Biological Effects and Applications of Bulk and Surface Acoustic Waves on In Vitro Cultured Mammal Cells: New Insights
Abstract
:1. Introduction
2. Review Method
2.1. Research Design
2.2. Selection and Extraction of the Studies
2.3. Analysis of the Studies
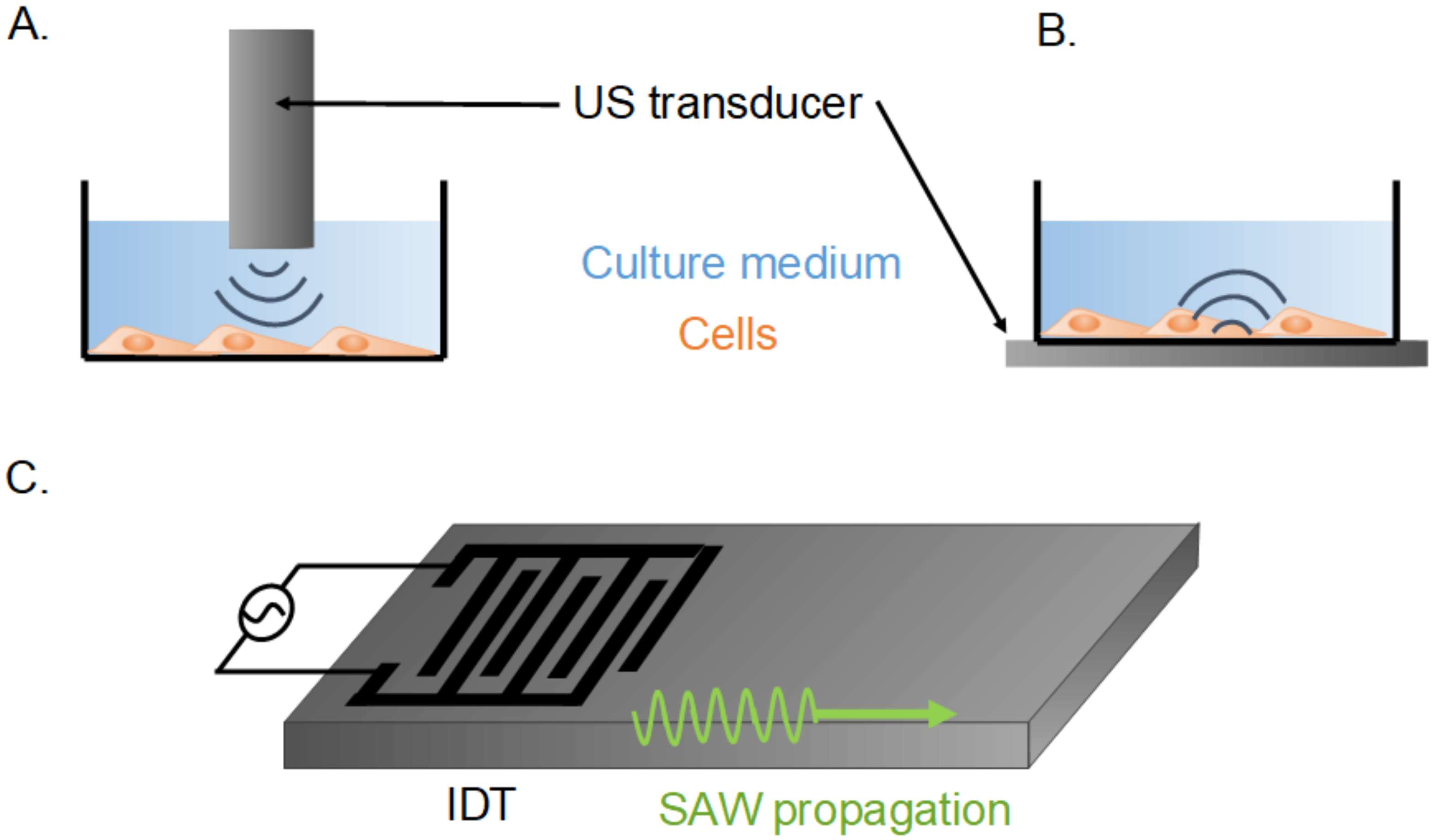
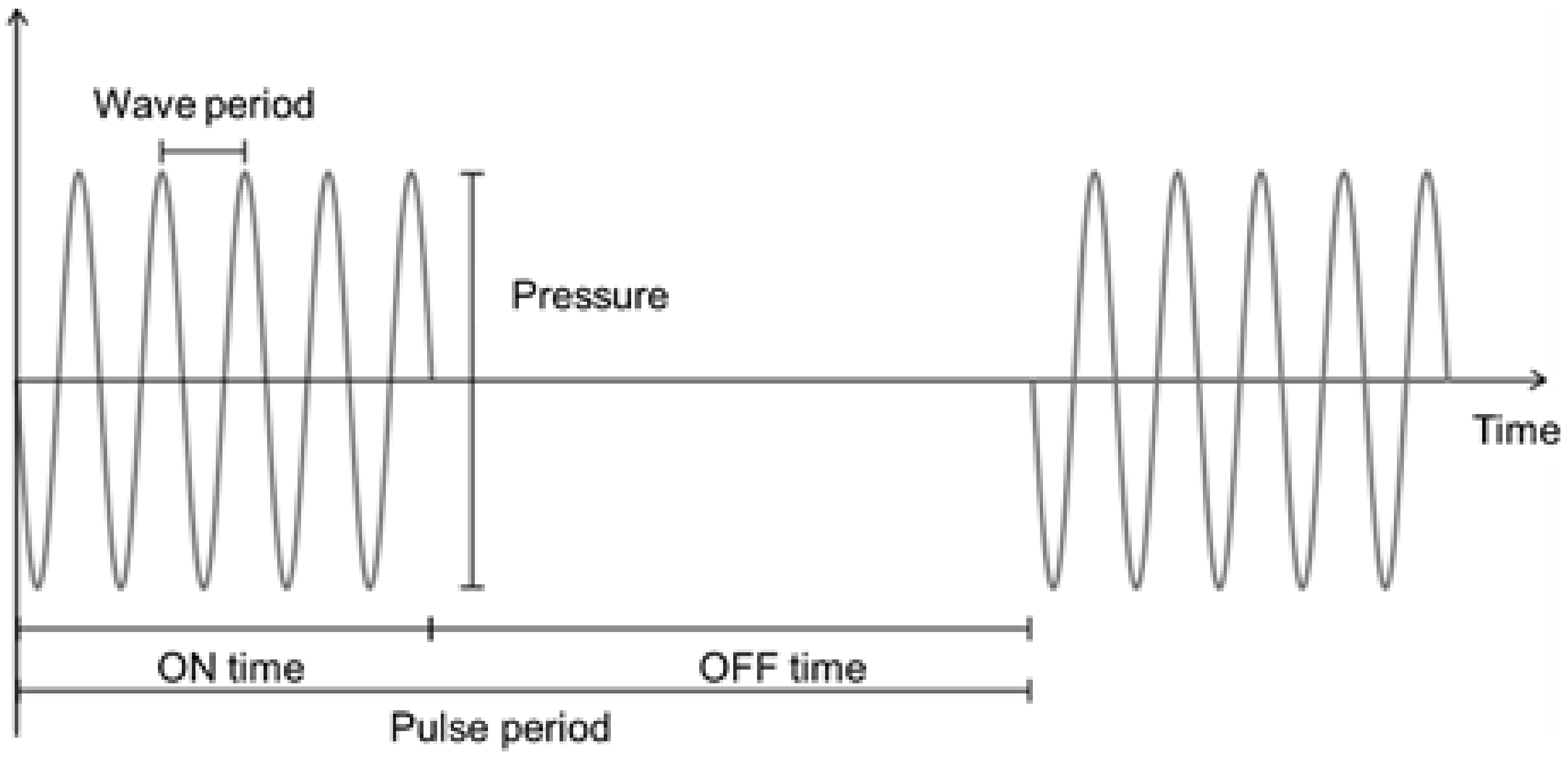
3. Ultrasounds at Low Frequencies (<10 MHz)
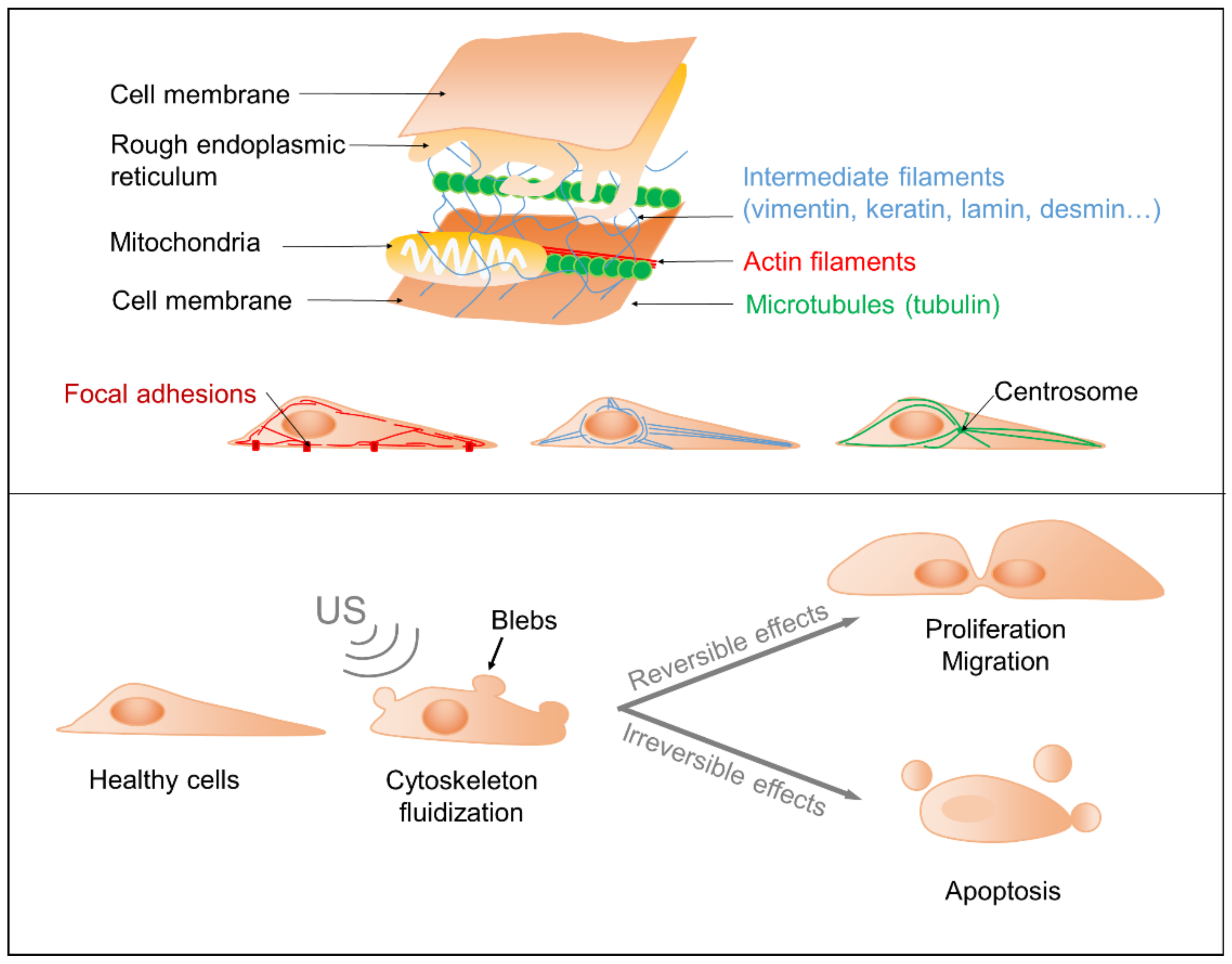
3.1. Adverse Effects on Cells
3.2. Proliferation, Cytoskeleton Rearrangement and Transfection
3.3. Towards an Understanding of the Physical Mechanisms of Action
4. Ultrasounds at High Frequencies (10–1000 MHz)
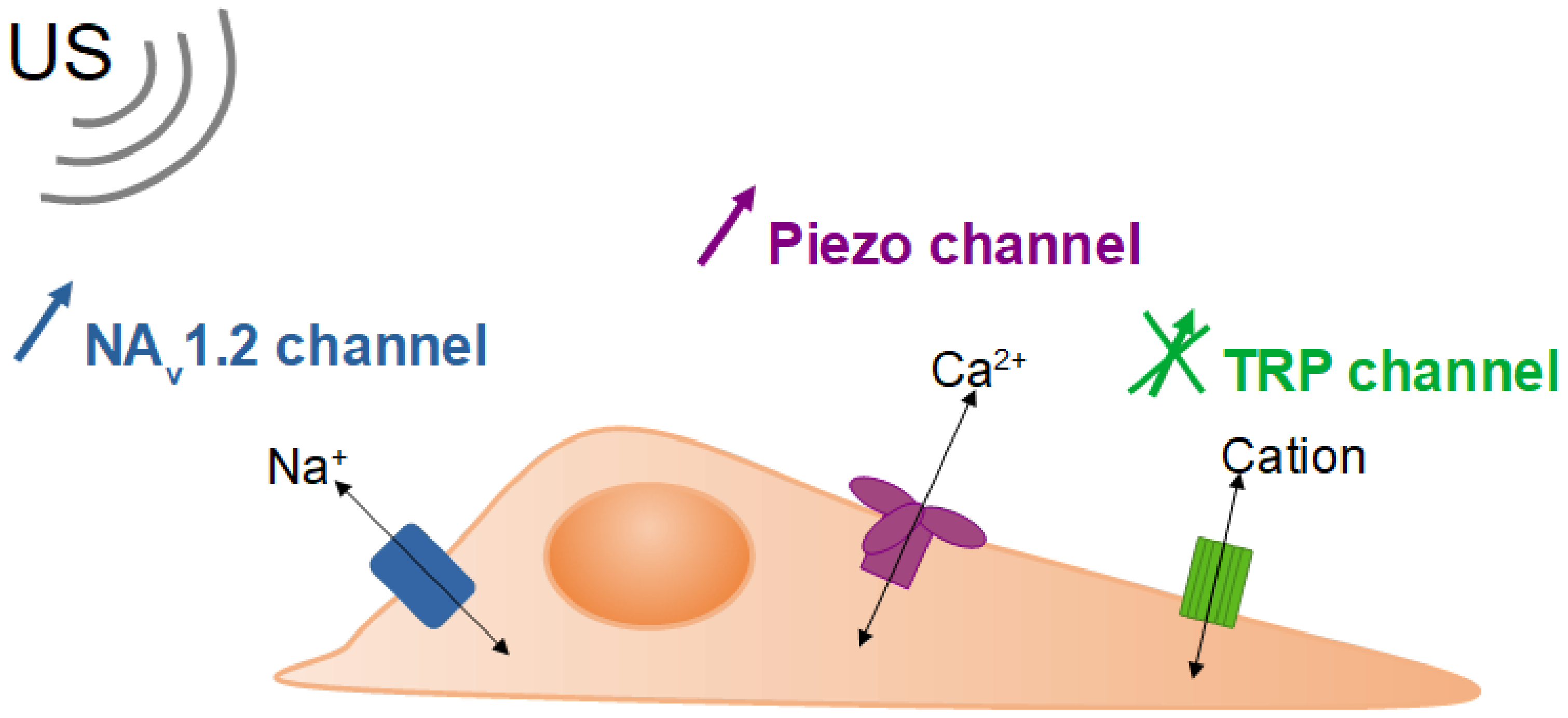
4.1. Activation of Ion Channels, Applications in Oncology and Neurostimulation
4.2. Increase in Permeability and Transfection
5. Ultrasounds Induced by Surface Acoustic Waves
5.1. Controlling Cell Detachment
5.2. From Cell Manipulation to Cell Sorting
5.3. Wound Healing: Cell Migration or Proliferation?
6. A Need for Experimental Standardization
7. Perspectives in Biomedicines
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dalecki, D. Mechanical Bioeffects of Ultrasound. Annu. Rev. Biomed. Eng. 2004, 6, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Willie, B. The Enhancement of Bone Regeneration by Ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Lin, G.; Lei, H.; Lue, T.F.; Guo, Y. Clinical Applications of Low-Intensity Pulsed Ultrasound and Its Potential Role in Urology. Transl. Androl. Urol. 2016, 5, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, E.C.; Hersh, E.; Vannan, M.; Matsunaga, T.O.; McCreery, T. Local Drug and Gene Delivery through Microbubbles. Prog. Cardiovasc. Dis. 2001, 44, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Zhang, Q.; Tan, Y.; Lam, K.H.; Zheng, H.; Qian, M. Non-Contact High-Frequency Ultrasound Microbeam Stimulation: A Novel Finding and Potential Causes of Cell Responses. IEEE Trans. Biomed. Eng. 2020, 67, 1074–1082. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, M.G.; Chiu, C.T.; Hwang, J.Y.; Kim, H.H.; Wang, Y.; Shung, K.K. Direct and Sustained Intracellular Delivery of Exogenous Molecules Using Acoustic-Transfection with High Frequency Ultrasound. Sci. Rep. 2016, 6, 20477. [Google Scholar] [CrossRef] [Green Version]
- Brugger, M.S.; Baumgartner, K.; Mauritz, S.C.F.; Gerlach, S.C.; Röder, F.; Schlosser, C.; Fluhrer, R.; Wixforth, A.; Westerhausen, C. Vibration Enhanced Cell Growth Induced by Surface Acoustic Waves as in Vitro Wound-Healing Model. Proc. Natl. Acad. Sci. USA 2020, 117, 31603–31613. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, W.; Lin, Z.; Cai, F.; Li, F.; Wu, J.; Meng, L.; Niu, L.; Zheng, H. Sorting of Tumour Cells in a Microfluidic Device by Multi-Stage Surface Acoustic Waves. Sens. Actuators B Chem. 2018, 258, 1174–1183. [Google Scholar] [CrossRef]
- Brugger, M.S.; Grundeen, S.; Doyle, A.; Theogarajan, L.; Wixforth, A.; Westerhausen, C. Orchestrating Cells on a Chip: Employing Surface Acoustic Waves towards the Formation of Neural Networks. Phys. Rev. E 2018, 98, 012411. [Google Scholar] [CrossRef]
- Devendran, C.; Carthew, J.; Frith, J.E.; Neild, A. Cell Adhesion, Morphology, and Metabolism Variation via Acoustic Exposure within Microfluidic Cell Handling Systems. Adv. Sci. 2019, 6, 1902326. [Google Scholar] [CrossRef] [Green Version]
- Levario-Diaz, V.; Bhaskar, P.; Carmen Galan, M.; Barnes, A.C. Effect of Acoustic Standing Waves on Cellular Viability and Metabolic Activity. Sci. Rep. 2020, 10, 8493. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Babyn, P.; Chapman, D. Mechanical and Biological Effects of Ultrasound: A Review of Present Knowledge. Ultrasound Med. Biol. 2017, 43, 1085–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, A.; Nelmes, R.T.C.; Gougoulias, N.; Maffulli, N.; Gray, J. The Effects of LIPUS on Soft-Tissue Healing: A Review of Literature. Br. Med. Bull. 2009, 89, 169–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, T.J. Therapeutic Ultrasound an Overview. Ultrason. Sonochem. 2011, 18, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.M.; Anderton, N.; Smalberger, C.; Polliack, J.; Nathan, M.; Postema, M. On the Behaviour of Living Cells under the Influence of Ultrasound. Fluids 2018, 3, 82. [Google Scholar] [CrossRef] [Green Version]
- Doan, N.; Reher, P.; Meghji, S.; Harris, M. In Vitro Effects of Therapeutic Ultrasound on Cell Proliferation, Protein Synthesis, and Cytokine Production by Human Fibroblasts, Osteoblasts, and Monocytes. J. Oral Maxillofac. Surg. 1999, 57, 409–419. [Google Scholar] [CrossRef]
- Furusawa, Y.; Fujiwara, Y.; Campbell, P.; Zhao, Q.-L.; Ogawa, R.; Hassan, M.A.; Tabuchi, Y.; Takasaki, I.; Takahashi, A.; Kondo, T. DNA Double-Strand Breaks Induced by Cavitational Mechanical Effects of Ultrasound in Cancer Cell Lines. PLoS ONE 2012, 7, e29012. [Google Scholar] [CrossRef]
- Hassan, M.A.; Ahmed, I.S.; Campbell, P.; Kondo, T. Enhanced Gene Transfection Using Calcium Phosphate Co-Precipitates and Low-Intensity Pulsed Ultrasound. Eur. J. Pharm. Sci. 2012, 47, 768–773. [Google Scholar] [CrossRef]
- Lagneaux, L.; de Meulenaer, E.C.; Delforge, A.; Dejeneffe, M.; Massy, M.; Moerman, C.; Hannecart, B.; Canivet, Y.; Lepeltier, M.F.; Bron, D. Ultrasonic Low-Energy Treatment: A Novel Approach to Induce Apoptosis in Human Leukemic Cells. Exp. Hematol. 2002, 30, 1293–1301. [Google Scholar] [CrossRef]
- Lee, W.; Yoo, S.; Jung, J.; Kang, W.; Wang, W.; Moon, C.; Choi, H. All-in-One Low-Intensity Pulsed Ultrasound Stimulation System Using Piezoelectric Micromachined Ultrasonic Transducer (PMUT) Arrays for Targeted Cell Stimulation. Biomed. Microdevices 2017, 19, 86. [Google Scholar] [CrossRef]
- Man, J.; Shelton, R.M.; Cooper, P.R.; Landini, G.; Scheven, B.A. Low Intensity Ultrasound Stimulates Osteoblast Migration at Different Frequencies. J. Bone Miner. Metab. 2012, 30, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, N.; Zhou, E.H.; Lenormand, G.; Krishnan, R.; Weihs, D.; Butler, J.P.; Weitz, D.A.; Fredberg, J.J.; Kimmel, E. Low Intensity Ultrasound Perturbs Cytoskeleton Dynamics. Soft Matter 2012, 8, 2438–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narihira, K.; Watanabe, A.; Sheng, H.; Endo, H.; Feril, L.B.; Irie, Y.; Ogawa, K.; Moosavi-Nejad, S.; Kondo, S.; Kikuta, T.; et al. Enhanced Cell Killing and Apoptosis of Oral Squamous Cell Carcinoma Cells with Ultrasound in Combination with Cetuximab Coated Albumin Microbubbles. J. Drug Target. 2018, 26, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Raz, D.; Zaretsky, U.; Einav, S.; Elad, D. Cellular Alterations in Cultured Endothelial Cells Exposed to Therapeutic Ultrasound Irradiation. Endothelium 2005, 12, 201–213. [Google Scholar] [CrossRef]
- Salgarella, A.R.; Cafarelli, A.; Ricotti, L.; Capineri, L.; Dario, P.; Menciassi, A. Optimal Ultrasound Exposure Conditions for Maximizing C2C12 Muscle Cell Proliferation and Differentiation. Ultrasound Med. Biol. 2017, 43, 1452–1465. [Google Scholar] [CrossRef]
- Samandari, M.; Abrinia, K.; Mokhtari-Dizaji, M.; Tamayol, A. Ultrasound Induced Strain Cytoskeleton Rearrangement: An Experimental and Simulation Study. J. Biomech. 2017, 60, 39–47. [Google Scholar] [CrossRef]
- Schuster, A.; Schwab, T.; Bischof, M.; Klotz, M.; Lemor, R.; Degel, C.; Schäfer, K.-H. Cell Specific Ultrasound Effects Are Dose and Frequency Dependent. Ann. Anat. Anat. Anz. 2013, 195, 57–67. [Google Scholar] [CrossRef]
- Schuster, A.; Rabe, H.; Schwab, T.; Bischof, M.; Degel, C.; Klotz, M.; Schäfer, K.-H. Neural Stem Cells Influenced by Ultrasound: Frequency and Energy Density Dependencies. Phys. Med. 2017, 4, 8–16. [Google Scholar] [CrossRef]
- Udroiu, I.; Coluzzi, E.; Bedini, A.; Giliberti, C.; Palomba, R.; Sgura, A. In Vitro Effects of 1-MHz Ultrasound on the Mitotic Spindle. Environ. Mol. Mutagen. 2019, 60, 568–575. [Google Scholar] [CrossRef]
- Miller, A.D.; Subramanian, A.; Viljoen, H.J. Theoretically Proposed Optimal Frequency for Ultrasound Induced Cartilage Restoration. Theor. Biol. Med. Model. 2017, 14, 21. [Google Scholar] [CrossRef] [Green Version]
- Trepat, X.; Deng, L.; An, S.S.; Navajas, D.; Tschumperlin, D.J.; Gerthoffer, W.T.; Butler, J.P.; Fredberg, J.J. Universal Physical Responses to Stretch in the Living Cell. Nature 2007, 447, 592–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns, L.D. Nonthermal Effects of Therapeutic Ultrasound: The Frequency Resonance Hypothesis. J. Athl. Train. 2002, 37, 293–299. [Google Scholar] [PubMed]
- Krasovitski, B.; Frenkel, V.; Shoham, S.; Kimmel, E. Intramembrane Cavitation as a Unifying Mechanism for Ultrasound-Induced Bioeffects. Proc. Natl. Acad. Sci. USA 2011, 108, 3258–3263. [Google Scholar] [CrossRef] [Green Version]
- Or, M.; Kimmel, E. Modeling Linear Vibration of Cell Nucleus in Low Intensity Ultrasound Field. Ultrasound Med. Biol. 2009, 35, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, W.D. Ultrasound–Biophysics Mechanisms. Prog. Biophys. Mol. Biol. 2007, 93, 212–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strohm, E.M.; Moore, M.J.; Kolios, M.C. High Resolution Ultrasound and Photoacoustic Imaging of Single Cells. Photoacoustics 2016, 4, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Strohm, E.M.; Berndl, E.S.L.; Kolios, M.C. High Frequency Label-Free Photoacoustic Microscopy of Single Cells. Photoacoustics 2013, 1, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Choe, S.-W.; Choi, H. Suppression Technique of HeLa Cell Proliferation Using Ultrasonic Power Amplifiers Integrated with a Series-Diode Linearizer. Sensors 2018, 18, 4248. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.Y.; Lee, J.; Lee, C.; Jakob, A.; Lemor, R.; Medina-Kauwe, L.K.; Shung, K.K. Fluorescence Response of Human HER2+ Cancer- and MCF-12F Normal Cells to 200MHz Ultrasound Microbeam Stimulation: A Preliminary Study of Membrane Permeability Variation. Ultrasonics 2012, 52, 803–808. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.Y.; Lee, N.S.; Lee, C.; Lam, K.H.; Kim, H.H.; Woo, J.; Lin, M.Y.; Kisler, K.; Choi, H.; Zhou, Q.; et al. Investigating Contactless High Frequency Ultrasound Microbeam Stimulation for Determination of Invasion Potential of Breast Cancer Cells. Biotechnol. Bioeng. 2013, 110, 2697–2705. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.Y.; Lim, H.G.; Yoon, C.W.; Lam, K.H.; Yoon, S.; Lee, C.; Chiu, C.T.; Kang, B.J.; Kim, H.H.; Shung, K.K. Non-Contact High-Frequency Ultrasound Microbeam Stimulation for Studying Mechanotransduction in Human Umbilical Vein Endothelial Cells. Ultrasound Med. Biol. 2014, 40, 2172–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto, M.L.; Firouzi, K.; Khuri-Yakub, B.T.; Maduke, M. Activation of Piezo1 but Not NaV1.2 Channels by Ultrasound at 43 MHz. Ultrasound Med. Biol. 2018, 44, 1217–1232. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Wang, P.; Peng, Q.; Wang, Y.; Shung, K.K. Acoustic-Transfection for Genomic Manipulation of Single-Cells Using High Frequency Ultrasound. Sci. Rep. 2017, 7, 5275. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Ryu, J.-M.; Choe, S. A Novel Therapeutic Instrument Using an Ultrasound-Light-Emitting Diode with an Adjustable Telephoto Lens for Suppression of Tumor Cell Proliferation. Measurements 2019, 147, 106865. [Google Scholar] [CrossRef]
- Choi, H.; Choe, S.-W. Acoustic Stimulation by Shunt-Diode Pre-Linearizer Using Very High Frequency Piezoelectric Transducer for Cancer Therapeutics. Sensors 2019, 19, 357. [Google Scholar] [CrossRef] [Green Version]
- Barani, A.; Paktinat, H.; Janmaleki, M.; Mohammadi, A.; Mosaddegh, P.; Fadaei-Tehrani, A.; Sanati-Nezhad, A. Microfluidic Integrated Acoustic Waving for Manipulation of Cells and Molecules. Biosens. Bioelectron. 2016, 85, 714–725. [Google Scholar] [CrossRef]
- Carey, T.R.; Cotner, K.L.; Li, B.; Sohn, L.L. Developments in Label-Free Microfluidic Methods for Single-Cell Analysis and Sorting. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1529. [Google Scholar] [CrossRef] [Green Version]
- Bourquin, Y.; Syed, A.; Reboud, J.; Ranford-Cartwright, L.C.; Barrett, M.P.; Cooper, J.M. Rare-Cell Enrichment by a Rapid, Label-Free, Ultrasonic Isopycnic Technique for Medical Diagnostics. Angew. Chem. Int. Ed. Engl. 2014, 53, 5587–5590. [Google Scholar] [CrossRef] [Green Version]
- Collins, D.J.; Morahan, B.; Garcia-Bustos, J.; Doerig, C.; Plebanski, M.; Neild, A. Two-Dimensional Single-Cell Patterning with One Cell per Well Driven by Surface Acoustic Waves. Nat. Commun. 2015, 6, 8686. [Google Scholar] [CrossRef] [Green Version]
- Greco, G.; Agostini, M.; Tonazzini, I.; Sallemi, D.; Barone, S.; Cecchini, M. Surface-Acoustic-Wave (SAW)-Driven Device for Dynamic Cell Cultures. Anal. Chem. 2018, 90, 7450–7457. [Google Scholar] [CrossRef]
- Imashiro, C.; Kang, B.; Lee, Y.; Hwang, Y.-H.; Im, S.; Kim, D.-E.; Takemura, K.; Lee, H. Propagating Acoustic Waves on a Culture Substrate Regulate the Directional Collective Cell Migration. Microsyst. Nanoeng. 2021, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Jötten, A.M.; Angermann, S.; Stamp, M.E.M.; Breyer, D.; Strobl, F.G.; Wixforth, A.; Westerhausen, C. Correlation of in Vitro Cell Adhesion, Local Shear Flow and Cell Density. RSC Adv. 2018, 9, 543–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Friend, J.R.; Yeo, L.Y. A Scaffold Cell Seeding Method Driven by Surface Acoustic Waves. Biomaterials 2007, 28, 4098–4104. [Google Scholar] [CrossRef] [Green Version]
- Schmid, L.; Weitz, D.A.; Franke, T. Sorting Drops and Cells with Acoustics: Acoustic Microfluidic Fluorescence-Activated Cell Sorter. Lab Chip 2014, 14, 3710–3718. [Google Scholar] [CrossRef]
- Senveli, S.U.; Ao, Z.; Rawal, S.; Datar, R.H.; Cote, R.J.; Tigli, O. A Surface Acoustic Wave Biosensor for Interrogation of Single Tumour Cells in Microcavities. Lab Chip 2016, 16, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sivanantha, N.; Ma, C.; Collins, D.J.; Sesen, M.; Brenker, J.; Coppel, R.L.; Neild, A.; Alan, T. Characterization of Adhesive Properties of Red Blood Cells Using Surface Acoustic Wave Induced Flows for Rapid Diagnostics. Appl. Phys. Lett. 2014, 105, 103704. [Google Scholar] [CrossRef]
- Stamp, M.E.M.; Brugger, M.S.; Wixforth, A.; Westerhausen, C. Acoustotaxis -in Vitro Stimulation in a Wound Healing Assay Employing Surface Acoustic Waves. Biomater. Sci. 2016, 4, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Stamp, M.E.M.; Jötten, A.M.; Kudella, P.W.; Breyer, D.; Strobl, F.G.; Geislinger, T.M.; Wixforth, A.; Westerhausen, C. Exploring the Limits of Cell Adhesion under Shear Stress within Physiological Conditions and beyond on a Chip. Diagnostics 2016, 6, 38. [Google Scholar] [CrossRef]
- Inui, T.; Mei, J.; Imashiro, C.; Kurashina, Y.; Friend, J.; Takemura, K. Focused Surface Acoustic Wave Locally Removes Cells from Culture Surface. Lab Chip 2021, 21, 1299–1306. [Google Scholar] [CrossRef]
- Wang, S.; Lv, X.; Su, Y.; Fan, Z.; Fang, W.; Duan, J.; Zhang, S.; Ma, B.; Liu, F.; Chen, H.; et al. Piezoelectric Microchip for Cell Lysis through Cell–Microparticle Collision within a Microdroplet Driven by Surface Acoustic Wave Oscillation. Small 2019, 15, 1804593. [Google Scholar] [CrossRef]
- Li, H.; Friend, J.; Yeo, L.; Dasvarma, A.; Traianedes, K. Effect of Surface Acoustic Waves on the Viability, Proliferation and Differentiation of Primary Osteoblast-like Cells. Biomicrofluidics 2009, 3, 34102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliton. Soliton Planar Acoustic Wave Device System for Dermal Tattoo Clearing Human Trial. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02877667 (accessed on 5 May 2022).
- Soliton. Soliton Planar Acoustic Wave Device System for Dermal Clearing Human Trial Protocol. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03125824 (accessed on 5 May 2022).
- Ho, D.D.-M.; London, R.; Zimmerman, G.B.; Young, D.A. Laser-Tattoo Removal—A Study of the Mechanism and the Optimal Treatment Strategy via Computer Simulations. Lasers Surg. Med. 2002, 30, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, J.I. DPM An Evaluation of the Effect of a Surface Acoustic Wave Patch Device on the Symptons of Trigeminal Neuralgia. 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02801630 (accessed on 5 May 2022).
- Zwecker, M. Examining the Efficacy of Low Intensity Low Frequency Surface Acoustic Wave Ultrasound(LILF/SAWU) in Trigeminal Neuralgia Pain; Sheba Medical Center: Ramat Gan, Israel, 2012. [Google Scholar]
- Gai, J.; Dervisevic, E.; Devendran, C.; Cadarso, V.J.; O’Bryan, M.K.; Nosrati, R.; Neild, A. High-Frequency Ultrasound Boosts Bull and Human Sperm Motility. Adv. Sci. 2022, 9, 2104362. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, J.I.; Gazes, M.I.; Greenberg, N. Surface Acoustic Wave Patch Therapy Affects Tissue Oxygenation in Ischemic Feet. Wounds 2014, 26, 301–305. [Google Scholar]
- Zhou, W.; Chen, M.; Liu, X.; Zhang, W.; Cai, F.; Li, F.; Wu, J.; Wang, J.; Wang, Y.; Huang, X.; et al. Selective Photothermal Ablation of Cancer Cells by Patterned Gold Nanocages Using Surface Acoustic Waves. Lab Chip 2019, 19, 3387–3396. [Google Scholar] [CrossRef]
- Stewart, E.A.; Gedroyc, W.M.W.; Tempany, C.M.C.; Quade, B.J.; Inbar, Y.; Ehrenstein, T.; Shushan, A.; Hindley, J.T.; Goldin, R.D.; David, M.; et al. Focused Ultrasound Treatment of Uterine Fibroid Tumors: Safety and Feasibility of a Noninvasive Thermoablative Technique. Am. J. Obstet. Gynecol. 2003, 189, 48–54. [Google Scholar] [CrossRef]
- University College London Hospitals. An Evaluation of Lesion Control Using Focal Ablation With High Intensity Focused Ultrasound in the Treatment of Non-Metastatic Progressive Prostate Cancer. 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT00987675 (accessed on 5 May 2022).


| Reference | Frequency (MHz) | Intensity or Pressure | Duty Cycle (%) | Pulse Time (min) | Dose (J cm−2) | Cells | Temperature Control | Biological Effects | Hypothesis |
|---|---|---|---|---|---|---|---|---|---|
| [16] | 0.045, 1 | 10–400 mW cm−2 | 25 | 5 | 7.5–75 | Primary fibroblasts Primary osteoblasts Primary monocytes | Rise ≤ 1.8 °C | ↗ proliferation ↗ collagen synthesis | N.A. |
| [17] | 1 | 100–400 mW cm−2 | 10 | 1 | 0.6–2.4 | Human monocytes (U-937) T lymphoblasts (Molt-4) Lymphocytes (Jurkat) Leukemia cell line (HL 60) | Rise ≤ 1 °C | ↗ DNA double strand breaks if I > 200 mW cm−2 | Free radicals formation, due to cavitation. |
| [18] | 1 | 300 mW cm−2 | 50 | 0.5–15 | 4.5–135 | Human adenocarcinoma epithelial cells (HeLa) | None | ↗ membrane permeabilization ↗ intracellular transport | N.A. |
| [19] | 1.8 | 7 mW mL−1 | 65 | 0.33 | 91 J mL−1 | Human leukemia bone marrow cells (K562, KG1a) HL-60, human B cell precursor leukemia cells (Nalm-6) | None | ↗ apoptosis Mild necrosis Virulent leukemic cells more sensitive | Oxygen singlet formation, due to cavitation. |
| [20] | 1.48 | 0.045 MPa | 15–70 | 5–30 | N.A. | Rat pheochromocytoma adrenal medulla cells (PC-12) | None | ↗ proliferation | N.A. |
| [21] | 1 | 250 mW cm−2 | 20 | 30 | 90 | Mouse osteoblasts (MC3T3-E1) | Pre-heated water tank | ↗ proliferation ↗ migration | N.A. |
| [22] | 1 | 1000–2000 mW cm−2 | 20 | 0.5 | 6–12 | Human aortic smooth muscle cells (HASM) | Rise ≤ 1 °C | Reversible fluidization for I = 1000 mW cm−2 Damages to the actin filaments for I = 2 W cm−2 | Fluidization due to the compression wave causing a local cell deformation |
| [23] | 1 | 800–1000 mW cm−2 | 50 | 0.25 | 6–7.5 | Human oral squamous carcinoma cells (HSC-2) U-937 | None | ↘ HSC-2 viability with microbubbles. No effect on U-937. No effect without microbubble. | N.A. |
| [24] | 0.5, 1, 3.5, 5 | 1600–2000 mW cm−2 | 10–100 | 30 | 288–3600 | Endothelial cells | Measured temperature “excluded the possibility that thermal effects may cause changes in the cultured cells” | ↗ proliferation ↗ cytoskeleton disorganization ↗ tissue repair. | direct mechanical action |
| [25] | 0.5, 1, 3, 5 | 250–1000 mW cm−2 | 20 | 5 | 15–60 | Mouse myoblasts (C2C12) | Room temperature (28 °C) water tank | ↗ proliferation ↗ differentiation | Mechanical constraints |
| [26] | 0.8, 1.5 | 150, 250 kPa | 100 | 0.17–0.5 | N.A. | C2C12 | Rise ≤ 1 °C | Induce cytoskeleton fluidization ↗ cell mortality | Cell deformation with acoustic pressure |
| [27] | 0.51, 0.994, 4.36 | N.A. | N.A. | N.A. | 3, 25, 50 | Human cardiac microvascular endothelial cells (hcMEC) Madin–Darby Canine Kidney cells (MDCK) Mouse neuroblastoma cells (Neuro2A) Human colon cancer cells (HT29) | Perfused water tank at 37 °C | ↗ proliferation at low I Not anymore at high intensity | N.A. |
| [28] | 0.51, 4.36 | N.A. | N.A. | N.A. | 3, 25 | Neural stem cells | Perfused water tank at 37 °C | ↗ proliferation no increase in neurogenesis or gliogenesis | N.A. |
| [29] | 1 | 70–300 mW cm−2 | 100 | 30 | 126–540 | HeLa Human fetal lung fibroblasts (MCR-5) Human breast cancer cells (MCF-7) | Rise ≤ 1 °C | ↗ mitotic abnormalities as a function of I disassembly of focal adhesions and microtubules. | N.A. |
| Reference | Frequency (MHz) | Voltage, Intensity or Electrical Power | Duty Cycle (%) | Pulse Time (s) | Dose (J cm−2) | Cells (Adherent) | Temperature Control | Biological Effects | Hypothesis |
|---|---|---|---|---|---|---|---|---|---|
| [38] | 15 + LED | 47.9, 82.15, 128.11 mW cm−2 | 100 | 1800 (daily) | 126,000– 230,600 | Human cervix carcinoma cells (HeLa) | None | ↘ proliferation | N.A. |
| [39] | 200 | 16, 32, 47 V 110, 230, 330 mW * | 2.5 | 10 | N.A. | Human breast cells (MCF-12F) Human breast cancer cells (MDA-MB-435) | Thermally controlled chamber | ↗ cell permeability higher in non-cancerous cells | N.A. |
| [40] | 200–1000 | 4, 8, 16, 32 V 30, 60, 110, 230 mW * | 0.0025–1 | 0.3–150 | N.A. | Highly invasive human breast cancer cells (MDA-MB-231) Weakly invasive human breast cancer cells (MCF-7, SKBR3, and BT-474) | None | ↗ Ca2+ influx as a function of invasiveness | N.A. |
| [41] | 193 | 1.8–3.6 MPa | 0.1, 0.25, 0.5, 0.75, 1 | 0.5 | N.A. | Endothelial cells (HUVEC) | Thermally controlled chamber | ↗ Ca2+ influx | N.A. |
| [42] | 43 | 50,000, 90,000 mW cm−2 3.2, 5.7 mW focused on 1 cell | 100 | 0.7 | 35, 63 | Chinese hamster ovary cells (CHO) expressing rat Nav1.2 or mouse piezo 1 channels Human embryonic kidney cells (HEK) expressing mouse piezo 1 channels | Estimated rise of 0.8 °C | Stimulation of the Nav1.2 and piezo channels | US through acoustic radiation and shear stimulate the piezo channel Thermal heating stimulates the Nav1.2 channel |
| [5] | 50 | 0.43–1.97 MPa | 33 | 3.3 | N.A. | Human breast cells (MCF-10A) MDA-MB-231 MCF-7 | Rise ≤ 0.5 °C | ↗ Ca2+ influx, as a function of invasiveness | US stimulate the piezo channel |
| [6] | 150, 215 | 22–43 V 160–300 mW * | 100 | 0.016, 0.023 | N.A. | HeLa | None | Size and amount of transfected elements depend on the voltage, duration, frequency and number of US pulsation. No impact on viability | N.A. |
| [43] | 150, 215 | 22 V 160 mW * | 0.0036 | 0.5–1.5 | N.A. | HeLa | None | Genomic transfection facilitated by US | N.A. |
| Reference | Frequency (MHz) | Intensity or Electrical Power | Duty Cycle (%) | Time | Shear Flow | Device | Cells | Temperature Control | Biological Effects | Hypothesis |
|---|---|---|---|---|---|---|---|---|---|---|
| [48] | 10 | 65–250 mW | N.A. | N.A. | N.A. | Slanted IDT, LiNbO3 chip | Human red blood cells (RBC) RBC infected by the malarial parasite Plasmodium falciparum | None | Enrichment, separation of the cells depending on their pathological state | Cell density impacts their displacement with the shear flow |
| [7] | 77–164 | 80–1000 mW cm−2 up to 13.6 mW | 100 or 0.00077 | 5 min–27 h | N.A. | LiNbO3 chip covered with a SiO2 layer (= substrate), PDMS well | Madin–Darby canine kidney (MDCK-II) Human osteosarcoma sarcoma osteogenic (SaOs-2) Human embryonic kidney (T-REx-293) | Estimated rise of 2.4 °C | Wound healing ↗ cell migration ↗ cell proliferation | Direct mechanical stimulation > flow field, or electrical field |
| [49] | 101–204 | 380 mW | 100 | seconds | N.A. | 4 IDT, LiNbO3 chip | Human lymphocytes RBC infected by the malarial parasite Plasmodium falciparum | Thermally controlled chamber | Patterning of spatially isolated individual cells in an acoustic field defined in 2D | N.A. |
| [50] | 48.8 | 467 mW | 2.5 | 48 h | Shear stress 120–280 mN m−2 Shear velocity 600 ± 250 μm s−1 | LiNbO3 chip, titanium substrate, PDMS well | Human monocytes (U-937) | Rise ≤ 0.5 °C | ↗ cell proliferation (+36%) | Shear stress linked to SAW has a more positive impact than stirring |
| [51] | 14 | Up to18 V, 59.3 mW cm−2 and 0.23 µW for a single cell (400 µm2) order of magnitude up to 100 mW * | 100 | 4–8 h | Velocity up to 56 µm s−1, shear stress 3.8 mPa | LiNbO3 chip, glycerol as a coupling liquid with the PDMS cell culture chamber | Mouse embryonic fibroblasts (NIH-3T3) | Feedback loop to maintain the temperature of the medium flow | Cell migration first enhanced, then suppressed as the intensity rose No reduction in cell viability Thicker actin bundles | Cell orientation alignment along the propagating wave, high traction forces activated the Rho signaling pathway |
| [52] | 160 | 631 mW | 100 | 60 min | Shear rate distribution 1750–6900 s−1 | Gold IDT, LiNbO3 chip, a cylindrical PDMS chamber on top filled with culture medium, cells attached to a titanium implant on top | SaOs-2 | Temperature maintained at 37 °C, no precision | Correlation between shear flow and cell detachment from an implant | Cell density plays a key role |
| [53] | 19.35 | 325–575 mW | 100 | 10 s | Velocity 0–9 mm s−1 | LiNbO3 chip, titanium layer, aluminum substrate, | none | / | ↗ penetration rate into a porous scaffold | N.A. |
| [54] | 161–171 | 31.6 mW | N.A. | >330 µs per pulse | N.A. | Gold and titan LiNbO3 chip, covered with glass, PDMS microchannel device | Mouse melanoma cells (B16F10) | None. | Sorting rate of 3000 cells s−1 depending on their fluorescence (Calcein-AM) | N.A. |
| [55] | 196.7 | 1 mW 10–20 kPa | 100 | 3–10 min | N.A. | Quartz (SiO2) chip, cells suspended in glycerin, SU-8 microprobe | Chondrosarcoma (JJ012) Breast cancer cells (MDA-MB-231, SKBR3, MCF7) | None | US velocity measurement for single cell analysis 106 sensitivity in elasticity compared to AFM | Cell elastic moduli is a possible biomarker for aggressiveness or metastatic potential |
| [56] | 132 | 55–500 mW | 100 | 100 s | Velocity 0.42–1.80 m s−1 Shear stress 0.01–0.045 Pa | Concentric gold IDT, LiNbO3 chip | Untreated, and non-infected human RBC Glutaraldehyde- treated RBC RBC infected by the malarial parasite | None | Cell detachment behavior was different according to the RBC state of infection. | Specific mechanotransduction might be a biomarker |
| [57] | 159 | 2–4 mW | 100 | 48 h | N.A. | LiNbO3 chip, SiO2 substrate, PDMS well | SaOs-2 | Rise ≤ 0.32 °C | ↗ wound healing as a function of US intensity no significant necrosis no preferred direction for migration/proliferation | Unclear if the effect is due to mechanical or electrical stimulation, or a combination of both |
| [58] | N.A. | 316–501 mW | 100 | 0–60 min | Shear flow 2 Pa | LiNbO3 chip, titanium substrate | SaOs-2 | Thermally controlled chamber | No significant impact on cell adhesion, when T ≤ 37 °C | Decrease in cell adhesion is due to increase in temperature or decrease in pH |
| [8] | 38.74 | 125.6 mW | 80 | 2 h | N.A. | Two circular IDT (and two straight IDT for SSAW), LiNbO3 chip, covered with Al, and PDMS channels | Human glioma cell lines (U87) Rat RBC | None | Cell sorting depending on their virulence | Sorting of particles is dependent on their size |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figarol, A.; Olive, L.; Joubert, O.; Ferrari, L.; Rihn, B.H.; Sarry, F.; Beyssen, D. Biological Effects and Applications of Bulk and Surface Acoustic Waves on In Vitro Cultured Mammal Cells: New Insights. Biomedicines 2022, 10, 1166. https://doi.org/10.3390/biomedicines10051166
Figarol A, Olive L, Joubert O, Ferrari L, Rihn BH, Sarry F, Beyssen D. Biological Effects and Applications of Bulk and Surface Acoustic Waves on In Vitro Cultured Mammal Cells: New Insights. Biomedicines. 2022; 10(5):1166. https://doi.org/10.3390/biomedicines10051166
Chicago/Turabian StyleFigarol, Agathe, Lucile Olive, Olivier Joubert, Luc Ferrari, Bertrand H. Rihn, Frédéric Sarry, and Denis Beyssen. 2022. "Biological Effects and Applications of Bulk and Surface Acoustic Waves on In Vitro Cultured Mammal Cells: New Insights" Biomedicines 10, no. 5: 1166. https://doi.org/10.3390/biomedicines10051166
APA StyleFigarol, A., Olive, L., Joubert, O., Ferrari, L., Rihn, B. H., Sarry, F., & Beyssen, D. (2022). Biological Effects and Applications of Bulk and Surface Acoustic Waves on In Vitro Cultured Mammal Cells: New Insights. Biomedicines, 10(5), 1166. https://doi.org/10.3390/biomedicines10051166









