BMP4 Exerts Anti-Neurogenic Effect via Inducing Id3 during Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Animal Ethics Statement
2.3. Animals
2.4. Drugs, Antibodies and Devices
2.5. Brain Tissue Processing
2.6. Protein Isolation and Western Blotting
2.7. Quantitative RT-PCR
2.8. In Situ Hybridization
2.9. Immunofluorescence
2.10. Recombinant Lentivirus Production
2.11. In Vivo Overexpression via Lentivirus Stereotaxic Injections to SVZ or SGZ
2.12. Osmotic Pump Grafting
2.13. BrdU Labeling Experiments
2.14. Microscopy and Imaging
2.15. Statistical Analysis
3. Results
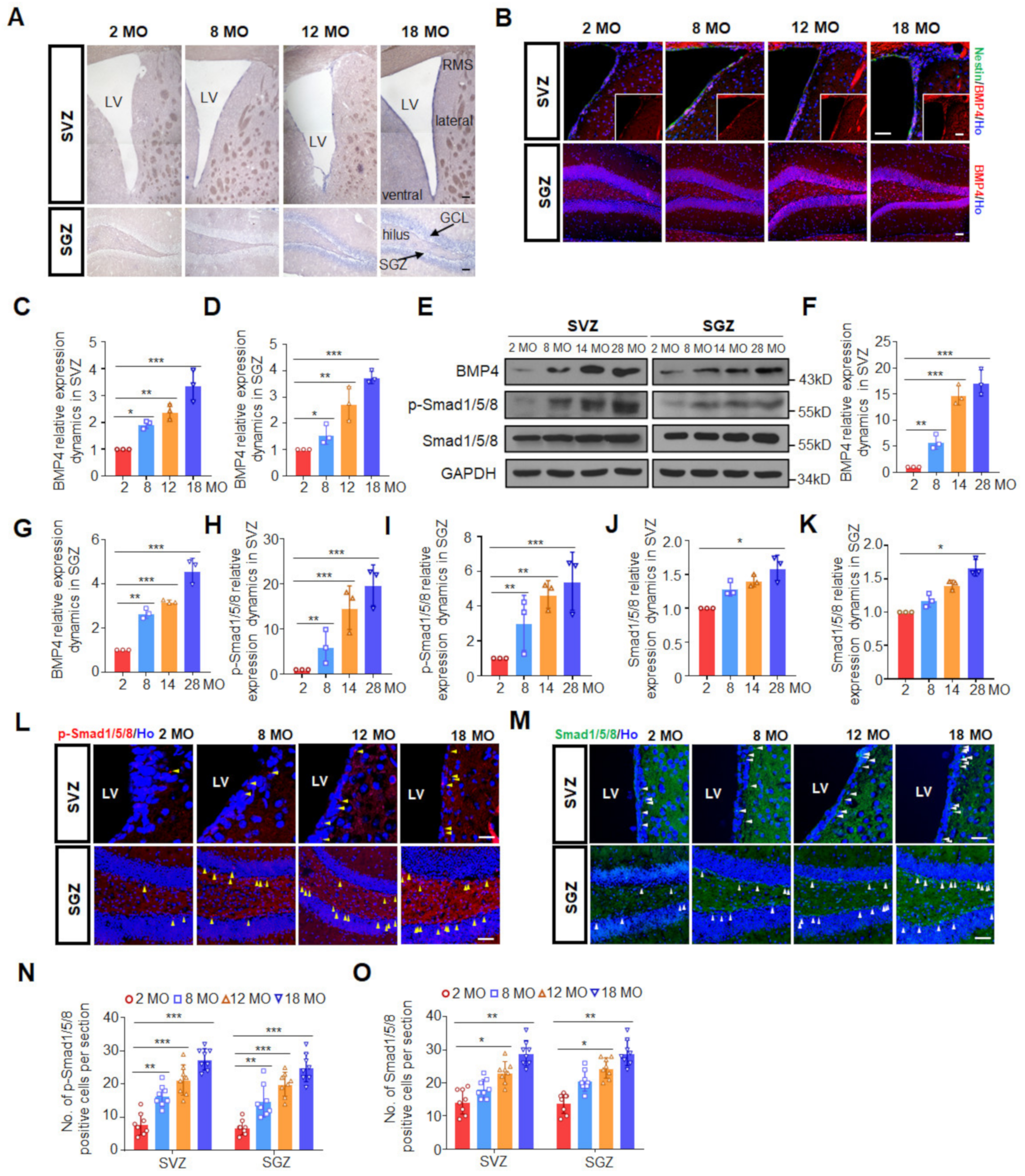
3.1. Canonical BMP Signaling Increases with Age in Both Neurogenic Regions
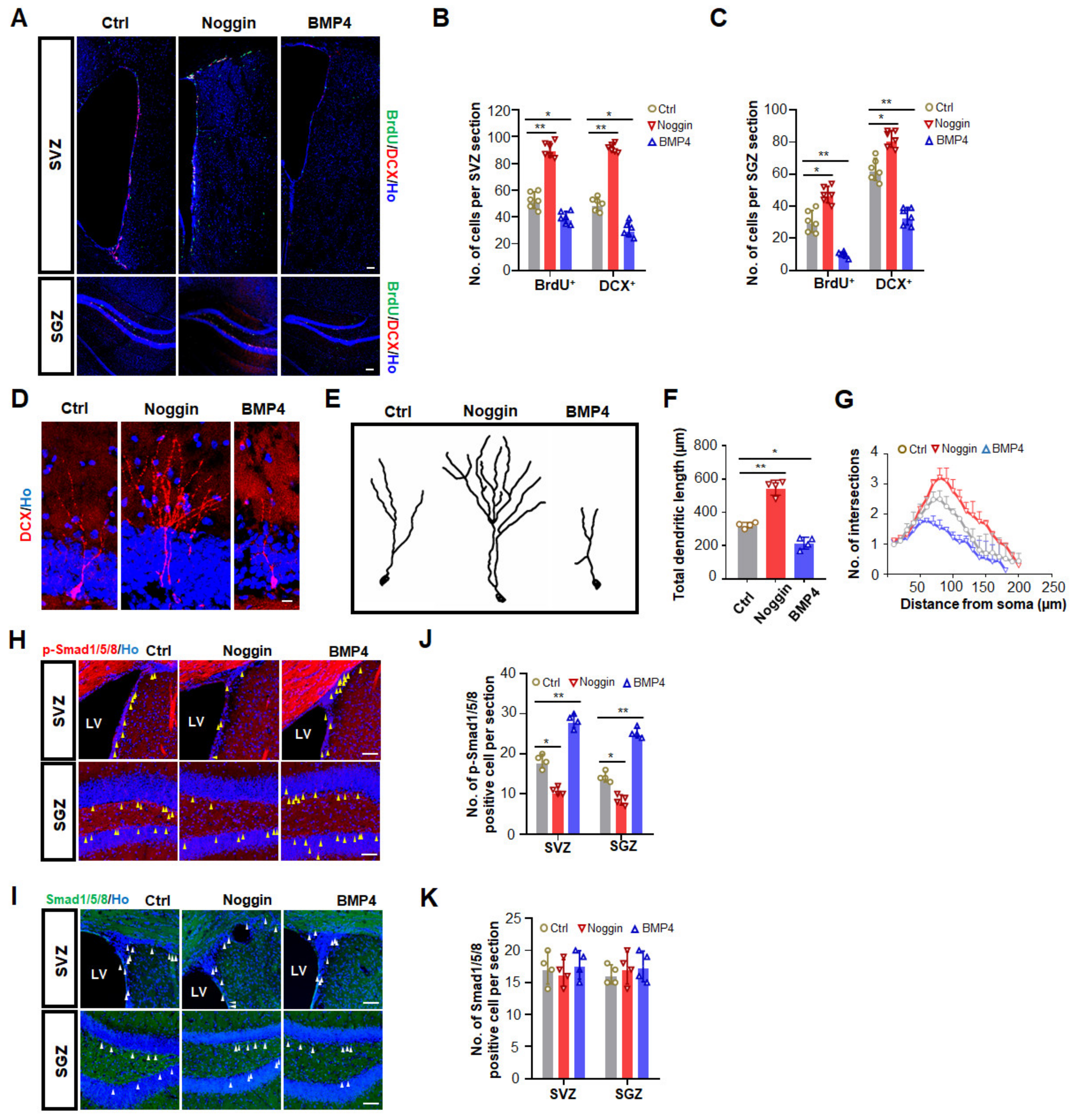
3.2. Increased BMP Signaling Impairs Adult Neurogenesis in SVZ and SGZ
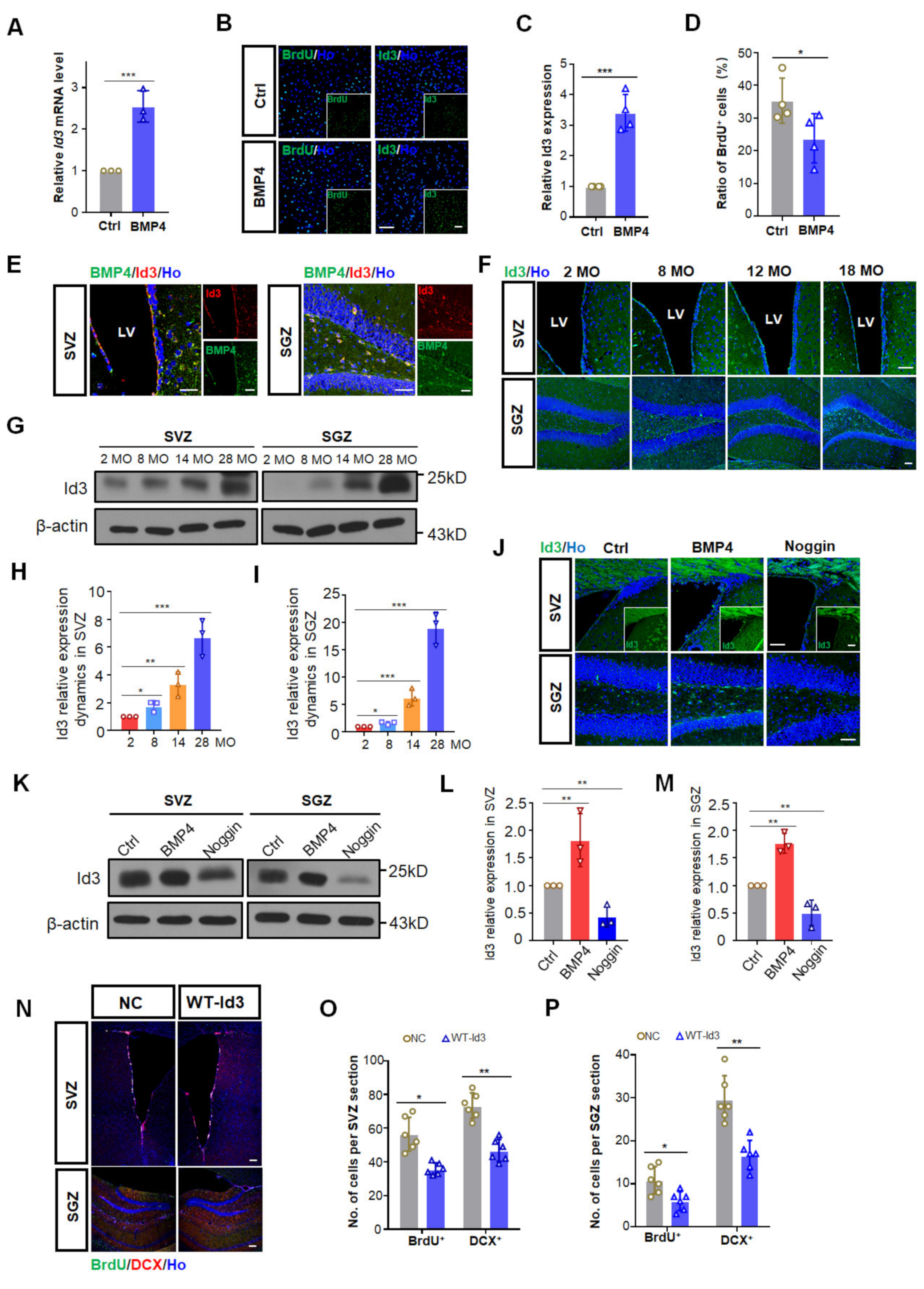
3.3. BMP4 Inhibited Adult Neurogenesis via Up-Regulating Id3
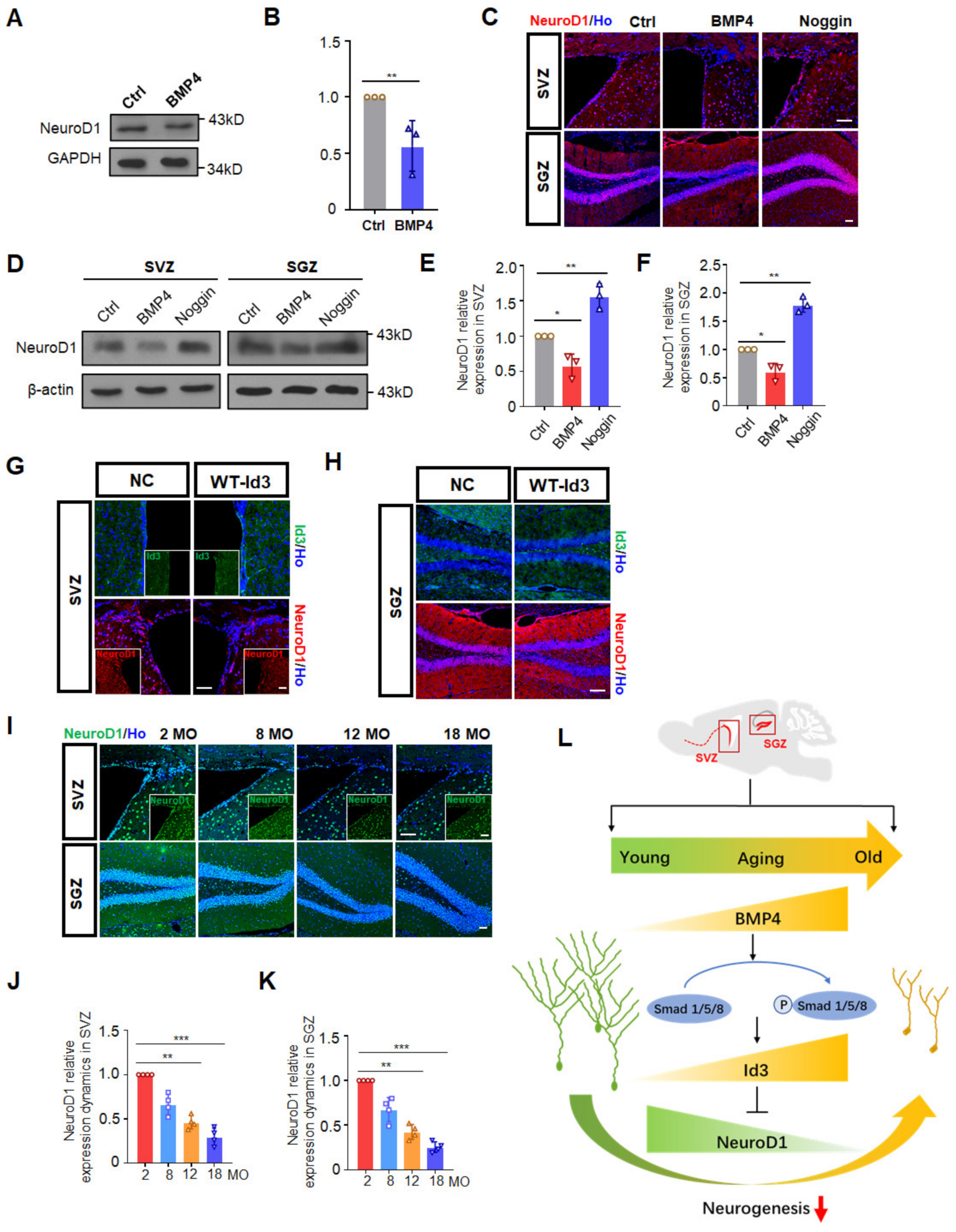
3.4. Id3 Inhibited NeuroD1 to Restrain Progenitor Cell Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.L.; Bergmann, O.; Alkass, K.; Bernard, S.; Salehpour, M.; Huttner, H.B.; Bostrom, E.; Westerlund, I.; Vial, C.; Buchholz, B.A.; et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013, 153, 1219–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucassen, P.J.; Fitzsimons, C.P.; Salta, E.; Maletic-Savatic, M. Adult neurogenesis, human after all (again): Classic, optimized, and future approaches. Behav. Brain Res. 2020, 381, 112458. [Google Scholar] [CrossRef]
- Doetsch, F.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997, 17, 5046–5061. [Google Scholar] [CrossRef]
- Belarbi, K.; Rosi, S. Modulation of adult-born neurons in the inflamed hippocampus. Front. Cell Neurosci. 2013, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.K.; White, C.W., 3rd; Villeda, S.A. The systemic environment: At the interface of aging and adult neurogenesis. Cell Tissue Res. 2018, 371, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Navarro Negredo, P.; Yeo, R.W.; Brunet, A. Aging and Rejuvenation of Neural Stem Cells and Their Niches. Cell Stem Cell 2020, 27, 202–223. [Google Scholar] [CrossRef]
- Aimone, J.B.; Li, Y.; Lee, S.W.; Clemenson, G.D.; Deng, W.; Gage, F.H. Regulation and function of adult neurogenesis: From genes to cognition. Physiol. Rev. 2014, 94, 991–1026. [Google Scholar] [CrossRef] [Green Version]
- Shohayeb, B.; Diab, M.; Ahmed, M.; Ng, D.C.H. Factors that influence adult neurogenesis as potential therapy. Transl. Neurodegener. 2018, 7, 4. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.; Patel, D.K.; et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/beta-catenin pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef]
- Palma, V.; Lim, D.A.; Dahmane, N.; Sanchez, P.; Brionne, T.C.; Herzberg, C.D.; Gitton, Y.; Carleton, A.; Alvarez-Buylla, A.; Ruiz i Altaba, A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 2005, 132, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Woodbury, M.E.; Ikezu, T. Fibroblast growth factor-2 signaling in neurogenesis and neurodegeneration. J. Neuroimmune Pharmacol. 2014, 9, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Faigle, R.; Song, H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta 2013, 1830, 2435–2448. [Google Scholar] [CrossRef] [Green Version]
- Choe, Y.; Pleasure, S.J.; Mira, H. Control of Adult Neurogenesis by Short-Range Morphogenic-Signaling Molecules. Cold Spring Harb. Perspect. Biol. 2015, 8, a018887. [Google Scholar] [CrossRef] [Green Version]
- Navarro Quiroz, E.; Navarro Quiroz, R.; Ahmad, M.; Gomez Escorcia, L.; Villarreal, J.L.; Fernandez Ponce, C.; Aroca Martinez, G. Cell Signaling in Neuronal Stem Cells. Cells 2018, 7, 75. [Google Scholar] [CrossRef] [Green Version]
- Meyers, E.A.; Gobeske, K.T.; Bond, A.M.; Jarrett, J.C.; Peng, C.Y.; Kessler, J.A. Increased bone morphogenetic protein signaling contributes to age-related declines in neurogenesis and cognition. Neurobiol. Aging 2016, 38, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Cole, A.E.; Murray, S.S.; Xiao, J. Bone Morphogenetic Protein 4 Signalling in Neural Stem and Progenitor Cells during Development and after Injury. Stem Cells Int. 2016, 2016, 9260592. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Huang, W.; Wang, Y.; Sun, W.; Tang, J.; Li, D.; Xu, P.; Guo, L.; Yin, Z.Q.; Fan, X. The function of BMP4 during neurogenesis in the adult hippocampus in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 157–164. [Google Scholar] [CrossRef]
- Bond, A.M.; Peng, C.Y.; Meyers, E.A.; McGuire, T.; Ewaleifoh, O.; Kessler, J.A. BMP signaling regulates the tempo of adult hippocampal progenitor maturation at multiple stages of the lineage. Stem Cells 2014, 32, 2201–2214. [Google Scholar] [CrossRef]
- Yousef, H.; Morgenthaler, A.; Schlesinger, C.; Bugaj, L.; Conboy, I.M.; Schaffer, D.V. Age-Associated Increase in BMP Signaling Inhibits Hippocampal Neurogenesis. Stem Cells 2015, 33, 1577–1588. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Song, M.; Wang, Y.; Fan, X.; Xu, H.; Bai, Y. Noggin and BMP4 co-modulate adult hippocampal neurogenesis in the APPswe/PS1ΔE9 transgenic mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2009, 385, 341–345. [Google Scholar] [CrossRef]
- Lim, D.A.; Tramontin, A.D.; Trevejo, J.M.; Herrera, D.G.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 2000, 28, 713–726. [Google Scholar] [CrossRef] [Green Version]
- Bonaguidi, M.A.; McGuire, T.; Hu, M.; Kan, L.; Samanta, J.; Kessler, J.A. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development 2005, 132, 5503–5514. [Google Scholar] [CrossRef] [Green Version]
- Mercier, F.; Douet, V. Bone morphogenetic protein-4 inhibits adult neurogenesis and is regulated by fractone-associated heparan sulfates in the subventricular zone. J. Chem. Neuroanat. 2014, 57–58, 54–61. [Google Scholar] [CrossRef]
- Colak, D.; Mori, T.; Brill, M.S.; Pfeifer, A.; Falk, S.; Deng, C.; Monteiro, R.; Mummery, C.; Sommer, L.; Gotz, M. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J. Neurosci. 2008, 28, 434–446. [Google Scholar] [CrossRef]
- Niola, F.; Zhao, X.; Singh, D.; Castano, A.; Sullivan, R.; Lauria, M.; Nam, H.S.; Zhuang, Y.; Benezra, R.; Di Bernardo, D.; et al. Id proteins synchronize stemness and anchorage to the niche of neural stem cells. Nat. Cell Biol. 2012, 14, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Blomfield, I.M.; Rocamonde, B.; Masdeu, M.D.M.; Mulugeta, E.; Vaga, S.; van den Berg, D.L.; Huillard, E.; Guillemot, F.; Urban, N. Id4 promotes the elimination of the pro-activation factor Ascl1 to maintain quiescence of adult hippocampal stem cells. Elife 2019, 8, e48561. [Google Scholar] [CrossRef]
- Chen, H.L.; Lein, P.J.; Wang, J.Y.; Gash, D.; Hoffer, B.J.; Chiang, Y.H. Expression of bone morphogenetic proteins in the brain during normal aging and in 6-hydroxydopamine-lesioned animals. Brain Res. 2003, 994, 81–90. [Google Scholar] [CrossRef]
- Mu, Y.; Lee, S.W.; Gage, F.H. Signaling in adult neurogenesis. Curr. Opin. Neurobiol. 2010, 20, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, X.; Yang, S.; Zhang, J. The Wnt/β-catenin signaling pathway in the adult neurogenesis. Eur. J. Neurosci. 2011, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Coskun, V.; Venkatraman, G.; Yang, H.; Rao, M.S.; Luskin, M.B. Retroviral manipulation of the expression of bone morphogenetic protein receptor Ia by SVZa progenitor cells leads to changes in their p19INK4d expression but not in their neuronal commitment. Int. J. Dev. Neurosci. 2001, 19, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Kiewitz, S.D.; Cabrele, C. Synthesis and conformational properties of protein fragments based on the Id family of DNA-binding and cell-differentiation inhibitors. Biopolymers 2005, 80, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Theil, T.; Aydin, S.; Koch, S.; Grotewold, L.; Ruther, U. Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development 2002, 129, 3045–3054. [Google Scholar] [CrossRef]
- Hussein, S.M.; Duff, E.K.; Sirard, C. Smad4 and beta-catenin co-activators functionally interact with lymphoid-enhancing factor to regulate graded expression of Msx2. J. Biol. Chem. 2003, 278, 48805–48814. [Google Scholar] [CrossRef] [Green Version]
- Armenteros, T.; Andreu, Z.; Hortiguela, R.; Lie, D.C.; Mira, H. BMP and WNT signalling cooperate through LEF1 in the neuronal specification of adult hippocampal neural stem and progenitor cells. Sci. Rep. 2018, 8, 9241. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Ure, K.; Ables, J.L.; Lagace, D.C.; Nave, K.A.; Goebbels, S.; Eisch, A.J.; Hsieh, J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat. Neurosci. 2009, 12, 1090–1092. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, T.; Hsieh, J.; Muotri, A.; Yeo, G.; Warashina, M.; Lie, D.C.; Moore, L.; Nakashima, K.; Asashima, M.; Gage, F.H. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci. 2009, 12, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.E.; Greenberg, M.E.; Stiles, C.D. Basic helix-loop-helix factors in cortical development. Neuron 2003, 39, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Boareto, M.; Iber, D.; Taylor, V. Differential interactions between Notch and ID factors control neurogenesis by modulating Hes factor autoregulation. Development 2017, 144, 3465–3474. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, V.; Cornelius, C.; Mancuso, C.; Pennisi, G.; Calafato, S.; Bellia, F.; Bates, T.E.; Giuffrida Stella, A.M.; Schapira, T.; Dinkova Kostova, A.T.; et al. Cellular stress response: A novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem. Res. 2008, 33, 2444–2471. [Google Scholar] [CrossRef]
- Sikder, H.A.; Devlin, M.K.; Dunlap, S.; Ryu, B.; Alani, R.M. Id proteins in cell growth and tumorigenesis. Cancer Cell 2003, 3, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Norton, J.D. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J. Cell Sci. 2000, 113 Pt 22, 3897–3905. [Google Scholar] [CrossRef]
- Roschger, C.; Cabrele, C. The Id-protein family in developmental and cancer-associated pathways. Cell Commun. Signal. 2017, 15, 7. [Google Scholar] [CrossRef] [Green Version]
- Asp, J.; Thornemo, M.; Inerot, S.; Lindahl, A. The helix-loop-helix transcription factors Id1 and Id3 have a functional role in control of cell division in human normal and neoplastic chondrocytes. FEBS Lett. 1998, 438, 85–90. [Google Scholar] [CrossRef] [Green Version]
| Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
| Id1 | TGAACGTCCTGCTCTACGAC | TTGCTCACTTTGCGGTTCTG |
| Id2 | GAAAGCCTTCAGTCCGGTGA | TGGTCCGACAGGCTGTTTTT |
| Id3 | GCCCGAGAGAAGGACTGAAC | CGACACCCCATTCTCGGAAA |
| Id4 | TCCCGCCCAACAAGAAAGTC | CTGTCTCAGCAAAGCAGGGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Liu, H.; Jiang, D.; Yang, K.; Shen, J.; Feng, H.; Wang, S.; Zhang, Y.; Wang, Y.; Tang, T.-S. BMP4 Exerts Anti-Neurogenic Effect via Inducing Id3 during Aging. Biomedicines 2022, 10, 1147. https://doi.org/10.3390/biomedicines10051147
Li T, Liu H, Jiang D, Yang K, Shen J, Feng H, Wang S, Zhang Y, Wang Y, Tang T-S. BMP4 Exerts Anti-Neurogenic Effect via Inducing Id3 during Aging. Biomedicines. 2022; 10(5):1147. https://doi.org/10.3390/biomedicines10051147
Chicago/Turabian StyleLi, Tingting, Hongmei Liu, Dongfang Jiang, Keyan Yang, Jiaqi Shen, Haiping Feng, Sijia Wang, Yuxin Zhang, Yun Wang, and Tie-Shan Tang. 2022. "BMP4 Exerts Anti-Neurogenic Effect via Inducing Id3 during Aging" Biomedicines 10, no. 5: 1147. https://doi.org/10.3390/biomedicines10051147
APA StyleLi, T., Liu, H., Jiang, D., Yang, K., Shen, J., Feng, H., Wang, S., Zhang, Y., Wang, Y., & Tang, T.-S. (2022). BMP4 Exerts Anti-Neurogenic Effect via Inducing Id3 during Aging. Biomedicines, 10(5), 1147. https://doi.org/10.3390/biomedicines10051147






