neurosci. 2004, 25, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Enhanced Neuroprotective Effects of Panax Ginseng G115® and Ginkgo Biloba GK501® Combinations In Vitro Models of Excitotoxicity. Int. J. Mol. Sci. 2019, 20, 5872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, P.; Pinto, A.; Tamborini, L.; Madsen, U.; Nielsen, B.; Bräuner-Osborne, H.; Hansen, K.B.; Landucci, E.; Pellegrini-Giampietro, D.E.; De Sarro, G.; et al. Novel 3-Carboxy- and 3-Phosphonopyrazoline Amino Acids as Potent and Selective NMDA Receptor Antagonists: Design, Synthesis, and Pharmacological Characterization. ChemMedChem 2010, 5, 1465–1475. [Google Scholar] [CrossRef]
- Bigagli, E.; Luceri, C.; Scartabelli, T.; Dolara, P.; Casamenti, F.; Pellegrini-Giampietro, D.E.; Giovannelli, L. Long-term Neuroglial Cocultures as a Brain Aging Model: Hallmarks of Senescence, MicroRNA Expression Profiles, and Comparison with In Vivo Models. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Boscia, F.; Annunziato, L.; Taglialatela, M. Retigabine and flupirtine exert neuroprotective actions in organotypic hippocampal cultures. Neuropharmacology 2006, 51, 283–294. [Google Scholar] [CrossRef]
- Formisano, L.; Guida, N.; Valsecchi, V.; Pignataro, G.; Vinciguerra, A.; Pannaccione, A.; Secondo, A.; Boscia, F.; Molinaro, P.; Sisalli, M.J.; et al. NCX1 is a new rest target gene: Role in cerebral ischemia. Neurobiol. Dis. 2013, 50, 76–85. [Google Scholar] [CrossRef]
- Landucci, E.; Llorente, I.; Anuncibay-Soto, B.; Pellegrini-Giampietro, D.; Fernández-López, A. Using organotypic hippocampal slice cultures to gain insight into mechanisms responsible for the neuroprotective effects of meloxicam: A role for gamma aminobutyric and endoplasmic reticulum stress. Neural Regen. Res. 2019, 14, 65–66. [Google Scholar]
- Cerullo, P.; Brancaccio, P.; Anzilotti, S.; Vinciguerra, A.; Cuomo, O.; Fiorino, F.; Severino, B.; Di Vaio, P.; Di Renzo, G.; Annunziato, L.; et al. Acute and long-term NCX activation reduces brain injury and restores behavioral functions in mice subjected to neonatal brain ischemia. Neuropharmacology 2018, 135, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Borjini, N.; Sivilia, S.; Giuliani, A.; Fernandez, M.; Giardino, L.; Facchinetti, F.; Calzà, L. Potential biomarkers for neuroinflammation and neurodegeneration at short and long term after neonatal hypoxic-ischemic insult in rat. J. Neuroinflamm. 2019, 16, 194. [Google Scholar] [CrossRef] [PubMed]
- Carloni, S.; Balduini, W. Simvastatin preconditioning confers neuroprotection against hypoxia-ischemia induced brain damage in neonatal rats via autophagy and silent information regulator 1 (SIRT1) activation. Exp. Neurol. 2020, 324, 113117. [Google Scholar] [CrossRef] [PubMed]
- Balduini, W.; Carloni, S.; Perrone, S.; Bertrando, S.; Tataranno, M.L.; Negro, S.; Proietti, F.; Longini, M.; Buonocore, G. The use of melatonin in hypoxic-ischemic brain damage: An experimental study. J. Matern. Fetal Neonatal Med. 2012, 25 (Suppl. S1), 119–124. [Google Scholar] [CrossRef] [PubMed]
- Garofoli, F.; Longo, S.; Pisoni, C.; Accorsi, P.; Angelini, M.; Aversa, S.; Caporali, C.; Cociglio, S.; De Silvestri, A.; Fazzi, E.; et al. Oral melatonin as a new tool for neuroprotection in preterm newborns: Study protocol for a randomized controlled trial. Trials 2021, 22, 82. [Google Scholar] [CrossRef] [PubMed]
| Model | Cell/Tissue/Animal | Exposure | Strengths | Limitations |
|---|---|---|---|---|
| OGD in vitro | ||||
| Immortalized cell lines | NT2, SY5Y | 4 h OGD (95% N2, 5% CO2) | Simple, reproducible | Distant from normal CNS cells |
| Primary neuronal cultures | 7 DIV neurons from E17 rodents | 3 h OGD | CNS-like homogeneous cells | No neuron-glia interactions, low response to OGD |
| Mixed cortical cells | 21 DIV astrocytes from P1 mice+ 14 DIV neurons from E17 mice | 1 h OGD | Neuron–glia interactions, selective neuronal vulnerability to OGD | Artificial architecture |
| Organotypic hippocampal slices | 14 DIV slices from P8 rats | 30 min OGD | CNS-like structural and synaptic organization, CA1 vulnerability to OGD | No vessels |
| HIE in vivo | ||||
| Rice–Vannucci model | P7 rats or mice | MCAO + 2 h 92% N2, 8% O2 | Most convenient, cost-effective, and widely used to study effects of drugs and hypothermia | Lissencephalic brain, variability, mild neurological deficits |
| Piglet model | P2 piglets | 45 min 10% O2 | Gyrencephalic brain, i.v. drugs + hypothermia, accurate PK | No follow-up on neurological deficits, variability, requires ICU |
| Intra-uterine models | E29 rabbits | 40 min uterine ischemia | Dystonic hypertonia post-natally, complementary to rodents | Limited accessibility |
| Fetal sheep | BCAO or UCO | Hypothermia, intrauterine pathophysiology | Maternal/placenta metabolism, cost, and complexity | |
| Pre-term Macaca nemestrina | 15 min UCO | Hypothermia, cerebral palsy-like abnormalities | Cost and complexity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landucci, E.; Pellegrini-Giampietro, D.E.; Facchinetti, F. Experimental Models for Testing the Efficacy of Pharmacological Treatments for Neonatal Hypoxic-Ischemic Encephalopathy. Biomedicines 2022, 10, 937. https://doi.org/10.3390/biomedicines10050937
Landucci E, Pellegrini-Giampietro DE, Facchinetti F. Experimental Models for Testing the Efficacy of Pharmacological Treatments for Neonatal Hypoxic-Ischemic Encephalopathy. Biomedicines. 2022; 10(5):937. https://doi.org/10.3390/biomedicines10050937
Chicago/Turabian StyleLanducci, Elisa, Domenico E. Pellegrini-Giampietro, and Fabrizio Facchinetti. 2022. "Experimental Models for Testing the Efficacy of Pharmacological Treatments for Neonatal Hypoxic-Ischemic Encephalopathy" Biomedicines 10, no. 5: 937. https://doi.org/10.3390/biomedicines10050937
APA StyleLanducci, E., Pellegrini-Giampietro, D. E., & Facchinetti, F. (2022). Experimental Models for Testing the Efficacy of Pharmacological Treatments for Neonatal Hypoxic-Ischemic Encephalopathy. Biomedicines, 10(5), 937. https://doi.org/10.3390/biomedicines10050937







