Inflammatory Mechanisms of Diabetes and Its Vascular Complications
Abstract
1. Introduction
2. Inflammation in Insulin Resistance and Diabetes Mellitus
3. Macrophages in Type 2 Diabetes
4. The Role of Mitochondria in the Development and Progression of Type 2 Diabetes and Its Vascular Complications
5. Endothelial Dysfunction in Type 2 Diabetes and Its Vascular Complications
6. Current Therapeutic Strategies for the Treatment of Type 2 Diabetes and Its Vascular Complications
6.1. Biguanides
6.2. Alpha-Glucosidase Inhibitors
6.3. GLP-1 Agonists
6.4. PPAR Agonists
6.5. SGLT-2 Inhibitors
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IDF Diabetes Atlas. Tenth Edition. Available online: https://diabetesatlas.org/ (accessed on 22 February 2022).
- Bloomgarden, Z. Does Glycemic Control Affect Outcome of COVID-19? J. Diabetes 2020, 12, 868–869. [Google Scholar] [CrossRef] [PubMed]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of Type 1 and Type 2 Diabetes with COVID-19-Related Mortality in England: A Whole-Population Study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef]
- Rana, J.S.; Dunning, A.; Achenbach, S.; Al-Mallah, M.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; Chang, H.J.; Cheng, V.Y.; Chinnaiyan, K.; et al. Differences in Prevalence, Extent, Severity, and Prognosis of Coronary Artery Disease among Patients with and without Diabetes Undergoing Coronary Computed Tomography Angiography: Results from 10,110 Individuals from the CONFIRM (COronary CT Angiography Evaluation for Clinical Outcomes): An InteRnational Multicenter Registry. Diabetes Care 2012, 35, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and Vascular Disease: Pathophysiology, Clinical Consequences, and Medical Therapy: Part I. Eur. Heart J. 2013, 34, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Marinopoulos, S.; Berkenblit, G.; Rami, T.; Brancati, F.L.; Powe, N.R.; Golden, S.H. Meta-Analysis: Glycosylated Hemoglobin and Cardiovascular Disease in Diabetes Mellitus. Ann. Intern. Med. 2004, 141, 421–431. [Google Scholar] [CrossRef]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef]
- Santilli, F.; D’Ardes, D.; Davì, G. Oxidative Stress in Chronic Vascular Disease: From Prediction to Prevention. Vasc. Pharm. 2015, 74, 23–37. [Google Scholar] [CrossRef]
- Williamson, R.T. On the Treatment of Glycosuria and Diabetes Mellitus with Sodium Salicylate. Br. Med. J. 1901, 1, 760–762. [Google Scholar] [CrossRef]
- Reid, J.; Macdougall, A.I.; Andrews, M.M. Aspirin and Diabetes Mellitus. Br. Med. J. 1957, 2, 1071–1074. [Google Scholar] [CrossRef][Green Version]
- Shimobayashi, M.; Albert, V.; Woelnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin Resistance Causes Inflammation in Adipose Tissue. J. Clin. Investig. 2018, 128, 1538–1550. [Google Scholar] [CrossRef]
- Johnson, A.M.F.; Olefsky, J.M. The Origins and Drivers of Insulin Resistance. Cell 2013, 152, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Skeletal Muscle Inflammation and Insulin Resistance in Obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, A.; de Souza, C.N.; Saraiva Câmara, N.O.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2016, 6, 637. [Google Scholar] [CrossRef] [PubMed]
- Drareni, K.; Gautier, J.F.; Venteclef, N.; Alzaid, F. Transcriptional Control of Macrophage Polarisation in Type 2 Diabetes. Semin. Immunopathol. 2019, 41, 515–529. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Ozawa, K.; Miyazaki, M.; Matsuhisa, M.; Takano, K.; Nakatani, Y.; Hatazaki, M.; Tamatani, T.; Yamagata, K.; Miyagawa, J.I.; Kitao, Y.; et al. The Endoplasmic Reticulum Chaperone Improves Insulin Resistance in Type 2 Diabetes. Diabetes 2005, 54, 657–663. [Google Scholar] [CrossRef]
- Lemmer, I.L.; Willemsen, N.; Hilal, N.; Bartelt, A. A Guide to Understanding Endoplasmic Reticulum Stress in Metabolic Disorders. Mol. Metab. 2021, 47, 101169. [Google Scholar] [CrossRef]
- Lin, Y.; Berg, A.H.; Iyengar, P.; Lam, T.K.T.; Giacca, A.; Combs, T.P.; Rajala, M.W.; Du, X.; Rollman, B.; Li, W.; et al. The Hyperglycemia-Induced Inflammatory Response in Adipocytes: The Role of Reactive Oxygen Species. J. Biol. Chem. 2005, 280, 4617–4626. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Markina, Y.V.; Sukhorukov, V.N.; Khotina, V.A.; Wu, W.K.; Orekhov, A.N. A Novel Insight at Atherogenesis: The Role of Microbiome. Front. Cell Dev. Biol. 2020, 8, 586189. [Google Scholar] [CrossRef]
- Tanti, J.F.; Grémeaux, T.; van Obberghen, E.; le Marchand-Brustel, Y. Serine/Threonine Phosphorylation of Insulin Receptor Substrate 1 Modulates Insulin Receptor Signaling. J. Biol. Chem. 1994, 269, 6051–6057. [Google Scholar] [CrossRef]
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Biochemical and Cellular Properties of Insulin Receptor Signalling. Nat. Rev. Mol. Cell Biol. 2018, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-like Receptors in Innate Immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yaglova, N.V.; Obernikhin, S.S.; Yaglov, V.V.; Nazimova, S.V. Role of Skin Dendritic and Mast Cells Communications in Triggering Immune Reactions. Clin. Exp. Morphol. 2021, 10, 5–10. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Taylor, E.B. The Complex Role of Adipokines in Obesity, Inflammation, and Autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef]
- Leal, V.d.O.; Mafra, D. Adipokines in Obesity. Clin. Chim. Acta 2013, 419, 87–94. [Google Scholar] [CrossRef]
- Kraakman, M.J.; Murphy, A.J.; Jandeleit-Dahm, K.; Kammoun, H.L. Macrophage Polarization in Obesity and Type 2 Diabetes: Weighing down Our Understanding of Macrophage Function? Front. Immunol. 2014, 5, 470. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and Insulin Resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Herck, M.A.V.; Weyler, J.; Kwanten, W.J.; Dirinck, E.L.; Winter, B.Y.D.; Francque, S.M.; Vonghia, L. The Differential Roles of T Cells in Non-Alcoholic Fatty Liver Disease and Obesity. Front. Immunol. 2019, 10, 82. [Google Scholar] [CrossRef]
- Vinué, Á.; Herrero-Cervera, A.; González-Navarro, H. Understanding the Impact of Dietary Cholesterol on Chronic Metabolic Diseases through Studies in Rodent Models. Nutrients 2018, 10, 939. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to Diabetes Mellitus, Cardiovascular Disease or Cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Vonghia, L.; van Herck, M.A.; Weyler, J.; Francque, S. Targeting Myeloid-Derived Cells: New Frontiers in the Treatment of Non-Alcoholic and Alcoholic Liver Disease. Front. Immunol. 2019, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Liu, M.; Guo, W.; Yu, L. Microglia NLRP3 Inflammasomes Activation Involving Diabetic Neuroinflammation in Diabetic Mice and BV2 Cells. Curr. Pharm. Des. 2021, 27, 2802–2816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Deng, C.; Han, Q.; Xu, H.; Chen, Y. Carvacrol May Alleviate Vascular Inflammation in Diabetic Db/Db Mice. Int. J. Mol. Med. 2020, 46, 977–988. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Saito, T.; Ogihara, T.; Ishigaki, Y.; Yamada, T.; Imai, J.; Uno, K.; Gao, J.; Kaneko, K.; Shimosawa, T.; et al. Blockade of the Nuclear Factor-ΚB Pathway in the Endothelium Prevents Insulin Resistance and Prolongs Life Spans. Circulation 2012, 125, 1122–1133. [Google Scholar] [CrossRef]
- Wang, A.; Gong, Y.; Pei, Z.; Jiang, L.; Xia, L.; Wu, Y. Paeoniflorin Ameliorates Diabetic Liver Injury by Targeting the TXNIP-Mediated NLRP3 Inflammasome in Db/Db Mice. Int. Immunopharmacol. 2022, 109, 108792. [Google Scholar] [CrossRef]
- Kampschulte, M.; Stöckl, C.; Langheinrich, A.C.; Althöhn, U.; Bohle, R.M.; Krombach, G.A.; Stieger, P.; Churin, Y.; Kremer, S.; Dierkes, C.; et al. Western Diet in ApoE-LDLR Double-Deficient Mouse Model of Atherosclerosis Leads to Hepatic Steatosis, Fibrosis, and Tumorigenesis. Lab. Investig. 2014, 94, 1273–1282. [Google Scholar] [CrossRef]
- Schneider, J.L.; Suh, Y.; Cuervo, A.M. Deficient Chaperone-Mediated Autophagy in Liver Leads to Metabolic Dysregulation. Cell Metab. 2014, 20, 417–432. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Boutens, L.; Stienstra, R. Adipose Tissue Macrophages: Going off Track during Obesity. Diabetologia 2016, 59, 879–894. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 Links Innate Immunity and Fatty Acid-Induced Insulin Resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.C.; Hwang, I.; Choe, S.S.; Park, J.; Ji, Y.; Kim, J.I.; Lee, G.Y.; Choi, S.H.; Ching, J.; Kovalik, J.P.; et al. Macrophage VLDLR Mediates Obesity-Induced Insulin Resistance with Adipose Tissue Inflammation. Nat. Commun. 2017, 8, 1087. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, C.M.; Hövelmeyer, N.; Wunderlich, F.T. Mechanisms of Chronic JAK-STAT3-SOCS3 Signaling in Obesity. JAKSTAT 2013, 2, e23878. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-Mediated Inhibition of Insulin Receptor Tyrosine Kinase Activity in TNF-Alpha- and Obesity-Induced Insulin Resistance. Science 1996, 271, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Pessin, J.E. Insulin Signaling Pathways in Time and Space. Trends Cell Biol. 2002, 12, 65–71. [Google Scholar] [CrossRef]
- Aguirre, V.; Werner, E.D.; Giraud, J.; Lee, Y.H.; Shoelson, S.E.; White, M.F. Phosphorylation of Ser307 in Insulin Receptor Substrate-1 Blocks Interactions with the Insulin Receptor and Inhibits Insulin Action. J. Biol. Chem. 2002, 277, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Jager, J.; Grémeaux, T.; Cormont, M.; le Marchand-Brustel, Y.; Tanti, J.F. Interleukin-1beta-Induced Insulin Resistance in Adipocytes through down-Regulation of Insulin Receptor Substrate-1 Expression. Endocrinology 2007, 148, 241. [Google Scholar] [CrossRef]
- Simsek, S.; van den Oever, I.A.M.; Raterman, H.G.; Nurmohamed, M.T. Endothelial Dysfunction, Inflammation, and Apoptosis in Diabetes Mellitus. Mediat. Inflamm. 2010, 2010, 15. [Google Scholar] [CrossRef]
- Pangare, M.; Makino, A. Mitochondrial Function in Vascular Endothelial Cell in Diabetes. J. Smooth Muscle Res. 2012, 48, 1–26. [Google Scholar] [CrossRef]
- Tang, X.; Luo, Y.X.; Chen, H.Z.; Liu, D.P. Mitochondria, Endothelial Cell Function, and Vascular Diseases. Front. Physiol. 2014, 5, 175. [Google Scholar] [CrossRef]
- Salnikova, D.; Orekhova, V.; Grechko, A.; Starodubova, A.; Bezsonov, E.; Popkova, T.; Orekhov, A. Mitochondrial Dysfunction in Vascular Wall Cells and Its Role in Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 8990. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Talaverón-Rey, M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; Sánchez-Alcázar, J.A. From Mitochondria to Atherosclerosis: The Inflammation Path. Biomedicines 2021, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Markin, A.M.; Khotina, V.A.; Zabudskaya, X.G.; Bogatyreva, A.I.; Starodubova, A.V.; Ivanova, E.; Nikiforov, N.G.; Orekhov, A.N. Disturbance of Mitochondrial Dynamics and Mitochondrial Therapies in Atherosclerosis. Life 2021, 11, 165. [Google Scholar] [CrossRef]
- Newsholme, P.; Haber, E.P.; Hirabara, S.M.; Rebelato, E.L.O.; Procopio, J.; Morgan, D.; Oliveira-Emilio, H.C.; Carpinelli, A.R.; Curi, R. Diabetes Associated Cell Stress and Dysfunction: Role of Mitochondrial and Non-Mitochondrial ROS Production and Activity. J. Physiol. 2007, 583, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Gaudel, C.; Krause, M. Mitochondria and Diabetes. An Intriguing Pathogenetic Role. Adv. Exp. Med. Biol. 2012, 942, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Ngo, J.; Osto, C.; Villalobos, F.; Shirihai, O.S. Mitochondrial Heterogeneity in Metabolic Diseases. Biology 2021, 10, 927. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; Hernandez-Mijares, A.; Rocha, M.; Victor, V.M. Mitochondrial Dynamics in Type 2 Diabetes: Pathophysiological Implications. Redox Biol. 2017, 11, 637–645. [Google Scholar] [CrossRef]
- Wada, J.; Nakatsuka, A. Mitochondrial Dynamics and Mitochondrial Dysfunction in Diabetes. Acta Med. Okayama 2016, 70, 151–158. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Jaiswal, S.K.; Liu, N.F. Mitochondria Homeostasis and Vascular Medial Calcification. Calcif. Tissue Int. 2021, 109, 113–120. [Google Scholar] [CrossRef]
- Permana Maksum, I.; Saputra, S.R.; Indrayati, N.; Yusuf, M.; Subroto, T. Bioinformatics Study of m.9053G>A Mutation at the ATP6 Gene in Relation to Type 2 Diabetes Mellitus and Cataract Diseases. Bioinform. Biol. Insights 2017, 11, 1177932217728515. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Ragino, Y.I.; Voevoda, M.I.; Urazalina, S.J.; Khasanova, Z.B.; Orekhova, V.A.; Sinyov, V.V.; Sazonova, M.A.; Orekhov, A.N.; Sobenin, I.A. Data on Association of Mitochondrial Heteroplasmy with Carotid Intima-Media Thickness in Subjects from Russian and Kazakh Populations. Data Brief 2020, 29, 105136. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, T.V.; Ryzhkova, A.I.; Sinyov, V.V.; Sazonova, M.D.; Orekhova, V.A.; Karagodin, V.P.; Gerasimova, E.V.; Voevoda, M.I.; Orekhov, A.N.; Ragino, Y.I.; et al. Impact of Mitochondrial DNA Mutations on Carotid Intima-Media Thickness in the Novosibirsk Region. Life 2020, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Markin, A.M.; Sobenin, I.A.; Grechko, A.V.; Zhang, D.; Orekhov, A.N. Cellular Mechanisms of Human Atherogenesis: Focus on Chronification of Inflammation and Mitochondrial Mutations. Front. Pharm. 2020, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Kytövuori, L.; Lipponen, J.; Rusanen, H.; Komulainen, T.; Martikainen, M.H.; Majamaa, K. A Novel Mutation m.8561C>G in MT-ATP6/8 Causing a Mitochondrial Syndrome with Ataxia, Peripheral Neuropathy, Diabetes Mellitus, and Hypergonadotropic Hypogonadism. J. Neurol. 2016, 263, 2188–2195. [Google Scholar] [CrossRef]
- Maude, H.; Lau, W.; Maniatis, N.; Andrew, T. New Insights into Mitochondrial Dysfunction at Disease Susceptibility Loci in the Development of Type 2 Diabetes. Front. Endocrinol. 2021, 12, 694893. [Google Scholar] [CrossRef]
- Hasheminasabgorji, E.; Jha, J.C. Dyslipidemia, Diabetes and Atherosclerosis: Role of Inflammation and ROS-Redox-Sensitive Factors. Biomedicines 2021, 9, 1602. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Jing, L.L.; Yan, J.C.; Sun, Z.; Bao, Z.Y.; Shao, C.; Pang, Q.W.; Geng, Y.; Zhang, L.L.; Li, L.H. Role of AGEs in the Progression and Regression of Atherosclerotic Plaques. Glycoconj. J. 2018, 35, 443–450. [Google Scholar] [CrossRef]
- Markina, Y.V.; Gerasimova, E.V.; Markin, A.M.; Glanz, V.Y.; Wu, W.K.; Sobenin, I.A.; Orekhov, A.N. Sialylated Immunoglobulins for the Treatment of Immuno-Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 5472. [Google Scholar] [CrossRef]
- Bubb, K.J.; Drummond, G.R.; Figtree, G.A. New Opportunities for Targeting Redox Dysregulation in Cardiovascular Disease. Cardiovasc. Res. 2020, 116, 532–544. [Google Scholar] [CrossRef]
- Ling, P.; Shan, W.; Zhai, G.; Qiu, C.; Liu, Y.; Xu, Y.; Yang, X. Association between Glutathione Peroxidase-3 Activity and Carotid Atherosclerosis in Patients with Type 2 Diabetes Mellitus. Brain Behav. 2020, 10, e01773. [Google Scholar] [CrossRef]
- Yamagishi, S.; Matsui, T. Role of Hyperglycemia-Induced Advanced Glycation End Product (AGE) Accumulation in Atherosclerosis. Ann. Vasc. Dis. 2018, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M.; Kuusisto, J. Insulin Resistance and Hyperglycaemia in Cardiovascular Disease Development. Nat. Rev. Endocrinol. 2014, 10, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Ji, H.H.; Li, Y.J.; Guo, S.D. Macrophage Plasticity and Atherosclerosis Therapy. Front. Mol. Biosci. 2021, 8, 679797. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Markin, A.M.; Markina, Y.V.; Sukhorukov, V.N.; Khaylov, A.M.; Orekhov, A.N. The Role of Physical Activity in the Development of Atherosclerotic Lesions of the Vascular Wall. Clin. Exp. Morphol. 2019, 8, 25–31. [Google Scholar] [CrossRef]
- Aberdeen, H.; Battles, K.; Taylor, A.; Garner-Donald, J.; Davis-Wilson, A.; Rogers, B.T.; Cavalier, C.; Williams, E.D. The Aging Vasculature: Glucose Tolerance, Hypoglycemia and the Role of the Serum Response Factor. J. Cardiovasc. Dev. Dis. 2021, 8, 58. [Google Scholar] [CrossRef]
- Luna, P.; Guarner, V.; Farías, J.M.; Hernández-Pacheco, G.; Martínez, M. Importance of Metabolic Memory in the Development of Vascular Complications in Diabetic Patients. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1369–1378. [Google Scholar] [CrossRef]
- Colliva, A.; Braga, L.; Giacca, M.; Zacchigna, S. Endothelial Cell-Cardiomyocyte Crosstalk in Heart Development and Disease. J. Physiol. 2020, 598, 2923–2939. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Li, S.; Lv, J. Endothelial Dysfunction and Diabetic Cardiomyopathy. Front. Endocrinol. 2022, 13, 851941. [Google Scholar] [CrossRef]
- Horton, W.B.; Barrett, E.J. Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease. Endocr. Rev. 2021, 42, 29–55. [Google Scholar] [CrossRef]
- Salvador, D.B.; Gamba, M.R.; Gonzalez-Jaramillo, N.; Gonzalez-Jaramillo, V.; Raguindin, P.F.N.; Minder, B.; Gräni, C.; Wilhelm, M.; Stettler, C.; Doria, A.; et al. Diabetes and Myocardial Fibrosis: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2022, 15, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Haque, M. Insulin Resistance Is Cheerfully Hitched with Hypertension. Life 2022, 12, 564. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Iacoviello, M. Diabetes Leading to Heart Failure and Heart Failure Leading to Diabetes: Epidemiological and Clinical Evidence. Heart Fail. Rev. 2022, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II Diabetes Mellitus: A Review on Recent Drug Based Therapeutics. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef]
- Drzewoski, J.; Hanefeld, M. The Current and Potential Therapeutic Use of Metformin—The Good Old Drug. Pharmaceuticals 2021, 14, 122. [Google Scholar] [CrossRef]
- Malin, S.K.; Stewart, N.R. Metformin May Contribute to Inter-Individual Variability for Glycemic Responses to Exercise. Front. Endocrinol. 2020, 11, 519. [Google Scholar] [CrossRef]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical Use in Type 2 Diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Tan, S.Y.; Mei Wong, J.L.; Sim, Y.J.; Wong, S.S.; Mohamed Elhassan, S.A.; Tan, S.H.; Ling Lim, G.P.; Rong Tay, N.W.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 Diabetes Mellitus: A Review on Current Treatment Approach and Gene Therapy as Potential Intervention. Diabetes Metab. Syndr. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Wehmeier, U.F.; Piepersberg, W. Biotechnology and Molecular Biology of the α-Glucosidase Inhibitor Acarbose. Appl. Microbiol. Biotechnol. 2004, 63, 613–625. [Google Scholar] [CrossRef]
- Gribble, F.M.; Meek, C.L.; Reimann, F. Targeted Intestinal Delivery of Incretin Secretagogues-towards New Diabetes and Obesity Therapies. Peptides 2018, 100, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An Overview on the Role of Bioactive α-Glucosidase Inhibitors in Ameliorating Diabetic Complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Yokoyama, H.; Yamashita, R.; Sato, T.; Hosoba, M.; Morii, T.; Fujita, H.; Tsukiyama, K.; Yamada, Y. Comparisons of the Effects of 12-Week Administration of Miglitol and Voglibose on the Responses of Plasma Incretins after a Mixed Meal in Japanese Type 2 Diabetic Patients. Diabetes Obes. Metab. 2012, 14, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P. α-Glucosidase Inhibitors and Their Use in Clinical Practice. Arch. Med. Sci. 2012, 8, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 Receptor Agonists in the Treatment of Type 2 Diabetes—State-of-the-Art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Htike, Z.Z.; Zaccardi, F.; Papamargaritis, D.; Webb, D.R.; Khunti, K.; Davies, M.J. Efficacy and Safety of Glucagon-like Peptide-1 Receptor Agonists in Type 2 Diabetes: A Systematic Review and Mixed-Treatment Comparison Analysis. Diabetes Obes. Metab. 2017, 19, 524–536. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycaemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018, 61, 2461–2498. [Google Scholar] [CrossRef]
- Meier, J.J. GLP-1 Receptor Agonists for Individualized Treatment of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742. [Google Scholar] [CrossRef]
- Drucker, D.J.; Buse, J.B.; Taylor, K.; Kendall, D.M.; Trautmann, M.; Zhuang, D.; Porter, L. Exenatide Once Weekly versus Twice Daily for the Treatment of Type 2 Diabetes: A Randomised, Open-Label, Non-Inferiority Study. Lancet 2008, 372, 1240–1250. [Google Scholar] [CrossRef]
- Uppal, S.; Italiya, K.S.; Chitkara, D.; Mittal, A. Nanoparticulate-Based Drug Delivery Systems for Small Molecule Anti-Diabetic Drugs: An Emerging Paradigm for Effective Therapy. Acta Biomater. 2018, 81, 20–42. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Management of endocrine disease: Are All GLP-1 Agonists Equal in the Treatment of Type 2 Diabetes? Eur. J. Endocrinol. 2019, 181, R211–R234. [Google Scholar] [CrossRef] [PubMed]
- Alharby, H.; Abdelati, T.; Rizk, M.; Youssef, E.; Gaber, N.; Moghazy, K.; Yafei, S. Association of Fasting Glucagon-like Peptide-1 with Oxidative Stress and Subclinical Atherosclerosis in Type 2 Diabetes. Diabetes Metab. Syndr. 2019, 13, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Crepaldi, G.; Carruba, M.; Comaschi, M.; del Prato, S.; Frajese, G.; Paolisso, G. Dipeptidyl Peptidase 4 (DPP-4) Inhibitors and Their Role in Type 2 Diabetes Management. J. Endocrinol. Investig. 2007, 30, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B.; Schmitz, O. GLP-1 Receptor Agonists and DPP-4 Inhibitors in the Treatment of Type 2 Diabetes. Horm. Metab. Res. Horm. Und Stoffwechs. Horm. Metab. 2004, 36, 867–876. [Google Scholar] [CrossRef]
- Deacon, C.F.; Ahrén, B.; Holst, J.J. Inhibitors of Dipeptidyl Peptidase IV: A Novel Approach for the Prevention and Treatment of Type 2 Diabetes? Expert Opin. Investig. Drugs 2004, 13, 1091–1102. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Maiorino, M.I.; Bellastella, G.; Capuano, A.; Giugliano, D. Glycaemic Durability with Dipeptidyl Peptidase-4 Inhibitors in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Long-Term Randomised Controlled Trials. BMJ Open 2014, 4, e005442. [Google Scholar] [CrossRef]
- Aroda, V.R.; Henry, R.R.; Han, J.; Huang, W.; DeYoung, M.B.; Darsow, T.; Hoogwerf, B.J. Efficacy of GLP-1 Receptor Agonists and DPP-4 Inhibitors: Meta-Analysis and Systematic Review. Clin. Ther. 2012, 34, 1247–1258.e22. [Google Scholar] [CrossRef]
- Samms, R.J.; Christe, M.E.; Collins, K.A.L.; Pirro, V.; Droz, B.A.; Holland, A.K.; Friedrich, J.L.; Wojnicki, S.; Konkol, D.L.; Cosgrove, R.; et al. GIPR Agonism Mediates Weight-Independent Insulin Sensitization by Tirzepatide in Obese Mice. J. Clin. Investig. 2021, 131, e146353. [Google Scholar] [CrossRef]
- Campbell, J.E. Targeting the GIPR for Obesity: To Agonize or Antagonize? Potential Mechanisms. Mol. Metab. 2021, 46, 101139. [Google Scholar] [CrossRef]
- Matza, L.S.; Stewart, K.D.; Landó, L.F.; Patel, H.; Boye, K.S. Exit Interviews Examining the Patient Experience in Clinical Trials of Tirzepatide for Treatment of Type 2 Diabetes. Patient 2022, 15, 367–377. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A.F.H. The Evolving Story of Incretins (GIP and GLP-1) in Metabolic and Cardiovascular Disease: A Pathophysiological Update. Diabetes Obes. Metab. 2021, 23 (Suppl. S3), 5–29. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, N.; al Bratty, M.; Javed, S.A.; Ahsan, W.; Alhazmi, H.A. Targeting Peroxisome Proliferator-Activated Receptors Using Thiazolidinediones: Strategy for Design of Novel Antidiabetic Drugs. Int. J. Med. Chem. 2017, 2017, 1069718. [Google Scholar] [CrossRef] [PubMed]
- Kroker, A.J.; Bruning, J.B. Review of the Structural and Dynamic Mechanisms of PPARγ Partial Agonism. PPAR Res. 2015, 2015, 1069718. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B. Peroxisome Proliferator-Activated Receptors and Their Ligands: Nutritional and Clinical Implications—A Review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef]
- Lamichane, S.; Lamichane, B.D.; Kwon, S.M. Pivotal Roles of Peroxisome Proliferator-Activated Receptors (PPARs) and Their Signal Cascade for Cellular and Whole-Body Energy Homeostasis. Int. J. Mol. Sci. 2018, 19, 949. [Google Scholar] [CrossRef]
- Zoete, V.; Grosdidier, A.; Michielin, O. Peroxisome Proliferator-Activated Receptor Structures: Ligand Specificity, Molecular Switch and Interactions with Regulators. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2007, 1771, 915–925. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diabetes Rep. 2019, 19, 151. [Google Scholar] [CrossRef]
- Hsueh, W.A.; Law, R. The Central Role of Fat and Effect of Peroxisome Proliferator-Activated Receptor–γ on Progression of Insulin Resistance and Cardiovascular Disease. Am. J. Cardiol. 2003, 92, 3–9. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Parolari, A.; Myasoedova, V.; Melnichenko, A.A.; Bobryshev, Y.V.; Orekhov, A.N. Peroxisome Proliferator-Activated Receptor (PPAR) Gamma in Cardiovascular Disorders and Cardiovascular Surgery. J. Cardiol. 2015, 66, 271–278. [Google Scholar] [CrossRef]
- Ricote, M.; Glass, C.K. PPARs and Molecular Mechanisms of Transrepression. Biochim. Biophys. Acta 2007, 1771, 926. [Google Scholar] [CrossRef]
- Krishnaswami, A.; Ravi-Kumar, S.; Lewis, J.M. Thiazolidinediones: A 2010 Perspective. Perm. J. 2010, 14, 64–72. [Google Scholar] [CrossRef] [PubMed]
- McGuire, D.K.; Inzucchi, S.E. New Drugs for the Treatment of Diabetes Mellitus: Part I: Thiazolidinediones and Their Evolving Cardiovascular Implications. Circulation 2008, 117, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 Diabetes: Principles of Pathogenesis and Therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Ahmed, I.; Furlong, K.; Flood, J.; Treat, V.P.; Goldstein, B.J. Dual PPAR Alpha/Gamma Agonists: Promises and Pitfalls in Type 2 Diabetes. Am. J. Ther. 2007, 14, 49–62. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Althagafi, I.I.; Shamshad, H. Role of PPAR Receptor in Different Diseases and Their Ligands: Physiological Importance and Clinical Implications. Eur. J. Med. Chem. 2019, 166, 502–513. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Katakami, N.; Furukado, S.; Kitagawa, K.; Nagatsuka, K.; Kashiwagi, A.; Daida, H.; Kawamori, R.; Kaku, K. Long-Term Effects of Pioglitazone on Carotid Atherosclerosis in Japanese Patients with Type 2 Diabetes without a Recent History of Macrovascular Morbidity. J. Atheroscler. Thromb. 2010, 17, 1132–1140. [Google Scholar] [CrossRef]
- Saremi, A.; Schwenke, D.C.; Buchanan, T.A.; Hodis, H.N.; MacK, W.J.; Banerji, M.; Bray, G.A.; Clement, S.C.; Henry, R.R.; Kitabchi, A.E.; et al. Pioglitazone Slows Progression of Atherosclerosis in Prediabetes Independent of Changes in Cardiovascular Risk Factors. Arter. Thromb Vasc. Biol. 2013, 33, 393. [Google Scholar] [CrossRef]
- Tilinca, M.C.; Tiuca, R.A.; Tilea, I.; Varga, A. The Sglt-2 Inhibitors in Personalized Therapy of Diabetes Mellitus Patients. J. Pers. Med. 2021, 11, 1249. [Google Scholar] [CrossRef]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An Update on Sodium-Glucose Co-Transporter-2 Inhibitors for the Treatment of Diabetes Mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef]
- Brown, E.; Rajeev, S.P.; Cuthbertson, D.J.; Wilding, J.P.H. A Review of the Mechanism of Action, Metabolic Profile and Haemodynamic Effects of Sodium-Glucose Co-Transporter-2 Inhibitors. Diabetes Obes. Metab. 2019, 21 (Suppl. S2), 9–18. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.J.; Eriksson, J.W. Emerging Role of SGLT-2 Inhibitors for the Treatment of Obesity. Drugs 2019, 79, 219. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Pharmacodynamics, Efficacy and Safety of Sodium-Glucose Co-Transporter Type 2 (SGLT2) Inhibitors for the Treatment of Type 2 Diabetes Mellitus. Drugs 2015, 75, 33–59. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.M.; Alsahli, M.; Gerich, J.E. SGLT2 Inhibitors in the Treatment of Type 2 Diabetes. Diabetes Res. Clin. Pract. 2014, 104, 297–322. [Google Scholar] [CrossRef] [PubMed]
- Lambers Heerspink, H.J.; de Zeeuw, D.; Wie, L.; Leslie, B.; List, J. Dapagliflozin a Glucose-Regulating Drug with Diuretic Properties in Subjects with Type 2 Diabetes. Diabetes Obes. Metab. 2013, 15, 853. [Google Scholar] [CrossRef]
- Sjöström, C.D.; Hashemi, M.; Sugg, J.; Ptaszynska, A.; Johnsson, E. Dapagliflozin-Induced Weight Loss Affects 24-Week Glycated Haemoglobin and Blood Pressure Levels. Diabetes Obes. Metab. 2015, 17, 809–812. [Google Scholar] [CrossRef]
- Tikkanen, I.; Narko, K.; Zeller, C.; Green, A.; Salsali, A.; Broedl, U.C.; Woerle, H.J. Empagliflozin Reduces Blood Pressure in Patients with Type 2 Diabetes and Hypertension. Diabetes Care 2015, 38, 420–428. [Google Scholar] [CrossRef]
- Kaplan, A.; Abidi, E.; El-Yazbi, A.; Eid, A.; Booz, G.W.; Zouein, F.A. Direct Cardiovascular Impact of SGLT2 Inhibitors: Mechanisms and Effects. Heart Fail. Rev. 2018, 23, 419–437. [Google Scholar] [CrossRef]
- Ojima, A.; Matsui, T.; Nishino, Y.; Nakamura, N.; Yamagishi, S. Empagliflozin, an Inhibitor of Sodium-Glucose Cotransporter 2 Exerts Anti-Inflammatory and Antifibrotic Effects on Experimental Diabetic Nephropathy Partly by Suppressing AGEs-Receptor Axis. Horm. Metab. Res. 2015, 47, 686–692. [Google Scholar] [CrossRef]
- Kothari, V.; Galdo, J.A.; Mathews, S.T. Hypoglycemic Agents and Potential Anti-Inflammatory Activity. J. Inflamm. Res. 2016, 9, 27–38. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.H.; Shah, K.; Mitchell, S.J.; Cho, K.; Hoe, H.S. The Anti-Diabetic Drug Gliquidone Modulates Lipopolysaccharide-Mediated Microglial Neuroinflammatory Responses by Inhibiting the NLRP3 Inflammasome. Front. Aging Neurosci. 2021, 13, 754123. [Google Scholar] [CrossRef] [PubMed]
- Jahan, H.; Choudhary, M.I. Gliclazide Alters Macrophages Polarization State in Diabetic Atherosclerosis in Vitro via Blocking AGE-RAGE/TLR4-Reactive Oxygen Species-Activated NF-Kβ Nexus. Eur. J. Pharm. 2021, 894, 173874. [Google Scholar] [CrossRef]
- Postler, T.S.; Peng, V.; Bhatt, D.M.; Ghosh, S. Metformin Selectively Dampens the Acute Inflammatory Response through an AMPK-Dependent Mechanism. Sci. Rep. 2021, 11, 18721. [Google Scholar] [CrossRef] [PubMed]
- Kristófi, R.; Eriksson, J.W. Metformin as an Anti-Inflammatory Agent: A Short Review. J. Endocrinol. 2021, 251, R11–R22. [Google Scholar] [CrossRef] [PubMed]
- Zangiabadian, M.; Nejadghaderi, S.A.; Zahmatkesh, M.M.; Hajikhani, B.; Mirsaeidi, M.; Nasiri, M.J. The Efficacy and Potential Mechanisms of Metformin in the Treatment of COVID-19 in the Diabetics: A Systematic Review. Front. Endocrinol. 2021, 12, 645194. [Google Scholar] [CrossRef] [PubMed]
- Bassaganya-Riera, J.; Song, R.; Roberts, P.C.; Hontecillas, R. PPAR-Gamma Activation as an Anti-Inflammatory Therapy for Respiratory Virus Infections. Viral Immunol. 2010, 23, 343–352. [Google Scholar] [CrossRef]
- Klimcakova, E.; Moro, C.; Mazzucotelli, A.; Lolmède, K.; Viguerie, N.; Galitzky, J.; Stich, V.; Langin, D. Profiling of Adipokines Secreted from Human Subcutaneous Adipose Tissue in Response to PPAR Agonists. Biochem. Biophys. Res. Commun. 2007, 358, 897–902. [Google Scholar] [CrossRef]
- Della Pepa, G.; Russo, M.; Vitale, M.; Carli, F.; Vetrani, C.; Masulli, M.; Riccardi, G.; Vaccaro, O.; Gastaldelli, A.; Rivellese, A.A.; et al. Pioglitazone Even at Low Dosage Improves NAFLD in Type 2 Diabetes: Clinical and Pathophysiological Insights from a Subgroup of the TOSCA.IT Randomised Trial. Diabetes Res. Clin. Pract. 2021, 178, 108984. [Google Scholar] [CrossRef]
- Wu, W.; Liu, L.; Zhu, H.; Sun, Y.; Wu, Y.; Liao, H.; Gui, Y.; Li, L.; Liu, L.; Sun, F.; et al. Butyrolactone-I, an Efficient α-Glucosidase Inhibitor, Improves Type 2 Diabetes with Potent TNF-α-Lowering Properties through Modulating Gut Microbiota in Db/Db Mice. FASEB J. 2019, 33, 12616–12629. [Google Scholar] [CrossRef]
- Emoto, T.; Sawada, T.; Hashimoto, M.; Kageyama, H.; Terashita, D.; Mizoguchi, T.; Mizuguchi, T.; Motodi, Y.; Iwasaki, M.; Taira, K.; et al. Effect of 3-Month Repeated Administration of Miglitol on Vascular Endothelial Function in Patients with Diabetes Mellitus and Coronary Artery Disease. Am. J. Cardiol. 2012, 109, 42–46. [Google Scholar] [CrossRef]
- Tahara, A.; Kurosaki, E.; Yokono, M.; Yamajuku, D.; Kihara, R.; Hayashizaki, Y.; Takasu, T.; Imamura, M.; Li, Q.; Tomiyama, H.; et al. Effects of Sodium-Glucose Cotransporter 2 Selective Inhibitor Ipragliflozin on Hyperglycaemia, Oxidative Stress, Inflammation and Liver Injury in Streptozotocin-Induced Type 1 Diabetic Rats. J. Pharm. Pharm. 2014, 66, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Pawlos, A.; Broncel, M.; Woźniak, E.; Gorzelak-Pabiś, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Park, M.S.; Choung, J.S.; Kim, S.S.; Oh, H.H.; Choi, C.S.; Ha, S.Y.; Kang, Y.; Kim, Y.; Jun, H.S. Glucagon-like Peptide-1 Inhibits Adipose Tissue Macrophage Infiltration and Inflammation in an Obese Mouse Model of Diabetes. Diabetologia 2012, 55, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Gusdon, A.M.; Liu, H.; Qu, S. Effects of Glucagon-like Peptide-1 Receptor Agonists on Non-Alcoholic Fatty Liver Disease and Inflammation. World J. Gastroenterol. 2014, 20, 14821–14830. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Zempo, H.; Ogawa, M.; Watanabe, R.; Suzuki, J.I.; Akazawa, H.; Komuro, I.; Isobe, M. A DPP-4 Inhibitor Suppresses Fibrosis and Inflammation on Experimental Autoimmune Myocarditis in Mice. PLoS ONE 2015, 10, e0119360. [Google Scholar] [CrossRef]
- Dai, Y.; Dai, D.; Wang, X.; Ding, Z.; Mehta, J.L. DPP-4 Inhibitors Repress NLRP3 Inflammasome and Interleukin-1beta via GLP-1 Receptor in Macrophages through Protein Kinase C Pathway. Cardiovasc. Drugs Ther. 2014, 28, 425–432. [Google Scholar] [CrossRef]
- Shinjo, T.; Nakatsu, Y.; Iwashita, M.; Sano, T.; Sakoda, H.; Ishihara, H.; Kushiyama, A.; Fujishiro, M.; Fukushima, T.; Tsuchiya, Y.; et al. DPP-IV Inhibitor Anagliptin Exerts Anti-Inflammatory Effects on Macrophages, Adipocytes, and Mouse Livers by Suppressing NF-ΚB Activation. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E214–E223. [Google Scholar] [CrossRef]
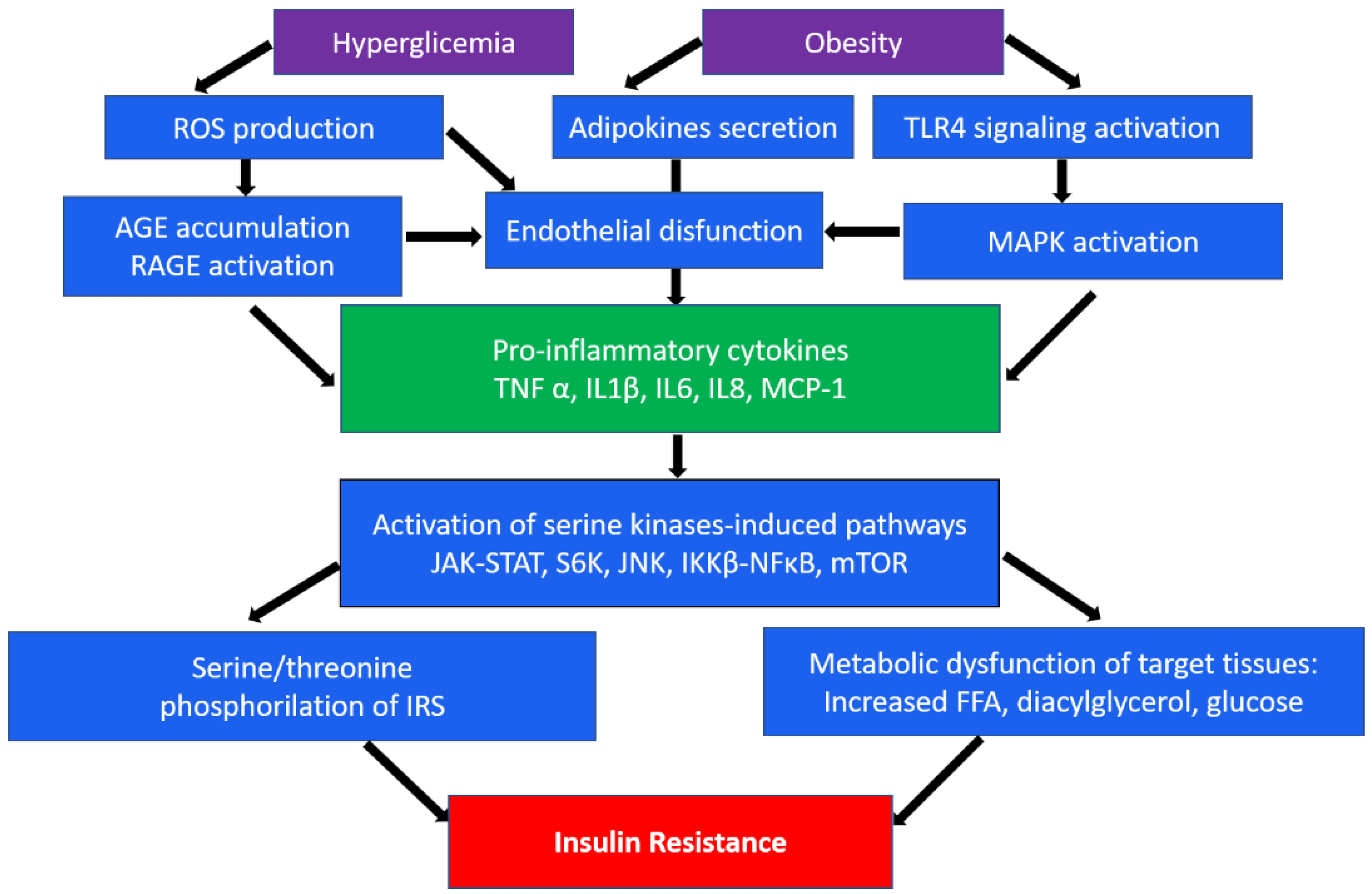
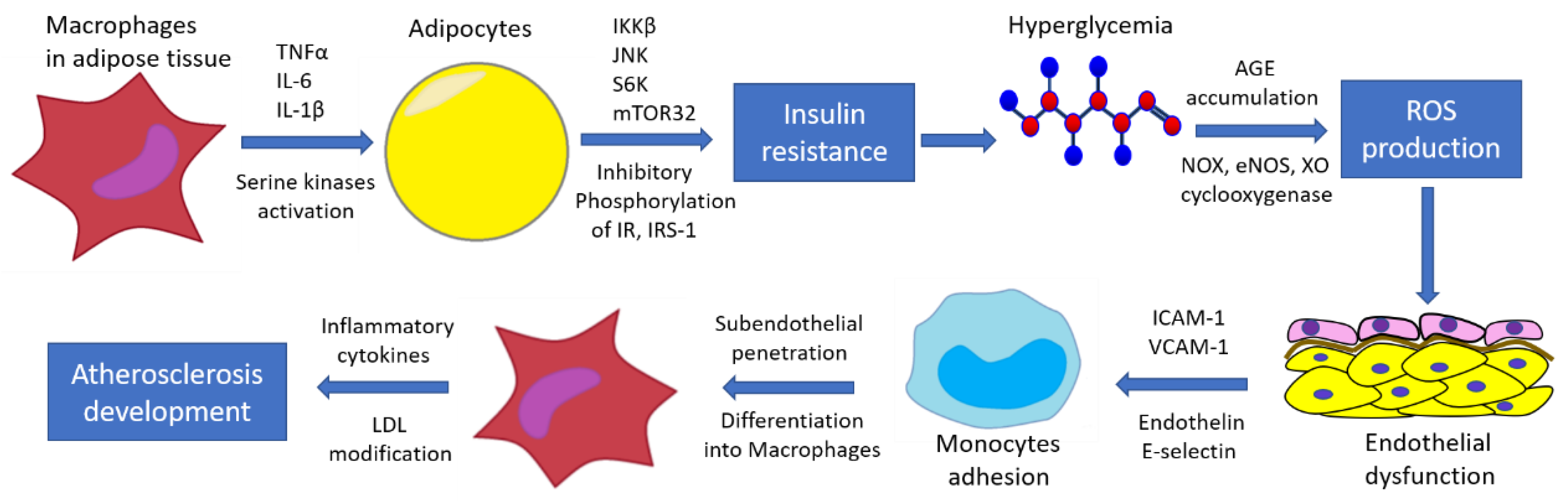
| Pathway | Model | Findings |
|---|---|---|
| NOD-, LRR- and pyrin-domain-containing protein 3 (NLRP3) inflammasome activation: protein complex triggering inflammatory mediator production | Streptozotocin-induced diabetic mice | Microglia NLRP3 proteins were highly expressed, and serum cytokines IL-1β, IL6, IL18, and TNFα were increased in streptozotocin-induced diabetic mice [34] |
| Endothelial NF-κB signaling: complex of transcription factors that regulate cytokine gene expression and the inflammatory response | Diabetic C57BL/KsJ db/db mice compared to healthy mice | The mRNA and protein levels of NF-κB and TLR4 were significantly higher in the db/db mice compared to normal control group [35] |
| E-DNIκB mice (transgenic mice expressing dominant-negative IκB under the Tie2 promoter/enhancer) | Endothelial NF-κB inhibition ameliorates insulin resistance and improves glucose homeostasis via reduced aortic expression of adhesion molecules, upregulation of eNOS signaling, reduced macrophage infiltration, and iNOS expression in adipose tissue [36] | |
| Major inflammatory cytokines secretion: IL-1β, IL-6, IL-18, and TNF-α | Diabetic C57BL/KsJ db/db mice compared to healthy mice | The serum levels of IL-1β, IL-6, IL-18, and TNF-α in the db/db mice were significantly higher compared to healthy control group [35] |
| Male db/db mice | IL-1β, IL-18, and TNF-α levels were increased in liver of db/db mice compared to healthy mice [37] | |
| JNK pathway activation: one of the major signaling cassettes of the mitogen-activated protein kinase (MAPK) signaling pathway | Apolipoprotein E/low-density lipoprotein receptor double-knockout (AL) mice | Hepatic inflammation and dyslipidemia were increased in AL mice on 35-week Western diet (WD) compared with wild-type mice on WD through activation of NF-κB, Stat3, JNK signaling pathways [38] |
| Chaperone-mediated autophagy (CMA): catabolic pathway for selective degradation of cytosolic proteins in lysosomes | Knockout mice 4–6 months old with selectively blocked CMA in liver | Key enzymes in carbohydrate and lipid metabolism are normally degraded by CMA while CMA block leads to peripheral adiposity, increased energy expenditure, and altered glucose homeostasis [39] |
| Group of Preparation | Mechanism of Action | Metabolic Effects | Anti-Inflammatory Effects |
|---|---|---|---|
| Sulfonylurea preparations | Bind to the sulfonylurea receptor (SUR) of ATP-sensitive potassium channel on pancreatic β cells | Enhance the release of insulin from the pancreatic islets | - Inhibit the NLRP3 inflammasome [142], decrease production of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) [143]; - Inhibit AGEs-induced pro-inflammatory mediators (NO, reactive oxygen species, i-NOS) [143]; - Enhance production of anti-inflammatory cytokines (IL-10 and TGF-β) [143]. |
| Biguanides | Block the breakdown of fatty acids through activation of AMP-dependent protein kinase | Reduce glucose production in liver by decreasing gluconeogenesis and stimulating glycolysis | - Activation of AMP-activated protein kinase (AMPK) [144,145]; - Inhibit mTOR and NF-κB pro-inflammatory signaling [145]; - Reduce inflammatory cytokines IL-6 and TNF-α [146]. |
| PPAR agonists | Activate PPARα/γ/δ receptors | Enhance insulin effects, decrease insulin resistance, decrease dyslipidemia | - Downregulate the inflammatory pathway NF-κB [147]; - Regulate adipokine production and secretion [148]; - Inhibit of pro-inflammatory molecules in liver [149]. |
| α-Glucosidase inhibitors | Inhibit enzymes in the small intestine | Prevent the absorption of glucose in the intestine | - Decrease TNF-α and other inflammatory mediators [150]; - Ameliorate vascular endothelial dysfunction [151]; - Decrease C-reactive protein (CRP) [151]. |
| SGLT2 inhibitors | inhibit SGLT-2 | Promote the excretion of glucose in the urine by inhibiting the reabsorption of glucose from the urine in the proximal tubules of the kidneys | - Improve endothelial function [12]; - Reduce inflammatory mediators IL-6, TNF-α, MCP-1, and CRP in plasma and liver [152]; - Inhibit NLRP3 inflammasome [153]; - Cause M2 macrophage polarization [153]. |
| GLP-1 agonists (GLP-1RA) | Activate GLP-1 receptor | Increase insulin secretion in a glucose-dependent manner and suppress glucagon secretion | - Reduce production of IL-6, TNF-α, and MCP-1 in adipose tissue [154]; - Inhibit NF-κB and JNK pathways [155]; - Decrease CRP [154]. |
| DPP-4 inhibitors | Inhibit DPP-4 receptor | Stimulate insulin secretion and decrease glucagon secretion, improve B-cell function and regeneration | - Reduce inflammatory cytokines IL-2, TNF-α, IL-1β, and IL-6 gene expression [156]; - Decrease NLRP3 inflammasome and TLR-4 activity [157]; - Suppress NF-κB activation [158]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedosugova, L.V.; Markina, Y.V.; Bochkareva, L.A.; Kuzina, I.A.; Petunina, N.A.; Yudina, I.Y.; Kirichenko, T.V. Inflammatory Mechanisms of Diabetes and Its Vascular Complications. Biomedicines 2022, 10, 1168. https://doi.org/10.3390/biomedicines10051168
Nedosugova LV, Markina YV, Bochkareva LA, Kuzina IA, Petunina NA, Yudina IY, Kirichenko TV. Inflammatory Mechanisms of Diabetes and Its Vascular Complications. Biomedicines. 2022; 10(5):1168. https://doi.org/10.3390/biomedicines10051168
Chicago/Turabian StyleNedosugova, Lyudmila V., Yuliya V. Markina, Leyla A. Bochkareva, Irina A. Kuzina, Nina A. Petunina, Irina Y. Yudina, and Tatiana V. Kirichenko. 2022. "Inflammatory Mechanisms of Diabetes and Its Vascular Complications" Biomedicines 10, no. 5: 1168. https://doi.org/10.3390/biomedicines10051168
APA StyleNedosugova, L. V., Markina, Y. V., Bochkareva, L. A., Kuzina, I. A., Petunina, N. A., Yudina, I. Y., & Kirichenko, T. V. (2022). Inflammatory Mechanisms of Diabetes and Its Vascular Complications. Biomedicines, 10(5), 1168. https://doi.org/10.3390/biomedicines10051168






