Brain-Derived Neurotropic Factor in Neurodegenerative Disorders
Abstract
:1. Introduction
2. BDNF Expression
3. Pathological Mechanism of Action
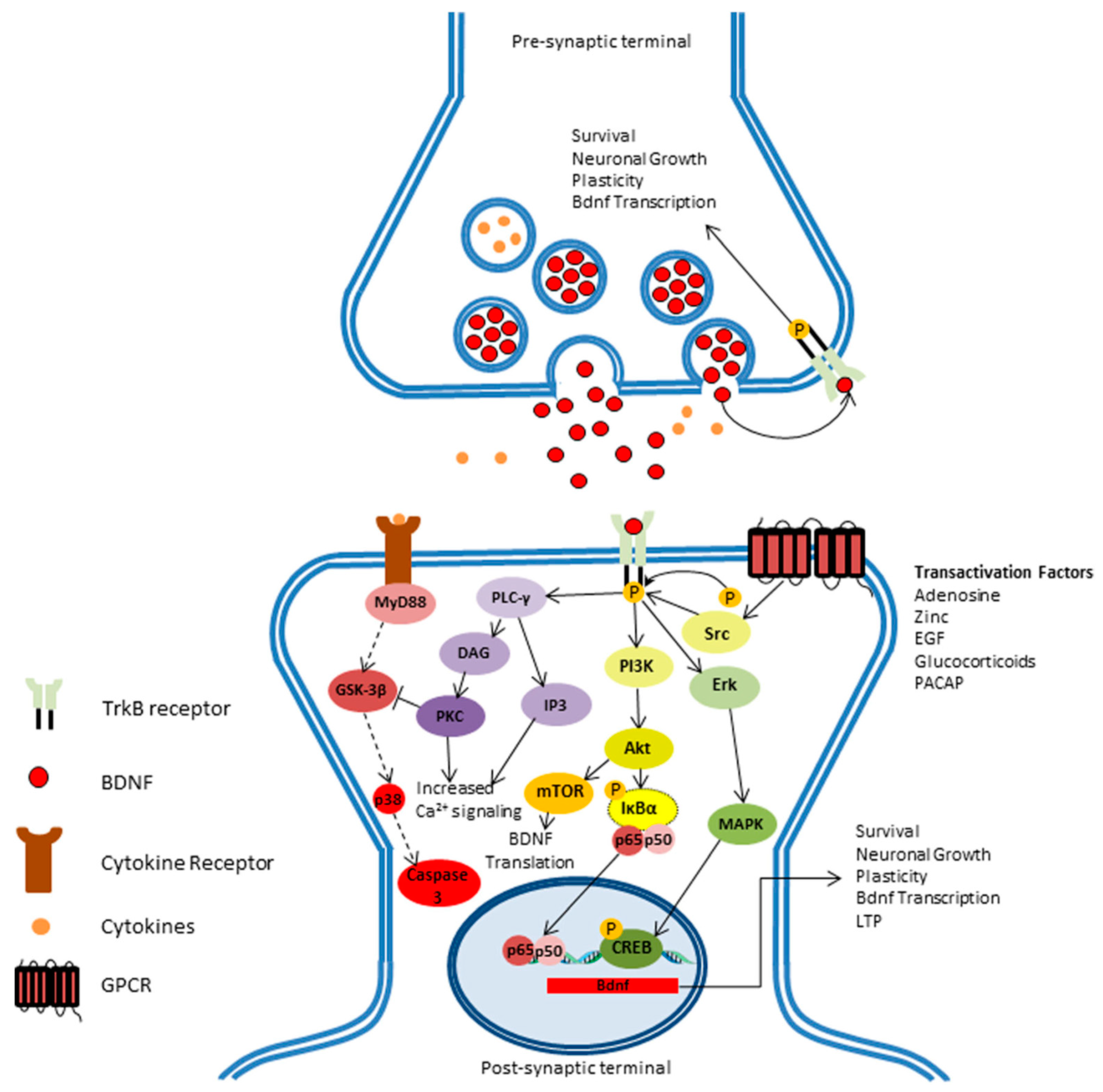
3.1. Activation of TrkB
3.2. Secondary Messengers Activation
3.3. Signaling Cascade in BDNF
3.4. Ras/MAPK/ERK Pathway
3.5. IRS-1/PI3K/AKT Pathway
3.6. PLC/DAG/IP3 Pathway
4. Functions of BDNF
4.1. BDNF and Aging
4.2. The Role of BDNF and Alzheimer’s Disease
4.3. The Role of BDNF in Parkinson’s Disease
4.4. Potential Biological Impact of BDNF Markers
5. Recent Advancements and Challenges
6. Conclusions
Funding
Conflicts of Interest
References
- Acheson, A.; Conover, J.C.; Fandl, J.P.; DeChiara, T.M.; Russell, M.; Thadani, A.; Squinto, S.P.; Yancopoulos, G.D.; Lindsay, R.M. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 1995, 374, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisonpierre, P.C.; Le Beau, M.M.; Espinosa, R., III; Ip, N.Y.; Belluscio, L.; Suzanne, M.; Squinto, S.; Furth, M.E.; Yancopoulos, G.D. Human and rat brain-derived neurotrophic factor and neurotrophin-3: Gene structures, distributions, and chromosomal localizations. Genomics 1991, 10, 558–568. [Google Scholar] [CrossRef]
- Zigova, T.; Pencea, V.; Wiegand, S.J.; Luskin, M.B. Intraventricular Administration of BDNF Increases the Number of Newly Generated Neurons in the Adult Olfactory Bulb. Mol. Cell. Neurosci. 1998, 11, 234–245. [Google Scholar] [CrossRef]
- Scalzo, P.; Kümmer, A.; Bretas, T.L.; Cardoso, F.; Teixeira, A.L. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J. Neurol. 2010, 257, 540–545. [Google Scholar] [CrossRef]
- Sohrabji, F.; Lewis, D.K. Estrogen–BDNF interactions: Implications for neurodegenerative diseases. Front. Neuroendocrinol. 2006, 27, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Mughal, M.R.; Baharani, A.; Chigurupati, S.; Son, T.G.; Chen, E.; Yang, P.; Okun, E.; Arumugam, T.; Chan, S.L.; Mattson, M.P. Electroconvulsive shock ameliorates disease processes and extends survival in huntingtin mutant mice. Hum. Mol. Genet. 2011, 20, 659–669. [Google Scholar] [CrossRef]
- Monteleone, P.; Serritella, C.; Martiadis, V.; Maj, M. Decreased levels of serum brain-derived neurotrophic factor in both depressed and euthymic patients with unipolar depression and in euthymic patients with bipolar I and II disorders. Bipolar Disord. 2008, 10, 95–100. [Google Scholar] [CrossRef]
- Gravesteijn, E. Effects of nutritional interventions on BDNF concentrations in humans: A systematic review. Nutr. Neurosci. 2021, 56, 3295–3312. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [Green Version]
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- McEachan, R.; Taylor, N.; Harrison, R.; Lawton, R.; Gardner, P.; Conner, M. Meta-Analysis of the Reasoned Action Approach (RAA) to Understanding Health Behaviors. Ann. Behav. Med. 2016, 50, 592–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, V.H.; Teeling, J. Microglia and macrophages of the central nervous system: The contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 2013, 35, 601–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyman, C.; Hofer, M.; Barde, Y.A.; Juhasz, M.; Yancopoulos, G.D.; Squinto, S.P.; Lindsay, R.M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 1991, 350, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Aid, T.; Kazantseva, A.; Piirsoo, M.; Palm, K.; Timmusk, T. Mouse and rat BDNF gene structure and expression revisited. J. Neurosci. Res. 2007, 85, 525–535. [Google Scholar] [CrossRef]
- Timmusk, T.; Palm, K.; Metsis, M.; Reintam, T.; Paalme, V.; Saarma, M.; Persson, H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron 1993, 10, 475–489. [Google Scholar] [CrossRef]
- Lauterborn, J.C.; Rivera, S.; Stinis, C.T.; Hayes, V.Y.; Isackson, P.J.; Gall, C.M. Differential Effects of Protein Synthesis Inhibition on the Activity-Dependent Expression of BDNF Transcripts: Evidence for Immediate-Early Gene Responses from Specific Promoters. J. Neurosci. 1996, 16, 7428–7436. [Google Scholar] [CrossRef] [Green Version]
- Keifer, J. Comparative Genomics of the BDNF Gene, Non-Canonical Modes of Transcriptional Regulation, and Neurological Disease. Mol. Neurobiol. 2021, 58, 2851–2861. [Google Scholar] [CrossRef]
- Tapia-Arancibia, L.; Aliaga, E.; Silhol, M.; Arancibia, S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res. Rev. 2008, 59, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Camuso, S. Pleiotropic effects of BDNF on the cerebellum and hippocampus: Implications for neurodevelopmental disorders. Neurobiol. Dis. 2022, 163, 105606. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Begni, V.; Pariante, C.M.; Riva, M.A. The human BDNF gene: Peripheral gene expression and protein levels as biomarkers for psychiatric disorders. Transl. Psychiatry 2016, 6, e958. [Google Scholar] [CrossRef]
- Kraemer, B.R.; Yoon, S.O.; Carter, B.D. The Biological Functions and Signaling Mechanisms of the p75 Neurotrophin Receptor. In Neurotrophic Factors; Lewin, G.R., Carter, B.D., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 121–164. [Google Scholar] [CrossRef]
- Barkat, M.A. Nanopaclitaxel therapy: An evidence based review on the battle for next-generation formulation challenges. Nanomedicine 2019, 14, 1323–1341. [Google Scholar]
- Minichiello, L. TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009, 10, 850–860. [Google Scholar] [CrossRef]
- Matsumoto, T.; Rauskolb, S.; Polack, M.; Klose, J.; Kolbeck, R.; Korte, M.; Barde, Y.A. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat. Neurosci. 2008, 11, 131–133. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Igaz, L.M.; Bevilaqua, L.R.; Izquierdo, I.; Medina, J.H. Persistence of long-term memory storage requires a late protein synthesis-and BDNF-dependent phase in the hippocampus. Neuron 2007, 53, 261–277. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Poo, M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Castrén, E.; Kojima, M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol. Dis. 2017, 97, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Kolbeck, R.; Jungbluth, S.; Barde, Y.A. Characterisation of Neurotrophin Dimers and Monomers. Eur. J. Biochem. 1994, 225, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.Y.; Jing, D.; Bath, K.G.; Ieraci, A.; Khan, T.; Siao, C.J.; Herrera, D.G.; Toth, M.; Yang, C.; McEwen, B.S.; et al. Genetic Variant BDNF (Val66Met) Polymorphism Alters Anxiety-Related Behavior. Science 2006, 314, 140–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninan, I.; Bath, K.G.; Dagar, K.; Perez-Castro, R.; Plummer, M.R.; Lee, F.S.; Chao, M.V. The BDNF Val66Met Polymorphism Impairs NMDA Receptor-Dependent Synaptic Plasticity in the Hippocampus. J. Neurosci. 2010, 30, 8866–8870. [Google Scholar] [CrossRef] [Green Version]
- Mizui, T.; Ishikawa, Y.; Kumanogoh, H.; Lume, M.; Matsumoto, T.; Hara, T.; Yamawaki, S.; Takahashi, M.; Shiosaka, S.; Itami, C.; et al. BDNF pro-peptide actions facilitate hippocampal LTD and are altered by the common BDNF polymorphism Val66Met. Proc. Natl. Acad. Sci. USA 2015, 112, E3067–E3074. [Google Scholar] [CrossRef] [Green Version]
- Akaneya, Y.; Tsumoto, T.; Kinoshita, S.; Hatanaka, H. Brain-Derived Neurotrophic Factor Enhances Long-Term Potentiation in Rat Visual Cortex. J. Neurosci. 1997, 17, 6707–6716. [Google Scholar] [CrossRef]
- Kang, H.; Schuman, E.M. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 1995, 267, 1658–1662. [Google Scholar] [CrossRef]
- Kang, H.; Welcher, A.A.; Shelton, D.; Schuman, E.M. Neurotrophins and Time: Different Roles for TrkB Signaling in Hippocampal Long-Term Potentiation. Neuron 1997, 19, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Patapoutian, A.; Reichardt, L.F. Trk receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef]
- Roback, J.D.; Marsh, H.N.; Downen, M.; Palfrey, H.C.; Wainer, B.H. BDNF-activated Sianal Transduction in Rat Cortical Glial Cells. Eur. J. Neurosci. 1995, 7, 849–862. [Google Scholar] [CrossRef]
- Fang, H.; Chartier, J.; Sodja, C.; Desbois, A.; Ribecco-Lutkiewicz, M.; Walker, P.R.; Sikorska, M. Transcriptional activation of the human brain-derived neurotrophic factor gene promoter III by dopamine signaling in NT2/N neurons. J. Biol. Chem. 2003, 278, 26401–26409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Podyma, B. Metabolic homeostasis via BDNF and its receptors. Trends Endocrinol. Metab. 2021, 32, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.P.; Barker, P.A. Neurotrophin signaling through the p75 neurotrophin receptor. Prog. Neurobiol. 2002, 67, 203–233. [Google Scholar] [CrossRef]
- Barnabé-Heider, F.; Miller, F.D. Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J. Neurosci. 2003, 23, 5149–5160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, M.P.; Maudsley, S.; Martin, B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004, 27, 589–594. [Google Scholar] [CrossRef]
- Rhee, S.G.; Bae, Y.S. Regulation of Phosphoinositide-specific Phospholipase C Isozymes. J. Biol. Chem. 1997, 272, 15045–15048. [Google Scholar] [CrossRef] [Green Version]
- Pezet, S.; Malcangio, M.; McMahon, S.B. BDNF: A neuromodulator in nociceptive pathways? Brain Res. Rev. 2002, 40, 240–249. [Google Scholar] [CrossRef]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Hansen, H.H.; Briem, T.; Dzietko, M.; Sifringer, M.; Voss, A.; Rzeski, W.; Zdzisinska, B.; Thor, F.; Heumann, R.; Stepulak, A.; et al. Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. Neurobiol. Dis. 2004, 16, 440–453. [Google Scholar] [CrossRef]
- Yuan, M. Recognition of the SARS-CoV-2 receptor binding domain by neutralizing antibodies. Biochem. Biophys. Res. Commun. 2021, 538, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, A.R.; Mazzoni, I.; Tudan, C.; Boudreau, M.; Kaplan, D.R.; Miller, F.D. Depolarization and Neurotrophins Converge on the Phosphatidylinositol 3-Kinase–Akt Pathway to Synergistically Regulate Neuronal Survival. J. Cell Biol. 1999, 146, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Grãos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.Z. Promotion on NLRC5 upregulating MHC-I expression by IFN-γ in MHC-I–deficient breast cancer cells. Immunol. Res. 2019, 67, 497–504. [Google Scholar] [CrossRef]
- Emamian, E.S.; Hall, D.; Birnbaum, M.J.; Karayiorgou, M.; Gogos, J.A. Convergent evidence for impaired AKT1-GSK3β signaling in schizophrenia. Nat. Genet. 2004, 36, 131–137. [Google Scholar] [CrossRef]
- Corbit, K.C.; Foster, D.A.; Rosner, M.R. Protein kinase Cδ mediates neurogenic but not mitogenic activation of mitogen-activated protein kinase in neuronal cells. Mol. Cell. Biol. 1999, 19, 4209–4218. [Google Scholar] [CrossRef] [Green Version]
- Barde, Y.A.; Edgar, D.; Thoenen, H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982, 1, 549–553. [Google Scholar] [CrossRef]
- Dimitropoulou, A.; Bixby, J.L. Regulation of retinal neurite growth by alterations in MAPK/ERK kinase (MEK) activity. Brain Res. 2000, 858, 205–214. [Google Scholar] [CrossRef]
- Cohen, S.; Levi-Montalcini, R.; Hamburger, V. A Nerve Growth-Stimulating Factor Isolated from Sarcomas 87 and 180. In The Saga of the Nerve Growth Factor: Preliminary Studies, Discovery, Further Development; World Scientific: Singapore, 1997; pp. 156–160. [Google Scholar]
- Soppet, D.; Escandon, E.; Maragos, J.; Middlemas, D.S.; Raid, S.W.; Blair, J.; Burton, L.E.; Stanton, B.R.; Kaplan, D.R.; Hunter, T.; et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 1991, 65, 895–903. [Google Scholar] [CrossRef]
- Ebadi, M.; Bashir, R.M.; Heidrick, M.L.; Hamada, F.M.; El Refaey, E.; Hamed, A.; Helal, G.; Baxi, M.D.; Cerutis, D.R.; Lassi, N.K. Neurotrophins and their receptors in nerve injury and repair. Neurochem. Int. 1997, 30, 347–374. [Google Scholar] [CrossRef]
- Eggert, S. Brothers in arms: proBDNF/BDNF and sAPPα/Aβ-signaling and their common interplay with ADAM10, TrkB, p75NTR, sortilin, and sorLA in the progression of Alzheimer’s disease. Biol. Chem. 2022, 403, 43–71. [Google Scholar] [CrossRef] [PubMed]
- Sendtner, M.; Holtmann, B.; Kolbeck, R.; Thoenen, H.; Barde, Y.A. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature 1992, 360, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.; Laurin, D.; Verreault, R.; Hébert, R.; Helliwell, B.; Hill, G.B.; McDowell, I. Risk Factors for Alzheimer’s Disease: A Prospective Analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikoletopoulou, V.; Lickert, H.; Frade, J.M.; Rencurel, C.; Giallonardo, P.; Zhang, L.; Bibel, M.; Barde, Y.A. Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature 2010, 467, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pintilie, S.R. Neuroprotective effects of physical exercise: Implications in health and disease. Rev. Med. Rom. 2021, 68, 383–389. [Google Scholar] [CrossRef]
- Pang, P.T.; Teng, H.K.; Zaitsev, E.; Woo, N.T.; Sakata, K.; Zhen, S.; Teng, K.K.; Yung, W.H.; Hempstead, B.L.; Lu, B. Cleavage of proBDNF by tPA/Plasmin Is Essential for Long-Term Hippocampal Plasticity. Science 2004, 306, 487–491. [Google Scholar] [CrossRef]
- Bamji, S.X.; Rico, B.; Kimes, N.; Reichardt, L.F. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin–β-catenin interactions. J. Cell Biol. 2006, 174, 289–299. [Google Scholar] [CrossRef]
- Lu, B.; Chow, A. Neurotrophins and hippocampal synaptic transmission and plasticity. J. Neurosci. Res. 1999, 58, 76–87. [Google Scholar] [CrossRef]
- Li, Y.; Yui, D.; Luikart, B.W.; McKay, R.M.; Li, Y.; Rubenstein, J.L.; Parada, L.F. Conditional ablation of brain-derived neurotrophic factor-TrkB signaling impairs striatal neuron development. Proc. Natl. Acad. Sci. USA 2012, 109, 15491–15496. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, L.R.; Charrin, B.C.; Borrell-Pagès, M.; Dompierre, J.P.; Rangone, H.; Cordelières, F.P.; De Mey, J.; MacDonald, M.E.; Leßmann, V.; Humbert, S.; et al. Huntingtin Controls Neurotrophic Support and Survival of Neurons by Enhancing BDNF Vesicular Transport along Microtubules. Cell 2004, 118, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Gurney, M.E. Human platelets contain brain-derived neurotrophic factor. J. Neurosci. 1990, 10, 3469–3478. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Greco, L.; Stipa, A.; Floridi, A.; Gallai, V. Brain-derived neurotrophic factor in patients with multiple sclerosis. J. Neuroimmunol. 2002, 132, 180–188. [Google Scholar] [CrossRef]
- Neves-Pereira, M.; Cheung, J.K.; Pasdar, A.; Zhang, F.; Breen, G.; Yates, P.; Sinclair, M.; Crombie, C.; Walker, N.; St Clair, D.M. BDNF gene is a risk factor for schizophrenia in a Scottish population. Mol. Psychiatry 2005, 10, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves-Pereira, M.; Mundo, E.; Muglia, P.; King, N.; Macciardi, F.; Kennedy, J.L. The Brain-Derived Neurotrophic Factor Gene Confers Susceptibility to Bipolar Disorder: Evidence from a Family-Based Association Study. Am. J. Hum. Genet. 2002, 71, 651–655. [Google Scholar] [CrossRef] [Green Version]
- Tacke, C.D.A.J. Role of a Novel TrkB Agonist Antibody in Positively Modulating the Architecture and Synaptic Plasticity of Hippocampal Neurons in Health and Disease. 2021. Available online: https://publikationsserver.tu-braunschweig.de/receive/dbbs_mods_00069356 (accessed on 1 January 2020).
- Katoh-Semba, R.; Takeuchi, I.K.; Semba, R.; Kato, K. Distribution of Brain-Derived Neurotrophic Factor in Rats and Its Changes with Development in the Brain. J. Neurochem. 1997, 69, 34–42. [Google Scholar] [CrossRef]
- Araki, T. The effects of microglia- and astrocyte-derived factors on neurogenesis in health and disease. Eur. J. Neurosci. 2021, 54, 5880–5901. [Google Scholar] [CrossRef]
- Eide, F.F.; Vining, E.R.; Eide, B.L.; Zang, K.; Wang, X.Y.; Reichardt, L.F. Naturally Occurring Truncated trkB Receptors Have Dominant Inhibitory Effects on Brain-Derived Neurotrophic Factor Signaling. J. Neurosci. 1996, 16, 3123–3129. [Google Scholar] [CrossRef]
- Schinder, A.F.; Poo, M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000, 23, 639–645. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C.M. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef] [Green Version]
- Hayek, L.E.; Khalifeh, M.; Zibara, V.; Assaad, R.A.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar]
- Cirulli, F.; Berry, A.; Chiarotti, F.; Alleva, E. Intrahippocampal administration of BDNF in adult rats affects short-term behavioral plasticity in the Morris water maze and performance in the elevated plus-maze. Hippocampus 2004, 14, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Bolton, M.M.; Pittman, A.J.; Lo, D.C. Brain-Derived Neurotrophic Factor Differentially Regulates Excitatory and Inhibitory Synaptic Transmission in Hippocampal Cultures. J. Neurosci. 2000, 20, 3221–3232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaaf, M.J.M.; Workel, J.O.; Lesscher, H.M.; Vreugdenhil, E.; Oitzl, M.S.; Ron de Kloet, E. Correlation between hippocampal BDNF mRNA expression and memory performance in senescent rats. Brain Res. 2001, 915, 227–233. [Google Scholar] [CrossRef]
- Guerra-Crespo, M.; Ubieta, R.; Joseph-Bravo, P.; Charli, J.L.; Pérez-Martínez, L. BDNF increases the early expression of TRH mRNA in fetal TrkB+ hypothalamic neurons in primary culture. Eur. J. Neurosci. 2001, 14, 483–494. [Google Scholar] [CrossRef]
- Marmigère, F.; Choby, C.; Rage, F.; Richard, S.; Tapia-Arancibia, L. Rapid Stimulatory Effects of Brain-Derived Neurotrophic Factor and Neurotrophin-3 on Somatostatin Release and Intracellular Calcium Rise in Primary Hypothalamic Cell Cultures. Neuroendocrinology 2001, 74, 43–54. [Google Scholar] [CrossRef]
- Givalois, L.; Arancibia, S.; Alonso, G.; Tapia-Arancibia, L. Expression of Brain-Derived Neurotrophic Factor and Its Receptors in the Median Eminence Cells with Sensitivity to Stress. Endocrinology 2004, 145, 4737–4747. [Google Scholar] [CrossRef] [Green Version]
- Crook, T.; Bartus, R.T.; Ferris, S.H.; Whitehouse, P.; Cohen, G.D.; Gershon, S. Age-associated memory impairment: Proposed diagnostic criteria and measures of clinical change—Report of a national institute of mental health work group. Dev. Neuropsychol. 1986, 2, 261–276. [Google Scholar] [CrossRef]
- Hebb, D.O. The Organization of Behavior: A Neuropsychological Theory; Psychology Press: London, UK, 2005; 379p. [Google Scholar]
- Thoenen, H. Neurotrophins and activity-dependent plasticity. In Progress in Brain Research; Neural Plasticity and Regeneration; Elsevier: Amsterdam, The Netherlands, 2000; Volume 128, pp. 183–191. Available online: http://www.sciencedirect.com/science/article/pii/S0079612300280163 (accessed on 12 January 2021).
- Burke, S.N.; Barnes, C.A. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef]
- Barnes, C.A. Normal aging: Regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994, 17, 13–18. [Google Scholar] [CrossRef]
- Gooney, M.; Messaoudi, E.; Maher, F.O.; Bramham, C.R.; Lynch, M.A. BDNF-induced LTP in dentate gyrus is impaired with age: Analysis of changes in cell signaling events. Neurobiol. Aging 2004, 25, 1323–1331. [Google Scholar] [CrossRef]
- Rex, C.S.; Lauterborn, J.C.; Lin, C.Y.; Kramár, E.A.; Rogers, G.A.; Gall, C.M.; Lynch, G. Restoration of Long-Term Potentiation in Middle-Aged Hippocampus After Induction of Brain-Derived Neurotrophic Factor. J. Neurophysiol. 2006, 96, 677–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, R.; Deadwyler, S.; Davis, M.; Moskovitz, B.; Kessler, M.; Rogers, G.; Lynch, G. Facilitation of glutamate receptors reverses an age-associated memory impairment in rats. Synapse 1996, 22, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Hattiangady, B.; Rao, M.S.; Shetty, G.A.; Shetty, A.K. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp. Neurol. 2005, 195, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Lommatzsch, M.; Zingler, D.; Schuhbaeck, K.; Schloetcke, K.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging 2005, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Mora, F.; Segovia, G.; del Arco, A. Aging, plasticity and environmental enrichment: Structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res. Rev. 2007, 55, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Silhol, M.; Arancibia, S.; Maurice, T.; Tapia-Arancibia, L. Spatial memory training modifies the expression of brain-derived neurotrophic factor tyrosine kinase receptors in young and aged rats. Neuroscience 2007, 146, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Marín, C.; Rey, M.J.; Ribalta, T.; Goutan, E.; Blanco, R.; Tolosa, E.; Martí, E. BDNF and Full-length and Truncated TrkB Expression in Alzheimer Disease. Implications in Therapeutic Strategies. J. Neuropathol. Exp. Neurol. 1999, 58, 729–739. [Google Scholar] [CrossRef]
- Peng, S.; Garzon, D.J.; Marchese, M.; Klein, W.; Ginsberg, S.D.; Francis, B.M.; Mount, H.T.; Mufson, E.J.; Salehi, A.; Fahnestock, M. Decreased Brain-Derived Neurotrophic Factor Depends on Amyloid Aggregation State in Transgenic Mouse Models of Alzheimer’s Disease. J. Neurosci. 2009, 29, 9321–9329. [Google Scholar] [CrossRef] [Green Version]
- Selkoe, D.J. Amyloid β-Protein and the Genetics of Alzheimer’s Disease. J. Biol. Chem. 1996, 271, 18295–18298. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.Z.; Ye, K. Deficiency in BDNF/TrkB Neurotrophic Activity Stimulates δ-Secretase by Upregulating C/EBPβ in Alzheimer’s Disease. Cell Rep. 2019, 28, 655–669.e5. [Google Scholar] [CrossRef] [Green Version]
- Rohe, M.; Synowitz, M.; Glass, R.; Paul, S.M.; Nykjaer, A.; Willnow, T.E. Brain-Derived Neurotrophic Factor Reduces Amyloidogenic Processing through Control of SORLA Gene Expression. J. Neurosci. 2009, 29, 15472–15478. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M. Emphasizing roles of BDNF promoters and inducers in Alzheimer’s disease for improving impaired cognition and memory. J. Basic Clin. Physiol. Pharmacol. 2022. [Google Scholar] [CrossRef]
- Matrone, C.; Ciotti, M.T.; Mercanti, D.; Marolda, R.; Calissano, P. NGF and BDNF signaling control amyloidogenic route and Aβ production in hippocampal neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 13139–13144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Numakawa, T.; Odaka, H. Brain-Derived Neurotrophic Factor Signaling in the Pathophysiology of Alzheimer’s Disease: Beneficial Effects of Flavonoids for Neuroprotection. Int. J. Mol. Sci. 2021, 22, 5719. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Malek-Ahmadi, M.H.; Alldred, M.J.; Chen, Y.; Chen, K.; Chao, M.V.; Counts, S.E.; Mufson, E.J. Brain-derived neurotrophic factor (BDNF) and TrkB hippocampal gene expression are putative predictors of neuritic plaque and neurofibrillary tangle pathology. Neurobiol. Dis. 2019, 132, 104540. [Google Scholar] [CrossRef]
- Lim, Y.Y. BDNF VAL66MET polymorphism and memory decline across the spectrum of Alzheimer’s disease. Genes Brain Behav. 2021, 20, e12724. [Google Scholar] [CrossRef]
- Kitiyanant, N.; Kitiyanant, Y.; Svendsen, C.N.; Thangnipon, W. BDNF-, IGF-1- and GDNF-Secreting Human Neural Progenitor Cells Rescue Amyloid β-Induced Toxicity in Cultured Rat Septal Neurons. Neurochem. Res. 2012, 37, 143–152. [Google Scholar] [CrossRef]
- Arancibia, S.; Silhol, M.; Moulière, F.; Meffre, J.; Höllinger, I.; Maurice, T.; Tapia-Arancibia, L. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol. Dis. 2008, 31, 316–326. [Google Scholar] [CrossRef]
- Wang, R.; Holsinger, R.M.D. Exercise-induced brain-derived neurotrophic factor expression: Therapeutic implications for Alzheimer’s dementia. Ageing Res. Rev. 2018, 48, 109–121. [Google Scholar] [CrossRef]
- Michalski, B.; Fahnestock, M. Pro-brain-derived neurotrophic factor is decreased in parietal cortex in Alzheimer’s disease. Mol. Brain Res. 2003, 111, 148–154. [Google Scholar] [CrossRef]
- Holsinger, R.M.D.; Schnarr, J.; Henry, P.; Castelo, V.T.; Fahnestock, M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: Decreased levels in Alzheimer’s disease. Mol. Brain Res. 2000, 76, 347–354. [Google Scholar] [CrossRef]
- Elahi, F.M.; Casaletto, K.B.; La Joie, R.; Walters, S.M.; Harvey, D.; Wolf, A.; Edwards, L.; Rivera-Contreras, W.; Karydas, A.; Cobigo, Y.; et al. Plasma Biomarkers of Astrocytic and Neuronal Dysfunction in Early- and Late-Onset Alzheimer’s Disease. Alzheimers Dement. 24 December 2019. Available online: https://www.sciencedirect.com/science/article/pii/S1552526019353737 (accessed on 28 March 2022).
- Ziegenhorn, A.A.; Schulte-Herbrüggen, O.; Danker-Hopfe, H.; Malbranc, M.; Hartung, H.D.; Anders, D.; Lang, U.E.; Steinhagen-Thiessen, E.; Schaub, R.T.; Hellweg, R. Serum neurotrophins—A study on the time course and influencing factors in a large old age sample. Neurobiol. Aging 2007, 28, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Laske, C.; Stransky, E.; Leyhe, T.; Eschweiler, G.W.; Wittorf, A.; Richartz, E.; Bartels, M.; Buchkremer, G.; Schott, K. Stage-dependent BDNF serum concentrations in Alzheimer’s disease. J. Neural Transm. 2006, 113, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Elkouzi, A.; Vedam-Mai, V.; Eisinger, R.S.; Okun, M.S. Emerging therapies in Parkinson disease—Repurposed drugs and new approaches. Nat. Rev. Neurol. 2019, 15, 204–223. [Google Scholar] [CrossRef] [PubMed]
- Parain, K.; Murer, M.G.; Yan, Q.; Faucheux, B.; Agid, Y.; Hirsch, E.; Raisman-Vozari, R. Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. Neuroreport 1999, 10, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y. Serum concentration and clinical significance of brain-derived neurotrophic factor in patients with Parkinson’s disease or essential tremor. J. Int. Med. Res. 2018, 46, 1477–1485. [Google Scholar] [CrossRef] [Green Version]
- Bruna, B.; Lobos, P.; Herrera-Molina, R.; Hidalgo, C.; Paula-Lima, A.; Adasme, T. The signaling pathways underlying BDNF-induced Nrf2 hippocampal nuclear translocation involve, R.O.S.; RyR-Mediated Ca2+ signals, ERK and PI3K. Biochem. Biophys. Res. Commun. 2018, 505, 201–207. [Google Scholar] [CrossRef]
- Ryu, E.J.; Harding, H.P.; Angelastro, J.M.; Vitolo, O.V.; Ron, D.; Greene, L.A. Endoplasmic Reticulum Stress and the Unfolded Protein Response in Cellular Models of Parkinson’s Disease. J. Neurosci. 2002, 22, 10690–10698. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, G.; Beiser, A.S.; Choi, S.H.; Preis, S.R.; Chen, T.C.; Vorgas, D.; Au, R.; Pikula, A.; Wolf, P.A.; DeStefano, A.L.; et al. Serum Brain-Derived Neurotrophic Factor and the Risk for Dementia: The Framingham Heart Study. JAMA Neurol. 2014, 71, 55–61. [Google Scholar] [CrossRef]
- Lotharius, J.; Brundin, P. Pathogenesis of parkinson’s disease: Dopamine, vesicles and α-synuclein. Nat. Rev. Neurosci. 2002, 3, 932–942. [Google Scholar] [CrossRef]
- Miller, K.M. Synucleinopathy-associated pathogenesis in Parkinson’s disease and the potential for brain-derived neurotrophic factor. NPJ Park. Dis. 2021, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kohno, R.; Sawada, H.; Kawamoto, Y.; Uemura, K.; Shibasaki, H.; Shimohama, S. BDNF is induced by wild-type α-synuclein but not by the two mutants, A30P or A53T, in glioma cell line. Biochem. Biophys. Res. Commun. 2004, 318, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli-Daley, L.A.; Gamble, K.L.; Schultheiss, C.E.; Riddle, D.M.; West, A.B.; Lee, V.M.Y. Formation of α-synuclein Lewy neurite–like aggregates in axons impedes the transport of distinct endosomes. Mol. Biol. Cell 2014, 25, 4010–4023. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Zhang, Z.; Liu, X.; Manfredsson, F.P.; Benskey, M.J.; Cao, X.; Xu, J.; Sun, Y.E.; Ye, K. TrkB neurotrophic activities are blocked by α-synuclein, triggering dopaminergic cell death in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 10773–10778. [Google Scholar] [CrossRef] [Green Version]
- Sortwell, C.E. BDNF rs6265 Genotype Influences Outcomes of Pharmacotherapy and Subthalamic Nucleus Deep Brain Stimulation in Parkinson’s Disease. Neuromodul. Malden Mass. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jin, W. Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease. J. Clin. Med. 2020, 9, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.M.; de Oliveira, G.L.; Fonseca, A.C.S.; de Lana, R.C.; Polese, J.C.; Pernambuco, A.P. Levels of cortisol and neurotrophic factor brain-derived in Parkinson’s disease. Neurosci. Lett. 2019, 708, 134359. [Google Scholar] [CrossRef]
- Roy, A.; Mondal, B.; Banerjee, R.; Choudhury, S.; Chatterjee, K.; Dey, S.; Kumar, H. Do peripheral immune and neurotrophic markers correlate with motor severity of Parkinson’s disease? J. Neuroimmunol. 2021, 354, 577545. [Google Scholar] [CrossRef]
- Hernández-Vara, J.; Sáez-Francàs, N.; Lorenzo-Bosquet, C.; Corominas-Roso, M.; Cuberas-Borròs, G.; Lucas-Del Pozo, S.; Carter, S.; Armengol-Bellapart, M.; Castell-Conesa, J. BDNF levels and nigrostriatal degeneration in “drug naïve” Parkinson’s disease patients. An “in vivo” study using I-123-FP-CIT SPECT. Parkinsonism Relat. Disord. 2020, 78, 31–35. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, C.; Yun, W. Peripheral BDNF/TrkB protein expression is decreased in Parkinson’s disease but not in Essential tremor. J. Clin. Neurosci. 2019, 63, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Wang, J. Brain-derived neurotrophic factor attenuates cognitive impairment and motor deficits in a mouse model of Parkinson’s disease. Brain Behav. 2021, 11, e2251. [Google Scholar] [CrossRef]
- Yoshida, T.; Ishikawa, M.; Iyo, M.; Hashimoto, K. Serum Levels of Mature Brain-Derived Neurotrophic Factor (BDNF) and Its Precursor proBDNF in Healthy Subjects. Open Clin. Chem. J. 2012, 5. Available online: https://benthamopen.com/ABSTRACT/TOCCHEMJ-5-7 (accessed on 7 January 2021). [CrossRef] [Green Version]
- Hashimoto, K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: Emerging links between cardiovascular disease and depression. Prog. Neurobiol. 2013, 100, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Miller, D.L.; Roecklein, K.A. The Aging Hippocampus: Interactions between Exercise, Depression, and BDNF. Neuroscientist 2012, 18, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Oliff, H.S.; Berchtold, N.C.; Isackson, P.; Cotman, C.W. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Mol. Brain Res. 1998, 61, 147–153. [Google Scholar] [CrossRef]
- Mori, Y. Serum BDNF as a Potential Biomarker of Alzheimer’s Disease: Verification Through Assessment of Serum, Cerebrospinal Fluid, and Medial Temporal Lobe Atrophy. Front. Neurol. 2021, 12, 511. [Google Scholar] [CrossRef]
- Karantali, E. Serum BDNF Levels in Acute Stroke: A Systematic Review and Meta-Analysis. Medicina 2021, 57, 297. [Google Scholar] [CrossRef]
- Nagahara, A.H.; Tuszynski, M.H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2011, 10, 209–219. [Google Scholar] [CrossRef]
- Kells, A.P.; Henry, R.A.; Connor, B. AAV–BDNF mediated attenuation of quinolinic acid-induced neuropathology and motor function impairment. Gene Ther. 2008, 15, 966–977. [Google Scholar] [CrossRef] [Green Version]
- Ankeny, D.P.; McTigue, D.M.; Guan, Z.; Yan, Q.; Kinstler, O.; Stokes, B.T.; Jakeman, L.B. Pegylated Brain-Derived Neurotrophic Factor Shows Improved Distribution into the Spinal Cord and Stimulates Locomotor Activity and Morphological Changes after Injury. Exp. Neurol. 2001, 170, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Ramirez, G.A.; Kuhn, H.G.; Peterson, D.A.; Day-Lollini, P.A.; Stewart, G.R.; Tuszynski, M.H.; Gage, F.H.; Thal, L.J. Reversible schwann cell hyperplasia and sprouting of sensory and sympathetic neurites after intraventricular administration of nerve growth factor. Ann. Neurol. 1997, 41, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Mucke, L.; Masliah, E.; Yu, G.Q.; Mallory, M.; Rockenstein, E.M.; Tatsuno, G.; Hu, K.; Kholodenko, D.; Johnson-Wood, K.; McConlogue, L. High-Level Neuronal Expression of Aβ1–42 in Wild-Type Human Amyloid Protein Precursor Transgenic Mice: Synaptotoxicity without Plaque Formation. J. Neurosci. 2000, 20, 4050–4058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutt, J.G.; Burchiel, K.J.; Comella, C.L.; Jankovic, J.; Lang, A.E.; Laws, E.R.; Lozano, A.M.; Penn, R.D.; Simpson, R.K.; Stacy, M.; et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 2003, 60, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Gill, S.; Patel, N.K.; Lozano, A.; Nutt, J.G.; Penn, R.; Brooks, D.J.; Hotton, G.; Moro, E.; Heywood, P.; et al. Randomized controlled trial of intraputamenal glial cell line–derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006, 59, 459–466. [Google Scholar] [CrossRef]
- Hovland, D.N.; Boyd, R.B.; Butt, M.T.; Engelhardt, J.A.; Moxness, M.S.; Ma, M.H.; Emery, M.G.; Ernst, N.B.; Reed, R.P.; Zeller, J.R.; et al. Reprint: Six-Month Continuous Intraputamenal Infusion Toxicity Study of Recombinant Methionyl Human Glial Cell Line-Derived Neurotrophic Factor (r-metHuGDNF) in Rhesus Monkeys. Toxicol. Pathol. 2007, 35, 1013–1029. [Google Scholar] [CrossRef]
- Gasmi, M.; Herzog, C.D.; Brandon, E.P.; Cunningham, J.J.; Ramirez, G.A.; Ketchum, E.T.; Bartus, R.T. Striatal Delivery of Neurturin by CERE-120, an AAV2 Vector for the Treatment of Dopaminergic Neuron Degeneration in Parkinson’s Disease. Mol. Ther. 2007, 15, 62–68. [Google Scholar] [CrossRef]
- Herzog, C.D.; Brown, L.; Gammon, D.; Kruegel, B.; Lin, R.; Wilson, A.; Bolton, A.; Printz, M.; Gasmi, M.; Bishop, K.M.; et al. Expression, bioactivity, and safety 1 year after adeno-associated viral vector type 2–mediated delivery of neurturin to the monkey nigrostriatal system support cere-120 for parkinson’s disease. Neurosurgery 2009, 64, 602–613. [Google Scholar] [CrossRef]
- Fukuchi, M.; Okuno, Y.; Nakayama, H.; Nakano, A.; Mori, H.; Mitazaki, S.; Nakano, Y.; Toume, K.; Jo, M.; Takasaki, I.; et al. Screening inducers of neuronal BDNF gene transcription using primary cortical cell cultures from BDNF-luciferase transgenic mice. Sci. Rep. 2019, 9, 11833. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef] [Green Version]
- Simmons, D.A.; Belichenko, N.P.; Yang, T.; Condon, C.; Monbureau, M.; Shamloo, M.; Jing, D.; Massa, S.M.; Longo, F.M. A Small Molecule TrkB Ligand Reduces Motor Impairment and Neuropathology in R6/2 and BACHD Mouse Models of Huntington’s Disease. J. Neurosci. 2013, 33, 18712–18727. [Google Scholar] [CrossRef] [PubMed]
- Diógenes, M.J.; Costenla, A.R.; Lopes, L.V.; Jerónimo-Santos, A.; Sousa, V.C.; Fontinha, B.M.; Ribeiro, J.A.; Sebastiao, A.M. Enhancement of LTP in Aged Rats is Dependent on Endogenous BDNF. Neuropsychopharmacology 2011, 36, 1823–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimes, M.L.; Zhou, J.; Beattie, E.C.; Yuen, E.C.; Hall, D.E.; Valletta, J.S.; Topp, K.S.; LaVail, J.H.; Bunnett, N.W.; Mobley, W.C. Endocytosis of Activated TrkA: Evidence that Nerve Growth Factor Induces Formation of Signaling Endosomes. J. Neurosci. 1996, 16, 7950–7964. [Google Scholar] [CrossRef] [PubMed]

| Study Objective | Sample Origin | BDNF Status | Assay Used | Conclusion | Ref. |
|---|---|---|---|---|---|
| To determine the stage in which BDNF reduced | Postmortem cortex | Declined | Western plot | The early stages were associated with decreased BDNF. | [103] |
| A meta-analysis to examine serum BDNF in patients with AD and mild cognitive impairment (MCI) compared to healthy controls | Peripheral serum of BDNF | Declined | NA | A systematic review and meta-analysis, comprising 15 studies, suggested that a significant decline in peripheral BDNF can only be detected in the late stages of Alzheimer’s disease. | [82] |
| To explain the selective vulnerability of certain neurons to AD | Postmortem cortex | Decreased | Western plot | Reduced BDNF may have a role in the selectivity in neuronal degeneration in AD | [115] |
| To confirm the relation between decreased BDNF and AD development | Postmortem cortex | Low BDNF mRNA | RT-PCR | A decrease in brain-derived neurotrophic factor synthesis could significantly affect hippocampal, cortical, and basal forebrain cholinergic neurons and may account for their selective vulnerability in Alzheimer’s disease. | [116] |
| Investigate plasma proteomic markers in early-onset versus late-onset AD | Plasma BDNF | Elevated | Ultra-sensitive immuno-based assay | BDNF levels were elevated in both early-onset and late-onset AD | [117] |
| Examination of BDNF serum level in elderly people | Serum samples | No significant change | ELISA | There was no association between gender, depression, and dementia on serum level of BDNF. | [118] |
| To assess BDNF serum and CSF concentrations in 30 patients at different stages of AD | Serum, CSF | Early stages increased BDNF serum, decreased level in late stage | ELISA | BDNF can be a good determinant in the assessment of the progression of AD. | [119] |
| Study Objective | Sample Origin | BDNF Status | Assay Used | Conclusion | Ref. |
|---|---|---|---|---|---|
| Investigating the effects of BDNF as a neuroprotective factor and as an adjunct therapy in PD | NA | Decreased | NA | In animal PD models, physical activity increased the levels of BDNF and TrkB, which acted as a neuroprotective factor and resulted in symptomatic improvement | [133] |
| Evaluate salivary cortisol and plasma BDNF levels in PD patients compared to healthy controls | Plasma BDNF | No significant difference in BDNF, but higher cortisol in PD | ELISA | PD patients were in the early stage of the disease, so BDNF is not a suitable biomarker for early cases of PD | [134] |
| Assess the association between neurotrophic changes and the clinical staging and motor severity of PD | Peripheral BDNF | Decreased | ELISA | A larger decrease in BDNF (and other immune markers) were associated with a higher severity of PD | [135] |
| Evaluate the levels of serum BDNF in recently diagnosed and untreated PD patients | Serum BDNF | Decreased | Sandwich ELISA | Serum BDNF levels were lower in recently diagnosed and untreated PD patients compared to healthy controls | [136] |
| To compare BDNF levels in PD, essential tremor (ET), and healthy controls | Peripheral blood lymphocytes | Decreased in PD | Western blot | BDNF levels were decreased in PD patients, but no significant difference in ET patients | [137] |
| Investigate the neuroprotective role of BDNF in PD mice | NA | NA | NA | Elevating BDNF levels reduced mitochondrial impairment via increasing electron transport chain (ETC) activity and alleviating dopaminergic loss in PD mice | [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.M.; Chauhan, L.; Bhardwaj, A.; Sharma, A.; Fayaz, F.; Kumar, B.; Alhashmi, M.; AlHajri, N.; Alam, M.S.; Pottoo, F.H. Brain-Derived Neurotropic Factor in Neurodegenerative Disorders. Biomedicines 2022, 10, 1143. https://doi.org/10.3390/biomedicines10051143
Ibrahim AM, Chauhan L, Bhardwaj A, Sharma A, Fayaz F, Kumar B, Alhashmi M, AlHajri N, Alam MS, Pottoo FH. Brain-Derived Neurotropic Factor in Neurodegenerative Disorders. Biomedicines. 2022; 10(5):1143. https://doi.org/10.3390/biomedicines10051143
Chicago/Turabian StyleIbrahim, Abdallah Mohammad, Lalita Chauhan, Aditi Bhardwaj, Anjali Sharma, Faizana Fayaz, Bhumika Kumar, Mohamed Alhashmi, Noora AlHajri, Md Sabir Alam, and Faheem Hyder Pottoo. 2022. "Brain-Derived Neurotropic Factor in Neurodegenerative Disorders" Biomedicines 10, no. 5: 1143. https://doi.org/10.3390/biomedicines10051143
APA StyleIbrahim, A. M., Chauhan, L., Bhardwaj, A., Sharma, A., Fayaz, F., Kumar, B., Alhashmi, M., AlHajri, N., Alam, M. S., & Pottoo, F. H. (2022). Brain-Derived Neurotropic Factor in Neurodegenerative Disorders. Biomedicines, 10(5), 1143. https://doi.org/10.3390/biomedicines10051143







